Abstract
Objectives
Recent work has identified different aspects of executive function that may underlie cognitive changes associated with age. The current study used a multifactorial design to investigate age sensitivity in the ability to shift between different task sets and the interaction of this ability with several specific aspects of executive control.
Method
A large, well-characterized sample of younger (n = 40) and clinically healthy older (n = 51) adults completed a task switching paradigm in which 3 aspects of executive control were manipulated between subjects: a) sensorimotor demand (the number of distinct stimulus-response options); b) stimulus-level interference (i.e., flanker effects); and c) updating/monitoring (the frequency of task switches).
Results
Unique age-related deficits were observed for different aspects of local task switching performance costs and updating/monitoring, but not for interference. Sensorimotor demand was also an important additional factor that interacted with task switching performance.
Discussion
Our findings suggest that task switching, coupled with infrequent and unexpected transitions from one task set to another, in the context of high motoric demands, is particularly difficult for older adults.
Keywords: Aging, Executive function, Sensorimotor control, Task switching, Updating
With the dramatic rise in the elderly population comes the need to understand the mechanisms that contribute to age-related changes to cognition. Described as “the most complex of behaviors, executive functions are intrinsic to the ability to respond in an adaptive manner to novel situations and are also the basis of many cognitive, emotional, and social skills” (Lezak, Howieson, & Loring, 2004, p. 68). Task switching paradigms have long been used by cognitive psychologists and neuroscientists to assess and tease apart different aspects of executive function. The key finding is that response times and error rates increase when individuals must switch between completing two simple tasks as compared to when each task is completed alone (see Kiesel et al., 2010, for a review). Older adults have particular difficulty on tasks that require executive control. Age-related performance deficits have been observed in situations that require the coordination of more than one task at a time (e.g., Hartley, 1992; Hartley & Little, 1999; Kramer, Larish, & Strayer, 1995; Salthouse & Miles, 2002) or the manipulation of stored information in working memory (e.g., Bopp & Verhaeghen, 2005; Kane & Engle, 2002; Myerson, Emery, White, & Hale, 2003). It is not surprising thus that older adults are affected in task switching situations (e.g., Kramer, Hahn, & Gopher, 1999; Kray & Lindenberger, 2000; Mayr, 2001; Verhaeghen & Cerella, 2002). Because they are flexible to context and component manipulations and have high ecological validity (the execution of most activities of daily life requires the ability to switch between different tasks), task switching paradigms represent a powerful tool for examining changes to executive control processes due to senescence.
In the current study, the basic paradigm consisted of two simple choice tasks: a letter category task in which participants decided if letter stimuli were consonants or vowels, and number category task in which participants decided if number stimuli were odd or even. Stimuli were presented to participants in either single task blocks (where participants only completed the letter or the number task) or dual task blocks (where the two tasks were intermixed). Univalent stimuli (those with features that are relevant to only one task), rather than bivalent stimuli (those with features that are relevant to more than one task), were used. Within the context of this task switching paradigm, three specific executive control functions were manipulated.
The first executive control demand that was manipulated was sensorimotor control, operationalized as stimulus-response mapping complexity. In this manipulation, we varied the number of distinct pairings of response-selections to motor-execution mappings, while maintaining the same number of conceptual responses. That is, across the two tasks, there were always four possible conceptual responses (vowel, consonant, odd, and even). However, in the two-response condition, participants used one finger to indicate vowels in the letter classification task and even numbers in the number classification task, and a second finger to indicate consonants and odd numbers. In the four-response condition, each response was tied to an individual finger. Thus, in this condition, each response was unique, and there was only one response for each finger.
In both the two- and four-response conditions, participants had to decide whether each stimulus was a letter or a number in order to determine which task they needed to perform. Once a participant had determined the task, however, they still had to map their decision as to whether the letter is a vowel or consonant, or whether the number is even or odd, to a motoric response. In the two-response condition, there were two alternative choices, whereas in the four-response condition, participants were faced with four possible responses. A bounty of research exploring the choice reaction-time paradigm has shown that as the number stimulus-response alternatives increase, so too do the decision latencies, in a logarithmic manner (Hick, 1952; Hyman, 1953; Smith, 1968). Increasing the set size of the number of response options, much like increasing the set size of items to be remembered in a typical Sternberg memory task (Sternberg, 1966), should therefore increase executive control demands. Longer reaction times were thus expected in the four- versus the two-response condition.
In addition, according to Reeve and Proctor (1988), response competition can result from responses from two fingers of the same hand. Due to the design of the current study, this form of competition could arise in the four-response condition and not in the two-response condition. In addition, Smid and colleagues suggested that choice reaction time involving responses between two hands would result in increased reaction time (Smid, Fiedler, & Heinze, 2000). Movement between hands occurs in both conditions; however, in the four-response condition, this movement only occurred on a task switch. In the two-response condition, movement across hands was balanced between task repetition and task switch trials. We posited that the four-response condition would cause greater disruption due to the combined effects of a larger number of response-selection to response-execution mappings, response competition of same-hand fingers, and movement across hands at switch trials. This disruption was expected to be greater in older adults.
The second executive function manipulation of the current study targeted input interference and was operationalized as stimulus-level interference due to irrelevant, but distracting flankers that surrounded the target letter or number stimulus. The effects of stimulus level interference on older adults’ performance have been explained by some as a process-specific deficit in older adults (Dennis & Cabeza, 2008; Zacks, Hasher, & Li, 2000), whereas others have argued that observed age differences are accounted for by general processing speed differences (Salthouse, 2010; Verhaeghen, 2011). For example, in the standard flanker effect, responses to a central target are slowed if flanking stimuli are incongruent with the correct response (Eriksen & Eriksen, 1974). A process-specific account proposes that the additional interference from flanking stimuli disrupts performance to a greater extent in older adults compared to younger adults, potentially due to impaired inhibitory control over the irrelevant, interfering information. In a young adult sample, Hubner, Dreisbach, Haider, and Kluwe (2003) tested for the effect of flankers on a current switch trial when the current flanker was the target stimulus on the preceding task compared to two control conditions (no flanker or flanker from a task that was not present on the trial immediately prior). General flanker interference effects were found such that any form of flanker increased reaction time relative to no flanker. It was also observed that reaction time was longer for trials in which the current flanker was the target stimulus on the preceding task. The key manipulation in the study by Hubner and colleagues (2003) was to test for backward inhibition (as described by Mayr & Keele, 2000) on those same trials but after a cue that the task would switch to a specified different task set. In these cases, they found reduced reaction times relative to control trials when the current flanker was the target stimulus on the preceding task and when the participant was given the task cue in advance. Thus, Hubner and colleagues found supporting evidence for backward inhibition effects, specifically when the participant was able to prepare in advance. In contrast, a domain-general processing speed account argues that the relative increase in difficulty due to interfering stimuli is the same in young and old after accounting for the effects of differences in speed of processing. Thus, domain-general accounts state there is no differential effect by age and thus no unique age-related deficit in inhibitory control. Regardless of the mechanism at work, older adults have been shown to be more susceptible to interference than their younger counterparts (Zacks et al., 2000; see also Dennis & Cabeza, 2008 for supporting neuroimaging evidence). To test the effect of an age-related deficit in the inhibition of interference on task performance, we incorporated stimuli from Eriksen and Eriksen’s (1974) flanker paradigm in a manner similar to the design of Hubner and colleagues (2003). We focused on the main finding of increased interference effects of flanking stimuli in general, and on directly preceding trials for uncued switches. We examined flanker effects on task repeat and task switch trials in two ways. First, we investigated differences in performance on target items when they were flanked by stimuli from the same task. For example, if the central stimulus was a letter and participants were to make a vowel consonant decision about it, the flanking stimuli were also letters. In this case, the flanking stimuli could be either congruent to the correct target response (e.g., EAE) or incongruent to it (e.g., CAC). Second, we investigated differences in task switching performance when the flanking stimuli were from a different task than the target stimuli, and thus less relevant to the target (e.g., 2A2). If switching performance is affected by proactive interference from previous task sets, then interference from flanking stimuli should affect switch trials relative to repetition trials if they are affecting a similar cognitive mechanism that resolves the interference from both sources.
The third executive control demand that we manipulated was updating/monitoring, operationalized as the frequency of switch versus repeat trials in dual task blocks. Switch frequency has been shown to influence performance costs in young adults, such that a higher switch frequency leads to lower performance costs (Mayr, Diedrichsen, Ivry, & Keele, 2006; Monsell & Mizon, 2006). The boost to performance that arises as switch frequency increases has been explained in terms of task-level adaptation. On each trial, an individual must decide if they will maintain or abandon the current task set, which constitutes the sensory mode and content of the information presented, a decision that is to be made about the information, a response to the information, and the execution of the responses in service of intentions and goal-directed behavior (Gopher, 2006). Mayr and colleagues (2006) hypothesized that high switch frequencies could lead an individual to prepare for a task switch after each trial (or more trials). In such a case, there would be an advantage for switch trials (i.e., lower switch costs) but also a disadvantage on repeat trials. Supporting this account, Philipp and Koch (2006) found that task-set inhibition was larger under high switch probability conditions compared to low switch probability conditions. In terms of age-related effects, Kray, Li, and Lindenberger (2002) did not find any interactions across switch percentages of 75, 50, and 37.5 with other experimental variables, including age. We hypothesized that a wider comparison of 10% versus 50% switch frequency may reveal a difference in switch cost. Braver and colleagues (2001) suggest that older adults are more reactive in their use of cognitive control and show less proactive control than younger adults. In accord with this position, older adults were expected show less task-level adaptation in response to the different switch frequencies and show greater switch costs.
The relative impact of these three executive control demands was assessed via two measures of local task switching performance costs: switch costs, or the increase in latency and decrease in accuracy when there is a task switch in a dual task block (e.g., AABAB) compared to a repetition trial within a dual task block (e.g., AABAB), and mixing costs, defined as the accuracy and reaction time performance costs associated with repetition trials in dual (e.g., AABAB), versus single task blocks (e.g., AAAA). Age-related differences in task switching have been shown to be greater for mixing costs than for switch costs (Kray & Lindenberger, 2000; Meiran, Gotler, & Perlman, 2001). There is converging evidence that the underlying cognitive mechanism responsible for age differences in switch costs is simply slowed processing speed, while the age differences in mixing costs may be due to an age-related deficit in the executive control processes responsible for memory maintenance and manipulation of task sets (Kray & Lindenberger, 2000; Wasylyshyn, Verhaeghen, & Sliwinski, 2011). In the current study, we sought to examine how different executive control manipulations affected these two types of task switching performance costs.
In summary, we hypothesized that (a) the four-response condition would cause greater disruption than the two-response condition, and this disruption was expected to be greater in older adults; (b) flanking stimuli would add additional interference and disrupt performance on switch trials across age groups; (c) older adults would show less task-level adaptation in response to the different switch frequencies; and finally (d) older adults would show greater performance costs relative to younger adults, with mixing cost in particular being deleteriously affected.
Method
Participants
Ninety-one individuals, 40 younger adults and 51 older adults, participated in the study. Participants were all native English speakers with no history of major medical disease (i.e., cancer, cardiac disease, or stroke), psychiatric illness, traumatic brain injury, or concussion with loss of consciousness. All participants were administered the Dementia Rating Scale (DRS; Mattis, 1988), a test of verbal learning (Buschke Selective Reminding Test [SRT]; Buschke, 1973), and the Trail Making Test (TMT; Lezak et al., 2004). As can be seen in Table 1, which presents age, gender, education, and neuropsychological measures by group, younger and older adults attained equivalent levels of education and did not differ on a test designed to screen for dementia (i.e., DRS). Older adults learned and recalled fewer items on a measure of verbal learning (SRT) and were slower to complete a test of speeded visual-motor sequencing (TMT-A) and sequencing plus switching (TMT-B) compared to the younger adults (all ps < .001). Aside from expected main effects of Age (described above), neuropsychological test performance was equivalent across experimental conditions (all ps > .05). Written informed consent was obtained from all participants before participation in the research. All experimental procedures were approved by the Institutional Review Board of Columbia University Medical Center and complied with these regulations.
Table 1.
Demographic and Neuropsychological Measures for Younger and Older Adults (Mean [SD; Range])
| Young | Old | |
|---|---|---|
| Age* (years) | 24.00 (2.07; 19–30) | 72.65 (7.45; 59–90) |
| Education (years) | 15.80 (1.96; 12–20) | 15.27 (2.41; 8–18) |
| Gender (% female) | 75 | 69 |
| DRS | 140.83 (2.80) | 140.55 (2.75) |
| SRT | ||
| Total recall* | 61.20 (5.31) | 48.45 (12.55) |
| Delayed recall* | 10.77 (1.27) | 7.53 (2.98) |
| TMT | ||
| A* | 22.75 (8.34) | 42.57 (13.96) |
| B* | 50.20 (18.20) | 114.39 (51.90) |
Notes: DRS = Dementia Rating Scale; SRT = Buschke Selective Reminding Test; TMT = Trail Making Test.
*p < .05 for age group comparison.
Procedure
The experiment was conducted in one 2-hr session. At the beginning of each session, participants were administered the DRS and the SRT. These were followed by the experimental task switching paradigm, in which participants performed two types of tasks. The first was the letter classification task, in which participants decided if white letters, which were serially displayed on a blue background, were consonants or vowels. The second task was the number classification task. In this task, participants decided if white numbers, which were serially displayed on a green background, were even or odd. All stimuli were chosen from the set: {O, A, E, U, W, P, J, K, 2, 4, 6, 8, 1, 3, 7, 9}. The two tasks were presented on a Toshiba laptop computer with a 15-inch monitor and a screen resolution of 1,280×800 pixels. Each experimental session was divided into 20 blocks of 40 trials each. Each block began with a set of instructions for the upcoming task that remained on the screen until the participant clicked to continue. Each trial in the block began with the presentation of an asterisk in the center of the screen for 200ms, followed by a 100-ms delay and the presentation of the target stimulus. In all blocks, on 60% of trials, the target stimulus was flanked on either side by either numbers or letters. Each character subtended a visual angle of 0.71° horizontally and vertically. Fifty percent of the flanker trials included flanking stimuli from the same task (e.g., a letter on either side of the letter target), with half of these same task flankers being congruent to the cued task (either vowels when the target was a vowel, e.g., “AEA,” or a consonant when the target was a consonant, e.g., “PKP”) and the other half being incongruent (e.g., “PEP”). The other 50% of the flanker trials included flanking stimuli from the other task (e.g., numbers flanking a target letter, e.g., “2A2,” or letters flanking a target number “A2A”). In all cases, the instruction was to respond to the middle character and ignore the irrelevant characters. A schematic of the trial sequence is presented in Figure 1, which shows these different types of flankers in a single task block (Panel A) and a dual task block (Panel B).
Figure 1.
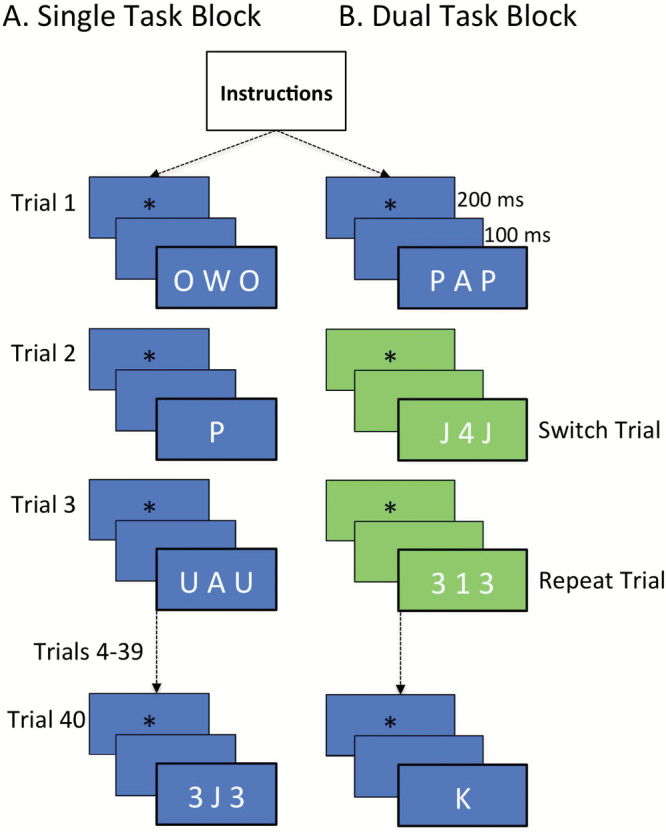
Schematic of a trial sequence in a single task block (Panel A) and a dual task block (Panel B). A repeat and a switch task trial are illustrated in the dual task block (note: all trials in single task blocks are repeat trials), along with the different types of Flankers. In the single task block (Panel A), Trial 1 is a same task incongruent flanker, Trial 2 is a no flanker, Trial 3 is a same task congruent flanker, and Trial 40 is a different task flanker. In the dual task block (Panel B), Trial 1 is a same task incongruent flanker, Trial 2 is a different task flanker, Trial 3 is a same task congruent flanker, and Trial 40 is a no flanker. The correct button presses for the four trials shown in the single task block (Panel A) are left index, left index, right index, left index for the single task block in the two-response condition, and left middle, left middle, left index, left middle in the four-response condition. For the dual task block (Panel B), the correct responses are right index, right index, left index, left index in the two-response condition, and left index, right middle, right index, left middle in the four-response condition.
The first two blocks of the experiment were single task blocks in which the letter classification task was performed alone (80 trials). The third and fourth blocks of the experiment were single task blocks in which participants completed the number classification task alone. The next block was a dual task block (40 trials), in which targets could be letters or numbers. The dual task block was followed by two single task blocks (40 trials of the letter task, then 40 trials of the number task), and then another dual task block. This block sequence was repeated 5 times, for a total of 20 blocks (800 trials) including the four initial single task blocks at the beginning of the experiment. In all trials, the target stimulus remained on the screen until a response was made. Participants were told to respond to each stimulus as quickly and as accurately as they could. The experiment was self-paced, with a response initiating the next trial. After the participant completed the block of 40 trials, feedback about number of errors was presented and a new block sequence was initiated.
Participants were pseudorandomly assigned by age to one of four experimental conditions, based on two between-subjects design factors. The first factor was Switch Frequency, which is the proportion of task switch trials to task repeat trials within dual task blocks. Table 2 presents sample sizes by age group in the 10% and 50% switch frequency groups. The manner in which responses were made represents the second between-subjects manipulation (Response Number), illustrated in Figure 2 (sample sizes in Table 2). In the two-response condition, participants were told to press the “F” key with their left index finger for stimuli that were either vowels or even, and to press the “J” key with their right index finger for either consonants or an odd numbers. In the four-response condition, both the index and middle finger of each hand were used. If the stimulus was a letter, the response was made with the left hand: For vowels the participant pressed the “F” key (with the index finger) and for consonants the participant pressed the “D” key (with the middle finger). If the stimulus was a digit, the response was made with the right hand: For odd numbers the participant pressed the “J” key (with the index finger) and for even numbers the participant pressed the “K” key (with the middle finger).
Table 2.
Between-Subject Experimental Conditions: Sample Size by Age Group
| Response Number | |||||
|---|---|---|---|---|---|
| Two responses | Four responses | ||||
| Young | Old | Young | Old | ||
| Switch Frequency | 10% switches | 10 | 15 | 10 | 14 |
| 50% switches | 10 | 11 | 10 | 11 | |
Note: There were no significant effects among the neuropsychological variables and between-subject conditions of Response Number, Switch Frequency, Response Number × Age, or Switch Frequency × Age.
Figure 2.
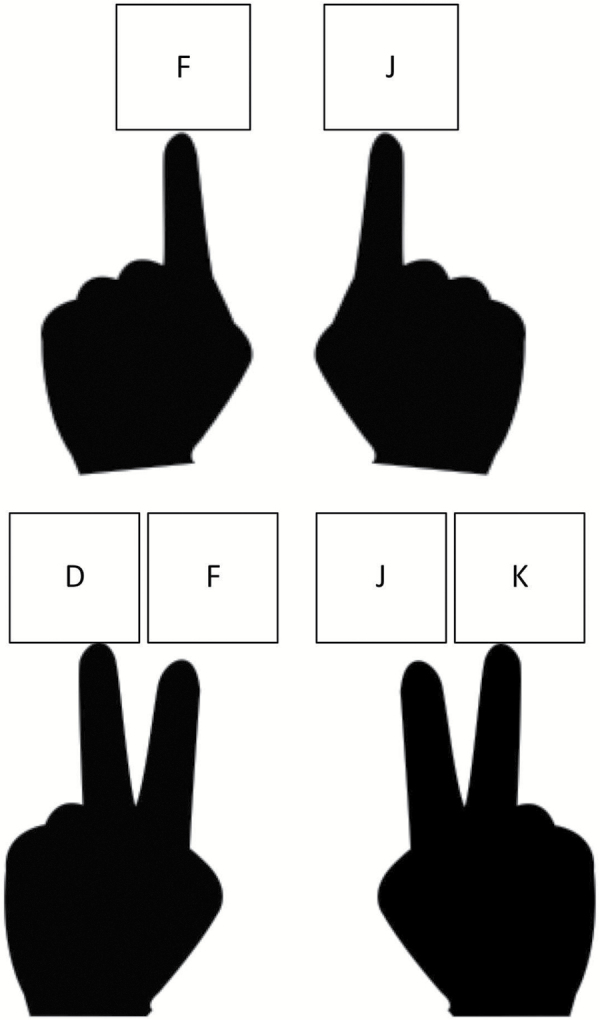
Schematic of sensorimotor response mapping. Two or four responses were mapped to either the index finger of each hand (two-response condition; top) or the index and middle finger of each hand (four-response condition; bottom). In the two-response condition, the “F” key was pressed for consonants or odd numbers, whereas the “J” key was pressed for vowels or even numbers. In the four-response condition, “D” = consonants; “F” = vowels; “J” = odd numbers; and “K” = even numbers.
Data Analysis
To simplify the reporting of age-related interactions with executive demand conditions and mitigate any effects that could be driven by larger scale age-related differences in response times, z-transformed Reaction Time (zRT) were used for analyses. Z-transformations, rather than log-transformations, were carried out so that any effect remaining above and beyond any effects that could be driven by the larger scale group differences in RT could be more readily interpreted (see Faust, Balota, Spieler, & Ferraro, 1999). zRT scores were based upon each participant’s overall mean RT for correct trials. In addition, trials with an RT ≥ 3 SDs above the mean for each participant across conditions were removed. Accuracy (percent correct) was also analyzed.
Repeated measures analyses of variance were conducted using SPSS (version 19). Between-subjects factors included Age (young vs old), Switch Frequency (10% vs 50%), and Response Number (2 vs 4). Within-subjects performance factors included Trial Type; either repetition trials within dual task blocks versus switch trials within dual task blocks to assay switch costs, or repetition trials in single task blocks versus repetition trials in dual task blocks to assay mixing costs. zRT and error rate means for younger and older participants in each of the three executive demand conditions are shown in Table 3. Due to the fact that error rates in some executive demand conditions were high, and that there were few total switch trials in the 10% switch frequency condition, the effect of flankers was examined collapsing across the between-subjects factors of Response Number and Switch Frequency. For the analyses that examined the between-subjects factors of Response Number and Switch Frequency, the results reported collapsed across levels of the flanker condition were confirmed in the subset of trials that did not include flankers.
Table 3.
Mean z-Transformed Reaction Times (Standard Error [SE])/% Correct (SE)/Average Number of Correct Trials for Younger and Older Adults as a Function of Response Number and Switch Frequency
| Two responses | Four responses | |||||
|---|---|---|---|---|---|---|
| Young | Old | Young | Old | |||
| Type of Trial and Switch Frequency | Single (Repeat) | 10% | −0.03 (0.02)/97.2 (0.01)/492.5 | −0.07 (0.02)/95.4 (0.01)/475.3 | −0.07 (0.02)/96.5 (0.01)/493.3 | −0.13 (0.02)/96.3 (0.01)/483.1 |
| 50% | −0.15 (0.02)/97.5 (0.01)/501.1 | −0.12 (0.02)/96.8 (0.01)/483.8 | −0.22 (0.02)/96.2 (0.01)/491.5 | −0.26 (0.02)/97.5 (0.01)/491.9 | ||
| Dual Repeat | 10% | −0.05 (0.05)/97.9 (0.02)/190.6 | 0.06 (0.04)/97.8 (0.02)/184.5 | 0.01 (0.5)/97.1 (0.02)/187.1 | 0.19 (0.04)/91.8 (0.02)/169.6 | |
| 50% | 0.16 (0.05)/97.8 (0.02)/104.9 | 0.12 (0.05)/97.6 (0.02)/103.9 | 0.12 (0.05)/97.7 (0.02)/169.6 | 0.38 (0.05)/91.4 (0.02)/91.8 | ||
| Dual Switch | 10% | 1.18 (0.18)/95.9 (0.03)/17.8 | 1.40 (0.15)/97.8 (0.03)/17.4 | 1.56 (0.18)/97.9 (0.03)/19 | 2.68 (0.15)/84.2 (0.02)/11.4 | |
| 50% | 0.50 (0.18)/97.7 (0.03)/103.9 | 0.51 (0.17)/95.0 (0.03)/95.1 | 1.07 (0.18)/96.0 (0.03)/91.6 | 1.12 (0.17)/90.2 (0.03)/85.45 | ||
Results
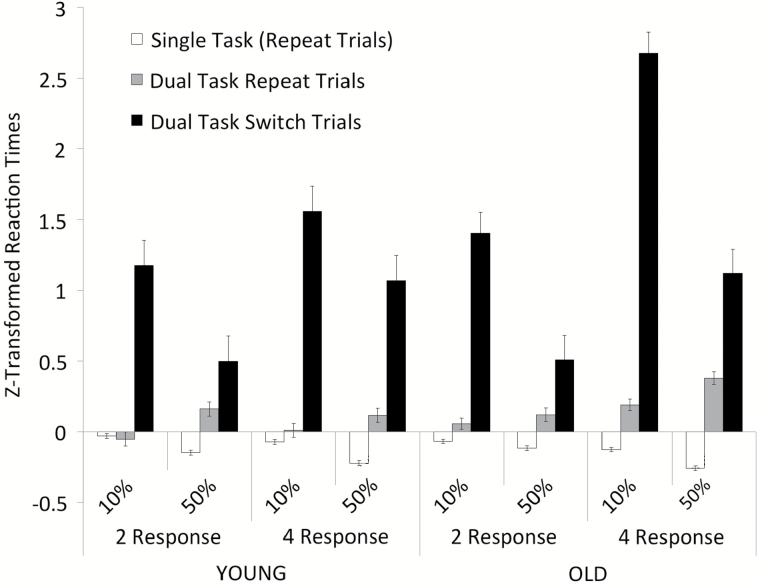
The first model we created examined age-related effects of executive control demands on switch costs, operationalized as the difference between performance on switch trials and repeat trials within the dual task block, using zRTs. As can be seen in Figure 3, there was a significant main effect of Trial Type, such that responses on dual task switch trials (black bars) were slower overall compared to dual task repeat trials (gray bars), F(1, 83) = 333.92, p < .001, = .80. Older adults were slower to respond than younger adults, F(1, 83) = 14.73, p < .001, = .15. Overall, four responses were slower than two responses, F(1, 83) = 42.00, p < .001, = .24, and the lower percent switch frequency produced longer latencies overall than did the higher percent switch frequency, F(1, 83) = 37.09, p < .001, = .31.
Figure 3.
Mean z-transformed Reaction Time for task repeat trials (in single task and dual task blocks) and for task switch trials (in dual task blocks) across task conditions of Response Number (two vs four responses) and Switch Frequency (10% vs 50% switches) in young and older adult groups. Error bars indicate standard error of the mean.
Interactions were observed between the Trial Type and Response Number, F(1, 83) = 24.10, p < .001, = .23; and Trial Type and Switch Frequency, F(1, 83) = 71.96, p < .001, = .46. Larger switch costs were observed in the four-response condition relative to the two-response condition and in 10% switch frequency compared to the 50% switch frequency. The interaction between Trial Type and Age was not significant, F(1, 83) = 3.29, p = .073. Nor was the interaction among Trial Type, Age, and Response Number, F(1, 83) = 1.28, p = .262. However, the three-way interaction between Trial Type, Age, and Switch Frequency was significant, F(1, 83) = 6.08, p = .016, = .08. Post hoc t test comparing younger and older adults on Trial Type within the 10% and 50% switch frequency conditions revealed significant age-related difference for both repeat trials t(47) = −3.17, p = .003, 95% CI (−0.23, −0.05) and switch trials t(47) = −2.60, p = .012, 95% CI (−1.16, −0.15) in dual task blocks in the 10% Switch Frequency condition, such that in both cases older adults showed longer latencies, but nonsignificant zRT differences between old and young on switch and repeat trials in the 50% condition, both ts(47) < 1. However, the main effects and interactions were qualified by a four-way interaction of Trial Type × Age × Switch Frequency × Response Number, F(1, 83) = 4.79, p = .032, = .06. Post hoc t tests comparing younger to older adults performance on repeat and switch trials in the two-response condition for 10% and 50% Switch Frequency revealed no significant differences (ts ranged from −1.9 to 1.62). For the four-response, 10% Frequency condition, however, we found a significant difference between older and younger adults on dual task repeat trials t(22) = −2.79, p = .011, 95% CI (−0.31, −0.05) as well as switch trials t(22) = −4.37, p < .001, 95% CI (−1.65, −0.59), such that older adults had longer latencies. The analogous analysis for the 50% Switch Frequency revealed an age-related difference on repeat trials t(19) = −3.73, p = .001, 95% CI (−0.41, −0.12), but not on switch trials t(19) < 1. Thus, when collapsing over high and low Switch Frequency, Response Number did not interact with Age and Trial Type. However, for older adults, when Switch Frequency was teased apart and executive control demands were high, Response Number had a marked effect on performance. As can be seen in Figure 3, this differential age-related effect on switch costs was observed under conditions of high sensorimotor demand (four vs two responses) and less frequent switching (10% vs 50%).
The results of the same analysis using Accuracy were consistent with the zRT data. We found a four-way interaction of Age × Trial Type × Response Number × Switch Frequency, F(1, 83) = 6.91, p = .010, = .08, such that the most errors (15.79%) were committed by older adults on dual task switch trials with four responses in the 10% switch frequency condition.
We next investigated age and executive demand impacts on mixing costs, first with zRTs. Trial Type in this analysis consisted of repeat trials within single versus dual task blocks. As can be seen in Figure 3, responses were slower for repetition trials in dual task blocks (gray bars) relative to single task blocks (white bars), F(1, 83) = 139.32, p < .001, = .63. Older adults were also slower to respond than younger adults, F(1, 83) = 18.52, p < .001, = .18. There were no main effects of Response Number, F(1, 83) < 1 or Switch Frequency, F(1, 83) = 1.54, p > .2.
We found several significant interactions. The first was a two-way interaction between Age and Trial Type, F(1, 83) = 12.56, p = .001, = .12, which stemmed from older adults responding more slowly than younger adults on repetition trials in dual task blocks, t(89) = −3.07, p = .003, 95% CI (−0.19, −0.04) compared to single task blocks, t(89) < 1, ns. We also found significant interactions between Trial Type and (a) Response Number, F(1, 83) = 18.05, p < .001, = .18, and (b) Switching Frequency, F(1, 83) = 35.59, p < .001, = .30, as well as a two-way interaction between Age and Response Number, F(1, 83) = 8.74, p = .004, = .10. The highest level interaction was a three-way interaction of Age × Trial Type × Response Number, F(1, 83) = 7.09, p = .009, = .08. Independent post hoc t tests for the two-response condition revealed nonsignificant differences between older and younger adults on both single task repeat trials, t(44) < −1, and dual task repeat trials t(44) < −1. For the four-response condition, however, whereas the difference between older and younger adults was not significant for single task (repetition) trials, t(43) = 1.39, p = .169, there was a significant difference between age groups for the dual task repeat trials, t(43) = −4.02, p < .001, 95% CI (−0.31, −0.10), such that older adults had longer latencies. In other words, the older adults showed increased latencies on repetition trials in dual task blocks in the four-response condition, but not in the two-response condition. The four-way interaction between Trial Type, Age, Response Number, and Switch Frequency was not significant, F(1, 83) = 2.73, p = .103.
For mixing cost accuracy, we found a trend for a two-way interaction of Age × Trial Type, F(1, 83) = 3.75, p = .056, = .04, such that more errors were made by older adults in dual task blocks than in single task blocks, and a significant two-way interaction between Trial Type and Response Number, F(1, 83) = 5.58, p = .020, = .06. The three-way interaction of Age × Trial Type × Response Number that we found for zRT was also observed for accuracy, F(1, 83) = 7.88, p = .006, = .09, such that more errors were made by older adults in the four-response condition on repetition trials within dual task blocks.
Finally, we examined the effect of interference on performance, operationalized as the effect of flankers. The standard flanker effect was examined in the within-task-set analysis (i.e., flanking stimuli of the same type as the target stimulus and either congruent vs incongruent to target response). Interference across task sets (i.e., flanking stimuli of the other task set on current target response) was also examined. The standard flanker effect of slower responding on incongruent versus congruent trials was confirmed in repetition trials both within single task blocks, F(1, 89) = 13.30, p < .001, = .13, and dual task blocks, F(1, 89) = 5.07, p < .05, = .05. There was no effect of flanker on switch trials, F(1, 89) = 2.10, and there were no Flanker × Age interactions across repetition or switch trials for single or dual task blocks (all Fs < 1). The accuracy data were consistent with the zRT findings and showed no differential disruption of performance costs due to flanker interference among older adults. We also performed an across-task-set analysis, comparing trials in which there were different flankers to trials in which no flanker was present. We found no significant interactions with Age, in either zRT or accuracy (all Fs < 1). We did, however, find a main effect of the type of flanker trial (different flanker vs no flanker) on repeat trials in dual task blocks, F(1, 89) = 13.94, p < .001, = .14.
Discussion
In the current study, we examined the age sensitivity of several separable aspects of executive control, including the number of distinct stimuli-response options (sensorimotor demand); stimulus-level interference (flanker effects); and the frequency of task switches (updating/monitoring). To our surprise, we found no unique age-related differences in stimulus level interference in the form of flankers. Age-related differences were found, however, in switch costs in the context of high monitoring/updating (lower—10%—switch frequency), as well as in the context of high monitoring/updating demands and increased sensorimotor demand (four vs two responses). In contrast, mixing costs were age sensitive to monitoring/updating even in the absence of increased sensorimotor demand. Although sensorimotor demand did not account for all observed age effects, the interaction of the mental processing demands of monitoring/updating in the context of high sensorimotor demand yielded a pronounced difficulty for older adults (see Figure 3). This study reinforces the robust effect of aging on general executive control processes that are clearly distinguishable in age-related performance differences between dual task blocks that include task switches and single task blocks that do not.
A novel contribution of this study was the identification of an age-related disruption in performance in switch costs under the conditions of low switch frequency (10% switching) and high response number (four responses) in both zRT data, as well as for accuracy, with greater latencies and errors for older adults in high response and updating/monitoring conditions. Although these results stemmed from a four-way interaction, given that the RT data were cleaned for outliers, it is unlikely that the results were biased in this way. Consistent with past studies that have reported age invariance in switch costs after accounting for differences in processing speed, there was no two-way interaction between Age and Trial Type for switch costs. In addition, we examined the updating and monitoring demands of high and low switch frequency. We observed that switch costs, often not observed to reveal unique age-related effects, were differentially greater in older adults in the low switch frequency condition. This finding supports our hypothesis that infrequent switching may result in greater switch costs in older adults compared to younger adults due to the increased monitoring and preparation demands. An earlier study did not find age-related effects across switch frequencies of 75%, 50%, and 37.5% (Kray et al., 2002). The effect we observed was at a lower switch percentage (10%) and only emerged in the context of high sensorimotor demand.
Our findings also converge with result from a recent meta-analysis of aging and task switching studies which showed differential age effects for mixing costs (Wasylyshyn et al., 2011). We found in addition that mixing costs interacted with executive control demands in specific ways. We examined the hypothesis that response-execution complexity contributes to differential age effects in mixing costs (Hartley, 2001). We found that four responses (with a larger number of response-execution mappings) were differentially disruptive to older adults compared to two responses (with high motor program overlap and fewer mappings) collapsing across Switch Frequency. Although both conditions required switching between hands, switches only occurred at times of a task switch in the four-response condition and so this additional step at a crucial processing stage is an important factor. The interaction of mental processing demands required to maintain and manage multiple task sets and the sensorimotor demands of response mapping combined with the motor execution placed at a crucial processing stage may account for the synergistic disruption to performance among older adults in this multitasking context. Interestingly, the age-related effects of increased sensorimotor demand collapsing across the frequency of task switch was not significant for the analogous analysis of switch costs, and instead only emerged in the context of switch costs when updating/monitoring processes were taken into account, suggesting differential impacts of the executive control manipulations on switch versus mixing costs.
We also tested the hypothesis that stimulus-level interference in the form of flankers would disrupt performance in older adults to a greater degree, based on the hypothesis that older adults are relatively more susceptible to this form of interference (Zacks et al., 2000). We hypothesized an alternative account that the effects of flanking interference are not differentially disruptive to older adults and if any group differences are observed, those can be accounted for by general differences in processing speed. As we hypothesized, interference effects that were observed in young and older adults were comparable after adjusting for age-related declines in speed of processing. This finding supports reports of age invariance across tasks of selective attention broadly (Verhaeghen, 2011) and flanker-based interference specifically (Lien, Ruthruff, & Kuhns, 2008; Salthouse, 2010). An important limitation of this study was that we were not able to examine the interactive effects of flanker interference under the control demands of switch frequency or response number due to the high error rate by condition. Despite this limitation, there is evidence to support the age-invariant findings observed in this study (see also Salthouse, 2010). Further, the absence of an age effect on flankers, coupled with the findings of age-related effects on switching frequency especially in the context of elevated sensorimotor demands, may be related to the proposed distinction between top-down and bottom-up attention and executive control (Buschman & Miller, 2007; Miller & Buschman, 2014). Bottom-up are salient and alerting cues of the environment that capture attention and demand control. Mayr’s (2001) retroactive inhibition may be one example. Task switching and adopting a task set are active top-down executive control processes. In our study, flanker influences are clearly bottom-up initiated, whereas the ability to switch between the different tasks (Switch Frequency) and map responses to specific motoric outputs (Response Number) are clearly top-down and active executive control of intentions. Thus, age-related differences in bottom-up versus top-down control processes, from our data, appear to be affected differentially.
Overall, age and executive control-specific impairments in mixing and switch costs were observed in both zRT data and accuracy data. Our study suggests that age-sensitive switch costs were particularity affected in the context of additive, high general executive control demands; specifically, when task switches occurred less frequently and when there were a greater number response mappings. These two executive demands, in combination, appear to pose a particular challenge for older adults. Age effects were observed for mixing costs overall and in interaction with greater sensorimotor demand. Evidence for larger decrements in performance in older adults due to increased load on working memory is also reported in previous studies of task switching (Gopher, Armony, & Greenshpan, 2000; Meiran et al., 2001) and multitasking (Craik & Bialystok, 2006). Similar to these studies, we also observed that selective increases in memory load on single items resulted in larger age differences in local switch costs.
Taken together, the most important outcome of the present study is that decrements with age are evident for executive control manipulations that impose high demands on working memory, with maintenance over longer durations, across a large number of trials. Age differences for local and discrete executive operations are smaller and harder to observe, but emerge when additional control demands (such as working memory load) are incorporated into the task.
The practical implication of performance disruption in the face of combined and heightened executive control requirements is apparent in the potential hazards associated with the increasing multitask demands placed on younger and older adults as we incorporate technologies such as cell phones, navigation systems, and personal portable technologies into our everyday lives. A recent study by Neider and colleagues (2011) used a virtual reality environment and demonstrated that technology use (talking on a cell phone) was particularly disruptive for older adults, even during a relatively routine activity like crossing the street. Likewise, our results suggest that particular combinations of cognitive and motor control demands present a significant burden to older adults. Although some of these effects can be attributed to a domain-general slowing of cognitive processing or to motor response interference, we found strong support for age-sensitive deficits in executive control processes such as the maintenance, manipulation, and monitoring of task sets.
Funding
This research was supported in part by grant T32 5T32MH020004, Research Training in Late-Life Neuropsychiatric Disorders from the National Institute of Mental Health to T. S. Eich and A. MacKay-Brandt, and grant R01 AG-026158 from the National Institutes of Health-National Institute on Aging to Y. Stern.
Acknowledgments
Thanks to Brittany Cerbone for assistance with data collection. T. S. Eich and A. MacKay-Brandt contributed equally to the study.
References
- Bopp K. L., & Verhaeghen P (2005). Aging and verbal memory span: A meta-analysis. The Journals of Gerontology, Series B: Psychological Sciences, 60, 223–233. doi:10.1093/geronb/60.5.p223 [DOI] [PubMed] [Google Scholar]
- Braver T. S. Barch D. M. Keys B. A. Carter C. S. Cohen J. D. Kaye J. A., … Reed B. R (2001). Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General, 130, 746–763. doi:10.1037/0096-3445.130.4.746 [PubMed] [Google Scholar]
- Buschke H. (1973). Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior, 12, 543–550. doi:10.1016/s0022- 5371(73)80034-9 [Google Scholar]
- Buschman T. J., & Miller E. K (2007). Prefrontal and posterior parietal cortices top-down versus bottom-up control of attention. Science, 315, 1860–1862. doi:10.1126/science.1138071 [DOI] [PubMed] [Google Scholar]
- Craik F., & Bialystok E (2006). Planning and task management in older adults: Cooking breakfast. Memory and Cognition, 34, 1236–1249. doi:10.3758/bf03193268 [DOI] [PubMed] [Google Scholar]
- Dennis N. A., & Cabeza R (2008). Neuroimaging of healthy cognitive aging. In Salthouse T. A., Craik F. E. M. (Eds.), Handbook of aging and cognition (3rd ed., pp. 1–54). New York and Hove: Psychology Press. doi:10.4324/9780203837665.ch1 [Google Scholar]
- Eriksen B. A., & Eriksen C,W (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16, 143–149. doi:10.3758/bf03203267 [Google Scholar]
- Faust M. E. Balota D. A. Spieler D. H., & Ferraro F. R (1999). Individual differences in information processing rate and amount: Implications for group differences in response latency. Psychological Bulletin, 125, 777–799. doi:10.1037/0033-2909.125.6.777 [DOI] [PubMed] [Google Scholar]
- Gopher D. (2006). Control processes in the formation of task units. In Jing Q. (Ed.), Progress in psychological science around the world, volume 2, social and applied issues (pp. 385–404). London, UK: Oxford Psychology Press. A chapter based on a keynote address given at the 28th International Congress of Psychology. doi:10.4324/9780203783122 [Google Scholar]
- Gopher D. Armony L., & Greenshpan Y (2000). Switching tasks and attention policies. Journal of Experimental Psychology: General, 109, 306–339. doi:10.1037/0096-3445.129.3.308 [DOI] [PubMed] [Google Scholar]
- Hartley A. A. (1992). Attention. In Craik F. I. M., Salthouse T. A. (Eds.), Handbook of aging and cognition (pp. 3–49). Hillsdale, NJ: Erlbaum. doi:10.1017/s0714980800012332 [Google Scholar]
- Hartley A. A. (2001). Age differences in dual-task interference are localized to response-generation processes. Psychology and Aging, 16, 47–54. doi:10.1037/0882-7974.16.1.47 [DOI] [PubMed] [Google Scholar]
- Hartley A. A., & Little D. M (1999). Age-related differences and similarities in dual-task interference. Journal of Experimental Psychology: General, 128, 416–449. doi:10.1037/0096-3445.128.4.416 [DOI] [PubMed] [Google Scholar]
- Hick W. E. (1952). On the rate of gain of information. Quarterly Journal of Experimental Psychology, 4, 11–26. doi:10.1080/17470215208416600 [Google Scholar]
- Hubner R. Dreisbach G. Haider H., & Kluwe R. H (2003). Backward inhibition as a means of sequential task-set control: Evidence for reduction of task competition. Journal of Experimental Psychology: Learning Memory, & Cognition, 29, 289–297. doi:10.1037/0278-7393.29.2.289 [DOI] [PubMed] [Google Scholar]
- Hyman R.(1953). Stimulus information as a determinant of reaction time. Journal of Experimental Psychology, 45, 188–196. doi:10.1037/h0056940 [DOI] [PubMed] [Google Scholar]
- Kane M. J., & Engle R. W (2002). The role of prefrontal cortex in working-memory capacity,executive attention, and general fluid intelligence: An individual differences perspective. Psychonomic Bulletin & Review, 9, 637–671. doi:10.3758/bf03196323 [DOI] [PubMed] [Google Scholar]
- Kiesel A. Steinhauser M. Wendt M. Falkenstein M. Jost K. Philipp A. M., & Koch I (2010). Control and interference in task switching - A review. Psychological Bulletin, 136, 849–874. doi:10.1037/a0019842 [DOI] [PubMed] [Google Scholar]
- Kramer A. F. Hahn S., & Gopher D (1999). Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychologica, 101, 339–378. doi:10.1016/s0001-6918(99)00011-6 [DOI] [PubMed] [Google Scholar]
- Kramer A. F. Larish J. F., & Strayer D. L (1995). Training for attentional control in dual task settings: A comparison of young and old adults. Journal of Experimental Psychology: Applied, 1, 50–76. doi:10.1037/1076-898x.1.1.50 [Google Scholar]
- Kray J., & Lindenberger U (2000). Adult age differences in task switching. Psychology and Aging, 15, 126–147. doi:10.1037/0882-7974.15.1.126 [DOI] [PubMed] [Google Scholar]
- Kray J. Li K. Z. H., & Lindenberger U (2002). Age-specific changes in task-switching components: The role of task uncertainty. Brain and Cognition, 49, 363–381. doi:10.1006/brcg.2001.1505 [DOI] [PubMed] [Google Scholar]
- Lezak M. D. Howieson D. B., & Loring D. W (2004). Neuropsychological assessment (4th ed.). New York, NY: Oxford University Press. [Google Scholar]
- Lien M.-C. Ruthruff E., & Kuhns D (2008). Age-related differences in switching between cognitive tasks: Does internal control ability decline with age?Psychology and Aging, 23, 330–341. doi:10.1037/0882-7974.23.2.330 [DOI] [PubMed] [Google Scholar]
- Mattis S. (1988). Dementia Rating Scale: Professional manual. Odessa, FL: Psychological Assessment Resources. doi:10.1007/springerreference_183336 [Google Scholar]
- Mayr U. (2001). Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging, 16, 96–109. doi:10.1037/0882-7974.16.1.96 [DOI] [PubMed] [Google Scholar]
- Mayr U. Diedrichsen J. Ivry R., & Keele S. W (2006). Dissociating task-set selection from task-set inhibition in the prefrontal cortex. Journal of Cognitive Neuroscience, 18, 14–21. doi:10.1162/089892906775250085 [DOI] [PubMed] [Google Scholar]
- Mayr U., & Keele S (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General, 129, 4–26. doi:10.1037/0096-3445.129.1.4 [DOI] [PubMed] [Google Scholar]
- Meiran N. Gotler A., & Perlman A (2001). Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. The Journals of Gerontology, Series B: Psychological Sciences, 56, 88–102. doi:10.1093/geronb/56.2.p88 [DOI] [PubMed] [Google Scholar]
- Miller E. K., & Buschman T. J (2014). Chapter 27: Neural mechanisms for the executive control of attention. In Nobre A., Kastner S. (Eds.), The Oxford Handbook of Attention. Oxford, UK: Oxford University Press. doi:10.1093/oxfordhb/9780199675111.013.017 [Google Scholar]
- Monsell S., & Mizon G. A (2006). Can the task-cuing paradigm measure an endogenous task- set reconfiguration process?Journal of Experimental Psychology: Human Perception and Performance, 32, 493–516. doi:10.1037/0096-1523.32.3.493 [DOI] [PubMed] [Google Scholar]
- Myerson J. Emery L. White D. A., & Hale S (2003). Effects of age, domain, and processing demands on memory span: Evidence for differential decline. Aging, Neuropsychology, and Cognition, 10, 20–27. doi:10.1076/anec.10.1.20.13454 [Google Scholar]
- Neider M. B. Gaspar J. G. McCarley J. S. Crowell J. A. Kaczmarski H., & Kramer A. F (2011). Walking and talking: dual-task effects on street crossing behavior in older adults. Psychology and Aging, 26, 260–268. doi:10.1037/a0021566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp A. M., & Koch I (2006). Task inhibition and task repetition in task switching. European Journal of Cognitive Psychology, 18, 624–639. doi:10.1037/e520602012-405 [Google Scholar]
- Reeve T. G., & Proctor R. W (1988). Determinants of two- choice reaction-time patterns for same-hand and different-hand finger pairings. Journal of Motor Behavior, 20, 317–340. doi:10.1080/00222895.1988.10735448 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2010). Is flanker-based inhibition related to age? Identifying specific influences of individual differences on neurocognitive variables. Brain and Cognition, 73, 51–61. doi:10.1016/j.bandc.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A., & Miles J. D (2002). Aging and time-sharing aspects of executive control. Memory and Cognition, 30, 572–582. doi:10.1016/j.bandc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Smid H. G. O. M. Fiedler R., & Heinze H. J (2000). An electrophysiological study of the insertion of overt response choice. Journal of Experimental Psychology: Human Perception and Performance, 26,1053–1071. doi:10.1037/0096-1523.26.3.1053 [DOI] [PubMed] [Google Scholar]
- Smith E. E. (1968). Choice reaction time: an analysis of the major theoretical positions. Psychological Bulletin, 59, 77–110. doi:10.1037/h0020189 [DOI] [PubMed] [Google Scholar]
- Sternberg S. (1966). High-speed scanning in human memory. Science, 153, 652–654. doi:10.1126/science.153.3736.652 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. (2011). Aging and executive control: Reports of a demise greatly exaggerated. Current Directions in Psychological Science, 20, 174–180. doi:10.1177/0963721411408772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P., & Cerella J (2002). Aging, executive control, and attention: A review of meta- analyses. Neuroscience & Biobehavioral Reviews, 26, 849–857. doi:10.1016/s0149-7634(02)00071-4 [DOI] [PubMed] [Google Scholar]
- Wasylyshyn C., Verhaeghen P., Sliwinski M. J. (2011). Aging and task switching: A meta- analysis. Psychology and Aging, 26, 15–20. doi:10.1037/a0020912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks R. T. Hasher L., & Li K. Z. H (2000). Human memory. In Salthouse T. A., Craik F. I. M. (Eds.), Handbook of aging and cognition (2nd ed., pp. 293–357). Mahwah, NJ: Lawrence Erlbaum. doi:10.1017/s0714980800012332 [Google Scholar]