Abstract
Objectives
The incidence of patients with refractory angina (RA) is increasing. Medical therapy for RA is limited and prognosis is poor. Experimental data suggest that the use of Extracorporeal shockwave myocardial revascularization (ESMR) may contribute to angiogenesis and improve symptoms of angina in patients with RA. Purpose of our study is to determine the efficacy of cardiac shock wave therapy (ESMR) in the management of patients with nonrevascolarized coronary artery disease (CAD).
Methods
We performed a prospective cohort study to examine the efficacy of ESMR applcation in patients with RA despite optimal medical therapy, not suitable for further PCI or CABG. Characteristics such as angina class scores (CCS class score), nitroglycerin consumption and hospitalization rate among cases (patients with RA who received ESMR) and controls (patients with RA who did not receive ESMR) were compared at baseline and 6 months after ESMR therapy. In patients receiveing d ESMR the effect of on cardiac perfusion was assessed.
Results
There were 43 patients in the case group and 29 patients in the control group. The mean age of the patients was 70 ± 9.5 years in the case group and 71 ± 5.3 years in the control group. Other characteristics (diabetes, coronary artery bypass graft, percutaneus coronary intervention, baseline CCS class score) were similar in both groups. There was a significant improvement in CCS class score (1.33 ± 0.57 in cases and 1.92 ± 0.69 in controls; p = 0.0002), nitroglycerin consumption (20% in case cases, and 44.8% in controls; P < 0.03) and hospitalization rate significantly reduced (13.9% in case cases, and 37.9% in controls; P < 0.03). The patients who received ESMR, there was a significantly improvement in myocardial perfusion after 6 months with a 33% relative reduction of summed stress score (SSS) (p = 0.002).
Conclusion
This case control study demonstrates the beneficial effect of ESMR therapy on cardiac symptoms, myocardial perfusion and reduced hospitalization in patients with refractory angina. Ther current study supports a role for ESMR as a non-invasive therapuetic option for patients with RA.
Keywords: Refractory angina, Shock wave therapy, Echocardiography
1. Introduction
Refractory angina in patients without revascularization options (RA) is a growing clinical concern,. The prognosis and the quality of life in these patients is reduced and conventional medical therapy is frequently inadeguate for symptom relief. According to the last ACC/AHA guidelines on the management of chronic stable angina, the goal of the treatment should be the elimination of chest pain reduce hospitatlizations, cost and the restoration of normal activities [1]. Despite the continued growth in myocardial revascularization most patients continue to require antianginal drugs; furthermore, despite both revascularization and optimal pharmacotherapy, up to 26% of patients still experience limiting symptoms [2–5].
Previous studies showed other modalities of treatment such as transmyocardial laser revascularization during CABG with proved reduction of symptoms in patients with diffuse coronary disease not amenable to conventional revascularization. Newer approaches such as gene therapy promoting angiogenesis and stem cell transplantation so far failed or are still at in a pre-clinical stage and are invasive in nature [6–9].
Shockwave therapy has been used in the last decades in other medical fields such urology and in the treatment of several orthopedic settings [10,11]. Extracorporeal Shockwave Myocardial Revascularization therapy (ESMR) is a new non-invasive treatment that may improve myocardial perfusion and reduce symptoms of myocardial ischemia, through the application of shockwaves (SW), i.e. special acoustic waves that can be targeted and focused on a selected area of the heart under echocardiographic guidance [12].
High-energy extracorporeal shock-wave therapy (ESWT) was introduced for medical use 30 years ago as a treatment for ureteral stones [13]. ESWT has changed the treatment of urolithiasis, and even today remains the primary treatment for most uncomplicated upper urinary tract calculi [14]. The ‘destroyer-use’ of high-energy SWs is different from the more recent ‘regenerative-use’ of low-energy SWs. Low-energy ESWT has been developed as a treatment standard or alternative therapy for a variety of orthopaedic and soft tissue diseases [15,16]. The observed immediate increase in blood flow due to local vasodilation and the formation of new capillaries in the treated tissue [17,18] has led to one of its more promising application in cardiovascular medicine as a possible therapy for patients with refractory angina. Shock waves consist of acoustic energy that can be transmitted in a liquid medium and focused with precision of several millimeters to any intended treatment area inside the body. The energy density describes the maximum amount of acoustical energy which is transmitted per pulse, and varies among different uses of shock waves, from 0.09 mJ/mm2 in cardiology up to 0.9–1.8 mJ/mm2 in lithotripsy.
Shock waves can be artificially generated by discharge of a high voltage spark under water. Cardiac shock wave therapy (ESMR) is performed using a generator system designed to address the clinical anatomical requirements of the chest cavity. A cardiac ultrasound imaging system is used to locate the treatment area with documented ischemia. Shock waves are then delivered via a special applicator through the anatomical acoustic window to the treatment area under electrocardiographic R-wave gating. Several treatment sessions are required. At each session, shock waves are delivered to the border of the ischemic area, to potentially induce neovascularization from the healthy area to the ischemic area.
The precise mechanisms remain to be elucidated, two major effects may contribute to the previous observations: immediate vasodilatation, and the induction of neovascularization in the treated tissue, which most likely accounts for the observed longterm effects. It has been described that shock wave may induce tissue cavitation, generating highly localized physical forces which could produce localized stress on cell membranes. This would lead to a variety of biochemical effects including: shear stress on cell membranes [19], hyperpolarization and Ras activation [20], an increase in nitric oxide synthesis [21–23], an up-regulation of VEGF, its receptor Flt-1 and PGF [24–26], in addition to an enhance expression of stromal-derived factor-1 [27]. Another potential cellular mechanism may involve the recruitment of progenitor cells to the site of the ischemia undergoing ESMR [28–29]. Thus, we can conclude that there are probably multiples angiogenic pathways involved in the beneficial effects of ESMR.
The purpose of our study is to determine the efficacy of cardiac shock wave therapy as an adjunct therapy in the management of patients with refractory angina compared to standard therapy.
Our study can be considered an extension of a previous multicenter study performed by the group at the Mayo Clinic, in which, however, there was no control group [30].
2. Methods
The study was approved by the institutional review board, and informed consent was obtained from all participants prior to inclusion.
2.1. Patient selection
Entry criteria included age > 18 years, documentated history of CAD with at least three months of refractory angina, coronary disease not amenable for revascularization, as was determined by an interventional cardioogust and cardiac surgeon and stable maximal medical therapy for at least 6 weeks.
Patients were excluded if they had a history of myocardial infarction or unstable angina within previous 3 months, active acute myocarditis, periarditis, or left ventricular thrombus, significant valve disease, cardiac malignanancyies, chronic pulmonary disease (included emphysema and pulmonary fibrosis), endocarditis, and pregnancy.
The options were discussed with each patient in order to exclude any options of revascularization and to reject patients with a non-adequate chest acoustic window.
72 patients were included in the prospective study, 43 patients in the treatment group; patients who received ESMR and 29 patients in the controls group, patients who did not receive ESMR. Clinical and demographic characteristics such as angina class scores (CCS class score), nitroglycerin consumption (consuming nitrates occasionally as oral spray or sublingual tablets) and hospitalization rate (presentation to the emergency room or hospital admissions) among cases and controls were recorded and compared at baseline and 6 months after ESMR therapy between the groups. In all 72 patients we also evaluated the improvement in cardiac perfusion as the reduction in single-photon emission computed tomography (SPECT) summed stress score (SSS) and summed rest score (SRS) at baseline. In patients who received shock wave therapy we also evaluated the improvement in cardiac perfusion (SPECT) summed stress score (SSS) and summed rest score (SRS) at 6 months. The control group was composed of patients who met the inlusin/excusion cretiria but for for different reasons could not undergo the treatment (poor acoustic window, declined to participate, and 2 patiens with absence of significant inducible ischemia at SPECT). The two patients with myocardial ischemia are not significant, however, were included in the control group because they still had a small positive scintigraphy; we decided not to treat them because the territory was not extended to at least two myocardial segments.
This study is not randomized, but case–control study. We followed the patients treated and those who were excluded from treatment, to see if there were any differences in follow-up between the two groups. The two groups (although numerically different) were, however, uniform.
2.2. Single-photon emission computed tomography (SPECT)
All patients underwent a single-photon emission computed tomography (SPECT) at the start of treatment and the 43 patients treated with shock wave therapy after 6 months. The 29 patients who did not receive the shock waves not performed SPECT control after 6 months for ethical questions. All images were acquired and processed according to the American Society of Nuclear Cardiology guidelines using either single-day or 2-day (depending on body weight) stress/rest–technetium-99 m sestamibi. Imaging was started 60 min after the tracer injection at rest or exercise. Identical doses of the tracer were used for the baseline and follow-up studies. All raw data of gated SPECT images (2 sets per patient before and 2 sets for patient after 6 months) were reconstructed using standard back-projection and identical filtering (Butterworth filter with a critical frequency of 0.4 cycles/s, order 5). Motion correction was applied before image reconstruction if > 1 pixel of x or y axis deviation was observed over the 180° acquisition. No attenuation correction was used.
Quantitative SPECT was performed using a previously validated automated program that determines the extent and severity of LV perfusion defect size and the extent of reversible (ischemia) or fixed (scar) perfusion defects. Severity of ischemia was calculated as the percentage of count improvement toward normal values within ischemic pixels and defined as minimal (0–25% improvement), moderate (2–50% improvement), or marked (> 50% improvement).
In addition, the automated program was used to derive the summed stress score, summed rest score, and summed difference score (SDS) based on conventional 17-segment model. The program assigned a score of 0 to 5 to each segment based on activity level: 0 = normal and 5 = absent. All data reported here are based on automated analysis of the data. In addition to perfusion data, the LV ejection fraction, end-diastolic volume, and end-systolic volume were measured from the gated SPECT as previously described.
At the conclusion of the protocol, the images were again interpreted side by side in a blinded fashion by 1 reader to qualitatively grade the perfusion pattern as improved, worsened, or no change.
2.3. The cardiac shock wave therapy (CSWT): treatment protocol
The CSWT were applicated with a commercially available cardiac shock wave generator system (Cardiospect™, Medispec, Germantown, MD) under echocardiographic guidance. The inizial step of ESMR is to locate the ischemic region of interest after which, a full cardiac cycle is recorded with echocardioraphiy system. These measurements are calibrated into the shockwave applicator head to ensure the position of the focal treatment zone on the ischemic zone and shockwaves were then applied.
The ischemic area of interest was divided into 3 zones, corresponding to the three weeks of treatments. The treatment was divided into three sessions with 3 treatments for week every 4 weeks. Each treatment for each target spot consisted of 100 pulses gated by R wave trigger. Up to 10 target spots (total of 1000 pulses) were treated at each individual session. We applied a low energy of shockwaves (0.09 mJ/mm2, ≈ 10% of the energy for the lithotripsy treatment. Each session lasted about 20 minutes.
During the treatment symptoms and vital signs were continously monitored.
2.4. Endpoints
The endpoints was to examine the effects of ESMR application:
Changes in cardiac perfusion at 6 months from the treatment (in patients who received ESMR)
Evaluation angina class scores (CCS class score), nitroglycerin consumption and hospitalization rate among cases (patients with RA who received ESMR) and controls (patients with RA who did not receive ESMR) at 6 months after shock wave therapy,
2.5. Statistical analysis
Data are expressed as percentages for discrete variables and mean ± SD for continuous variables. Variables with normal distribution have been analyzed using parametric tests while variables with a non-normal distribution have been analyzed using non-parametric tests; categorical variables and proportions have been compared with the chi-square test; p values <0.05 are considered statistically significant. Comparisons have been performed between the baseline and follow up. All the statistical analysis have been performed using MedCalc Software for Windows, version 11.2.1.0 (Mariakerke, Belgium).
3. Results
We screened 78 patients for 72 potential candidates; (3 did not have a follow-up study, 1 died before starting treatment, 2 patients died, the first secondary to septic shock 3 months after the treatment and the second one died of sudden death 5 month after the treatment). The treatment was well tolerated; no patient complained for any discomfort, neither any adverse effects were recorded and none of the patients needed to discontinue the therapy secondary to side effects.
There were 43 patients in the treatment group and 29 patients in the control group. The mean age of the patients was 70 ± 9.5 years in the case group and 71 ± 5.3 years in the control group. Other characteristics (diabetes, coronary artery bypass graft, percutaneus coronary intervention, baseline CCS class score) were similar in both groups. The baseline characteristics of two groups are displayed in Table 1.
Table 1.
Clinical characteristics of the patients population (n = 72).
| Cases group (n 43) | Control group (n 29) | p | |
|---|---|---|---|
| Mean age (years) | 70 ± 5.3 | 71 ± 5.3 | 0.4 |
| M/F | 36/9 (83.7%) | 24/5 (79%) | 0.9 |
| Hypertension | 43 (100%) | 29 (100%) | 1 |
| Diabetes mellitus | 14 (32.5%) | 8 (27%) | 0.65 |
| Hyperlipidemia | 41 (95.3%) | 28 (96%) | 0.8 |
| Previous STEMI | 22 (51.1%) | 11 (38%) | 0.26 |
| Previous NSTEMI | 16 (37.2%) | 12 (41%) | 0.7 |
| Previuous PCI | 38 (88.4%) | 21 (72%) | 0.08 |
| Prevoius CABG | 21 (48.8%) | 9 (31%) | 0.13 |
| Previous stroke | 3 (7%) | 1 (3%) | 0.5 |
| Beta blockers | 39 (90%) | 26 (89%) | 0.8 |
| Clopidogrel | 18 (41.8%) | 11 (37%) | 0.7 |
| ASA | 40 (93%) | 28 (96%) | 0.5 |
| Statins | 39 (90%) | 27 (93%) | 0.7 |
| chronic therapy with nitrates | 31 (72%) | 20 (69%) | 0.7 |
| Ranolazine | 11 (25.8%) | 8 (27%) | 0.8 |
| Mean CCS class Score | 2.67 ± 0.75 | 2.52 ± 0.78 | 0.41 |
| Mean NYHA score | 2.51 ± 0.74 | 2.32 ± 0.79 | 0.3 |
| LV ejection fraction (%) by echocardiography | 56.40 ± 10.3 | 57.3 ± 9.6 | 0.7 |
| Nitrates up-take | 26 (60.5%) | 18 (41%) | 0.8 |
| Previous Hospitalization | 14 (32.5%) | 9 (31%) | 0.8 |
ASA = acetil-salicilic acid, PCI = Percutaneous coronary intervention, CABG = coronary artery bypass graft, Nitrates ut-take = use of nitrates for angina attacks.
Clinical follow up of both group demonstrated a significant improvement CCS class score at six months (1.33 ± 0.57 in treatment and 1.92 ± 0.69 in controls; p < 0.0002); a significant improvement NYHA class score (1.23 ± 0.42 in cases and 1.73 ± 0.59 in controls; p < 0.0001) also nitroglycerin consumption (20% in case cases, and 44.8% in controls; P < 0.03) and hospitalization rate significantly reduced I in the treatment group compoared to control (13.9% vs.37.9%; P < 0.03) (Table 2, Fig. 1).
Table 2.
Clinical outcome of the two study groups at 6 months follow up (n = 72).
| Treatment group (n = 43) | Control group (n = 29) | P | |
|---|---|---|---|
| CCS score | 1.33 ± 0.57 | 1.92 ± 0.69 | 0.0002 |
| NYHA score | 1.23 ± 0.42 | 1.73 ± 0.59 | 0.0001 |
| Nitrates uptake | 9 (20%) | 13 (44.8%) | 0.03 |
| Hospitalization | 6 (13.9%) | 11 (37.9%) | 0.01 |
Fig. 1.
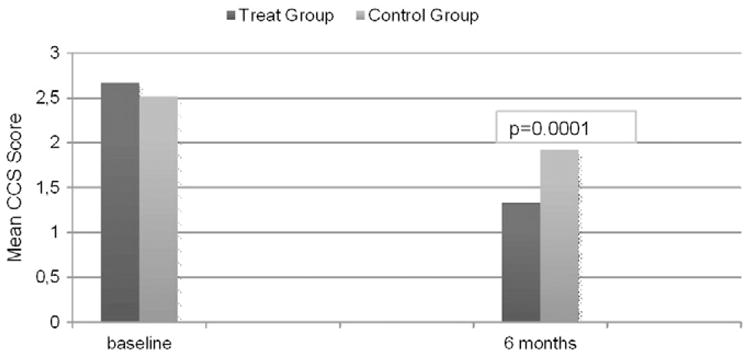
Clinical outcome CCS Class Score of the two study groups at 6 months follow up.
In the group of patients who received ESMR 60.5% used nitrates occasionally as oral spray or sublingual tablets because of unexpected symptoms (a mean of 7 ± 3 nitroglycerin intakes/month) before treatment. That proportion decreased in the the first month following treatment, to only 16.2% patients still consumed occasionally these drugs at the end of the follow-up to one year (p = 0.02) (Fig. 2).
Fig. 2.
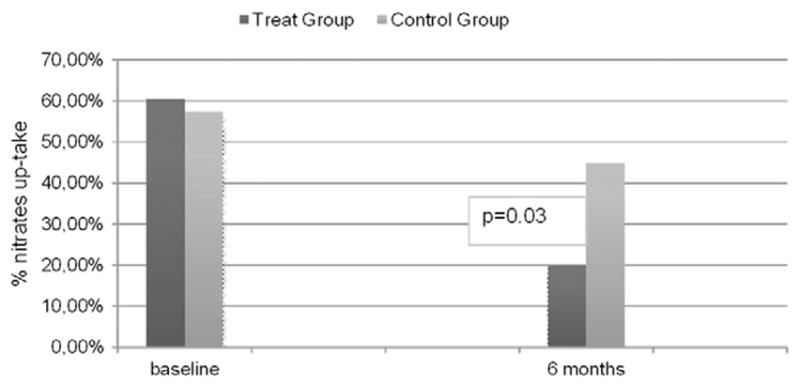
Clinical outcome nitrates up-take of the two study groups at 6 months follow up.
The reduction of angina severity was associated with a reduction in the hospitalization rates after the treatment. 32.5% of patients were referring to the medical emergency services in the 3 months previuous to the ESMR while only 11.6% needed to be hospitalized because of anginal symptoms in the following 3 months (p = 0.001, Fig. 3). The cumulative hospitalization rate at the end of the 1 year follow up was only 13.9%.
Fig. 3.
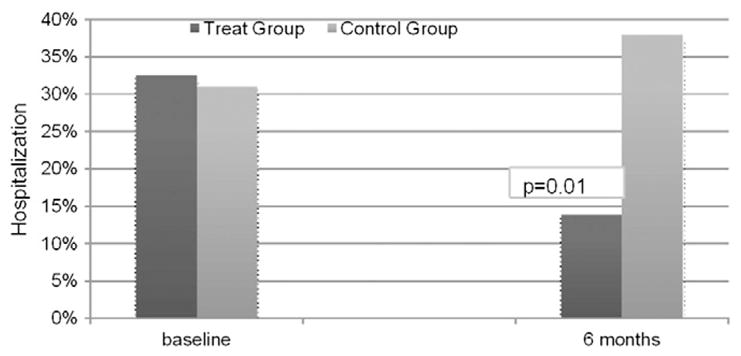
Clinical outcome hospitalization of the two study groups at 6 months follow up.
3.1. Evaluation myocardial perfusion by SPECT
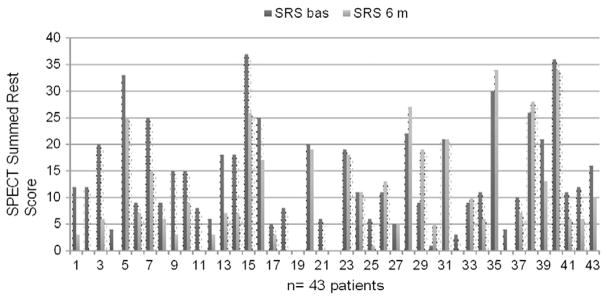
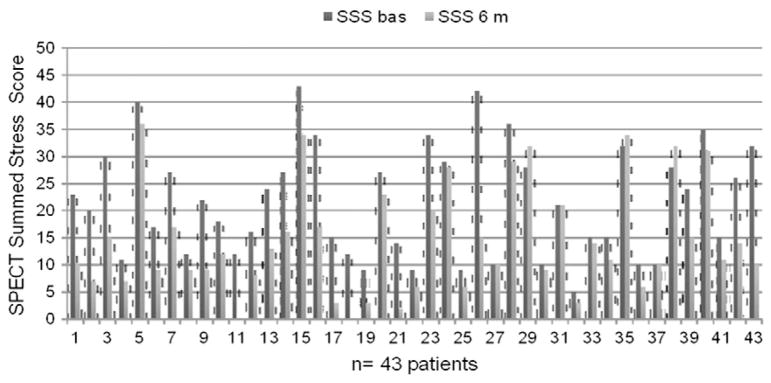
43 patients underwent SPECT at 6 months. SPECT performed before the ESMR treatment highlighted 3 to 9 ischemic segments per patient (mean 5.02 ± 1.8 segments/patient). The SPECT study performed 6 months after the ESMR treatment demonstrated a statistically significant improvement of myocardial perfusion: mean SSS was reduced from 21.2 ± 10.3 to 14.05 ± 10.05, with a 33% relative reduction (p = 0.003), and mean SRS score was reduced from 13.4 ± 9.28 to 9.5 ± 9.48 with a 29% relative reduction (p = 0.06) (Table 3). The reductions of SSS and SRS were consistent in all the treated patients (Figs. 4,5 and 6).
Table 3.
SPECT results at 6 months (n = 43).
| Baseline (n = 43) | After treatment (n = 43) | P | |
|---|---|---|---|
| Summed Stress Score | 21.2 ± 10.3 | 14.3 ± 10 | 0.002 |
| Summed Rest Score | 13.4 ± 9.28 | 10 ± 9.8 | 0.1 |
| LV ejection fraction (%) by Rest SPECT | 0.56 ± 0.09 | 0.55 ± 0.09 | ns |
Fig. 4.
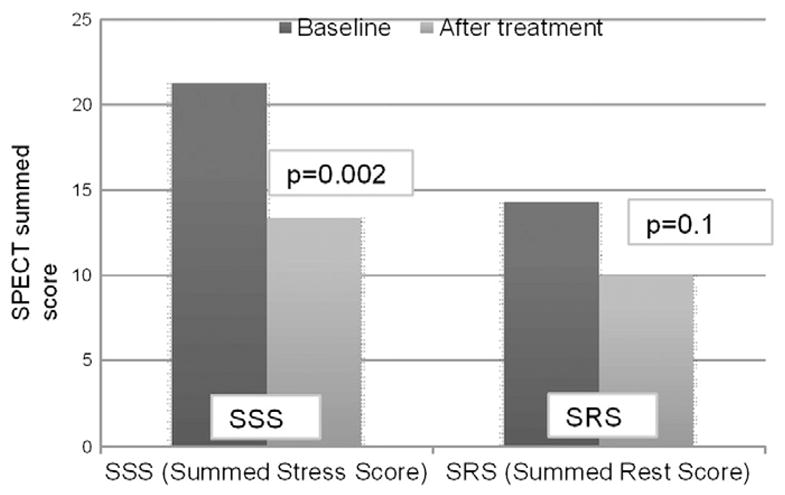
Evaluation myocardial perfusion by SPECT before and after the treatment.
Fig. 5.
Evaluation myocardial perfusion by SPECT (Summed Rest Score) before and after the treatment to single patients.
Fig. 6.
Evaluation myocardial perfusion by SPECT (Summed Stress Score) before and after the treatment to single patients.
4. Discussion
The current study demonstrates the ESMR significantly reduced symptoms in association with improvement in myocardial perfusion and reduced hospitalization in patients with refractory angina not amendable for revascularization. Their current study strongly suggest that ESMR is an adjuvant non-invasive therapy for patients with refractory angina.
The current management of chronic coronary artery disease (CAD) includes medical treatment, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). The improvement in pharmacological therapies and revascularization techniques has led to an increase in life expectancy of patients suffering from CAD. Nevertheless also the number of patients suffering from chronic CAD not amenable to further revascularization is growing worldwide.
The findings of our study extend and are in accord with data presented in previous reports, which demonstrated that low energy shock wave improve myocardial vascularization in ischemic settings and ameliorates anginal symptoms (30, 31, 32, 33, 34). Moreover, the current demonstrated the beneficial effect compared to a well matched control group.
The mechanism by which shock wave therapy may exert its effect is multifactorial. A number of studies have revealed that extracorporeal shock wave produces a series of biological changes. It enhances vascular endothelial growth factor (VEGF) mRNA expression as well as promotes bone marrow cells differentiation into cells with endothelial phenotype [35]. Hence, by applying appropriate energy to ischemic tissues, ESMR can attenuate inflammatory response and induce angiogenesis [36]. In the myocardium, the ESMR increases endothelial progenitor cells (EPC) concentrations and EPC homing-related chemokines in LV ischemic areas, reduces inflammatory response, oxidative stress, cellular apoptosis and fibrotic change [37].
There is a growing evidence that this treatment may promote angiogenesis, and imaging studies demonstrated increased myocardial blood flow in ischemic myocardium [31,38,39]. In vitro and in animal studies showed that ESMR, at 10% of the energy needed for lithotripsy, promote angiogenesis and improve myocardial function without any adverse effect in a model of chronic myocardial ischemia [40]. Another study suggested that shock wave therapy improves LV remodeling after acute myocardial infarction in pigs [39]. Angiogenic factors (such as vascular endothelial growth factor – VEGF – and its receptors, Flt-1, PlGF) are overexpressed, endogenous angiogenic pathways are upregulated, and the vascular density increases in the treated areas [40,41]. Furthermore, the significantly reduced oxidative stress and the suppression of CD40-positive cells in myocardium after ESMR imply significant roles of ESMR in attenuating inflammation and suppressing oxidative stress in ischemic myocardium [37]. These effects may contribute to the improvement in LV function and significantly reduced symptoms of ischemic heart failure [42–44]. Thus, in patients with non revascularized CAD, neoangiogenesis may be one of the mechanism by which ESMR improve myocardial vascularization and reduce ischemia.
The current study extends previous smaller clinical trials and showed that ESMR therapy is an effective and safe option for patients with refractory angina pectoris and improves exercise capacity and reduces nitroglycerin use in patients with end-stage ischemic heart disease with no procedural complications or side effects [30,33,34,37–39]. The clinical improvement of myocardial ischemia correlates with the SPECT finding that shows a significantly improved myocardial perfusion after the ESMR. The correlation between improvement of perfusion and clinical improvement seems to overcome doubts about a possible placebo effect of the ESMR. To our knowledge, this is the first report that directly evaluates the direct effects on local perfusion, through the objective analysis of SPECT images in association with symptoms and reduction of hospitalization and potentially reduction of cost of care.
Most of the benefits of the treatment can be evidenced as early the first month, probably suggesting an early local vasodilatatory effect produced by acoustic waves; those beneficial effects persist during the follow-up period for 1 year, demonstrating that there is also a long term effect, probably related to neoangiogenesis.
The current study also confirms that ESMR is a safe procedure. None of patients suffered from procedural complications, arrhythmias, pericardial disease, skin damage or patient discomfort.
Spinal cord stimulation (SCS) had been suggested as an alternative treatment for these patients [1,45]. However recent randomized study failed to show a beneficial effect of SCS on symptoms as well as functional capacity [30,33]. Moreover SCS is an invasive strategy that can carry some complications such as infections or lead migration or fracture [46].
The short and long term effect of shock wave therapy should be considered also for other cardiac indications, such as post myocardial infarction remodeling, improving the ventricular function in heart failure, silent ischemia, reperfusion injury and to enhance stem cell recruitment to the ischemic myocardium [47].
4.1. Limitations
One of the limitations of the current study is the lack of randomization between the two groups. However, the two groups in the current study presented with similar charateristics and recieved a similar medical therapy.
It is important to note that ESMR is still an experimental therapy, with very few patients currently treated with this modality compared with other strategies used in refractory angina, its a longterm prognosis still remains unknown.
The partial placebo effect cannot be totaly excluded, however those benefits were associated and supported by objective data such as the improvement in SPECT perfusion score.
A longer follow-up and the assessment of long term effect on quality of life in a large randomized study should be considered.
5. Conclusion
This current study demonstrates the potential efficacy and safety of ESMR therapy in patients with refractory angina. The possibility of a noninvasive revascularization is exciting, as is the absence of significant side effects with its use.
Further data support the potential use of this non-invasive therapy as adjuvant to medical therapy for patients with angina and non-revascularization options. If the previous data and its angiogenic effects are confirmed, ESMR may play a potential role in other conditions of is-chemic heart disease such for example diastolic dysfunction; more research into these potential indications is warranted.
References
- 1.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina–summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41(1):159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary artery bypass surgery and stentng for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–24. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 3.Andréll P, Ekre O, Grip L, Währborg P, Albertsson P, Eliasson T, et al. Fatality, morbidity and quality of life in patients with refractory angina pectoris. Int J Cardiol. 2009;147(3):377–82. doi: 10.1016/j.ijcard.2009.09.538. [DOI] [PubMed] [Google Scholar]
- 4.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23(5):355–70. doi: 10.1053/euhj.2001.2706. [DOI] [PubMed] [Google Scholar]
- 5.Arnold Suzanne V, Morrow David A, Yang Lei, Cohen David J, Mahoney Elizabeth M, Eugene Braunwald, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–53. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 6.Horvath KA. Transmyocardial laser revascularization. J Card Surg. 2008;23:266–76. doi: 10.1111/j.1540-8191.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KB, Kelly J, Borkon AM, Stuart RS, Daon E, Pak AF, et al. Transmyocardial revascularization: from randomized trials to clinical practice. A review of techniques, evidence-based outcomes, and future directions. Anesthesiol Clin. 2008;26:501–19. doi: 10.1016/j.anclin.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 10.Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, et al. American Urological Association Education and Research, Inc. European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–31. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Zelle BA, Gollwitzer H, Zlowodzki M, Buhren V. Extracorporeal shock wave therapy: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S66–70. doi: 10.1097/BOT.0b013e3181cad510. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Garcia J, Lerman A. Cardiac shock-wave therapy in the treatment of refractive angina pectoris. Interv Cardiol. 2011;3(2):191–201. [11] [Google Scholar]
- 13.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265–8. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 14.Preminger GM, Tiselius HG, Assimos DG, et al. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–31. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Zelle BA, Gollwitzer H, Zlowodzki M, Buhren V. Extracorporeal shock wave therapy: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S66–70. doi: 10.1097/BOT.0b013e3181cad510. [DOI] [PubMed] [Google Scholar]
- 16.Manganotti P, Amelio E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke. 2005;36:1967–71. doi: 10.1161/01.STR.0000177880.06663.5c. [DOI] [PubMed] [Google Scholar]
- 17.Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990;16:261–9. doi: 10.1016/0301-5629(90)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Wang CJ, Huang HY, Pai CH. Shock wave-enhanced neovascularization at the tendon-bone junction: an experiment in dogs. J Foot Ankle Surg. 2002;41:16–22. doi: 10.1016/s1067-2516(02)80005-9. [DOI] [PubMed] [Google Scholar]
- 19.Maisonhaute E, Prado C, White PC, Compton RG. Surface acoustic cavitation 86 understood via nanosecond electrochemistry. Part III: shear stress in ultrasonic cleaning. Ultrason Sonochem. 2002;9:297–303. doi: 10.1016/s1350-4177(02)00089-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical shock wave mediates membrane hyperpolarization and 87 Ras activation for osteogenesis in human bone marrow stromal cells. Biochem Biophys Res Commun. 2001;287:648–55. doi: 10.1006/bbrc.2001.5654. [DOI] [PubMed] [Google Scholar]
- 21.Gotte G, Amelio E, Russo S, Marlinghaus E, Musci G, Suzuki H. Short-time non-enzymatic nitric oxide synthesis from l-arginine and hydrogen peroxide induced by n shock waves treatment. FEBS Lett. 2002;520:153–5. doi: 10.1016/s0014-5793(02)02807-7. [DOI] [PubMed] [Google Scholar]
- 22.Mariotto S, Cavalieri E, Amelio E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 2005;12:89–96. doi: 10.1016/j.niox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16:2366–72. doi: 10.2174/092986709788682119. [DOI] [PubMed] [Google Scholar]
- 24.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine. 1999;11:416–23. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- 25.Gutersohn A, Caspari G. Shock waves upregulates vascular endothelial growth factor m-RNA in human umbilical vascular endothelial cells. Circulation. 2000;102:18. [Suppl] [Google Scholar]
- 26.Yoshida J, Ohmori K, Takeuchi H, et al. Treatment of ischemic limbs based on local recruitment of vascular endothelial growth factor-producing inflammatory cells with ultrasonic microbubble destruction. J Am Coll Cardiol. 2005;46:899–905. doi: 10.1016/j.jacc.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 27.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–30. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 28.Sheu JJ, Sun CK, Chang LT, et al. Shock wave-pretreated bone marrow cells further improve left ventricular function after myocardial infarction in rabbits. Ann Vasc Surg. 2010;24:809–21. doi: 10.1016/j.avsg.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325–35. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 30.Andrew Cassar, Megha Prasad, Martin-Rodriguez-Porcel, Reeder Guy S, Darshak Karia, DeMaria Anthony N, et al. Safety and efficacy of extracorporeal shock wave myocardial revascularization therapy for refractory angina pectoris. Mayo Clin Proc. 2014;89(3):346–54. doi: 10.1016/j.mayocp.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis. 2006;17(1):63–70. doi: 10.1097/00019501-200602000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi Y, Ito K, Ito Y, Shiroto T, Tsuburaya R, Aizawa K, et al. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J. 2010;74(3):589–91. doi: 10.1253/circj.cj-09-1028. [DOI] [PubMed] [Google Scholar]
- 33.Leibowitz D, Weiss AT, Rott D, et al. The efficacy of cardiac shock wave therapy in the treatment of refractory angina: A pilot prospective, randomized, double-bind trial. Int J Cardiol. 2012 Nov 30; doi: 10.1016/j.ijcard.2012.11.099. [DOI] [PubMed]
- 34.Kazmi WH, Rasheed SZ, Ahmed S, et al. Noninvasive therapy for the menagement of patients with advanced coronary artery disease. Coron Artery Dis. 2012;23(8):549–54. doi: 10.1097/MCA.0b013e328358a606. [DOI] [PubMed] [Google Scholar]
- 35.Yip HK, Chang LT, Sun CK, Youssef AA, Sheu JJ, Wang CJ. Shock wave therapy applied to rat bone marrow-derived mononuclear cells enhances formation of cells stained positive for CD31 and vascular endothelial growth factor. Circ J. 2008;72(1):150–6. doi: 10.1253/circj.72.150. [DOI] [PubMed] [Google Scholar]
- 36.CJ, Huang HY, Pai CH. Shock wave-enhanced neovascularization at the tendon-bone junction: an experiment in dogs. J Foot Ankle Surg. 2002;41:16–22. doi: 10.1016/s1067-2516(02)80005-9. [DOI] [PubMed] [Google Scholar]
- 37.Fu M, Sun CK, Lin YC, Wang CJ, Wu CJ, Ko SF, et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS One. 2011;6(9):e24342. doi: 10.1371/journal.pone.0024342. [1–14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Guo T, Cai HY, Ma TK, Tao SM, Sun S, et al. Cardiac shock wave therapy reduces angina and improves myocardial function in patients with refractory coronary artery disease. Clin Cardiol. 2010;33(11):693–9. doi: 10.1002/clc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110(19):3055–61. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 40.Uwatoku T, Ito K, Abe K, Oi K, Hizume T, Sunagawa K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coron Artery Dis. 2007;18(5):397–404. doi: 10.1097/MCA.0b013e328089f19b. [DOI] [PubMed] [Google Scholar]
- 41.Zimpfer D, Aharinejad S, Holfeld J, Thomas A, Dumfarth J, Rosenhek R, et al. Direct epicardial shock wave therapy improves ventricular function and induces angiogenesis in ischemic heart failure. J Thorac Cardiovasc Surg. 2009;137(4):963–70. doi: 10.1016/j.jtcvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Vasyuk YA, Hadzegova AB, Shkolnik EL, Kopeleva MV, Krikunova OV, Iouchtchouk EN, et al. Initial clinical experience with extracorporeal shockwave therapy in treatment of ischemic heart failure. Congest Heart Fail. 2010;16(5):226–30. doi: 10.1111/j.1751-7133.2010.00182.x. [DOI] [PubMed] [Google Scholar]
- 43.Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy as a new and non-invasive angiogenic strategy. Tohoku J Exp Med. 2009;219(1):1–9. doi: 10.1620/tjem.219.1. [DOI] [PubMed] [Google Scholar]
- 44.Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J. 1998;136(6):1114–20. doi: 10.1016/s0002-8703(98)70171-1. [DOI] [PubMed] [Google Scholar]
- 45.Zipes DP, Svorkdal N, Berman D, Boortz-Marx R, Henry T, Lerman A, et al. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation. 2012;15(6):550–9. doi: 10.1111/j.1525-1403.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RS, De Vries J, Buchser E, Dejongste MJ. Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2009;9:13. doi: 10.1186/1471-2261-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birgit Assmus, Walter Dirk H, Seeger Florian H, Leistner David M, Julia Steiner, Ina Ziegler, et al. Effect of shock wave–facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure. JAMA. 2013;309(15):1622–31. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]