Abstract
Objectives
Artificial light sources such as visual display units (VDUs) elicit a range of subconscious and reflex light responses, including increases in alertness and suppression of pineal melatonin. Such responses employ dedicated retinal circuits encompassing melanopsin photoreceptors. Here, we aimed to determine whether this arrangement can be exploited to modulate the impact of VDUs on melatonin onset and alertness without altering visual appearance.
Methods
We generated a five-primary VDU capable of presenting metameric movies (matched for color and luminance) but varying in melanopic-irradiance. Healthy human participants (n = 11) were exposed to the VDU from 18:00 to 23:00 hours at high- or low-melanopic setting in a randomized cross-over design and measured salivary melatonin and self-reported sleepiness at 30-minute intervals.
Results
Our VDU presented a 3× adjustment in melanopic-irradiance for images matched photometrically for color and luminance. Participants reported no significant difference in visual appearance (color and glare) between conditions. During the time in which the VDU was viewed, self-reported sleepiness and salivary melatonin levels increased significantly, as would be expected in this phase of the diurnal cycle. The magnitude of the increase in both parameters was significantly enhanced when melanopic-irradiance was reduced.
Conclusions
Our data demonstrate that melatonin onset and self-reported sleepiness can be modulated independent of photometric parameters (color and luminance) under a commonly encountered light exposure scenario (evening use of a VDU). They provide the first demonstration that the impact of light on alertness and melatonin production can be controlled independently of visual experience, and establish a VDU capable of achieving this objective.
Keywords: sleep/wake physiology, chronobiology, light therapy, melatonin, melanopsin, artificial light
Statement of Significance
Visual displays and artificial lighting elicit subconscious/reflex light responses, which realign human physiology and behavioral state. The inclusion of melanopsin in these pathways presents an opportunity to regulate reflex light responses without adjusting visual appearance. We have developed a new type of visual display, comprised of five distinct spectral channels, which allows us to regulate the activity of melanopsin independently of color and luminance. We find that modulating melanopsin activity in this way can regulate self-reported sleepiness and salivary melatonin in healthy human participants. This work thus establishes a new, practical approach to modulating the impact of displays on melatonin onset and self-reported alertness without impacting visual experience.
Introduction
Intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin drive a range of reflex/subconscious responses to light (sometimes also called non-image forming responses) [1–9] that together can constitute a fundamental realignment of behavioral and physiological state. The ability of light to elicit such responses is increasingly taken into consideration in the design and application of light emitting devices. Accepted methods of modulating reflex/subconscious light responses—changing light intensity (irradiance) or controlling energy over the blue portion of the spectrum [10–15]—also change visual appearance, often in unwanted ways (e.g. adjusting perceived color or brightness of an image). In the context of visual displays, this results in a fundamental conflict between the need to produce pleasing images and the wish to control other physiological impacts. The inclusion of melanopsin in the pathways that regulate many of these physiological functions, however, raises the exciting prospect of modulating reflex/subconscious light responses without altering visual appearance by using the concept of metamerism.
Metamers are stimuli with divergent spectral power distributions that have the same color and luminance. Metamers achieve this effect because each of the human cone photoreceptors responds to the total light across a wide range of wavelengths, weighted according to their spectral sensitivity. Accordingly, it is possible to make balanced changes in the intensity and wavelength of light without altering the effective photon flux for any given photoreceptor. The divergent spectral power distributions of metamers exploit this phenomenon by having equivalent effective radiance not just for one but for all three of the human cone photoreceptors. As cones are considered to define the important parameters of visual perception (color, brightness, lightness) under photopic conditions, it follows that metamers have the same visual appearance. This concept is employed by RGB (Red, Green, Blue) displays to recreate realistic images using a spectral composition that is very different from that of the real world. Metamerism is helpful in the context of subconscious/reflex visual functions because switching between metamers differing in energy over those wavelengths to which melanopsin is most sensitive could, in theory, allow modulation of subconscious/reflex light responses amplitude without changing visual appearance [16–19]. We have recently developed displays designed according to this principle capable of presenting still images and movies differing in melanopsin radiance suitable for use in mice (which have only two cone types) [20, 21]. Achieving an equivalent outcome for a human observer (with three cone types) provides an additional technological challenge and, to date, such displays have not been available. Even if available, it is not certain that such displays would have the hoped for effect on reflex light responses. ipRGCs receive input from rods and cones as well as melanopsin, and the degree to which reflex/subconscious responses under real-world scenarios are defined by the activity of each receptor class remains uncertain [1]. Given the similarity in spectral sensitivity between rods and melanopsin, metamers targeting differences in melanopsin would be equally appropriate for light-evoked responses driven by rods. On the other hand, if cones dominate reflex light responses, then metamers would, by definition, have nearly equivalent efficiency. Here we therefore set out to produce a visual display capable of presenting metameric images differing in melanopsin effective irradiance to humans, and to test the ability of this device to control two reflex light responses—melatonin onset and self-reported alertness—in the context of a typical real-world scenario of screen use: passive viewing of television content in the evening.
Methods
This study was approved by the Ethics Committee northwest/central Switzerland (EKNZ) and was conducted in accordance with the Swiss law and according to the Declaration of Helsinki.
Participants, eligibility criteria and setting
Participants were recruited at the University of Basel. The inclusion criteria were young healthy males with a regular sleep/wake rhythm and a good understanding of the English language. Females were excluded in this first study to maximize statistical power in view of reported sex-related differences in melatonin levels, sleepiness [22], and light sensitivity [23]. The screening process contained a general health questionnaire, the Pittsburgh Sleep Quality Index (PSQI), the Morningness-Eveningness Questionnaire (MEQ) and a questionnaire about caffeine intake. All participants (age range 21–30 years) were good sleepers (PSQI score between 2 and 5), intermediate or moderate morning types (MEQ scores between 35 and 68, with the exception of one moderately evening type) and low to moderate caffeine consumers (0–3 cups of coffee per day). Caffeine intake after 12:00 hours was forbidden during the laboratory days. Participants were asked to adhere to a regular sleep–wake cycle with bedtimes between 23:00 and 24:00 hours followed by 8 hours of sleep for at least 4 days prior study begin. Compliance was checked by sleep logs and actigraphy recordings. Average bed times were: 23:36 ± 1.1 hours (mean ± SD) and average rise time: 07:36 ± 1.2 hours (mean ± SD). Thirteen males were recruited, two were excluded as outliers, the first one based on the fact that melatonin decreased rather than increased over the course of the evening indicating inappropriate phasing of the melatonin rhythm, and the second because he had no measurable dim light melatonin onset (DLMO). The study was undertaken between April and July 2017.
Visual stimuli
A display device was generated by superimposing the output of two DMD projectors (model PA600X; NEC, Japan) to produce a composite 2 × 3 primary image on a projector screen. By inserting a notch filter (484–604 nm) into the light path of one projector, and bandpass filter (463/571 nm; both Pixelteq, United States) into the second, we were able to generate five distinct color planes: violet, cyan, green, yellow, and red. Adjusting the output of the five channels (0–255 resolution) allowed us to generate two stimuli that differed ~threefold in their excitation of melanopsin (24.7 vs. 77.7 melanopic lux) and twofold in excitation of rods (71.8 vs. 34.8 rhodopic lux), termed “low-melanopic” and “high-melanopic,” but were matched in terms of standard measures of color and brightness for visual displays (Commission Internationale de l’Eclairage [CIE] 10 degree xy coordinates = 0.40 and 0.36; photopic luminance = 79 cd/m2 and corneal photopic illuminance = 73.5 lux in both conditions). Differences in cone alpha-opic illuminance [1] were commensurately small (<5% Michelson contrast; 81.4 vs. 76.1 erythropic lux, 74.4 vs. 68.4 chloropic lux, 51.3 vs. 56.5 cyanopic lux in high- and low-melanopic, respectively). Our stimuli were also designed to be matched in their excitation of cones in the central retina, accounting for the filtering properties of the macular pigment (<2% contrast for erythropic, chloropic, and cyanopic lux in the central 2 degrees of the visual field [accounting for filtering of light by macular pigment]; CIE 2 degree xy coordinates = 0.40 and 0.37 and photopic luminance = 79 cd/m2). Measurements were made with a SpectroCAL spectroradiometer, Cambridge Research Systems UK. A gamma correction was measured for each stimulus, so that a range of isoluminant “greyscale” values could be produced in the low- or high-melanopic condition. Projectors were controlled independently with a PC running Processing v.3.3.1 (Processing Foundation), which converted RGB images into “greyscale” high- and low-melanopic images.
Questionnaires
Sleeping behavior and habits were retrieved from several questionnaires (Pittsburg Sleep Index, Epworth Sleepiness Scale, and the MEQ) that the participants got at arrival in the lab. Caffeine consumption was also assessed by means of a questionnaire.
During the experiment every 30 minutes we assessed the sleepiness by means of the Karolinska Sleepiness Scale (KSS). At the same time points, self-reported light comfort was rated on a scale from 1 to 7 regarding self-reported glare and observed light color (warm vs. cool).
Study design
Participants came to the lab twice on the same evening in two consecutive weeks for a period of 5.5 hours. In this timespan, the participants watched two movies and played some board games. The participants had access to several two-player games (Catan, Agricola, Yatzee, and Skipbo) and were free to choose the games they liked best. Every 30 minutes the participants would stop watching the movie or playing board games to answer questionnaires and give a saliva sample. At the moment of the measurements, a white screen with a fixation cross was displayed by the projectors. The participants would sit during the gaming phase, about 1.5 m from the projected screen (Supplementary Figure 1). During the movies, they would be seated about 2 meters in front of the screen, and during the gaming phase it differed between the participants, either faced directly toward the screen, or at an angle of 90 degrees. During the gaming phase, participants did not look directly at the screen.
Melatonin profiles
To assess the evening melatonin rise, participants collected saliva samples every 30 minutes using Salivettes (Sarstedt AG, Sevelen, Switzerland). Samples were refrigerated after collection at ~4° until melatonin measurements. Melatonin was measured by means of direct double-antibody radioimmunoassays (analytical sensitivity 0.2 pg/ml) and a functional minimum detectable dose of 0.65 pg/ml (Bühlmann Laboratories AG, Allschwil, Switzerland). During the day, salivary melatonin is normally under 3 pg/ml and can go up to 20 pg/ml at bedtime.
Statistical analysis
Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). All output variables (i.e. self-reported sleepiness, melatonin levels, light color, and glare) were statistically analyzed with mixed-model analyses of variance (PROC MIXED) with main repeated predictors “light condition” (low- and high-melanopic) and “time of day” (time points) and subject as random factor with a combined unstructured and autoregressive covariance structure [24]. All output variables showed a non-Gaussian distribution and different transformation approaches, based on the PROC TRANSREG, did not lead to a normal distribution. In addition, the data yielded high inter-individual variability, which could be reduced with a z-transformation per subject before entering the PROC MIXED. This led to best Akaike information criterion compared to models based on untransformed, log-transformed and square-root-transformed data. For graphical representation, the time course of un-transformed data was displayed in the figures. Post hoc comparisons between light conditions were based on paired Wilcoxon tests at different time points.
Results
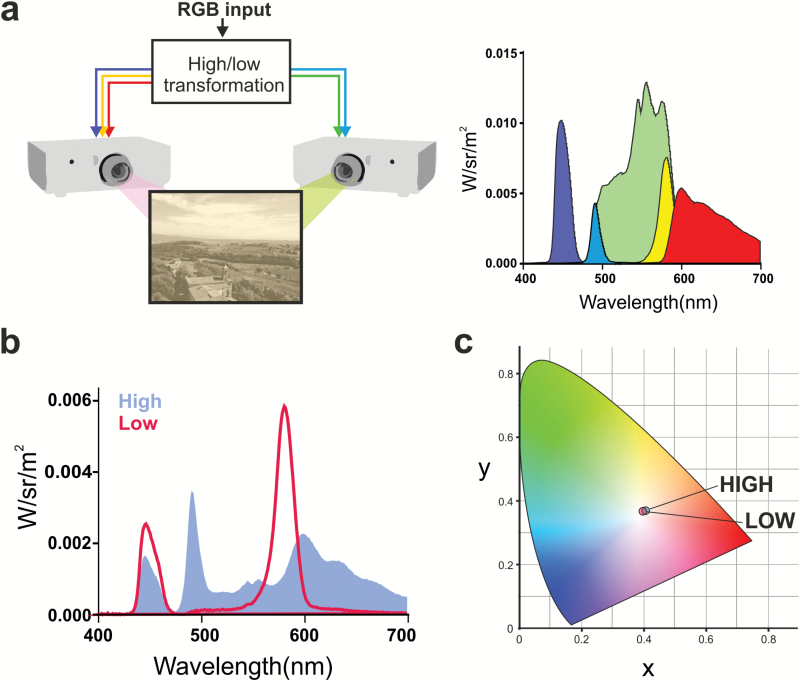
Standard RGB visual displays employ three independently controllable, spectrally distinct, light sources (primaries) to gain control over the three human cone photoreceptors and present patterns in luminance and color. One or more additional primaries are required to produce metameric versions of such images differing in effective intensity for melanopsin. Given the difficulties in constructing such a multi-primary “melanopic” visual display unit (VDU) from scratch, we did so by modifying existing technology. We took two ordinary, three-primary (RGB) digital mirror device projectors and superimposed their output to produce a composite 2 × 3 primary image on a projection screen (Figure 1a). We then introduced optical filters into the light path of each projector to change the spectral composition of the primaries. Although independent control of the three cones and melanopsin can be achieved with four color planes, additional color planes can provide enhanced control, and we, therefore, used the filters to produce a five-primary image (Figure 1a). Using this device we were able to produce monochrome images and movies in which the overall display (and each individual pixel) was matched for color and luminance (CIE xy coordinates: 0.40, 0.36; 79 cd/m2) while melanopic irradiance varied by 3.14 fold (Figure 1b). In order to maximize the difference in melanopic irradiance, we allowed the image to be slightly off-white (Figure 1c). We also ensured that images under high- and low-melanopic irradiance (hereinafter termed “high-melanopic” and “low-melanopic”) were matched for both central and peripheral cones (see Methods).
Figure 1.
Using a five-primary display to generate images varying in melanopic and irradiance. A melanopic display was produced by superimposing images from two projectors (shown in schematic, a; left hand panel) fitted with interference filters to modulate their spectral output in such a way as to produce a total of five independently controllable spectrally distinct output channels (primaries) across the two projectors (shown as blue, cyan, green, yellow, and red spectral power distributions in right hand panel of a). The five primaries were combined to produce high- and low-melanopic settings (blue and red spectral power distributions respectively in b), which were calculated to differ in melanopic irradiance (77.7 vs. 24.7 melanopic lux). High- and low-melanopic stimuli also differed in rhodopic irradiance (71.8 vs. 34.8 rhodopic lux). High- and low-melanopic settings were matched for color (CIE xy coordinates for both high- and low-melanopic = 0.40 and 0.36; shown in CIE 10-degree xy color space against approximate palate in c) and luminance (79 cd/m2).
We set the melanopic display to project a 100 × 70 cm image in a room in which it was the only source of light. Starting from 18:00 hours, participants were brought into the room and seated facing the displayed image at a distance at which it occupied a visual angle of around 23 × 16°. Over the subsequent 5.5 hours the display was used to present two movies (excerpts of The Jungle Book, 101 Dalmatians, Robin Hood, Peter Pan, and the Lion King) lasting 1.5 hours each, separated by 2.5 hours of playing a table-top board game with a maximum intensity “white screen” image as a source of ambient light. These were presented in either high- or low-melanopic modes on two occasions separated by 7 days in a randomized cross-over design. The visual appearance of displayed images was assessed by asking participants to rate the observed light color, and glare at 30-minute intervals until the end of the study at 23:30 hours. Self-reported assessments of alertness (Karolinska Sleepiness Scale) and a saliva sample for subsequent melatonin assay were taken at the same time.
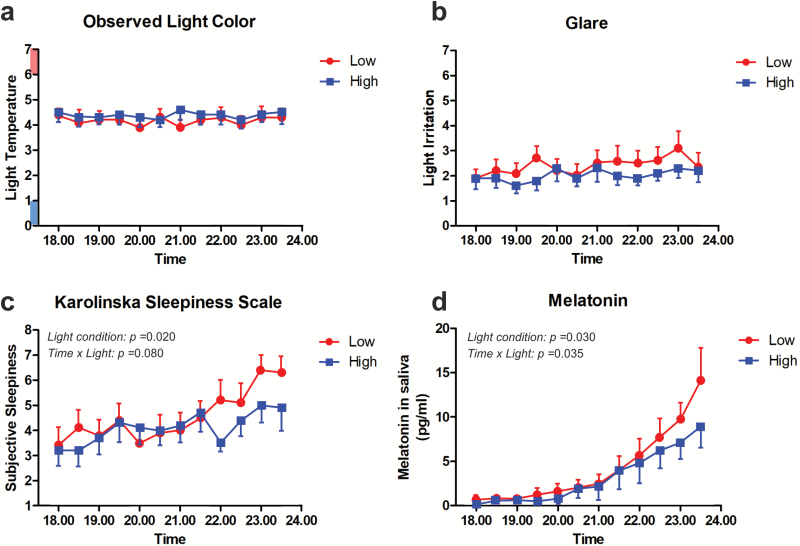
As well as stimuli being photometrically matched, self-reported ratings of visual appearance (Figure 2, a and b) were stable over time and did not significantly differ between light conditions (i.e. high- vs. low-melanopic; F1,11 = 1.61, p = 0.10 for glare and F1,11 = 2.11, p = 0.72 for observed light color; Table 1). Thus, from a visual perspective, both light conditions were well tolerated and sufficiently similar that the participants could not distinguish between them. Both self-reported sleepiness and salivary melatonin increased significantly over time across both conditions (Figure 2, c and d; time effect: self-reported sleepiness, F1,11 = 4.8, p ≤ 0.0001, salivary melatonin, F1,11 = 22.8, p ≤ 0.0001; Table 1) as expected over this part of the day. The magnitude of the increase in both parameters was, however, enhanced in the low-melanopic condition. Thus, compared with the high-melanopic condition, participants rated themselves more sleepy under the low-melanopic condition in the later part of the experiment (Figure 2b, condition: F1,11 = 7.3, p = 0.02; time × condition: F11,99 = 1.7, p = .08; post hoc comparison at 22:00 hours, p < 0.05). This difference in self-reported sleepiness was also reflected in the salivary melatonin measures. The evening rise in salivary melatonin levels in the high-melanopic condition was significantly attenuated compared to the low-melanopic condition (condition: F1,11 = 6.3, p = 0.03; time × condition: F11,99 = 2.0; p = 0.035, post hoc comparison at 23:30 hours, p < 0.001, Cohen’s d = 0.91), particularly in the late evening.
Figure 2.
Modifying melanopic irradiance alters sleepiness and melatonin production without impacting visual appearance. Time course of self-reported ratings of observed color temperature (a; lower ratings correspond to “bluer” and higher ratings more “orange” appearance), glare (b; higher ratings correspond to more discomfort), and sleepiness (c; Karolinska Sleepiness Scale, higher ratings correspond to more sleepy) and of salivary melatonin (d) levels as a function of clock time across the study period under low-melanopic (red) and high-melanopic (blue) conditions. Data were collected at half-hourly intervals and are depicted as mean ± SEM (n = 11).
Table 1.
Table summarizing results of statistical comparisons for each factor, showing how each outcome (salivary melatonin, KSS, observed color, glare) was impacted by time, light-condition, or the interaction between time and light-condition
| Light condition | Time | Time × light | |
|---|---|---|---|
| Salivary melatonin | 0.03 | <0.0001 | 0.035 |
| KSS | 0.02 | <0.0001 | 0.08 |
| Light color | 0.72 | 0.96 | 0.13 |
| Glare | 0.10 | 0.1 | 0.45 |
Values represent p values.
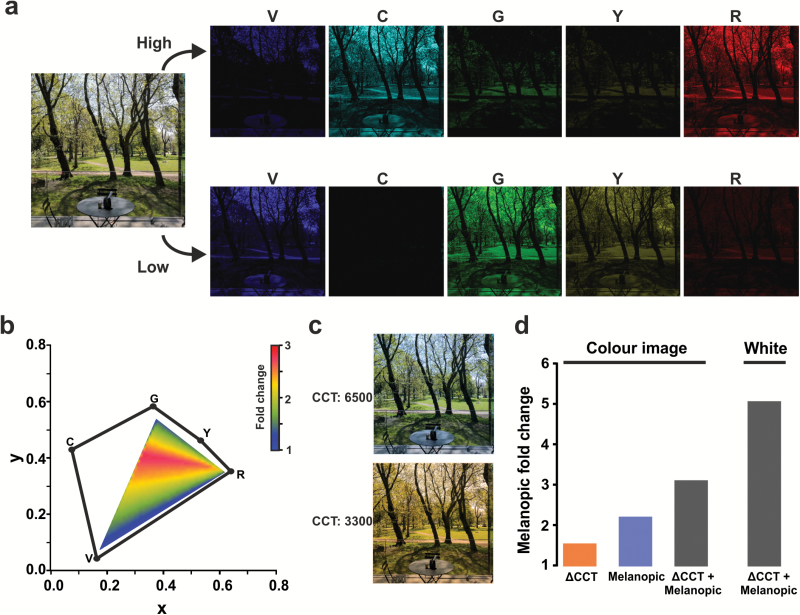
We finally explored the utility of the “melanopic display” for presenting full-color images. RGB values from all pixels within an image were quantified photometrically (in terms of CIE xy coordinates and luminance). Those values were then recreated with five primaries to generate a high- and low-melanopic version of this image, which again were designed to maximize the difference in melanopic irradiance. The difference in melanopic radiance between metameric pairs varies according to color, but across a range of 30 typical images, our current display achieves 2.26 ± 0.05 (mean ± SEM) fold difference in melanopic radiance (representative image shown in Figure 3, a and b). Screen “yellowing” functions are widely employed with the hope that they alleviate unwanted reflex light-responses by reducing melanopic irradiance. The modulation in melanopic radiance achievable with the melanopic display compares favorably with that produced by “yellowing” (Figure 3c). Moreover, if screen yellowing is combined with our “melanopic” display, it is possible to achieve more than threefold modulation in melanopic radiance for color images and >5× for “white” (Figure 3d).
Figure 3.
Application of the melanopic display to present a color image. (a) At left is a color image and to the right the five individual color planes that combine to render it in low- (top) and high-melanopic (bottom) radiance using the melanopic display in Figure 1. The color of each pixel is determined by its relative intensity at each color plane. Although melanopic radiance varies from pixel to pixel according to its color and intensity, there is an overall 2.5× difference in melanopic-irradiance between low- and high-melanopic images. The spectral power distribution of individual color planes determines the available gamut and the difference in melanopic-irradiance achievable between low- and high-melanopic settings. (b) An xy color space with the location of the five primaries (violet, cyan, green, yellow and red; VCGYR) in the melanopic display shown in black. The area encompassed by the pentagon comprises the available gamut for this display, and the triangle in the center is the RGB gamut of the unmodified projector. The fold-difference in melanopic radiance between high- and low-melanopic metamers (high melanopic/low melanopic) available with the display described in Figure 1 across the RGB gamut is depicted as a heat map (scale bar to side). Optimization of the color planes to maximize melanopic contrast could lead to increases in these values across the color gamut. (c) Adjustments in correlated color temperature (CCT; “screen yellowing”) are widely used in the hope of adjusting the impact of RGB displays on reflex light responses. The effect on appearance of a natural scene of a typical adjustment from CCT of 6500 to 3300 is shown (e.g. as used by f.lux; https://justgetflux.com/). (d) Bar graph plotting the fold change in melanopic excitation of the color image shown in (c) after: changing CCT; using the melanopic display at high- vs. low-melanopic settings (retaining color and luminance); and combining the melanopic display with a change in CCT. Also shown is the resultant change in melanopic excitation for white (xy coordinate 0.33, 0.33) when combining the two approaches.
Discussion
Our data confirm the potential of metamers for allowing control of reflex light responses independent of visual appearance, and the suitability of more than three-primary displays as a practical method for achieving this. We find that modulating melanopic irradiance can alter two reflex light responses—melatonin onset and self-reported alertness—under one of the most commonly encountered scenarios for real-world exposure to artificial light: passive viewing of visual display devices. We know from both human and animal studies that rods, cones, and melanopsin can all contribute to subconscious/reflex responses to light. The important question of which photoreceptor(s) play significant roles in defining response amplitude under real-world light exposure scenarios, however, remains largely unanswered [1]. Under some laboratory conditions of stylized light exposure, there is good evidence that melanopsin can dominate reflex light responses [13, 14, 25–27], but its significance under more realistic light exposure paradigms is less clear. To date, the evidence in favor of a strong melanopsin contribution under real-world conditions comes primarily from studies in which reflex responses have been related to modulations in the quantity of shorter “blue” wavelengths that change not only melanopic irradiance but also effective irradiance for short-wavelength sensitive cones (and therefore also color) [10–12, 14, 15]. Our findings reveal that it is possible to control evening melatonin production and self-reported alertness without changing cone activity, and thus confirm that photoreceptors other than cones can make a significant contribution to reflex light responses under a real-world light exposure scenario.
The design of the multi-primary display does not allow us to determine whether the effects we observe result from changes in the activity of melanopsin and/or rods. Thus, thanks to the similarity in the spectral sensitivity of rods vs. melanopsin our “high-melanopic” setting is also 2× brighter for rods (see Methods). Future work could address this distinction (although it may never be possible to modulate the activity of rods vs. melanopsin separately with sufficient magnitude to have a measurable impact on melatonin onset/self-reported alertness). From a practical perspective answering this question is not of paramount importance, as any realistic manipulation of melanopic irradiance would, in any case, have a corresponding effect on rhodopic irradiance.
The “melanopic display” employed here presented monochrome images for ease of image processing. We have also demonstrated that this display architecture can be used to present full-color images across a wide gamut, with differences in melanopic irradiance of ~2.5 fold. This change in melanopic radiance is comparable to that produced by common “yellowing” functions. Moreover, because the multi-primary approach modulates melanopic radiance without changing color or luminance, its effects are additive to changes in those parameters. Likewise, this effect is also multiplicative with changes in screen brightness, such that, e.g. a 5× modulation in screen brightness could be turned into a >10× difference in melanopic radiance using a melanopic display. These differences may be small in the context of the amplitude of the natural diurnal variation in irradiance, but our data show that they are sufficient to have a significant impact on evening sleepiness and salivary melatonin. Indeed, in the case of sleepiness, the “low-melanopic” condition left participants close to the point at which physiological signs of sleepiness are typically observed by the end of the study (KSS score of 7 [28]). This highlights the potential of multi-primary displays to contribute to achieving a desired level of alertness during evening display use. If preparing for sleep, one could choose the “low-melanopic” setting, while the “high-melanopic” setting could be helpful when performing tasks requiring high wakefulness. Such approaches could also be a valuable counter-measure for reducing the longer term negative consequences of exposure to artificial light in the evening [29].
Supplementary material
Supplementary material is available at SLEEP online.
Funding
This work was supported by a Proof-of-Concept award from the European Research Council (ERC; funder ID: 100010663) to RJL.
Acknowledgments
We thank Dr Sei-ichi Tsujimura of Kagoshima University for advice on the design of the melanopic display, Dr Ruta Lasauskaite for helping in the design and planning of the lab protocol and Drs. Corrado Garbazza and Stig Solbach for or medical screenings and Dr. Sarah Chellappa for statistical advice.
Notes
Conflict of interest statement. None declared.
References
- 1. Lucas RJ, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang AM, et al. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hatori M, et al. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ Aging Mech Dis. 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Czeisler CA. Perspective: casting light on sleep deficiency. Nature. 2013;497(7450):S13. [DOI] [PubMed] [Google Scholar]
- 5. Touitou Y, et al. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. [DOI] [PubMed] [Google Scholar]
- 6. Vandewalle G, et al. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–438. [DOI] [PubMed] [Google Scholar]
- 7. Gringras P, et al. Bigger, brighter, bluer-better? Current light-emitting devices - adverse sleep properties and preventative strategies. Front Public Health. 2015;3:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt TM, et al. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31(45):16094–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Do MT, et al. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90(4):1547–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chellappa SL, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22(5):573–580. [DOI] [PubMed] [Google Scholar]
- 11. Münch M, et al. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1421–R1428. [DOI] [PubMed] [Google Scholar]
- 12. Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 13. Papamichael C, et al. Human nonvisual responses to simultaneous presentation of blue and red monochromatic light. J Biol Rhythms. 2012;27(1):70–78. [DOI] [PubMed] [Google Scholar]
- 14. Brainard GC, et al. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 2015;58(3):352–361. [DOI] [PubMed] [Google Scholar]
- 15. Viola AU, et al. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34(4):297–306. [DOI] [PubMed] [Google Scholar]
- 16. Brown TM, et al. Melanopsin-based brightness discrimination in mice and humans. Curr Biol. 2012;22(12):1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spitschan M, et al. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc Natl Acad Sci U S A. 2014;111(43):15568–15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung SM, et al. Cerebral neural correlates of differential melanopic photic stimulation in humans. Neuroimage. 2017;146:763–769. [DOI] [PubMed] [Google Scholar]
- 19. Barrionuevo PA, et al. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest Ophthalmol Vis Sci. 2014;55(2):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen AE, et al. Melanopsin contributions to the representation of images in the early visual system. Curr Biol. 2017;27(11):1623–1632.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen AE, et al. Melanopsin-driven light adaptation in mouse vision. Curr Biol. 2014;24(21):2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birchler-Pedross A, et al. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. J Biol Rhythms. 2009;24(3):232–242. [DOI] [PubMed] [Google Scholar]
- 23. Chellappa SL, et al. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci Rep. 2017;7(1):14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kincaid C. Guidelines for selecting the covariance structure in mixed model analysis. In: COMSYS Information Technology Services I. Portage, MI:SAS; 2005: 1–8. [Google Scholar]
- 25. McDougal DH, et al. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50(1):72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2(31):31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cajochen C, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–1316. [DOI] [PubMed] [Google Scholar]
- 28. Cajochen C, et al. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277(3):R640–R649. [DOI] [PubMed] [Google Scholar]
- 29. Green A, et al. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 2017;34(7):855–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.