Abstract
Background and Purpose
We sought to determine the long-term risk of seizures after stroke according to age, sex, race, and stroke subtype.
Methods
We performed a retrospective cohort study using administrative claims from two complementary patient datasets. First, we analyzed data from all emergency department visits and hospitalizations in California, Florida, and New York from 2005–2013. Second, we evaluated inpatient and outpatient claims from a nationally representative 5% random sample of Medicare beneficiaries. Our cohort consisted of all adults at the time of acute stroke hospitalization without a prior history of seizures. Our outcome was seizure occurring after hospital discharge for stroke. Poisson regression and demographic data were used to calculate age-, sex-, and race-standardized incidence rate ratios (IRR).
Results
Among 777,276 patients in the multi-state cohort, the annual incidence of seizures was 1.68% (95% CI, 1.67%–1.70%) after stroke versus 0.15% (95% CI, 0.15%–0.15%) among the general population (IRR, 7.3; 95% CI, 7.3–7.4). By 8 years, the cumulative rate of any ED visit or hospitalization for seizure was 9.27% (95% CI, 9.16%–9.38%) after stroke versus 1.21% (95% CI, 1.21%–1.22%) in the general population. Stroke was more strongly associated with a subsequent seizure among patients <65 years of age (IRR, 12.0; 95% CI, 11.9–12.2) than in patients ≥65 years of age (IRR, 5.5; 95% CI, 5.4–5.5) and in the multi-state analysis, the association between stroke and seizure was stronger among non-white patients (IRR, 11.0; 95% CI, 10.8–11.2) than among white patients (IRR, 7.3; 95% CI, 7.2–7.4). Risks were especially elevated after ICH (IRR, 13.3; 95% CI, 13.0–13.6) and SAH (IRR, 13.2; 95% CI, 12.8–13.7). Our study of Medicare beneficiaries confirmed these findings.
Conclusions
Almost 10% of patients with stroke will develop seizures within a decade. Hemorrhagic stroke, non-white race, and younger age appear to confer the greatest risk of developing seizures.
Keywords: Stroke, Seizures, Epilepsy, Outcomes Research
Subject Terms: Cerebrovascular disease/stroke, Risk Factors
Stroke occurs in about 800,000 people annually in the United States and is a leading cause of long-term disability.1 Seizures commonly occur after stroke and are associated with increased mortality, higher odds of functional decline, and an overall lower quality of life.2–9 Based on previous population-based studies, estimates of the overall rate of post-stroke seizures vary from 2.6% to 13.5%.2, 10–16 It is well known that seizures can occur in the setting of acute stroke, but the long-term risk of seizures in survivors of stroke remains unclear. Furthermore, although previous studies have suggested a higher risk of seizures after hemorrhagic stroke compared to ischemic stroke, the long-term risk of seizures has not been rigorously assessed by stroke subtype or demographic characteristics.2, 12–14, 16–18 We therefore designed this study to evaluate the long-term risk of seizures according to age, sex, race, and stroke subtype in patients free of seizures prior to stroke hospitalization in two large, nationally representative cohorts of patients in the United States.
Methods
Design
We performed a population-based, retrospective study of two large cohorts of patients from across the United States. In the first analysis, we used administrative claims data from all nonfederal emergency department (ED) and acute care hospital discharges in California from 2005 through 2011, New York from 2006 through 2013, and Florida from 2005 through 2013; these dates were chosen as they represent the most recent data available for analysis. These data were collected by the California Office of Statewide Health Planning and Development, the New York State Department of Health, and the Florida Agency for Health Care Administration; and were provided to the Agency for Healthcare Research and Quality for its Healthcare Cost and Utilization Project (HCUP).19
In the second analysis, we used both inpatient and outpatient claims data from a 5% random sample of nationwide Medicare beneficiaries from 2008 through 2014. The U.S. federal government’s Centers for Medicare and Medicaid Services (CMS) provides health insurance to a large majority of U.S. residents once they reach 65 years of age. CMS makes available to researchers deidentified datasets that include data on claims submitted by providers and hospitals in the course of Medicare beneficiaries’ clinical care.20 In keeping with standard practice in analyzing Medicare data,21 we limited our cohort to beneficiaries with continuous coverage in traditional fee-for-service Medicare (both Parts A and B) for at least 1 year.
In both datasets, all patients are assigned a deidentified personal linkage number that enables them to be followed anonymously over multiple years.22 Each claim includes the dates of service and up to 25 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes justifying the claim. The complementary nature of these two datasets facilitated study of patients regardless of age or insurance status through the HCUP data and analysis of outpatient as well as inpatient diagnoses drawn from Medicare data.
Standard Protocol Approval
In performing this study, we adhered to the Report of Studies Conducted Using Observational Routinely-Collected Health Data guidelines for analyses of administrative claims data.23 The data that support the findings of this study are available upon request. The institutional review board at Weill Cornell Medicine approved this study and waived the requirement for informed consent.
Subjects
In the multi-state analysis, we identified all patients 18 years or older at the time of a first-ever recorded hospitalization for stroke. Since Medicare eligibility generally begins at 65 years of age, in the analysis of claims data on Medicare beneficiaries, we included only patients ≥66 years of age in order to allow adequate time for beneficiaries to enter medical care and for their providers to document any pre-existing comorbidities. In both analyses, stroke was defined as the composite of ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH). In accordance with a validated algorithm, ischemic stroke was defined as ICD-9-CM codes 433.x1, 434.x1, or 436 in any hospital discharge diagnosis code position without a concurrent primary discharge code for rehabilitation (V57) or any codes for trauma (800–804 or 850–854), SAH (430) or ICH (431). ICH was defined as ICD-9-CM discharge code 431 without concomitant codes for rehabilitation, trauma, or SAH. Finally, SAH was defined as ICD-9-CM discharge code 430 without concomitant codes for rehabilitation or trauma. This algorithm has been previously validated to have a sensitivity of ≥82% and a specificity of ≥92% for correctly identifying both ischemic and hemorrhagic stroke subtypes.24 Patients who died during the index hospitalization were excluded. Patients coded as having multiple encounters for stroke were identified at the time of their first recorded hospital admission for stroke. To focus on new-onset seizures after stroke, patients with diagnoses of seizure prior to the index stroke hospitalization were excluded.
Measurements
Our primary outcome was a seizure occurring after hospital discharge for stroke. In the multi-state analysis, our primary outcome was an ED visit or hospitalization for seizure following discharge from the index stroke hospitalization, defined as any ICD-9-CM code for epilepsy (345.x) in any discharge diagnosis position; this approach has been shown to have a positive predictive value ranging from 84–98% in adults.25–27 Our secondary outcome was subsequent hospitalization for status epilepticus (ICD-9-CM code 345.3) documented as being present on admission. As Medicare claims data includes information on outpatient visits, in the second analysis, our outcome of seizure was defined as any inpatient or outpatient claim with an ICD-9-CM code for epilepsy (345.x); to avoid “rule out” diagnoses used to justify diagnostic tests (e.g. neuroimaging scans, electroencephalograms) we included only outpatient diagnoses associated with an evaluation and management claim.
Statistical Analysis
We used standard descriptive statistics with exact confidence intervals (CI) to report crude rates. Baseline characteristics were compared using the chi-square test and Student’s t-test. Survival statistics were used to calculate incidence rates of seizure per 100,000 patients per year.
Following the approach of prior studies on the long-term risk of seizures,28 we compared the risk of seizures in those with stroke versus the general population. For the multi-state analysis, we used publicly available demographic data from the three states included in this analysis to determine seizure risk in the general population;29 and for the Medicare analysis, we compared seizure risk in stroke patients versus the remaining beneficiaries. For both analyses, we used Poisson regression to calculate age-, sex-, and race-standardized incidence rate ratios (IRR). Subgroup analyses were performed stratified by patients’ age (<65 versus ≥65), gender, and race (white versus non-white). All statistical analyses were performed by HK using SAS version 9.3 (Cary, NC) and STATA/MP version 13 (College Station, TX). Statistical significance was set at α=0.05.
Results
Multi-State Patient Characteristics
We identified 777,276 patients with an acute stroke hospitalization in California, Florida, and New York between 2005 and 2013. Of these strokes, 653,564 (84.1%) were ischemic strokes, 89,173 (11.5%) were ICH, and 34,539 (4.4%) were SAH. Median patient follow-up time was 3.9 years (interquartile range 2.0–5.8). Among patients with stroke, 45,708 (5.88%) developed a seizure; those with subsequent seizures were on average younger than those without subsequent seizures (Supplemental Table I).
Multi-State Analysis
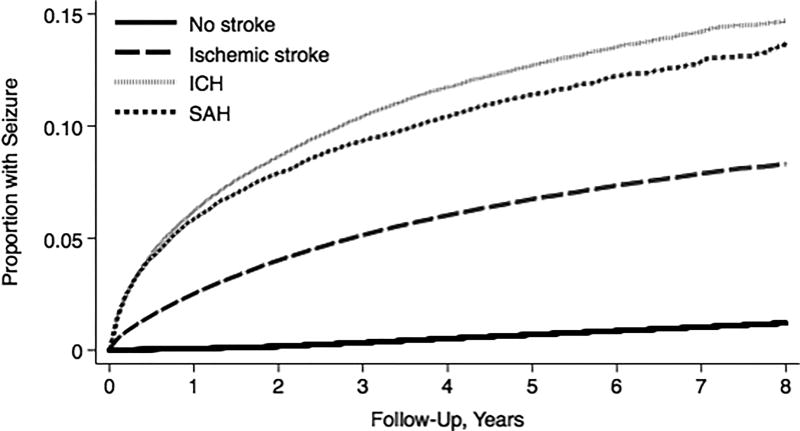
The annual incidence of seizures was 1.68% (95% CI, 1.67%–1.70%) in patients with stroke, as compared with 0.15% (95% CI, 0.15%-0.15%) among the general population of California, Florida, and New York (IRR, 7.3; 95% CI, 7.3–7.4) (Table 1). By 8 years, the cumulative rate of an ED visit or hospitalization for seizure was 9.27% (95% CI, 9.16%–9.38%) after discharge with stroke, as compared to 1.21% (95% CI, 1.21%–1.22%) in the general population. The annual incidence of seizures and the cumulative rate of seizures were highest in patients with hemorrhagic stroke subtypes (Figure 1). Stroke was also associated with our secondary outcome of subsequent status epilepticus resulting in hospitalization (IRR, 10.6; 95% CI, 9.9–11.5).
Table 1.
Seizures after Hospital Discharge for Stroke in California, Florida, and New York, Stratified by Stroke Type
| Stroke Status* | Annual Incidence |
8-Year Cumulative Rate |
Incidence Rate Ratio† |
|---|---|---|---|
| No stroke | 0.15% (0.15-0.15%) | 1.21% (1.21–1.22%) | — |
| All stroke | 1.68% (1.67–1.70%) | 9.27% (9.16–9.38%) | 7.3 (7.3–7.4) |
| Stroke type | |||
| Ischemic stroke | 1.46% (1.44–1.48%) | 8.31% (8.20–8.42%) | 6.3 (6.2–6.4) |
| SAH | 2.71% (2.62–2.81%) | 13.72% (13.08–14.38%) | 13.2 (12.8–13.7) |
| ICH | 3.01% (2.95–3.08%) | 14.73% (14.37–15.10%) | 13.3 (13.0–13.6) |
Abbreviations: ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Data are presented as percent or ratios with the 95% confidence interval in parentheses.
Poisson regression was used to calculate age-, sex-, and race-standardized incidence rate ratios.
Figure 1. Cumulative Rate of an Emergency Department Visit or Hospitalization for Seizures in California, Florida, and New York.
Kaplan-Meier curve showing cumulative rates of seizures in patients with ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) as compared to the general population of California, Florida, and New York.
In subgroup analyses, younger patients and non-white patients were more likely to develop seizures after stroke (Table 2). Among patients <65 years of age, stroke was more strongly associated with subsequent seizures (IRR, 12.0; 95% CI, 11.9–12.2) than in patients ≥65 years of age where the association between stroke and post-discharge seizures was similar to what we found in the Medicare population (IRR, 5.5; 95% CI, 5.4–5.5) (Table 3). A test of interaction confirmed that the association between stroke and seizures varied significantly by age (P < 0.001). The association between stroke and seizures was stronger among non-white patients (IRR, 11.0; 95% CI, 10.8–11.2) than among white patients (IRR, 7.3; 95% CI, 7.2–7.4) (Table 2). A test of interaction confirmed that the association between stroke and seizures varied significantly by race (P < 0.001). The association between stroke and subsequent seizures was similar among men and women (Table 2).
Table 2.
Seizures after Hospital Discharge for Stroke among in California, Florida, and New York, Stratified by Subgroup
| Characteristic* | Annual Incidence |
8-Year Cumulative Rate |
Incidence Rate Ratio† |
|---|---|---|---|
| Age | |||
| <65 years | 2.18% (2.15–2.21%) | 12.2% (12.0–12.5%) | 12.0 (11.9–12.2) |
| ≥65 years | 1.44% (1.43–1.46%) | 7.83% (7.72–7.95%) | 5.5 (5.4–5.5) |
| Sex | |||
| Female | 1.66% (1.64–1.69%) | 9.10% (8.96–9.24%) | 7.3 (7.3–7.4) |
| Male | 1.70% (1.68–1.72%) | 9.46% (9.30–9.63%) | 7.3 (7.2–7.4) |
| Race‡ | |||
| White | 1.42% (1.40–1.44%) | 7.95% (7.83–8.07%) | 7.3 (7.2–7.4) |
| Other | 2.16% (2.13–2.19%) | 11.66% (11.45–11.86%) | 11.0 (10.8–11.2) |
Data are presented as percent or ratios with the 95% confidence interval in parentheses.
Poisson regression was used to calculate age-, sex-, and race-standardized incidence rate ratios.
Self-reported by patients or their surrogate.
Table 3.
Seizures after Hospital Discharge for Stroke among Medicare Beneficiaries, Stratified by Stroke Type
| Stroke Status* | Annual Incidence |
6-Year Cumulative Rate |
Incidence Rate Ratio† |
|---|---|---|---|
| No stroke | 0.62% (0.61–0.62%) | 3.42% (3.39–3.45%) | — |
| All stroke | 3.45% (3.35–3.55%) | 13.37% (12.70–14.08%) | 4.1 (3.9–4.2) |
| Stroke type | |||
| Ischemic stroke | 3.05% (2.95–3.15%) | 12.20% (11.51–12.94%) | 3.6 (3.5–3.7) |
| SAH | 6.18% (5.50–6.95%) | 20.41% (16.39–25.25%) | 7.7 (6.8–8.7) |
| ICH | 6.31% (5.87–6.78%) | 21.50% (18.80–24.53%) | 7.4 (6.9–8.0) |
Abbreviations: ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Data are presented as percent or ratios with the 95% confidence interval in parentheses.
Poisson regression was used to calculate age-, sex-, and race-standardized incidence rate ratios.
Medicare Beneficiaries Patient Characteristics
We identified 1,672,886 Medicare beneficiaries of whom 81,984 had an acute stroke hospitalization. Of these strokes, 68,666 (83.7%) were ischemic strokes, 9,990 (12.2%) were ICH, and 3,328 (4.1%) were SAH. Median patient follow-up time was 5 years (interquartile range 2.6–6). Among patients with stroke, 4,530 (5.53%) developed a seizure; those with subsequent seizures were younger than those without subsequent seizures (Supplemental Table II).
Medicare Claims Analysis
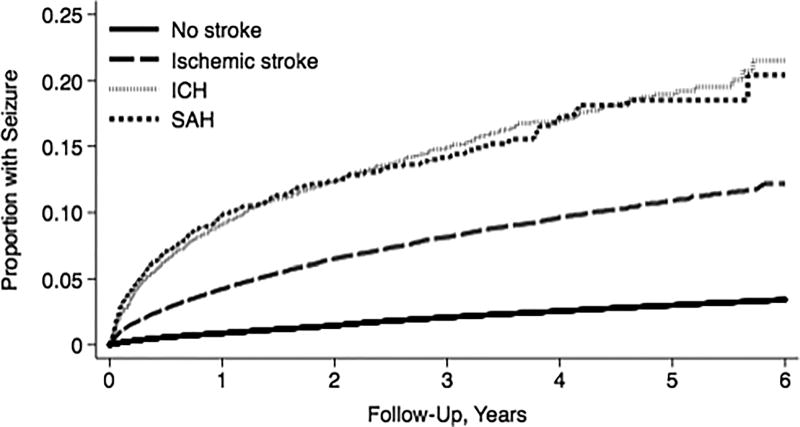
The annual incidence of seizures was 3.45% (95% CI, 3.35%–3.55%) in patients with stroke, as compared with 0.62% (95% CI, 0.61%–0.62%) among the remaining beneficiaries (IRR, 4.1; 95% CI, 3.9–4.2) (Table 3). By 6 years, the cumulative rate of seizure was 13.37% (95% CI, 12.70%–14.08%) after discharge with stroke, as compared to 3.42% (95% CI, 3.39%–3.45%) in the remaining beneficiaries. Similar to the multi-state analysis, the annual incidence of seizures and the cumulative rate of seizures were highest in patients with hemorrhagic stroke subtypes (Table 3) (Figure 2).
Figure 2. Cumulative Rate of Seizures in a 5% Sample of Medicare Beneficiaries.
Kaplan-Meier curve showing cumulative rates of seizures in patients with ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) as compared to the remaining beneficiaries.
In subgroup analyses of the Medicare claims data, the association between stroke and subsequent seizures was similar among white versus non-white patients and among men and women (Table 4).
Table 4.
Seizures after Hospital Discharge for Stroke among Medicare Beneficiaries, Stratified by Subgroup
| Characteristic* | Annual Incidence |
6-Year Cumulative Rate |
Incidence Rate Ratio† |
|---|---|---|---|
| Sex | |||
| Female | 3.47% (3.34–3.60%) | 13.73% (12.79–14.72%) | 4.1 (4.0–4.3) |
| Male | 3.42% (3.27–3.58%) | 12.89% (11.94–13.90%) | 4.0 (3.8–4.2) |
| Race‡ | |||
| White | 3.10% (3.0–3.20%) | 12.28% (11.52–13.08%) | 4.0 (3.9–4.2) |
| Other | 5.29% (5.0–5.6%) | 18.85% (17.48–20.32%) | 4.4 (4.1–4.7) |
Data are presented as percent or ratios with the 95% confidence interval in parentheses.
Poisson regression was used to calculate age-, sex-, and race-standardized incidence rate ratios.
Self-reported by patients or their surrogate.
Discussion
In two large, heterogeneous cohorts of patients, we found that patients who survived a stroke hospitalization faced a significant long-term risk of developing seizures, with a risk that was approximately seven-fold higher than the general population. The relative risk of developing seizures was higher after hemorrhagic stroke and in younger patients.
Prior studies found that between 2.6% and 13.5% of patients with stroke subsequently developed seizures and that the risk of post-stroke seizures was highest after hemorrhagic strokes.2, 10–16, 18 Our study extends these findings by elucidating the long-term risk of seizures according to age, sex, race, and stroke subtype in survivors of stroke.
Age has been previously evaluated as a risk factor for the development of post-stroke seizures with conflicting results.2, 3, 7, 8, 12, 13, 16 In this study, we found that younger age was strongly associated with an increased relative risk of development of seizures after stroke. In addition, we found that non-white race was associated with the development of post-stroke seizures in the multi-state analysis, but not in the Medicare claims analysis where all patients were ≥66 years of age. Although to our knowledge, no previous study has found an association between race and the development of seizures in patients with stroke, a recently published study found that the incidence of epilepsy was significantly higher in black patients.30 Prospective studies are needed to better understand the impact of race on the development of seizures.
Our study has several limitations. First, we relied on ICD-9-CM codes to identify patients with stroke and seizures, which may have led to misclassification of both our exposure and outcomes; however, we used previously validated, reliable diagnosis code algorithms to identify both stroke and seizures. Second, in our multi-state analysis, we only identified seizures resulting in an ED visit or hospitalization which may have led to an underestimation of the risk of seizures. However, the validity of our findings is supported by the similar findings from our separate analysis of Medicare claims which included data on outpatient diagnoses. Third, we lacked data on clinical characteristics such as stroke severity, size, and location—factors that may contribute to the risk of seizures. Likewise, we lacked information regarding the semiology, duration, and frequency of seizures. Fourth, as patients with stroke are often in close contact with a medical professional, the association between stroke and seizures may have been affected by ascertainment bias; however, we found a similar association between stroke and out-of-hospital status epilepticus, a severe form of seizure unlikely to be affected by ascertainment bias. Lastly, we were unable to account for medication use, including prophylactic antiepileptics, which may have influenced the risk of developing seizures and weakened the association between stroke and post-stroke seizures. However, since prophylactic antiepileptics in stroke patients are currently not recommended, the absence of data regarding medication use should not have significantly impacted the validity of our results.31
In two large, heterogeneous cohorts of patients, we found that approximately 10% of patients with stroke went on to develop a seizure within the following decade. Hemorrhagic stroke and younger age appear to be most strongly associated with the development of post-stroke seizures, while an association with non-white race was observed only in the multi-state analysis. Further study of the epidemiology and pathophysiology of post-stroke seizures may lead to improved methods of risk stratification and prevention.
Supplementary Material
Acknowledgments
The authors are grateful to Monica Chen for copyediting and clerical assistance.
Sources of Funding: Dr. Merkler is supported by NIH grant KL2TR0002385 and the Leon Levy Fellowship in Neuroscience. Dr. Parikh is supported by NINDS grant T32NS07153. Dr. Murthy is supported by the American Academy of Neurology, American Brain Foundation, and the Leon Levy Fellowship in Neuroscience. Dr. Navi is supported by NIH grant K23NS091395 and the Florence Gould Endowment for Discovery in Stroke. Dr. Iadecola is supported by NIH grants R37NS089323-02, R01NS034179-21, R01NS037853-19, R01NS100447, and R01NS073666-04. Dr. Kamel is supported by NIH grants K23NS082367, R01NS097443, U01NS095869, as well as the Michael Goldberg Research Fund.
Footnotes
Conflicts-of-Interest: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57:1617–1622. doi: 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- 3.Bryndziar T, Sedova P, Kramer NM, Mandrekar J, Mikulik R, Brown RD, Jr, et al. Seizures Following Ischemic Stroke: Frequency of Occurrence and Impact on Outcome in a Long-Term Population-Based Study. J Stroke Cerebrovasc Dis. 2016;25:150–156. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butzkueven H, Evans AH, Pitman A, Leopold C, Jolley DJ, Kaye AH, et al. Onset seizures independently predict poor outcome after subarachnoid hemorrhage. Neurology. 2000;55:1315–1320. doi: 10.1212/wnl.55.9.1315. [DOI] [PubMed] [Google Scholar]
- 5.Xu T, Ou S, Liu X, Yu X, Yuan J, Huang H, et al. Association between seizures after ischemic stroke and stroke outcome: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4117. doi: 10.1097/MD.0000000000004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology. 2014;82:768–776. doi: 10.1212/WNL.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 7.Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997;28:1590–1594. doi: 10.1161/01.str.28.8.1590. [DOI] [PubMed] [Google Scholar]
- 8.Biffi A, Rattani A, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al. Delayed seizures after intracerebral haemorrhage. Brain. 2016;139:2694–2705. doi: 10.1093/brain/aww199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi C, De Herdt V, Dequatre-Ponchelle N, Henon H, Leys D, Cordonnier C. Incidence and predictors of late seizures in intracerebral hemorrhages. Stroke. 2013;44:1723–1725. doi: 10.1161/STROKEAHA.111.000232. [DOI] [PubMed] [Google Scholar]
- 10.Olafsson E, Gudmundsson G, Hauser WA. Risk of epilepsy in long-term survivors of surgery for aneurysmal subarachnoid hemorrhage: a population-based study in Iceland. Epilepsia. 2000;41:1201–1205. doi: 10.1111/j.1528-1157.2000.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 11.Huttunen J, Kurki MI, von Und Zu Fraunberg M, Koivisto T, Ronkainen A, Rinne J, et al. Epilepsy after aneurysmal subarachnoid hemorrhage: A population-based, long-term follow-up study. Neurology. 2015;84:2229–2237. doi: 10.1212/WNL.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 12.Graham NS, Crichton S, Koutroumanidis M, Wolfe CD, Rudd AG. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke. 2013;44:605–611. doi: 10.1161/STROKEAHA.111.000220. [DOI] [PubMed] [Google Scholar]
- 13.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ. 1997;315:1582–1587. doi: 10.1136/bmj.315.7122.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TC, Chen YY, Cheng PY, Lai CH. The incidence rate of post-stroke epilepsy: a 5-year follow-up study in Taiwan. Epilepsy Res. 2012;102:188–194. doi: 10.1016/j.eplepsyres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 15.So EL, Annegers JF, Hauser WA, O'Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology. 1996;46:350–355. doi: 10.1212/wnl.46.2.350. [DOI] [PubMed] [Google Scholar]
- 16.Lahti AM, Saloheimo P, Huhtakangas J, Salminen H, Juvela S, Bode MK, et al. Poststroke epilepsy in long-term survivors of primary intracerebral hemorrhage. Neurology. 2017;88:2169–2175. doi: 10.1212/WNL.0000000000004009. [DOI] [PubMed] [Google Scholar]
- 17.Wang JZ, Vyas MV, Saposnik G, Burneo JG. Incidence and management of seizures after ischemic stroke: Systematic review and meta-analysis. Neurology. 2017;89:1220–1228. doi: 10.1212/WNL.0000000000004407. [DOI] [PubMed] [Google Scholar]
- 18.Zou S, Wu X, Zhu B, Yu J, Yang B, Shi J. The pooled incidence of post-stroke seizure in 102 008 patients. Top Stroke Rehabil. 2015;22:460–467. doi: 10.1179/1074935715Z.00000000062. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality. [Accessed August 30, 2017];Healthcare Cost and Utilization Project. http://hcupnet.ahrq.gov.
- 20.Centers for Medicare and Medicaid Services. [Accessed August 11, 2017];Medicare Limited Dataset Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/
- 21.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. HCUP Methods Series Report # 2011–01. U.S. Agency for Healthcare Research and Quality; 2011. [Accessed August 30, 2017]. Methodological issues when studying readmissions and revisits using hospital adminstrative data. http://www.hcupus.ahrq.gov/reports/methods/methods.jsp. [Google Scholar]
- 23.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 25.Kee VR, Gilchrist B, Granner MA, Sarrazin NR, Carnahan RM. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):183–193. doi: 10.1002/pds.2329. [DOI] [PubMed] [Google Scholar]
- 26.Pugh MJ, Van Cott AC, Cramer JA, Knoefel JE, Amuan ME, Tabares J, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000–2004. Neurology. 2008;70:2171–2178. doi: 10.1212/01.wnl.0000313157.15089.e6. [DOI] [PubMed] [Google Scholar]
- 27.Hardie NA, Garrard J, Gross CR, Bowers SE, Rarick JO, Bland P, et al. The validity of epilepsy or seizure documentation in nursing homes. Epilepsy Research. 2007;74:171–175. doi: 10.1016/j.eplepsyres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 29.United States Census Bureau. [Accessed August 30, 2017];American Communites Survey. http://factfinder.census.gov/
- 30.Choi H, Pack A, Elkind MS, Longstreth WT, Jr, Ton TG, Onchiri F. Predictors of incident epilepsy in older adults: The Cardiovascular Health Study. Neurology. 2017;88:870–877. doi: 10.1212/WNL.0000000000003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.