Abstract
Given the importance of identifying prodromes of dementia with specific etiologies, we assessed whether seven latent classes of mild cognitive impairment (MCI), defined empirically based on cognitive, functional, and neuropsychiatric information at initial visit, are associated with distinct clinical outcomes and neuropathological features. We separated 6,034 participants with a baseline diagnosis of MCI into seven latent classes using previously defined criteria. We found that these latent classes of MCI differed significantly in their clinical outcomes, survival time, and neuropathology. Two amnestic multi-domain subgroups, as well as two other subgroups with functional impairments and neuropsychiatric disturbances, were at higher risk of not only a ‘pure’ form of Alzheimer’s disease (AD) pathology, but also a ‘mixed’ pathology consisting of both AD and vascular features. Moreover, the seven latent classes had different risks of Lewy bodies, hippocampal sclerosis, and frontotemporal lobar degeneration (FTLD). This study indicates that data-driven subgroups of MCI are clinicopathologically informative and, with refinement, could lead to targeted interventions focused on each etiology.
Keywords: Alzheimer’s disease, competing risks, frontotemporal lobar degeneration, latent class analysis, Lewy bodies, mild cognitive impairment, prodromal dementia, survival analysis, vascular dementia
INTRODUCTION
Advances in AD drug development have led to late-stage clinical trials of potentially disease-modifying therapies. Unfortunately, to date, none of these trials has achieved their primary outcome measures (Cummings et al., 2017; Doody et al., 2014; Salloway et al., 2014). In parallel, biomarker studies in both sporadic and autosomal-dominant genetic forms of AD have yielded compelling evidence that dementia due to AD represents the product of a pathological process spanning two decades (Bateman et al., 2012; Jack et al., 2013). The primary target of current therapeutic development, the Aβ peptide that accumulates in senile plaques, is among the earliest lesions in the brains of individuals who develop AD. Since there is ample evidence that anti-amyloid therapeutics achieve target engagement and clearance of Aβ (Nicoll et al., 2003; Sevigny et al., 2016), a plausible explanation for the failure in these clinical trials is that Aβ must be eliminated earlier in the course of disease to produce clinical benefit.
MCI represents an important syndromic entity that encompasses early stages of decline associated with neurodegenerative diseases including AD (Abner et al., 2017; Morris et al., 2001; Petersen et al., 2001; Sperling et al., 2011). While specific criteria and definitions of MCI vary, they generally include significant decline in one or more cognitive domains with relative preservation of functional activities of daily living. When MCI is seen in conjunction with biological markers, such as CSF levels of Aβ and the microtubule-associated protein Tau, that reliably predict the presence of AD pathology, it represents a prodromal stage of AD. Importantly, efforts to develop effective disease-modifying treatments for AD have increasingly focused on identifying and enrolling participants with MCI due to prodromal AD. As symptoms of MCI can be produced by a broad range of etiologies, biomarker confirmation of underlying AD pathology is essential in screening appropriate participants for AD clinical trials. While effective, current methods based on CSF or PET tracers are invasive and costly. The ability to discriminate among individuals with MCI to accurately predict underlying pathology or predict clinical course could have a dramatic impact on the design and execution of disease-modifying clinical trials for AD.
By expanding the phenotype to include not only cognitive performance but also neuropsychiatric and functional features, we previously identified remarkable heterogeneity among persons with MCI, consisting of seven latent classes (Hanfelt et al., 2011). Moreover, two of these latent classes were more likely to have an elevated Rosen-Hachinski score, a marker of probable cerebrovascular disease, suggesting that there might be important differences in etiology among the latent classes (Hanfelt et al., 2011).
The goal of the current study was to investigate the clinicopathological relevance of these data-driven subgroups of MCI, by investigating whether these subgroups differed with regard to both clinical outcomes and neuropathological features. We separated individuals with MCI into one of seven MCI classes based on clinical features at the time of initial MCI diagnosis. The clinical course of these individuals and neuropathological findings at autopsy were analyzed to determine if distinct MCI subgroups followed characteristic clinical trajectories or demonstrated specific associations with pathological features.
MATERIALS AND METHODS
Participants
We used data from 39 past and present ADCs collected between September 2005 and the June 2015 data freeze of the UDS. Inclusion criteria for the current study required that participants had: 1) a diagnosis of MCI at initial visit from the clinicians at each center and 2) non-missing information on age, years of education, and race. In addition, we required that participants had a MMSE score of 22 or greater at initial visit, in order to exclude a small number of MCI subjects with suspiciously low MMSE scores. We considered the additional requirement that participants had a Clinical Dementia Rating score of 0.5 at initial visit. However, we rejected this cutoff since this would place the emphasis on a memory impaired sample and thus miss other cognitive subtypes.
Measures
Phenotypes of MCI at initial visit
Cognitive test scores were based on the UDS Neuropsychological Battery version 2.0, a core battery of measures collected by the ADCs evaluating overall cognitive status (MMSE), executive functioning (Trail Making Test), language (Boston Naming Test, category fluency), attention (Digit Span and Digit Symbol), and episodic memory (Logical Memory, Story A) (Weintraub et al., 2009). Raw scores were converted to standardized scores (z scores) by using the demographic characteristics, specifically age, years of education, and race, of the UDS cognitively normal participants as the reference group (Hanfelt et al., 2011). Functional abilities were evaluated by having the informant complete the Functional Activities Questionnaire, which measures dependence performing IADLs over the previous four weeks (Pfeffer et al., 1982). Informants also received the Neuropsychiatric Inventory Questionnaire to provide a reliable assessment of problematic behavioral changes in the last month (Kaufer et al., 2000). Participants provided a self-report of depressive symptoms via the short form of the Geriatric Depression Scale (GDS; 15 items) (Sheikh & Yesavage, 1986).
Neuropathological features
Neuropathological characteristics were extracted from several versions of the NACC Neuropathology Data Set using the July 2014 data dictionary (last modification 12/5/2016). Reported pathological elements have evolved over time with modifications and additions. Evaluation of classical elements, such as CERAD and Braak scores, was available in nearly all cases (n=410 and 406, respectively), while data on newer (e.g. TDP-43) or less common (e.g. neoplasm) features were more limited. In some instances, distinctions based on anatomic distribution (Brainstem vs. Limbic Lewy bodies) or pathological patterns (e.g., PSP vs. CBD vs. Pick FTLD-Tau subtypes) were grouped together to increase sample size. Combined sample size for Lewy Body included Absent (n=291), Brainstem or Limbic (n=57), and Neocortical (n=47), and combined FTLD-Tau cases included Absent (n=355) and Present (n=55).
Analysis
Participants were assigned objectively into one of seven subgroups of MCI based on characteristics at their initial visit using previously established criteria from our paper on latent class analysis (Hanfelt et al., 2011). We derived the following Classes based on the interpretation of cognitive test scores at least 1.5 SDs below the cognitively normal group as evidence of impairment: 1) “minimally impaired”, a group indistinguishable from the cognitively normal group; 2) “amnestic only” (AMN Only), characterized by a subtle impairment in delayed memory only; 3) “amnestic with functional impairments and neuropsychiatric features” (AMN+FX+NP), characterized by impairments in both immediate and delayed memory, difficulties performing IADL, and neuropsychiatric disturbances; 4) “amnestic multi-domain” (AMN Multi), characterized by impairments across cognitive domains including episodic and semantic memory, language, and executive function; 5) “amnestic multi-domain with functional impairments and neuropsychiatric features” (AMN Multi+FX+NP), a subtype that differed from the AMN Multi group in having difficulties performing IADL and also in having neuropsychiatric disturbances, as well as impairments across a broader spectrum of cognitive domains, including attention and visuomotor skills; 6) “functional impairments and neuropsychiatric features” (FX+NP Only), a group experiencing functional and behavioral impairments but with no cognitive impairment detected in the neuropsychological examination; and 7) “executive function and language impairments” (Exec FX+Lang), a subgroup distinguished neuropsychologically by impairment in nonmemory domains.
In all regression analyses, we adjusted for sex and age at first visit. Logistic regression analysis was used to compare the MCI subgroups with regard to cardiovascular comorbidity at initial visit. GEE with time x subgroup interaction terms, where the effect of time was modelled nonlinearly, was used to compare the longitudinal trajectories of cognitive decline across the MCI subgroups (Liang & Zeger, 1986). Given the relatively short follow-up time, a quadratic model sufficed to depict any nonlinear rates of decline. We adjusted for selective attrition in the GEE analysis by including stabilized inverse probability of attrition weights based on sex, age, and MCI subgroup (Weuve et al., 2012). Overall survival times were compared using proportional-hazards regression. Time to conversion from MCI to dementia, as diagnosed by clinicians at each center, were compared using the standard competing risks method of Fine and Gray (1999). Since neuropathology developed over time and was observed only at autopsy, to compare neuropathological features among the MCI subtypes it was important that we incorporated into the analysis the time to death: otherwise, naively ignoring the time to death would have led, for example, to the spurious conclusion that the least-impaired subtype had the highest proportion of lacunes, owing to the tangential fact that the least-impaired subtype lived the longest. To avoid such spurious conclusions, we compared the MCI subtypes with regard to neuropathological features, taking into account both the types of the neuropathological features and the time point of the observation (i.e., death). More specifically, we studied sets of neuropathological findings, defined so that death with one type of neuropathological feature excluded deaths with other neuropathological types. For example, when studying neuritic plaques, death with frequent plaques excluded the possibility of death with either moderate plaques or sparse/no plaques. As another example, when studying mixed neuropathologies, death with the combination of AD pathology and large vessel disease excluded three other possibilities: (i) death with AD pathology and without large vessel disease, (ii) death with large vessel disease and without AD pathology, and (iii) death with neither AD pathology nor large vessel disease. We formulated the outcome as time to death with each given type of neuropathological feature, subject to competing risks, which we summarized by subdistributions or subdistributional hazards (Fine & Gray, 1999). Since the autopsy consent rate was not 100%, the neuropathological information at death was missing for a considerable proportion of deceased subjects. Moreover, consent to autopsy was associated with race, age and follow-up time, and so the missing neuropathological information was not missing completely at random. For this reason, we applied a multiple imputation method that adjusted for race, age, and follow-up time, to help recover unobserved neuropathological information. We incorporated the multiple imputation results into the analysis of competing risks data, following the approach justified by Bakoyannis et al. (2010). Without the use of multiple imputation, the selective missing information on neuropathological features could have led to biased results. For example, without multiple imputation we would have found that the least impaired subtype of MCI had the highest risk of death with a moderate Braak stage of neurofibrillary degeneration, a spurious result owing to the composition of this MCI subtype in terms of race, time on study, and age.
P-values less than 0.05 were regarded as significant, as were hazard ratios with 95% confidence intervals that excluded the null value 1. In this exploratory study, we did not require adjustments for multiple comparisons, although in certain tables we highlight results that remained significant after Bonferroni correction, and in other supplemental tables we include the results of multivariate Wald tests for the multiple subtypes of MCI.
RESULTS
MCI participants’ demographic and clinical characteristics are given in Table 1. A total of 6,034 individuals were categorized as MCI, and mean follow-up period following the initial diagnosis of MCI was 2.6 years. There were 818 deaths among these individuals, and brain autopsy was performed on 411 individuals.
Table 1.
Demographic and clinical characteristics of 7 latent classes of MCI participants from the Uniform Data Set: Mean ± SD or frequency (%).
| Latent classes of MCI at first visit | |||||||
|---|---|---|---|---|---|---|---|
| Class 1: Minimally impaired | Class 2: Amnestic only | Class 3: Amnestic with functional impairment & neuropsychiatric features | Class 4: Amnestic multi-domain | Class 5: Amnestic multi-domain with functional impairment & neuropsychiatric features | Class 6: Functional impairment & neuropsychiatric features only | Class 7: Executive function & language impairments | |
| N | 472 (8%) | 1082 (18%) | 958 (16%) | 738 (12%) | 886 (15%) | 965 (16%) | 933 (15%) |
| Age at initial visit, y | 73.1 ± 8.9 | 73.5 ± 8.9 | 73.5 ± 9.1 | 75.4 ± 9.6 | 74.8 ± 9.7 | 72.5 ± 9.9 | 75.1 ± 9.1 |
| Sex: Female | 297 (63%) | 505 (47%) | 443 (46%) | 338 (46%) | 437 (49%) | 466 (48%) | 541 (58%) |
| Race: White | 350 (74%) | 935 (86%) | 886 (92%) | 623 (84%) | 676 (76%) | 803 (83%) | 618 (66%) |
| Black/African American | 98 (21%) | 110 (10%) | 53 (6%) | 82 (11%) | 183 (21%) | 128 (13%) | 287 (31%) |
| Other | 24 (5%) | 37 (3%) | 19 (2%) | 33 (4%) | 27 (3%) | 34 (4%) | 28 (3%) |
| Hispanic | 11 (2%) | 24 (2%) | 28 (3%) | 33 (4%) | 112 (13%) | 64 (7%) | 50 (5%) |
| Education, y | 15.2 ± 2.8 | 15.7 ± 2.9 | 15.6 ± 2.9 | 15.7 ± 3.2 | 14.2 ± 3.9 | 14.8 ± 3.2 | 14.8 ± 3.4 |
| MMSE at initial visit | 28.9 ± 1.1 | 28.3 ± 1.4 | 26.7 ± 2.1 | 26.3 ± 2.0 | 25.9 ± 2.2 | 28.1 ± 1.6 | 27.3 ± 2.0 |
| Follow-up time, y | 3.3 ± 2.9 | 2.8 ± 2.5 | 2.8 ± 2.5 | 2.4 ± 2.3 | 2.1 ± 2.1 | 2.5 ± 2.3 | 2.5 ± 2.6 |
| Died | 38 (8%) | 86 (8%) | 153 (16%) | 112 (15%) | 208 (23%) | 118 (12%) | 103 (11%) |
| Neuropathological exam | 27 (71%) | 34 (40%) | 80 (52%) | 63 (56%) | 97 (47%) | 67 (57%) | 43 (42%) |
Tests of differences between latent classes: age at initial visit, χ2(6) = 71.6, p < 0.001; sex, χ2(6) = 72.0, p < 0.001; race, χ2(12) = 320.2, p < 0.001; Hispanic, χ2(6) = 138.2, Monte-Carlo p < 0.001; education, χ2(6) = 128.9, p < 0.001; MMSE at initial visit, χ2(6) = 1349.0, p < 0.001; follow-up time, χ2(6) = 80.8, p < 0.001; died, χ2(6) = 128.6, p < 0.001; neuropathological exam, χ2(6) = 18.5, p = 0.005.
Cognitive decline
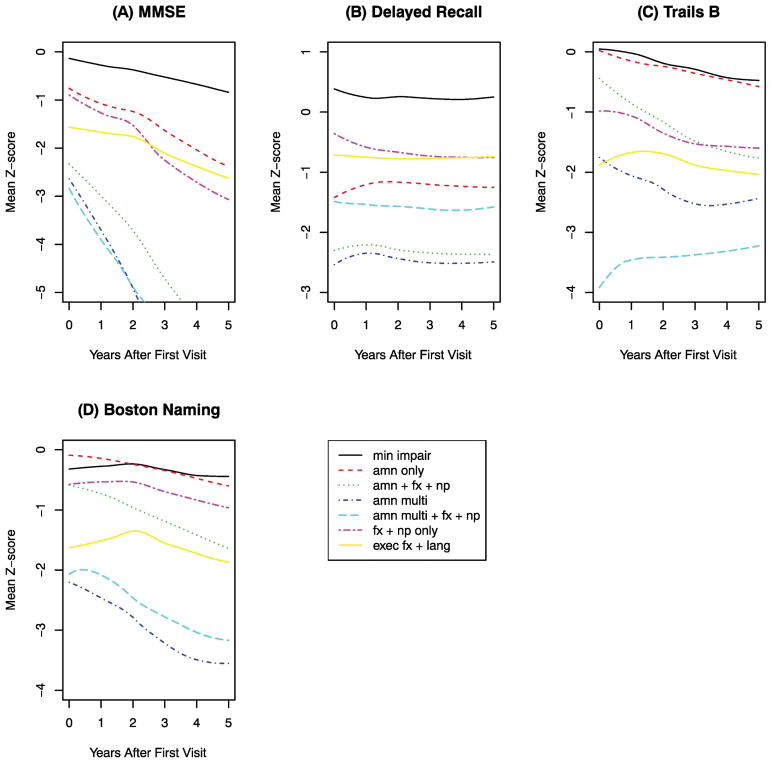
The four cognitive tests with the most separation across latent classes are shown in Figure 1. These included a measure of global cognitive performance (MMSE), and domain-specific measures of memory (Logical Memory – Delayed Recall), executive function (Trail Making Test – Part B), and language (Boston Naming Test). To explore the rapidity of cognitive decline, which was possibly nonlinear, we fitted regression models that included the quadratic effects of follow-up time and adjusted for sex and age at baseline.
Figure 1.
Longitudinal trajectories of cognitive performance for each of seven latent classes of MCI. After adjusting for sex and age at initial visit, the MCI classes had significant differences in the rate of cognitive decline. (A) Comparison of slopes of MMSE z-score: χ2(6) = 314.98, P < 0.001. (B) Comparison of slopes of Delayed Recall z-score: χ2(6) = 119.98, P < 0.001. (C) Comparison of slopes of Trails B z-score (reversed in sign so that a decrease in Trails B z-score indicates worse performance): χ2(6) = 186.92, P < 0.001. (D) Comparison of slopes of Boston Naming z-score: χ2(6) = 142.78, P < 0.001. For details on regression coefficients see Supplemental Table A1.
We found that the latent classes also differed with regard to the rapidity of cognitive decline. Except as noted below, nonlinear effects were minor (Supplemental Table A1), indicating that decline could generally be regarded as linear during the relatively short follow-up period. MCI individuals in the Minimally Impaired class showed mildest baseline impairments and slowest decline over time, and this group was used for statistical comparison with other MCI Classes. The MCI Minimally Impaired subgroup did not differ from cognitively normal individuals in any of the selected UDS variables (Hanfelt et al. 2011), but they were deemed to be not normal after expert review. It is possible that distinguishing clinical and neuropathological features of MCI subgroups might have been stronger if comparisons were made against normal controls. Latent class analysis identified the ‘minimally impaired’ subgroup as a distinct MCI Class, and given its features, this group served as a reasonable benchmark for other MCI classes. To aid in clinical interpretation, we regarded a given Class as having either a ‘steeper’ or ‘much steeper’ rate of decline than the Minimally Impaired class if the difference in slopes between the two classes exceeded either 0.15 z-units per year or 0.50 z-units per year, respectively; otherwise, the two classes were regarded as ‘similar’ in rate of decline. The AMN Only class and the FX+NP Only class experienced a steeper rate of decline in overall cognitive performance (MMSE), but were similar to the Minimally Impaired class in the rate of decline in each of the cognitive domain-specific measures. Three MCI classes (AMN+FX+NP, AMN Multi, and AMN Multi+FX+NP), characterized by memory loss with additional discriminating features, experienced much steeper decline in overall cognition (MMSE), as well as steeper decline in language (Boston Naming). The AMN+FX+NP and AMN Multi classes also showed steeper decline in executive function (Trails B), but curiously, the AMN Multi+FX+NP class – slightly improved in executive function. This latter result might be attributable to a floor effect, since the AMN Multi+FX+NP class remained much more impaired in executive function than any of the other classes. The AMN Multi+FX+NP class also exhibited a moderately large nonlinear effect with regard to MMSE: this class was more impaired than the other classes during the initial years of the study, and subsequently slightly improved in MMSE (Supplemental Table A1). Finally, the Exec FX+Lang class differed from the Minimally Impaired class in cognitive performance at initial visit but did not differ in the rate of cognitive decline in any of the measures. Surprisingly, none of the classes exhibited appreciable decline in delayed logical memory during the study period.
Clinical outcomes
During the follow-up period, 1730 (29%) participants converted from MCI to dementia, with symptoms attributed to a range of etiologies (Table 2). Clinically, the majority of incident dementia cases (81%) were felt to be caused by underlying AD.
Table 2.
Conversions to dementia, based on the most recent clinical diagnosis: frequency (%).
| Probable/possible AD | 1398 (81%) |
| Dementia with Lewy bodies | 79 (5%) |
| Vascular dementia | 65 (4%) |
| Primary progressive aphasia | 42 (2%) |
| Parkinson’s disease | 35 (2%) |
| Frontotemporal dementia | 26 (2%) |
| Other/undetermined dementia | 85 (5%) |
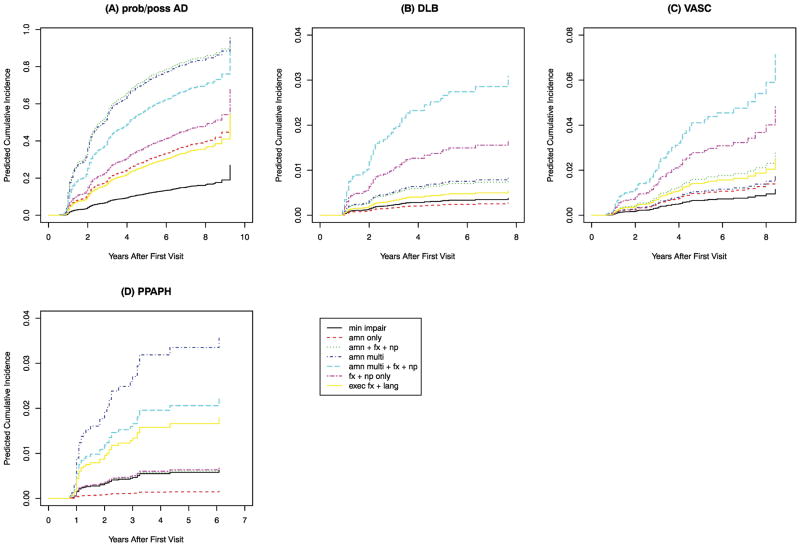
After adjusting for sex and age, the MCI latent classes differed significantly in their rates of conversion to dementia due to various etiologies (the findings for the four most frequently attributed etiologies are shown in Figure 2 and Supplemental Table A2). Compared to the Minimally Impaired class, three classes (AMN Only, AMN+FX+NP, and Exec FX+Lang) were more likely to convert to pAD, but were not associated with any other forms of dementia. The AMN Multi class was also significantly more likely to convert to pAD, and was the only MCI class at significantly higher risk of PPA. The AMN Multi+FX+NP class and the FX+NP Only class were both at significantly higher risk than the Minimally Impaired class to convert to pAD and DLB, and the AMN Multi+FX+NP class was the only class with significantly elevated risk of VaD. Although limited by the relatively small number of conversions to non-AD dementias, these observations reflect potential discriminating ability of MCI latent classes to predict subsequent clinical course.
Figure 2.
Subdistribution of time to conversion to a specific type of dementia for each of seven latent classes of MCI. Results are shown for females age 75. After adjusting for sex and age at initial visit, the MCI classes had significant differences in the times to conversion (see Supplemental Table A2).
Cardiovascular comorbidity
The MCI latent classes did not differ significantly in the odds of coronary artery disease, as measured by self-report of either myocardial infarction, cardiac arrest, cardiac bypass procedures or angioplasty (Table 3). However, the AMN Multi+FX+NP class and the FX+NP Only class each had significantly higher odds of atrial fibrillation or pacemaker procedures than the minimally impaired class. Moreover, the Exec FX+Lang class had significantly higher odds than the Minimally Impaired class of experiencing atrial fibrillation or pacemaker procedures -- but only for recent or active events, excluding the remote or inactive events. In our previous work defining MCI latent classes, we found that the AMN Multi+FX+NP class and the FX+NP Only class were also more likely to have modified Hachinski ischemic scale scores >4 and speculated that these groups might represent those that would correspond to clinical diagnoses of vascular cognitive impairment and vascular dementia (Hanfelt et al., 2011). However, neuropathological features of MCI Classes do not show particular enrichment of vascular events in these groups (Table 5). These findings suggest an unexpected relationship between MCI latent classes and cardiac dysrhythmias, but no specific relationship to coronary artery disease which is typically associated with atherosclerosis and strokes.
Table 3.
Odds ratios (95% confidence intervals) for cardiovascular comorbidity, based on self-reported information collected at first visit. Results are adjusted for age and sex. Confidence intervals displayed in red do not contain the value 1. No results would remain significant after Bonferroni adjustment when comparing six latent classes to the minimally impaired class.
| Latent classes of MCI at first visit | |||||||
|---|---|---|---|---|---|---|---|
| Class 1: Minimally impaired | Class 2: Amnestic only | Class 3: Amnestic with functional impairment & neuropsychiatric features | Class 4: Amnestic multi-domain | Class 5: Amnestic multi-domain with functional impairment & neuropsychiatric features | Class 6: Functional impairment & neuropsychiatric features only | Class 7: Executive function & language impairments | |
| Heart attack, cardiac arrest, cardiac bypass, or angioplasty | |||||||
| Recent | 1 | 1.25 (0.66, 2.35) | 1.42 (0.75, 2.68) | 1.16 (0.59, 2.26) | 1.31 (0.69, 2.51) | 1.65 (0.88, 3.09) | 1.52 (0.80, 2.87) |
| Recent or remote | 1 | 0.95 (0.68, 1.33) | 0.90 (0.64, 1.27) | 0.96 (0.68, 1.36) | 1.05 (0.75, 1.47) | 1.25 (0.90, 1.75) | 0.94 (0.66, 1.32) |
| Atrial fibrillation or pacemaker | |||||||
| Recent | 1 | 1.20 (0.73, 1.97) | 1.50 (0.92, 2.47) | 1.49 (0.90, 2.48) | 1.78 (1.09, 2.91) | 1.64 (1.001, 2.69) | 1.64 (1.004, 2.69) |
| Recent or remote | 1 | 1.14 (0.74, 1.75) | 1.36 (0.88, 2.08) | 1.49 (0.96, 2.30) | 1.67 (1.10, 2.56) | 1.59 (1.04, 2.43) | 1.37 (0.90, 2.11) |
Table 5.
Sub-distribution hazard ratios (95% confidence intervals) for time to death with specific neuropathological features. Results are adjusted for age and sex. Confidence intervals displayed in red or green do not contain the value 1. Entries displayed in green would remain significant under Bonferroni adjustment when comparing six classes versus the minimally impaired class.
| Latent classes of MCI at first visit | |||||||
|---|---|---|---|---|---|---|---|
| Class 1: Minimally impaired | Class 2: Amnestic only | Class 3: Amnestic with functional impairment & neuropsychiatric features | Class 4: Amnestic multi-domain | Class 5: Amnestic multi-domain with functional impairment & neuropsychiatric features | Class 6: Functional impairment & neuropsychiatric features only | Class 7: Executive function & language impairments | |
| Alzheimer’s features | |||||||
| Braak score | |||||||
| moderate | 1 | 0.95 (0.54, 1.67) | 1.53 (0.89, 2.63) | 1.55 (0.89, 2.71) | 2.66 (1.60, 4.45) | 1.52 (0.88, 2.63) | 1.22 (0.70, 2.12) |
| severe | 1 | 1.97 (0.84, 4.59) | 6.45 (2.94, 14.2) | 5.52 (2.46, 12.4) | 5.54 (2.48, 12.4) | 3.79 (1.67, 8.60) | 2.62 (1.14, 6.04) |
| CERAD score | |||||||
| moderate | 1 | 0.99 (0.44, 2.23) | 2.20 (1.05, 4.62) | 2.42 (1.14, 5.15) | 3.57 (1.74, 7.32) | 2.10 (0.99, 4.44) | 1.91 (0.91, 4.02) |
| frequent | 1 | 3.57 (1.24, 10.3) | 8.95 (3.23, 24.8) | 8.74 (3.11, 24.5) | 9.67 (3.48, 26.9) | 5.21 (1.83, 14.9) | 2.75 (0.93, 8.09) |
| Diffuse plaques | |||||||
| moderate | 1 | 1.12 (0.46, 2.69) | 1.70 (0.73, 3.96) | 1.58 (0.65, 3.83) | 3.23 (1.45, 7.18) | 2.00 (0.87, 4.63) | 1.68 (0.72, 3.91) |
| frequent | 1 | 1.67 (0.87, 3.18) | 4.43 (2.42, 8.10) | 3.94 (2.11, 7.36) | 4.98 (2.69, 9.20) | 3.57 (1.92, 6.64) | 2.19 (1.16, 4.14) |
| Vascular features | |||||||
| Large vessel disease | 1 | 1.24 (0.73, 2.12) | 2.28 (1.37, 3.79) | 2.56 (1.53, 4.27) | 4.16 (2.53, 6.84) | 1.97 (1.17, 3.31) | 2.21 (1.33, 3.69) |
| Amyloid angiopathy | 1 | 1.17 (0.52, 2.64) | 2.41 (1.13, 5.14) | 3.61 (1.70, 7.67) | 3.55 (1.68, 7.51) | 2.61 (1.22, 5.61) | 1.80 (0.82, 3.96) |
| Lacune | 1 | 0.65 (0.36, 1.19) | 1.13 (0.65, 1.96) | 1.15 (0.65, 2.03) | 1.95 (1.16, 3.29) | 1.25 (0.72, 2.18) | 1.09 (0.62, 1.90) |
| Small vessel disease | 1 | 1.09 (0.63, 1.89) | 2.00 (1.18, 3.37) | 2.18 (1.29, 3.70) | 3.63 (2.19, 6.01) | 2.20 (1.31, 3.71) | 1.49 (0.87, 2.55) |
| Other features | |||||||
| Lewy bodies | |||||||
| brainstem or limbic | 1 | 2.51 (0.87, 7.21) | 4.90 (1.78, 13.5) | 2.64 (0.88, 7.90) | 9.35 (3.44, 25.4) | 3.27 (1.14, 9.38) | 4.18 (1.49, 11.8) |
| neocortical | 1 | 1.87 (0.44, 7.92) | 3.46 (0.87, 13.8) | 4.13 (1.01, 16.9) | 7.30 (1.89, 28.2) | 3.68 (0.92, 14.8) | 1.91 (0.43, 8.48) |
| Hippocampal sclerosis | 1 | 3.90 (0.73, 20.8) | 6.16 (1.20, 31.5) | 7.55 (1.43, 39.7) | 5.54 (1.03, 29.7) | 3.53 (0.64, 19.6) | 3.31 (0.60, 18.2) |
| FTLD-tau | 1 | 1.49 (0.50, 4.43) | 3.15 (1.13, 8.75) | 3.03 (1.04, 8.81) | 5.16 (1.88, 14.2) | 2.67 (0.94, 7.64) | 1.85 (0.62, 5.50) |
| FTLD-TDP | 1 | 2.51 (0.44, 14.4) | 4.00 (0.74, 21.7) | 3.76 (0.64, 22.0) | 9.57 (1.83, 50.2) | 2.45 (0.42, 14.4) | 3.34 (0.58, 19.1) |
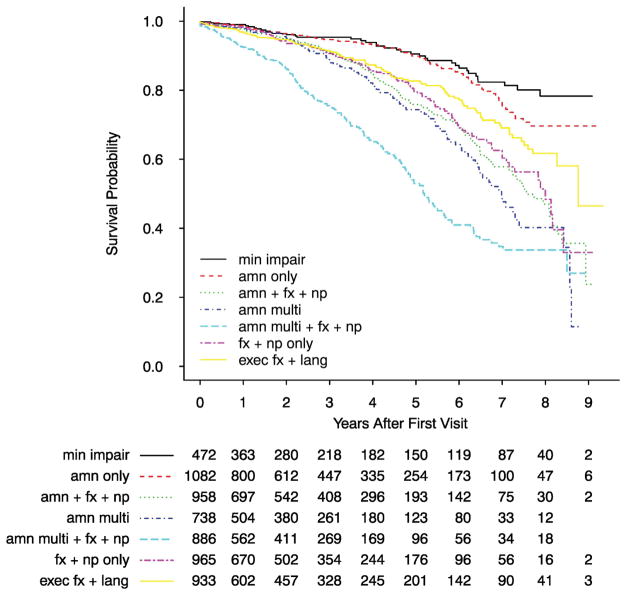
Overall survival
Adjusted for sex and age at initial visit, the AMN Only class had similar survival probability as the Minimally Impaired class while the other five classes each had significantly lower survival probability (Figure 3). In particular, the AMN Multi+FX+NP class had, by far, the worst survival rate, with a median survival time of approximately 5 years after initial visit.
Figure 3.
Survival time distribution for each of seven latent classes of MCI. After adjusting for sex, age at initial visit, and MMSE score at initial visit, the MCI classes had significant differences in survival time: χ2(6) = 165.5, P < 0.001. For details on regression coefficients see Supplemental Table A3.
Neuropathological features
The neuropathology of the AMN Only class resembled that of the Minimally Impaired class, except the AMN Only class was more likely to die with frequent neuritic plaques (Table 5 and Supplemental Figures A1–A11). Compared to the minimally impaired class, four classes (AMN+FX+NP, AMN Multi, AMN Multi+FX+NP, and FX+NP Only) were more likely to die with advanced AD pathology with severe neurofibrillary degeneration and frequent neuritic plaques, and were also more likely to die with various vascular features, including small vessel disease, large vessel disease, or amyloid angiopathy. Three amnestic classes (AMN+FX+NP, AMN Multi, and AMN Multi+FX+NP) had an elevated risk of hippocampal sclerosis, but the FX+NP Only class did not. Both the FX+NP Only class and the AMN+FX+NP class were more likely to have Lewy bodies in the brainstem or limbic region, but not in the neocortex. Neocortical Lewy bodies were seen more frequently only in the AMN Multi class and the AMN Multi+FX+NP class. Only the AMN Multi+FX+NP class had an elevated risk of either FTLD-Tau or FTLD-TDP. Finally, the Exec FX+NP class had ambiguous AD pathologies and vascular features, including severe neurofibrillary degeneration without frequent neuritic plaques and an elevated risk of large vessel disease but not small vessel disease.
We further examined ‘pure’ and ‘mixed’ combinations of AD pathology and vascular pathology, excluding 87 participants with ambiguous AD pathology (Table 6; Supplemental Figures A12–A15). Pathophysiologically distinct types of vascular pathology, including large vessel disease, small vessel disease, and amyloid angiopathy, were considered singly and in combination with AD pathology for relationships to specific MCI Classes. Four classes (AMN+FX+NP, AMN Multi, AMN Multi+FX+NP, and FX+NP Only) were at higher risk of death with not only a ‘pure’ form of AD pathology, but also a ‘mixed’ pathology consisting of the combination of AD with small vessel disease or amyloid angiopathy. The combination of AD and large vessel disease occurred more frequently only among the AMN Multi class and the AMN Multi+FX+NP class. The only latent class with an elevated risk of a ‘pure’ form of vascular pathology was the AMN Multi+FX+NP class, which had higher risk of small vessel disease without AD.
Table 6.
Sub-distribution hazard ratios (95% confidence intervals) for time to death with the combination of AD neuropathology and vascular neuropathology. Excludes 87 participants with ambiguous AD neuropathology. Results are adjusted for age and sex. Confidence intervals displayed in red or green do not contain the value 1. Entries displayed in green would remain significant under Bonferroni adjustment when comparing six classes versus the minimally impaired class.
| Latent classes of MCI at first visit | |||||||
|---|---|---|---|---|---|---|---|
| Class 1: Minimally impaired | Class 2: Amnestic only | Class 3: Amnestic with functional impairment & neuropsychiatric features | Class 4: Amnestic multi-domain | Class 5: Amnestic multi-domain with functional impairment & neuropsychiatric features | Class 6: Functional impairment & neuropsychiatric features only | Class 7: Executive function & language impairments | |
| AD and/or Large Vessel Disease | |||||||
| AD and large vessel disease | 1 | 0.84 (0.42, 1.67) | 1.62 (0.86, 3.04) | 2.16 (1.17, 3.99) | 2.05 (1.11, 3.80) | 1.62 (0.86, 3.05) | 1.24 (0.65, 2.36) |
| AD without large vessel disease | 1 | 1.57 (0.73, 3.38) | 4.53 (2.22, 9.24) | 3.51 (1.66, 7.39) | 4.58 (2.22, 9.43) | 3.04 (1.45, 6.37) | 1.66 (0.76, 3.62) |
| Large vessel disease without AD | 1 | 0.92 (0.29, 2.88) | 1.76 (0.61, 5.07) | 1.40 (0.46, 4.24) | 2.47 (0.88, 6.94) | 2.03 (0.70, 5.85) | 2.18 (0.77, 6.16) |
| AD and/or Amyloid Angiopathy | |||||||
| AD and amyloid angiopathy | 1 | 1.08 (0.47, 2.45) | 2.34 (1.09, 5.02) | 3.40 (1.59, 7.27) | 3.36 (1.58, 7.16) | 2.33 (1.07, 5.05) | 1.28 (0.56, 2.92) |
| AD without amyloid angiopathy | 1 | 1.31 (0.71, 2.40) | 3.20 (1.83, 5.61) | 2.24 (1.24, 4.06) | 4.17 (2.39, 7.31) | 2.08 (1.15, 3.75) | 1.92 (1.07, 3.45) |
| Amyloid angiopathy without AD | 1 | 0.12 (0.01, 1.26) | 1.52 (0.19, 12.0) | 1.27 (0.13, 12.4) | 2.27 (0.29, 17.7) | 1.60 (0.19, 13.4) | 2.46 (0.33, 18.3) |
| AD and/or Lacune | |||||||
| AD and lacune | 1 | 0.94 (0.41, 2.18) | 1.51 (0.68, 3.33) | 1.83 (0.83, 4.04) | 1.89 (0.87, 4.13) | 1.57 (0.70, 3.51) | 1.16 (0.51, 2.64) |
| AD without lacune | 1 | 1.34 (0.74, 2.43) | 3.42 (1.97, 5.94) | 3.09 (1.75, 5.47) | 4.39 (2.52, 7.64) | 2.43 (1.37, 4.32) | 1.78 (0.99, 3.19) |
| Lacune without AD | 1 | 0.96 (0.40, 2.30) | 1.93 (0.87, 4.29) | 1.19 (0.48, 2.95) | 3.50 (1.62, 7.54) | 1.60 (0.70, 3.66) | 1.67 (0.74, 3.81) |
| AD and/or Small Vessel Disease | |||||||
| AD and small vessel disease | 1 | 1.63 (0.79, 3.37) | 2.67 (1.32, 5.39) | 2.89 (1.41, 5.89) | 3.81 (1.90, 7.64) | 2.53 (1.24, 5.18) | 1.77 (0.86, 3.67) |
| AD without small vessel disease | 1 | 0.95 (0.49, 1.83) | 3.05 (1.70, 5.47) | 2.54 (1.38, 4.68) | 3.46 (1.92, 6.24) | 1.91 (1.03, 3.54) | 1.57 (0.84, 2.92) |
| Small vessel disease without AD | 1 | 0.94 (0.37, 2.40) | 1.56 (0.64, 3.77) | 1.12 (0.42, 2.98) | 3.31 (1.43, 7.67) | 2.00 (0.83, 4.79) | 1.83 (0.75, 4.45) |
DISCUSSION
We have employed latent class analytic techniques to define MCI subgroups using a broader array of relevant clinical information at baseline than could be captured by a single measure of impairment such as the MMSE. To further explore the extent to which our MCI latent classes were predictive of decline independent of a single baseline measure of cognitive impairment, we also conducted additional analyses that adjusted for not only sex and age, but also baseline impairment as measured by either the MMSE z-score, Delayed Recall z-score, Trails B z-score, or Boston Naming z-score. Specifically, we entered the baseline impairment variable into the model as a main effect and included interaction with time, where the effect of time was modelled as a quadratic polynomial. We found that, even after adjusting for baseline impairment, the MCI latent classes were strongly associated with cognitive decline (p < 0.001; data not shown) as well as survival time (p < 0.001; Supplemental Table A3). These results confirm the general advantage of latent class methods over single measures of impairment to identify MCI subgroups with differential types and rates of decline.
Prior studies have applied latent class analytic techniques to neuropsychological measures to determine whether empirically derived subtypes correspond to unique biomarker profiles and outcomes (Bangen et al., 2016; Bondi et al., 2014; Clark et al., 2013; Eppig et al., 2017). In general, these studies have demonstrated both the cognitive heterogeneity of MCI as well as differences in associated neuroradiologic, CSF, and genetic profiles among these subtypes. In a previous study, we combined cognitive, functional, and neuropsychiatric features at the first diagnosis of MCI to define seven distinct latent classes of MCI (Hanfelt et al., 2011), and in the current study, we show that these MCI subtypes predict clinically and neuropathologically meaningful outcomes. For example, our FX+NP Only class, characterized by isolated functional impairments and neuropsychiatric disturbances, exhibited a faster decline in cognitive performance on the MMSE, a faster conversion to possible/probable AD, worse survival, and greater associated cardiovascular comorbidity than the MCI minimally impaired group. In addition, compared to the MCI minimally impaired group, those in the FX+NP Only class and the AMN+FX+NP class were at higher risk of death with a ‘pure’ form of AD pathology, as well as a ‘mixed’ pathology consisting of AD combined with either small vessel disease or amyloid angiopathy. These two classes also had a heightened risk of death with Lewy bodies in the brainstem or limbic region, but not in the neocortical region. These findings highlight the value of conceptualizing MCI as a broader phenotypic entity encompassing functional and neuropsychiatric features as well as cognitive patterns.
The MCI Minimally Impaired class exhibited, at first visit, preserved cognitive functioning across all domains according to the UDS neuropsychological battery, and yet these individuals were diagnosed as MCI. While it might be argued that this minimally impaired class did not in fact have a neurodegenerative process, our finding that this class had neuropathology and survival probabilities that were very similar to the AMN Only class which, unlike the Minimally Impaired class, exhibited deficits in Logical Memory at first visit, confirms the accuracy of the clinicians’ initial diagnosis.
In contrast to the minimally impaired subgroup, the AMN Multi+FX+NP class demonstrated a more severe clinical and neuropathological phenotype. Individuals in the AMN Multi+FX+NP class showed more severe impairment at baseline, steeper rates of cognitive decline, and shorter survival time. At autopsy, the AMN Multi+FX+NP class had increased frequency of multiple pathologies including AD, vascular, Lewy bodies, hippocampal sclerosis, Tau, and TDP-43. Identification of MCI Classes is based on cognitive, functional, and neuropsychiatric features at the time an individual is first diagnosed as MCI. It is unclear if the AMN Multi+FX+NP class represents a more malignant subtype or if this group is the amalgamation of high risk groups for various mixed neuropathologies.
A number of studies examining the neuropathology of MCI have been reported (Stephan et al., 2012), and have mostly found that both amnestic and non-amnestic MCI are associated with AD and mixed pathologies at rates similar to those that we found in our study for individuals diagnosed with MCI at first visit to an ADC (Storandt et al., 2006; Markesbery et al., 2006; Schneider et al., 2009). A potential confounding element in our study is that the analysis is of diagnosed MCI cases from ADC, in which there may be an implicit bias toward recruitment and enrollment of those with underlying AD. This concern is alleviated by previous community-based studies which reported high rates of AD pathology similar to those found in the NACC neuropathology data (Lim et al., 1999; Bennett et al., 2006; Sonnen et al., 2007). In addition, the observed frequency of vascular, Lewy body, and other co-pathologies (Table 4) are quite similar to a recent detailed study of mixed pathologies from a community-based cohort (Kapasi et al., 2017). Several unexpected clinical and neuropathological associations with MCI classes were identified in our study. A strong relationship between dementia and cardiovascular disease has been well established in the literature, including associations with both coronary artery disease and atrial fibrillation (Alonso et al., 2017; de Bruijn et al. 2015; Kwok et al., 2011; Ott et al., 1997; Santangeli et al., 2012, Stefanidis et al., 2017). In our examination, none of the MCI classes had an increased risk of either recent or remote coronary artery disease, and only three classes (AMN Multi+FX+NP, FX+NP Only, and Exec FX+Lang) showed any association with atrial fibrillation. The mechanisms by which atrial fibrillation increases risk of cognitive decline and dementia are not fully understood, but some studies indicate that the association is, at least in part, independent of stroke. When examined in conjunction with AD pathologies, increased risk of concomitant large vessel disease was only found in those belonging to the AMN Multi and AMN Multi+FX+NP classes, and none of the MCI classes showed increased risk of AD with lacunar strokes. While our conclusions are constrained by relatively small numbers of autopsies of individuals in various MCI classes with different pathologies of interest, these findings suggest that cardiovascular co-morbidities and vascular co-pathologies may play a role in the genesis of distinct MCI classes.
Table 4.
Neuropathological features: frequency (%)
| Alzheimer’s features | |
| Braak score (N = 406) | |
| moderate | 143 (35%) |
| severe | 138 (34%) |
| CERAD score (N = 410) | |
| moderate | 102 (25%) |
| frequent | 137 (33%) |
| Diffuse plaques (N = 368) | |
| moderate | 73 (20%) |
| frequent | 177 (48%) |
| Vascular features | |
| Large vessel disease (N = 410) | 192 (47%) |
| Amyloid angiopathy (N = 407) | 110 (27%) |
| Lacune (N = 410) | 135 (33%) |
| Small vessel disease (N = 406) | 187 (46%) |
| Hemorrhage (N = 406) | 30 (7%) |
| Other features | |
| Lewy bodies (N = 395) | |
| brainstem or limbic | 57 (14%) |
| neocortical | 47 (12%) |
| Hippocampal sclerosis (N = 400) | 39 (10%) |
| FTLD-tau (N = 410) | 55 (13%) |
| FTLD-TDP (N = 356) | 24 (7%) |
Our finding of a lack of decline in logical memory across all groups was also surprising. We believe that basing impaired performance on delayed recall of a single prose passage has at least two problems. First, shortening a test and detaching it from its standardized administration may make it less sensitive (e.g., Bondi et al., 2014). Second, story recall may be less sensitive to an evolving dementia than tests of verbal list learning (De Jager et al, 2003; Rabin et al., 2009; Tierney et al., 2005).
In this study the MCI participants were not necessarily incident cases, since to enter the study the participant must have a baseline diagnosis of some form of MCI. A general concern with groups defined using prevalent cases is that the groups could differ in intercepts simply because some groups are further along in the disease process at the time of study entry. Nevertheless, we believe our results are valid, for the following reasons. First, the evidence strongly suggests that our MCI latent classes are not severity levels of a unidimensional construct but rather represent a multidimensional construct (p < 0.001; Hanfelt et al., 2011). Second, several community-based prospective studies (e.g., Graziane et al., 2016; Gottesman et al., 2014) have shown that trajectories of cognitive decline are unrelated to intercepts, which suggests that the time of entry of an MCI participant into the UDS affected the baseline cognitive performance but did not have much impact on the trajectory of cognitive decline, which is our main focus. This finding from community-based prospective studies is consistent with the results from our study, where we see that certain latent classes experienced steeper cognitive decline than other classes with similar baseline cognitive performance (Figure 1). Third, the age differences between the latent classes were statistically significant but were not large from a clinical perspective: the average ages of the groups differed by no more than 2.3 years (Table 1). Finally, all our results were adjusted by age and sex.
Other study limitations included a follow-up period that was relatively short, a 50% consent rate to autopsy, and the lack of biomarker data in the UDS.
In summary, we have demonstrated that latent class analysis to identify MCI subgroups combined with survival analysis subject to competing risks, provides a powerful analytical approach to investigate the heterogeneity among individuals diagnosed with MCI and can yield clinicopathologically informative subtypes. In future research, we will refine this approach by considering a large collection of longitudinal trajectories of both clinical and biological information when forming the subtypes, as well as incorporating the uncertainties of latent class membership into the survival analysis. For example, such refinements could help resolve the AMN Multi+FX+NP class plausibly an amalgamation of high risk groups for various mixed neuropathologies – into finer subtypes that are etiologically specific.
Supplementary Material
Highlights.
Heterogeneity of MCI is extensive and characterized by seven latent classes.
These MCI classes differ in their subsequent clinical courses and neuropathologies.
Two MCI classes with functional impairments and neuropsychiatric disturbances have higher risk of a ‘mixed’ pathology consisting of both Alzheimer’s and vascular features, as well as Lewy bodies.
With refinement, this analytical approach could lead to identification of prodromes of dementia with specific etiologies.
Acknowledgments
This study was supported in part by the US National Institutes of Health (grants R01 AG055634 and P50 AG025688). The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). We also thank the ADC participants for their willingness to devote their time to research.
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- ADC
Alzheimer’s Disease Center
- AMN
amnestic
- CSF
cerebrospinal fluid
- DLB
dementia with Lewy bodies
- FTLD
frontotemporal lobar degeneration
- FX
functional impairments
- GEE
generalized estimating equations
- IADL
instrumental activity of daily living
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NACC
National Alzheimer’s Coordinating Center
- NP
neuropsychiatric features
- pAD
probable or possible Alzheimer’s disease
- PET
positron-emission tomography
- PPA
primary progressive aphasia
- TDP-43
TAR DNA-binding protein 43
- UDS
Uniform Data Set
- VaD
vascular dementia
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abner E, Kryscio R, Schmitt F, Fardo D, Moga D, Ighodaro E, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81:549–559. doi: 10.1002/ana.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Knopman D, Gottesman R, Soliman E, Shah A, O’Neal W, et al. Correlates of dementia and mild cognitive impairment in patients with atrial fibrillation: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) J Amer Heart Assoc. 2017 doi: 10.1161/JAHA.117.006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakoyannis G, Siannis F, Touloumi G. Modelling competing risks data with missing cause of failure. Statist Med. 2010;29:3172–3185. doi: 10.1002/sim.4133. [DOI] [PubMed] [Google Scholar]
- Bangen K, Clark A, Werhane M, Edmonds E, Nation D, Evangelista N, et al. Cortical amyloid burden differences across empirically-derived mild cognitive impairment subtypes and interaction with APOE4 Genotype. J Alzheimer’s Dis. 2016;52:849–861. doi: 10.3233/JAD-150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman R, Xiong C, Benzinger T, Fagan A, Goate A, Fox N, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s Disease. New England J of Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Schneider J, Aggarwal N, Arvanitakis Z, Shah R, Kelly J, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bondi M, Edmonds E, Jak A, Clark L, Delano-Wood L, McDonald C, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimer’s Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Delano-Wood L, Libon D, McDonald C, Nation D, Bangen K, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19:635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s & Dementia: Transl Research & Clinical Interventions. 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn R, Heeringa J, Wolters F, Franco O, Stricker B, Hofman A, et al. Association between atrial fibrillation and dementia in the general population. J Amer Med Assoc Neurology. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- de Jager C, Hogervorst E, Combrinck M, Budge M. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer’s disease. Psychol Med. 2003;33:1039–1050. doi: 10.1017/s0033291703008031. [DOI] [PubMed] [Google Scholar]
- Doody R, Thomas R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s Disease. New England J of Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Eppig J, Edmonds E, Campbell L, Sanderson-Cimino M, Delano-Wood L, Bondi M. Statistically derived subtypes and associations with cerebrospinal fluid and genetic biomarkers in mild cognitive impairment: A latent profile analysis. J Intl Neuropsychol Soc. 2017;23:564–576. doi: 10.1017/S135561771700039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- Gottesman R, Rawlings A, Sharrett A, Albert M, Alonso A, Bandeen-Roche K, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Amer J Epidemiology. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane J, Beer J, Snitz B, Chang C-C, Ganguli M. Dual trajectories of depression and cognition: a longitudinal population-based study. Amer J Geriatr Psychiatry. 2016;24:364–373. doi: 10.1016/j.jagp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfelt J, Wuu J, Sollinger A, Greenaway M, Lah J, Levey A, Goldstein F. An exploration of subgroups of mild cognitive impairment based on cognitive, neuropsychiatric and functional features: Analysis of data from the National Alzheimer’s Coordinating Center. Am J Geriatr Psychiatry. 2011;19:940–950. doi: 10.1097/JGP.0b013e31820ee9d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C, Jr, Knopman D, Jagust W, Petersen R, Weiner M, Aisen P, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi A, DeCarli C, Schneider J. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–186. doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D, Cummings J, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kwok C, Loke Y, Hale R, Potter J, Myint P. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Amer Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- Markesbery W, Schmitt F, Kryscio R, Davis D, Smith C, Wekstein D. Neuropathologic substrate of mild cognitive impairment. Arch Neurology. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Morris J, Storandt M, Miller J, McKeel D, Price J, Rubin E, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nicoll J, Wilkinson D, Holmes C, Steart P, Markham H, Weller R. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Ott A, Breteler M, de Bruyne M, Martine C, van Harskamp F, Grobbee D, et al. Atrial Fibrillation and Dementia in a Population-Based Study: The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- Petersen R, Stevens J, Ganguli M, Tangalos E, Cummings J, DeKosky S. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) - Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurol. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pfeffer R, Kurosak T, Harrah C, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rabin L, Pare N, Saykin A, Brown M, Wishart H, Flashman L, et al. Differential memory test sensitivity for diagnostic amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Aging Neuropsychol Cogn. 2009;16:357–376. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox N, Blennow K, Klunk W, Raskind M, et al. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s Disease. New England J of Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangeli P, di Biase L, Bai R, Mohanty S, Pump A, Brantes M, et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm. 2012;9:1761–1768. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Schneider J, Arvanitakis Z, Leurgans S, Bennett D. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussière T, Weinreb P, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Yesavage J . Geriatric Depression Scale (GDS) Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Hawthorne Press; 1986. pp. 165–173. [Google Scholar]
- Sonnen J, Larson E, Crane P, Haneuse S, Li G, Schellenberg G, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurology. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sperling R, Aisen P, Beckett L, Bennett D, Craft S, Fagan A, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer”s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis K, Askew C, Greaves K, Summers M. The effect of non-stroke cardiovascular disease states on risk for cognitive decline and dementia: A systematic and meta-analytic review. Neuropsychol Rev. 2017 doi: 10.1007/s11065-017-9359-z. [DOI] [PubMed] [Google Scholar]
- Stephan B, Hunter S, Harris D, Llewellyn D, Siervo M, Matthews F, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17:1056–1076. doi: 10.1038/mp.2011.147. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant E, Miller J, Morris J. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Tierney M, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer’s disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford N, Chui J, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychologic Test Battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.