Abstract
Objectives
Reducing blood product utilization after cardiac surgery has become a focus of perioperative care as studies suggest improved outcomes. However, the relative impact of preoperative anemia versus packed red blood cell (PRBC) transfusion on outcomes remains poorly understood. The purpose of this study was to investigate the relative association between preoperative hematocrit (Hct) level versus PRBC transfusion on postoperative outcomes after coronary artery bypass grafting (CABG).
Methods
Patient records for primary, isolated CABG operations (1/2007–12/2017) from 19 cardiac surgery centers were evaluated. Hierarchical logistic regression modeling was utilized to estimate the relationship between baseline preoperative Hct level as well as PRBC transfusion and the likelihood for postoperative mortality and morbidity, adjusted for baseline patient risk. Variable and model performance characteristics were compared to determine relative strength of association between Hct level versus PRBC transfusion and primary outcomes.
Results
A total of 33,411 patients (median patient age = 65 [57–72] years, 26% female) were evaluated. Median preoperative hematocrit was 39% [36–42] while a mean STS PROM 1.8%±3.1%. Complications included: PRBC transfusion (31%), renal failure (2.8%), stroke (1.3%), and operative mortality (2.0%). As expected, a strong association was observed between preoperative Hct and the likelihood for PRBC transfusion (P<0.001). After risk-adjustment, PRBC transfusion (not Hct level) demonstrated stronger associations with postoperative mortality (OR 4.3, P<0.0001), renal failure (OR 6.3, P<0.0001) and stroke (OR 2.4, P<0.0001). A 1 point increase in preoperative Hct was associated with a decreased probability of mortality (OR 0.97, P=0.0001) and renal failure (OR 0.94, P<0.0001). The models with PRBC had superior predictive power with larger area under the curve compared to Hct for all outcomes (all p<0.01). Interestingly, preoperative anemia was associated with up to a four fold increase in the probability of PRBC transfusion, three fold increase in renal failure, and almost doubling of mortality.
Conclusions
PRBC transfusion appears more closely associated with risk-adjusted morbidity and mortality compared to the preoperative hematocrit level alone, supporting efforts to reduce unnecessary PRBC transfusions. Preoperative anemia does independently increase the risk of postoperative morbidity and mortality. These data suggest that preoperative hematocrit levels should be included in the STS risk calculators. Finally, efforts to optimize preoperative hematocrit should be investigated as a potentially modifiable risk factor for mortality and morbidity.
Keywords: Anemia, Hematocrit, Transfusion, Cardiac, CABG
INTRODUCTION
Coronary artery bypass grafting (CABG) remains one of the most commonly performed surgical operations in the United States and worldwide. Cardiac surgical procedures are estimated to be responsible for nearly 20% of the annual blood transfusions in the United States,1 and blood transfusion following CABG has been reported to occur in up to 60% of cases.2,3 Allogenic blood product transfusion has been associated with significant adverse patient effects, including the occurrence of transfusion reactions, transfusion related acute lung injury (TRALI), immunosuppression and immunomodulation, infectious complications, and even reduced long term survival. 2,4–15 While several series have investigated the independent effects of perioperative anemia or packed red blood cell (PRBC) transfusion, a fundamental question remains unanswered. Which factor (anemia vs. transfusion) contributes more to adverse cardiac surgical morbidity and mortality? Previous investigations have also been frequently limited by examination of single institution cohort analyses, heterogeneous surgical patient populations, and have failed to consider the non-linear relationship that exists between preoperative hematocrit (Hct) level and risk-adjusted mortality and morbidity.
The purpose of this study was to investigate the relative association between preoperative Hct versus PRBC transfusion on postoperative outcomes after CABG alone. We hypothesized that 1) a significant non-linear relationship exists between preoperative Hct level and patient outcomes as well as the need for PRBC transfusion, and 2) that PRBC transfusion would have a stronger relative strength of association with postoperative mortality and morbidity compared to preoperative Hct alone.
METHODS
The Virginia Cardiac Services Quality Initiative (VCSQI) consists of 19 cardiac surgical centers within the Commonwealth of Virginia and state of North Carolina. VCSQI centers perform approximately 99% of the Commonwealth’s cardiac operations, contributing patient data to the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database. This study was exempt from formal Institutional Review Board review at each participating hospital due to the secondary analysis of the VCSQI de-identified (absence of Health Insurance Portability and Accountability Act patient identifiers) data registry and because the data is collected for quality analysis and purposes other than research.
Patients and Data Acquisition
De-identified patient data was extracted from the VCSQI data registry for the study period 1/1/2007–12/31/2017. All records included patients undergoing primary (no previous sternotomy) isolated CABG operations (STS Procedure Type “CAB Alone”). Patients were excluded for missing preoperative hematocrit level, concomitant valve procedures or other cardiac surgery procedures. All CABG procedures represent standard open surgical approaches to myocardial surgical revascularization with and without the use of cardiopulmonary bypass support. Patient preoperative risk was assessed by prevalence of patient comorbid disease, operative status and individual calculated STS predicted risk scores.
The primary outcomes of interest included risk-adjusted association between preoperative Hct levels versus packed red blood cell (PRBC) transfusion and the probability of operative mortality and measures of major morbidity, including likelihood for PRBC transfusion, renal failure or stroke. PRBC transfusion was defined as either intraoperative or postoperative PRBC transfusion as both correlate with postoperative complications. Standard STS clinical definitions for all analyzed variables were utilized.16
Statistical Analysis
Descriptive Statistics
Categorical variables are expressed as group percentages, while continuous variables are expressed as either mean ± standard deviation (SD) or median [interquartile range (IQR) as 25th–75th percentile] depending upon overall variable distribution. Generalized linear mixed regression models were utilized to estimate the relationship between baseline preoperative Hct level as well as PRBC transfusion and the likelihood for postoperative mortality and morbidity, adjusted for baseline patient risk. Patients were excluded for preoperative hematocrit levels <17 or >54 and the remaining values modeled as a continuous function. Hematocrit values were centered at their mean, 38.855, to facilitate the convergence of the statistical models. The predicted associations between preoperative Hct level or PRBC transfusion and outcomes were adjusted for the confounding effects of preoperative patient risk profile (STS predicted risk scores), and year through the inclusion of these variables as model covariates. STS Predicted Risk scores were logit transformed which put them on the natural scale for inclusion as predictors in a logistic model. Hospital was included in the model as a random effect to account for clustering at the hospital level. The relative strength of association between Hct level versus PRBC transfusion and the probability for mortality or morbidity was determined by comparing adjusted odds ratios, p-values and the area under the curve (AUC) for each model. The AUC from the corresponding Hct and PRBC models were compared using DeLong’s test. In addition, the risk-adjusted functions of Hct level versus the probability of mortality and measures of major morbidity were graphically represented in order to provide better explanatory power. Patients with missing data were excluded from the corresponding summary statistic. For for regression analysis missing values were imputed with the lowest risk categorical predictors (egg no PRBC) or as non-events for outcome variables.. Two-sided P<0.05 defined statistically significant variable associations. All statistical analyses were conducted using R statistical software, version 3.4.3 (http://www.R-project.org).
RESULTS
Patient Characteristics and Operative Features for CABG Operations
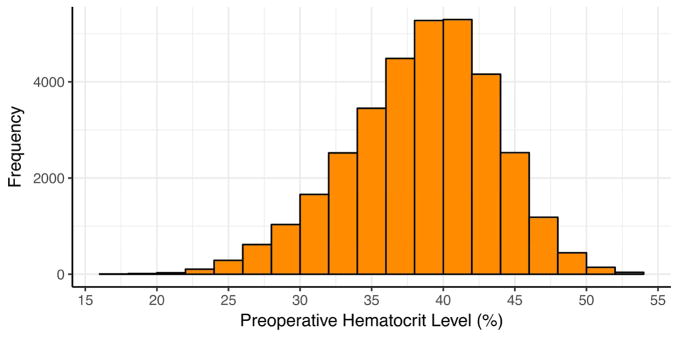
A total of 33,411 patients underwent primary, isolated CABG operations. Table 1 displays descriptive statistics and frequencies of patient preoperative and operative characteristics. The median patient age was 65 years (IQR 57–72 years), and females comprised 26% of patients. Mean STS Predicted Risk of Mortality (PROM) was 1.8% ± 3.1%. The most prevalent co-morbid disease states included hypertension, dyslipidemia, diabetes, and cerebrovascular disease. The majority of patients presented without heart failure symptoms. Median ejection fraction was 55% [IQR 45–60]. Most patients presented with 3-vessel coronary artery disease, and significant left main coronary artery disease was present in one-third of patients. The median preoperative hematocrit was 39% [IQR 36–42] for the study population. Figure 1 displays the distribution of preoperative hematocrit levels for the study population.
Table 1.
Descriptive statistics of preoperative and operative characteristics for patients undergoing primary, isolated CABG operations.
| Patient Characteristics | N = 33,411 |
|---|---|
| Patient Age | 65 [57–72] |
| Female | 8569 (25.7%) |
| Body Mass Index (kg/m2) | 29 [26–33] |
| Cerebrovascular Disease | 5586 (16.8%) |
| Stroke | 2054 (6.4%) |
| Chronic Lung Disease | |
| Mild | 4554 (13.9%) |
| Moderate | 1744 (5.3%) |
| Severe | 1283 (3.9%) |
| Hypertension | 28911 (86.6%) |
| Diabetes | 14824 (44.4%) |
| Peripheral Arterial Disease | 4526 (13.6%) |
| Renal Failure (Hemodialysis) | 994 (3.0%) |
| NYHA Class | |
| None | 27201 (82.7%) |
| I | 479 (1.5%) |
| II | 1921 (5.8%) |
| III | 2127 (6.5%) |
| IV | 1176 (3.6%) |
| Ejection Fraction (%; median [IQR]; n=21199) | 55 [45–60] |
| Left Main Disease > 50% | 7877 (33.2%) |
| Number of Disease Vessels | |
| 1 | 1368 (4.1%) |
| 2 | 6438 (19.3%) |
| 3 or more | 25490 (76.6%) |
| Last Preoperative Hematocrit (%) | 39 [36–42] |
| Predicted Risk of Mortality (%) | 1.8% ± 3.1 |
|
| |
| Operative Characteristics | |
|
| |
| Cardiopulmonary Bypass Support | |
| None | 2722 (8.2%) |
| Full | 30472 (91.2%) |
| Combination | 216 (0.7%) |
| Operative Status | |
| Elective | 11658 (34.9%) |
| Urgent | 20447 (61.2%) |
| Emergent | 1254 (3.8%) |
| Emergent Salvage | 46 (0.1%) |
| Cardiopulmonary Bypass Time (min) | 92 [72–115] |
| Aortic Cross Clamp Time (min) | 67 [51–85] |
| Lowest Intraoperative Hematocrit (%) | 25 [21–28] |
| Internal Mammary Artery (IMA) Use | |
| No IMA | 1646 (4.9%) |
| Left IMA | 30604 (91.7%) |
| Right IMA | 175 (0.5%) |
| Both IMA | 961 (2.9%) |
Figure 1.
Histogram displaying the distribution of preoperative hematocrit levels.
Most CABG operations were performed in either an elective (35%) or urgent (61%) setting with few being done on emergent basis. CABG operations were performed with cardiopulmonary bypass support in 91% of operations, off-pump CABG occurred in 8% of operations, and a combination of bypass support occurred in 1% of patients. The median cardiopulmonary bypass time was 92 minutes [IQR 72–115], and median aortic cross-clamp time was 67 minutes [IQR 51–85]. Left internal mammary artery (IMA) conduits were used in 95% of cases, with bilateral IMA use occurring in 3% of cases. Median lowest intraoperative hematocrit level was 25% [IQR 21–28].
Postoperative Outcomes Following CABG Operations
Table 2 displays the frequency of postoperative events and outcomes. Nearly one-third of patients received postoperative blood product transfusions, and when transfused the median number of PRBC transfusions was 2 units [IQR 1–4]. Detailed intraoperative and postoperative transfusion rates are available in eTable 1. The most common postoperative complication was atrial fibrillation (20%). Prolonged mechanical ventilation occurred in 9% of patients, while renal failure and stroke occurred in 3% and 1.3% of patients, respectively. Operative mortality was 2.0%.
Table 2.
Unadjusted incidence of postoperative events and complications following primary, isolated CABG operations.
| Outcome | N = 33,411 |
|---|---|
| Any PRBC transfusion | 10,747 (31.4%) |
| Transfused PRBC (units) | 2 [1–4] |
| Atrial Fibrillation | 6821 (20.4%) |
| Stroke | 431 (1.3%) |
| Cardiac Arrest | 461 (1.4%) |
| Pneumonia | 707 (2.1%) |
| Prolonged Mechanical Ventilation | 2887 (8.7%) |
| Renal Failure | 923 (2.8%) |
| Hemodialysis | 461 (1.4%) |
| Operative Mortality | 667 (2.0%) |
Adjusted Relationships Between Preoperative Hematocrit Level versus PRBC Transfusion and Mortality and Morbidity
The relative strengths of association between risk-adjusted outcomes and the effect of preoperative Hct level versus PRBC transfusion are displayed in Table 3 with the respective adjusted odds ratios (AOR), p-values and AUC estimated from each regression model for the outcomes of operative mortality, postoperative renal failure, and postoperative stroke. Complete covariate information is available for each model in eTables 2–4. A strong association was observed between preoperative Hct and the likelihood for PRBC transfusion (OR 0.82 [0.82–0.83], P<0.001). After risk-adjustment using the STS predicted risk of mortality, PRBC transfusion (not Hct) demonstrated stronger associations with postoperative mortality (OR 4.3, P<0.0001), renal failure (OR 6.3, P<0.0001) and stroke (OR 2.4, P<0.0001). While less strongly associated (compared to PRBC transfusion), a 1 point increase in preoperative Hct was associated with a significantly decreased probability of mortality (OR 0.97, P=0.00011) and renal failure (OR 0.94, P<2e-16). The PRBC models performed significantly better than the Hct models for mortality (AUC 0.82693 vs 0.78886, p<0.0001), renal failure (AUC 0.86063 vs 0.82067, p<0.0001), and stroke (0.72062 vs 0.69487, p=0.0013).
Table 3.
Risk-adjusted impact of preoperative hematocrit and packed red blood cell transfusion on postoperative mortality, renal failure and stroke.
| Preoperative Hct Models | PRBC Transfusion Models | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AOR [95% C.I.] | p-value | AUC | AOR [95% C.I.] | p-value | AUC | |
| Mortality | 0.97 [0.96–0.99] | 0.0001 | 0.789 | 4.25 [3.47–5.19] | <0.0001 | 0.827 |
| Renal Failure | 0.94 [0.93–0.96] | <0.0001 | 0.821 | 6.29 [5.24–7.56] | <0.0001 | 0.861 |
| Stroke | 1.02 [1.00–1.04] | 0.062 | 0.695 | 2.35 [1.90–2.92] | <0.0001 | 0.721 |
Denotes P<0.01.
Hct = hematocrit; PRBC = packed red blood cell; AOR = adjusted odds ratio; C.I. = confidence interval. Models adjusted for confounding influence of other covariates: Society of Thoracic Surgeons (STS) predicted risk indices (mortality, renal failure, stroke), operative year and hospital.
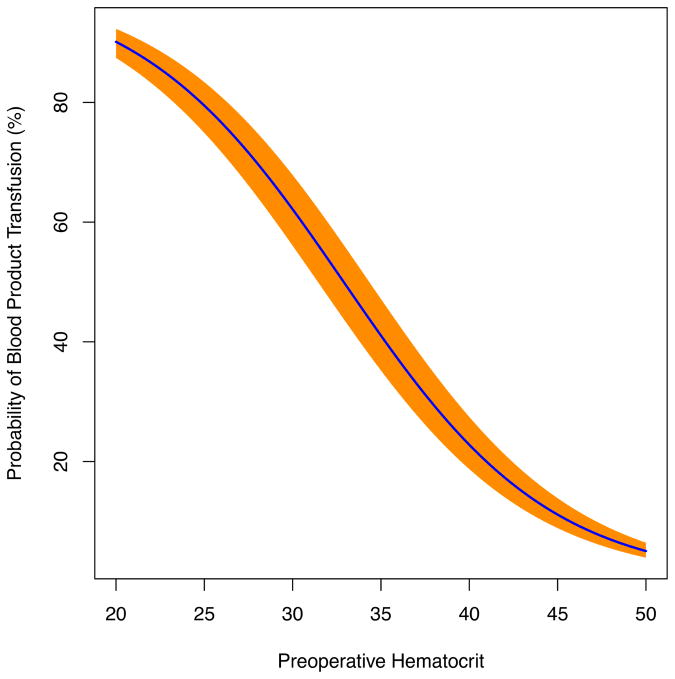
The adjusted associations between preoperative Hct and the probability of mortality (Figure 2a), postoperative PRBC transfusion (Figure 2b) and postoperative renal failure (Figure 2c) are demonstrated in the figures. Most importantly, these functions demonstrated that in an otherwise average patient preoperative anemia compared to a Hct level of 40% was associated with an almost 4 fold increase in the probability of PRBC transfusion, 3 fold increase in renal failure, and near doubling in risk of mortality (Table 4).
Figure 2.
Figure 2a. Risk-adjusted model of the impact of preoperative hematocrit versus the probability of mortality following primary, isolated CABG. The 95% confidence interval is represented in orange.
Figure 2b. Risk-adjusted model of the impact of preoperative hematocrit versus the probability of packed red blood cell (PRBC) transfusion following primary, isolated CABG.
Figure 2c. Risk-adjusted model of the impact of preoperative hematocrit versus the probability of postoperative renal failure following primary, isolated CABG.
Table 4.
Impact of preoperative anemia (compared to preoperative Hct =40%) on predicted probability of postoperative PRBC transfusion, renal failure, and mortality.
| Postoperative Outcome | Preoperative Hematocrit (20%) | Preoperative Hematocrit (30%) | Preoperative Hematocrit (40%) |
|---|---|---|---|
| PRBC Transfusion | 90.1% | 62.1% | 22.8% |
| Renal Failure | 3.9% | 2.3% | 1.3% |
| Mortality | 2.0% | 1.5% | 1.1% |
COMMENT
The present study reports upon risk-adjusted relationships between postoperative mortality and morbidity as a function of preoperative Hct versus PRBC transfusion to better determine which factor confers a greater risk to patients undergoing CABG procedures. In this multi-institution analysis of over 33,000 patient records, primary, isolated CABG procedures were evaluated to avoid the potential confounding effects on blood product requirements common during reoperations and combined procedures. As a result, the primary results demonstrate that while preoperative Hct and, more importantly preoperative anemia, are both associated with an increase in risk-adjusted mortality and morbidity, the effect of PRBC transfusion on patient outcomes is more profound. These results address an ill-defined issue within cardiac surgical critical care to identify which factor is the greater culprit for worse outcomes following cardiac surgery.
The risk-adjusted impact of preoperative Hct on mortality and morbidity in the present analysis is consistent with previous reports in cardiac surgical populations. Several studies have demonstrated a significant correlation between either preoperative or intraoperative anemia and adverse cardiac surgical outcomes.17–20 van Straten et al. demonstrated that preoperative anemia was a significant risk factor for early and late mortality in a heterogeneous cardiac surgical patient population of 10,025 patients.20 Similarly, Karkouti and colleagues demonstrated a 2-fold increase in the risk-adjusted odds of composite mortality, stroke, and renal injury in patients with preoperative anemia in an analysis of 3,500 patients.18 An international analysis of over 5,000 patients corroborated these findings in a multi-institution (70 centers) cohort of patients.19 In addition, the strong correlation, in this study, between preoperative Hct level and the likelihood for postoperative PRBC transfusion is consistent with the primary results of former analyses.17 The results of the present study extend those of former reports; however, they also address several limitations of previous analyses, including the risk-adjusted hierarchical modeling of preoperative Hct as a continuous function to better understand the relationship that exists between declining Hct level and perioperative mortality and morbidity. These results also provide an analysis of primary, isolated CABG operations alone to avoid inherent confounding that is often difficult to adjust for in heterogeneous patient populations. While the impact of intraoperative anemia was not specifically examined in this analyses, prior large cohort studies have demonstrated that nadir hematocrit levels during cardiopulmonary bypass significantly impacts postoperative mortality.21–23
The principle results of this study concern the demonstration that PRBC transfusion has a greater effect than that of preoperative Hct on the likelihood of postoperative mortality, stroke and renal failure after adjustment for the confounding influence of baseline patient risk profile, differences at the hospital level, and year of operation. These conclusions are apparent by the significantly higher strength of association (as determined by p-values and model AUC) estimated for the effect of PRBC transfusion (compared to preoperative Hct) with each outcome. While several previous analyses have demonstrated the adverse impact of either anemia or blood transfusions alone on mortality and morbidity,5,8,24–26 the study design of this analysis is unique in that it more directly addresses the question of which factor (anemia vs. transfusion) contributes more to poor patient outcomes. This methodology and results are important as they demonstrate that while anemia and PRBC transfusion are often clinically related events, the estimated negative impact of PRBC transfusion is greater and efforts to avoid transfusion when clinically feasible should be considered.
The present results have significant clinical implications related to the perioperative management of patients undergoing surgical myocardial revascularization. A fundamental limitation of any analysis of blood product utilization after cardiac surgery is the criticism that the occurrence of blood product transfusions likely represents a surrogate for sicker patient populations compared to those patients not requiring blood products. This is a factor that has been addressed in many former risk-adjusted and propensity score cohort matched analyses.5,8,24–26 While a legitimate concern, results of these analyses, as well as the present, have consistently demonstrated that transfusion is an independent risk factor for mortality. As a result, our demonstration that there exists a highly significant and strong association between preoperative Hct and the likelihood for PRBC transfusion further emphasizes the critical role of proper preoperative planning and correction of preoperative anemia when possible to avoid the untoward effects of PRBC transfusion following cardiac surgery. These results, therefore, further corroborate blood conservation efforts and guidelines advanced by the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists including: 1) efforts designed to increase preoperative blood volume (e.g. erythropoietin or autologous preoperative blood donation), 2) intraoperative blood conservation, cell-saving devices, 3) interventions to avoid operative stress, 4) implementation of institution-specific transfusion algorithms, 5) utilization of multimodality blood conservation efforts, and 6) use of miniaturized cardiopulmonary bypass circuits and modified ultrafiltration to maximize intraoperative and postoperative hematocrit levels.27,28 In addition, the results of this study further highlight the importance of focusing upon blood conservation efforts as prior reports have demonstrated the significantly increased economic burden of blood product transfusion on healthcare costs after cardiac surgery.8,25
This study has noteworthy limitations. The retrospective study design introduces inherent selection bias. The reported results describe observed associations between modeled factors and outcomes; the results do not demonstrate direct cause and effect relationships. An additional limitation concerns the methods required to measure and report preoperative Hct. Measurements of a single, preoperative blood Hct level as opposed to alternative measurements (e.g. average Hct) may over- or underestimate the estimated relationships between preoperative hematocrit or degree of anemia and measured outcomes. Furthermore, the impact of transfusions occurring in the preoperative setting in an effort to correct anemia and optimize preoperative status was not able to be determined or analyzed. The de-identified nature of a large data registry is inherently limited to the analysis of predefined variables, variable definitions, and variable granularity. As a result, it was not possible to directly analyze the impact of anemia or transfusion on the likelihood of TRALI, infectious complications, or transfusion reactions. Timing of blood transfusion was not captured in the VCSQI data registry, a limitation of currently captured data in the STS national database as well. While outside the scope of this analysis, additional investigation of the impact of other blood products on outcomes would be beneficial. The study is constrained by a lack of long-term reports of outcomes. Unrecognized miscoding of data must also be considered in any secondary analysis of a data registry. Finally, risk adjustment using PROM may not completely account for association of postoperative complications and transfusion requirements. Despite these limitations, this analysis provides a robust analysis of the largest reported, multi-institution cohort of CABG patients to date with the specific aim of determining the relative of the clinical impact of preoperative Hct level versus PRBC transfusion.
CONCLUSIONS
Herein, the primary results of this multi-institution analysis of CABG outcomes demonstrate that packed red blood cell transfusion appears more strongly associated with risk-adjusted morbidity and mortality compared to preoperative hematocrit level alone. Preoperative anemia does independently increase the risk of postoperative morbidity and mortality, but more strongly increases the likelihood of postoperative PRBC transfusion. Therefore, these data support efforts to reduce unnecessary PRBC transfusions. The present results suggest that preoperative hematocrit levels should be considered for inclusion in the STS risk calculators. Finally, efforts to optimize preoperative hematocrit to avoid anemia should be investigated as a potentially modifiable risk factor for mortality and morbidity.
Supplementary Material
CENTRAL MESSAGE.
PRBC transfusion was a stronger risk factor for morbidity and mortality than preoperative hematocrit level, supporting efforts to reduce unnecessary PRBC transfusions.
PERSPECTIVE STATEMENT.
Both preoperative anemia and PRBC transfusion are linked with poor outcomes after CABG. This study found PRBC transfusion was a stronger risk factor for worse mortality and morbidity. This supports both efforts to reduce unnecessary PRBC transfusions and investigation of optimization of hematocrit prior to surgery. Finally, hematocrit should be considered for inclusion in the STS risk models.
Acknowledgments
Funding: None
ABBREVIATIONS
- AOR
adjusted odds ratio
- CABG
coronary artery bypass grafting
- Hct
hematocrit
- IMA
internal mammary artery
- IQR
interquartile range
- LR
likelihood ratio
- PRBC
packed red blood cell
- RCS
restricted cubic spline
- SD
standard deviation
- STS
Society of Thoracic Surgeons
- TRALI
transfusion related acute lung injury
- VCSQI
Virginia Cardiac Services Quality Initiative
Footnotes
Presented at the 95th Annual Meeting of the American Association for Thoracic Surgery, Seattle, WA
Author Conflicts of Interest: There are no potential author conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Speiss BD. Transfusion and outcome in heart surgery. Ann Thorac Surg. 2002;74(4):986–987. doi: 10.1016/s0003-4975(02)03906-1. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MA, Blumberg N, Saint S, Langa KM, Nallamothu BK. Hospital variation in transfusion and infection after cardiac surgery: a cohort study. BMC Med. 2009;7:37. doi: 10.1186/1741-7015-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stover EP, Siegel LC, Parks R, et al. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Institutions of the Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology. 1998;88(2):327–333. doi: 10.1097/00000542-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Banbury MK, Brizzio ME, Rajeswaran J, Lytle BW, Blackstone EH. Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006;202(1):131–138. doi: 10.1016/j.jamcollsurg.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74(4):1180–1186. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 6.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34(6):1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 7.Koch CG, Li L, Duncan AI, et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81(5):1650–1657. doi: 10.1016/j.athoracsur.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 9.Scott BH, Seifert FC, Grimson R. Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Ann Card Anaesth. 2008;11(1):15–19. doi: 10.4103/0971-9784.38444. [DOI] [PubMed] [Google Scholar]
- 10.Surgenor SD, DeFoe GR, Fillinger MP, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114(1 Suppl):I43–48. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 11.Surgenor SD, Kramer RS, Olmstead EM, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108(6):1741–1746. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- 12.Spiess BD. Blood transfusion: the silent epidemic. Ann Thorac Surg. 2001;72(5):S1832–1837. doi: 10.1016/s0003-4975(01)03259-3. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34(9):2302–2308. doi: 10.1097/01.CCM.0000234034.51040.7F. quiz 2309. [DOI] [PubMed] [Google Scholar]
- 14.Toy P, Lowell C. TRALI--definition, mechanisms, incidence and clinical relevance. Best Pract Res Clin Anaesthesiol. 2007;21(2):183–193. doi: 10.1016/j.bpa.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch CG, Khandwala F, Li L, Estafanous FG, Loop FD, Blackstone EH. Persistent effect of red cell transfusion on health-related quality of life after cardiac surgery. Ann Thorac Surg. 2006;82(1):13–20. doi: 10.1016/j.athoracsur.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 16.Society of Thoracic Surgeons ESAahwsos-n-dd-me-sAO, 2013
- 17.Hung M, Besser M, Sharples LD, Nair SK, Klein AA. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients. Anaesthesia. 2011;66(9):812–818. doi: 10.1111/j.1365-2044.2011.06819.x. [DOI] [PubMed] [Google Scholar]
- 18.Karkouti K, Wijeysundera DN, Beattie WS Reducing Bleeding in Cardiac Surgery I. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478–484. doi: 10.1161/CIRCULATIONAHA.107.718353. [DOI] [PubMed] [Google Scholar]
- 19.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116(5):471–479. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 20.van Straten AH, Hamad MA, van Zundert AJ, Martens EJ, Schonberger JP, de Wolf AM. Preoperative hemoglobin level as a predictor of survival after coronary artery bypass grafting: a comparison with the matched general population. Circulation. 2009;120(2):118–125. doi: 10.1161/CIRCULATIONAHA.109.854216. [DOI] [PubMed] [Google Scholar]
- 21.DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71(3):769–776. doi: 10.1016/s0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80(4):1381–1387. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 23.Loor G, Li L, Sabik JF, 3rd, Rajeswaran J, Blackstone EH, Koch CG. Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. J Thorac Cardiovasc Surg. 2012;144(3):654–662. e654. doi: 10.1016/j.jtcvs.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 24.Kuduvalli M, Oo AY, Newall N, et al. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27(4):592–598. doi: 10.1016/j.ejcts.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 25.LaPar DJ, Crosby IK, Ailawadi G, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145(3):796–803. doi: 10.1016/j.jtcvs.2012.12.041. discussion 803–794. [DOI] [PubMed] [Google Scholar]
- 26.van Straten AH, Bekker MW, Soliman Hamad MA, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg. 2010;10(1):37–42. doi: 10.1510/icvts.2009.214551. [DOI] [PubMed] [Google Scholar]
- 27.Society of Thoracic Surgeons Blood Conservation Guideline Task F. Ferraris VA, Brown JR, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 28.Society of Thoracic Surgeons Blood Conservation Guideline Task F. Ferraris VA, Ferraris SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.