Abstract
Study Objectives
To assess the risks associated with the use of alcohol as a “sleep aid,” we evaluated tolerance development to pre-sleep ethanol’s sedative-hypnotic effects, and subsequent ethanol dose escalation.
Methods
Volunteers, 21–55 years old, with insomnia in otherwise good medical and psychiatric health and no history of alcoholism or drug abuse participated. In experiment 1 (n = 24) 0.0, 0.3, or 0.6 g/kg (n = 8 per dose) ethanol was administered before sleep and 8-hour nocturnal polysomnograms (NPSGs) were collected. In experiment 2, after six nights pretreatment with ethanol 0.45 g/kg (n = 6) versus placebo (n = 6), choice of pre-sleep ethanol or placebo was assessed over seven choice nights.
Results
The 0.6 g/kg ethanol dose increased total sleep time and stage 3–4 sleep on night 2, but these effects were lost by night 6 (p < .05). Six nights of ethanol pretreatment produced on the choice nights more self-administered ethanol refills than the placebo pretreatment (p < .03).
Conclusions
These are the first data to explicitly show the risks associated with the use of alcohol as a “sleep aid” among people with insomnia. Initially, a moderate dose of ethanol improved NPSG sleep, which was lost by night 6. Tolerance was associated with enhanced self-administration of pre-sleep ethanol.
Keywords: ethanol, insomnia, tolerance development, ethanol self-administration
Statement of Significance
In the population, 20%–30% of people with insomnia report use of alcohol as a sleep aid and 67% report it is effective. Studies in healthy volunteers have shown rapid tolerance to the sedative effects of high-dose ethanol. These studies assessed moderate dose pre-sleep ethanol tolerance and its risk in people with insomnia. These are the first data to explicitly show the risks associated with the use of alcohol as a “sleep aid” among people with insomnia.
Introduction
Chronic insomnia, the persistent difficulty falling asleep, maintaining sleep, or non-restorative sleep, which disrupts one’s ability to function during the day, is reported by 10%–20% of the general population [1]. Approximately 20%–30% of those individuals with chronic insomnia report using alcohol to help them sleep [2, 3] and 67% of users report the alcohol was effective [2]. A prior study compared the effects of low-dose ethanol (0.5 g/kg) on the sleep of individuals with chronic insomnia and age-matched healthy controls with similar moderate social drinking histories [4]. The ethanol, consumed 30 minutes before sleep, improved the sleep of those with insomnia relative to placebo. The second half of the night sleep disruption typically found in healthy volunteers at higher doses was not observed in either group at this lower dose. When given the opportunity to choose between a previously experienced color-coded ethanol or placebo beverage before sleep, those with insomnia chose ethanol and self-administered a 0.45 g/kg dose, while the controls chose placebo [4].
Several studies in healthy volunteers have assessed ethanol effects over repeated nights of administration [5, 6]. Tolerance to the effects of the high ethanol doses (>0.5 g/kg) used in these studies developed within three nights. Typically, in clinical reports with repeated nightly use of alcohol or other drugs of abuse that lead to tolerance development, dose is escalated following tolerance. Potential for dose escalation was not evaluated in the healthy volunteer tolerance studies. Tolerance development and dose escalation may be important signs of heightened risk of abuse when people with insomnia use alcohol as a “sleep aid.”
The current studies were conducted to assess tolerance development over seven nights to the sedative/hypnotic effects of a range of low ethanol doses in persons with insomnia. Further, the potential for dose escalation after nightly administration of ethanol was assessed by using the color-coded beverage choice procedures of our previous study following six nights of ethanol or placebo pre-choice exposure [4].
Methods
Participants
Healthy, volunteers aged 21–55 years old with primary chronic insomnia according to DSM-IVR (Diagnostic and Statistical Manual of Mental Disorders, 4th ed, revised) criteria and a standard sleep laboratory screening to confirm disturbed sleep, which was defined as a sleep efficiency of less than 85% on the 8-hour nocturnal polysomnogram (NPSG) and the absence of sleep disordered breathing and periodic leg movement disorders (apnea/hypopnea or periodic leg movement indices < 10) [7]. They were in otherwise good physical and psychological health as determined by medical, psychiatric, clinical laboratory, and drug/alcohol abuse screening. Participants were recruited using metropolitan Detroit area newspaper advertisements, on-line advertisements, university-medical school and community flyers, job fairs, and word of mouth.
The protocols were each approved by the Institutional Review Board and each participant signed an informed consent and was paid for participation. Each participant completed an exit interview at which sleep hygiene principles were discussed and the use of alcohol as a “sleeping aid” was strongly discouraged.
Medical and psychological screening
Each participant received a physical examination, the Cornell Medical Index, and clinical laboratory analyses of blood and urine samples for hematologic, hepatic, renal, and other major system functions. Volunteers with BMIs >30 were excluded. Any volunteer with positive laboratory findings was excluded from the study with special attention paid to liver function results to exclude persons with liver disease. Participants underwent the Structured Clinical Interview (SCID) for DSM-IVR to rule out major psychiatric disease.
Alcohol and drug use history
Participants were interviewed to quantify their current and past drug and alcohol use. No participant reporting current or past illicit drug use or alcoholism was admitted to the studies and a urine drug screen, which tested for use of amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, methadone, opiates, propoxyphene, and phencyclidine, was used to confirm the absence of current drug use. A breathalyzer was used to rule out current alcohol use.
Participants were also queried regarding use of alcohol as a sleep aid, which was an exclusion for all of the studies. Participants reported moderate social drinking defined as ≤14 standard alcohol drinks per week and <5 (men) or <4 (women) drinks per occasion.
Nocturnal Polysomnograms
The NPSGs consisted of central (C3-A2) and occipital (Oz-A2) electroencephalograms (EEGs), bilateral horizontal electro-occulograms, a submental electromyogram, and electrocardiogram recorded with a V5 lead [8]. In addition, on the screening night, leg movements and airflow was monitored [7]. The sleep recordings were scored in 30-second epochs according to the standards of Rechtschaffen and Kales by scorers who maintained a 90% scoring reliability [8]. Nasal-oral recordings were scored for apneas, defined as 10 seconds or longer cessations of airflow, and hypopneas, defined as 10 seconds or longer reductions (50% or greater) of airflow [7]. Tibialis muscle recordings were scored for leg movements associated with arousal, defined as 0.5 seconds or greater tibialis flexions coincident with brief EEG speeding [7]. Respiratory events (i.e. apnea and hypopnea) and leg movement events were tabulated and expressed as indices per hour of total sleep time. The subsequent NPSGs of the experimental nights for experiment 1 did not include respiratory and leg movement monitoring. On all nights, time-in-bed was fixed to 8 hours adjusted to the participant’s dairy reported sleep habits.
Ethanol administration and choice procedures
In the consents for these experiments, participants were told the effects of different alcohol beverages on their sleep and their preference for the different beverages was being evaluated. The ethanol was prepared in a 1:4 ratio with 80 proof vodka (Absolute) added to tonic water and the placebo consisted of tonic water (in equal volume to the ethanol) with five drops of ethanol floated on the surface for gustatory and olfactory cues. In fixed administration study phases the beverages were consumed over 30 minutes with the total volume divided into three separate cups and each consumed over 10 minutes.
For the six-night tolerance study (experiment 1) beverages were provided in white cups and each participant received the same dose nightly per the randomized dose assignment. For the dose escalation study (experiment 2) beverages were provided in color-coded cups with the colors associated with ethanol and placebo counterbalanced among participants. On the beverage sampling nights (nights 7 and 8) participants were told to attend to cup color because they would be given a choice of the two beverages on subsequent choice nights.
The ethanol drinking was done individually in a bedroom, while comfortably seated at a table. Movement about the room, except as necessary to void (each room has a bathroom) and go to bed (bed and bathroom are within 10 feet of the table), was restricted to minimize any ethanol-related kinesthetic cues. The beverage drinking was concluded 30 minutes before bedtime. For the reader not familiar with ethanol research, a Table comparing ethanol doses, the breath ethanol concentrations (BrEC) achieved at 30 minutes post consumption (i.e. estimated peak concentration), and 12 oz US beer equivalents can be seen in Roehrs and Roth [9]. Briefly for this study the 0.3 g/kg dose is approximately equivalent to two US beers and 0.6 g/kg to four beers.
Experiment 1
Twenty-four people (50% male–female) with insomnia were randomized to receive either 0.0, 0.3, or 0.6 g/kg (n = 8 per dose group) ethanol before sleep for six consecutive nights (see ethanol administration procedures above). The drinking began 1 hour before bedtime and was completed 30 minutes before bedtime. NPSGs were collected as described above on nights 2 and 6. Night 1 was used as an adaptation night for the pre-sleep ethanol drinking procedures.
Experiment 2
Twelve people (50% male–female) with insomnia as defined above were randomized to an ethanol (n = 6) or placebo (n = 6) pretreatment and received ethanol (0.45 g/kg) or placebo in the same color-coded cup on seven consecutive nights. For one half, the color associated with placebo was blue and for the other half red and the same was the case within the ethanol group. On night 8, they received the opposite beverage, ethanol for the placebo group and placebo for the ethanol group. The cup color on night 8 was the opposite color of that used on the first seven nights. This seven-night pretreatment fixed dose drinking was conducted as described above.
On the seven subsequent choice nights (nights 9 through 15) they choose their preferred beverage on each night based on cup color (i.e. the cup color of the first seven nights or the night 8 cup color). The choice on each of the seven nights was independent of the previous night’s choice. On a given night they then had an opportunity to request three refills of their chosen cup color beverage (0.2 g/kg ethanol or placebo refills). Thus, if ethanol was chosen on a given night, in addition to the initial 0.2 g/kg dose, if all three refills were taken, a total possible nightly ethanol dose of 0.8 g/kg could be self-administered. Initial and refill drinking opportunities were 15 minutes in duration. Bedtime was set at 30 minutes following the last refill opportunity. NPSGs were not collected in this study.
Analyses
NPSG measures of experiment 1 were submitted to mixed design MANOVAs with ethanol dose group the between subject variable and nights the within repeated variable. Night 2 was compared to night 6 as night 1 was considered an adaptation night to drinking procedures. Significant main effects and interactions were followed with post hoc comparisons to probe as to where differences existed. For experiment 2, the number of nights ethanol was chosen was compared between ethanol pretreatment groups by chi-square. The average number of ethanol refills on ethanol-choice nights was compared between pretreatment groups by between group t-tests.
Results
Experiment 1
Table 1 presents the NPSG measures (expressed as minutes and percentages) for the three ethanol doses on nights 2 and 6. Sleep efficiency showed a dose by night interaction (F2,21 = 5.61, p < .01). With the 0.6 g/kg dose sleep efficiency was higher on night 2 relative to the 0.0 and 0.3 g/kg doses (p < .02), but was worse on night 6 compared to night 2 (within dose group by night comparison, p < .04) and no longer differed significantly from the 0.0 and 0.3 g/kg dose sleep efficiency on night 6. Given the fixed time-in-bed, minutes of total sleep time showed the same interaction and pattern of effects. Minutes of stage 3–4 sleep showed a similar interaction (F2,21 = 3.46, p < .05). While on night 2 the 0.6 g/kg minutes of stage 3–4 was numerically higher than the 0.0 g/kg dose; it did not achieve statistical significance. But, within the 0.6 g/kg dose group minutes of stage 3–4 sleep on night 6 declined significantly versus that on night 2 (p < .04). Minutes of stage 1 sleep showed a dose-related increase (main effect, F2,21 = 3.48, p < .05) and no interaction across nights with the 0.6 g/kg differing from the 0.0 and 0.3 g/kg doses. No other NPSG parameters showed ethanol effects.
Table 1.
Polysomnographic sleep measures as a function of ethanol dose
| Dose | Night | 0.0 g/kg | 0.3 g/kg | 0.6 g/kg |
|---|---|---|---|---|
| Sleep efficiency (TST/TIB)a | 2 | 78.2 (7.3) | 79.6 (9.3) | 86.3 (10.3) |
| 6 | 79.2 (8.2) | 83.6 (5.3) | 70.6 (5.3) | |
| Total sleep time (minutes) a | 2 | 375.4 (33.6) | 382.1 (44.6) | 414.2 (49.4) |
| 6 | 380.2 (39.4) | 401.3 (25.4) | 338.9 (25.4) | |
| Stage 1 % | 2 | 10.9 (2.0) | 13.1 (2.0) | 7.1 (2.0) |
| 6 | 8.6 (1.5) | 10.2 (1.1) | 8.2 (1.5) | |
| Stage 1 minutesb | 2 | 40.9 (7.5) | 50.1 (7.6) | 29.4 (8.3) |
| 6 | 32.7 (0.6) | 40.9 (4.4) | 27.8 (5.1) | |
| Stage 3–4 % | 2 | 12.2 (2.5) | 14.5 (2.3) | 17.9 (2.1) |
| 6 | 12.6 (2.8) | 15.7 (4.0) | 15.2 (3.1) | |
| Stage 3–4 minutesc | 2 | 45.8 (9.4) | 55.3 (8.7) | 74.2 (8.8) |
| 6 | 47.9 (10.8) | 62.9 (10.0) | 51.5 (10.2) | |
| Stage REM % | 2 | 17.0 (1.6) | 18.0 (2.7) | 21.6 (2.0) |
| 6 | 21.2 (1.8) | 20.4 (2.2) | 20.6 (1.9) | |
| Stage REM minutes | 2 | 63.8 (6.0) | 68.8 (10.3) | 89.5 (8.3) |
| 6 | 80.6 (6.8) | 81.9 (8.8) | 69.9 (6.4) |
Data are means (SEM). Sleep efficiency (total sleep time/time in bed – fixed at 480 minutes).
a p < .01 dose × night interaction.
b p < .05 main effect of dose.
c p < .05 dose × night interaction.
Experiment 2
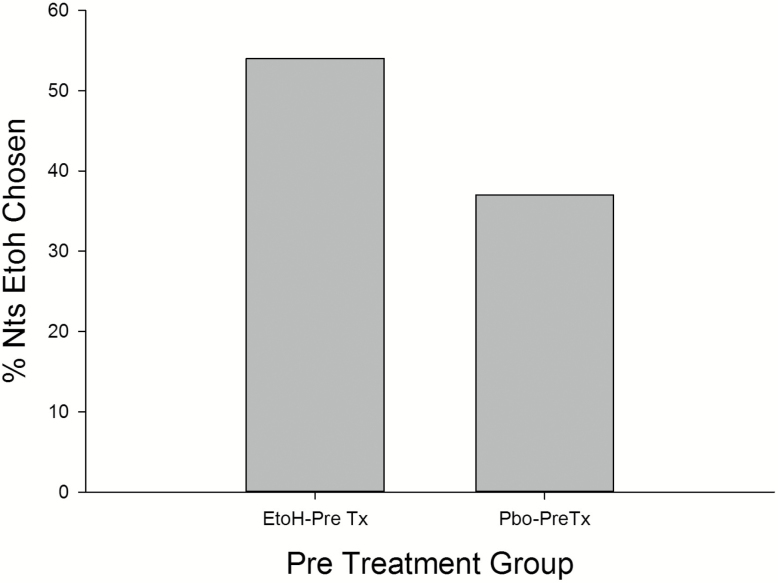
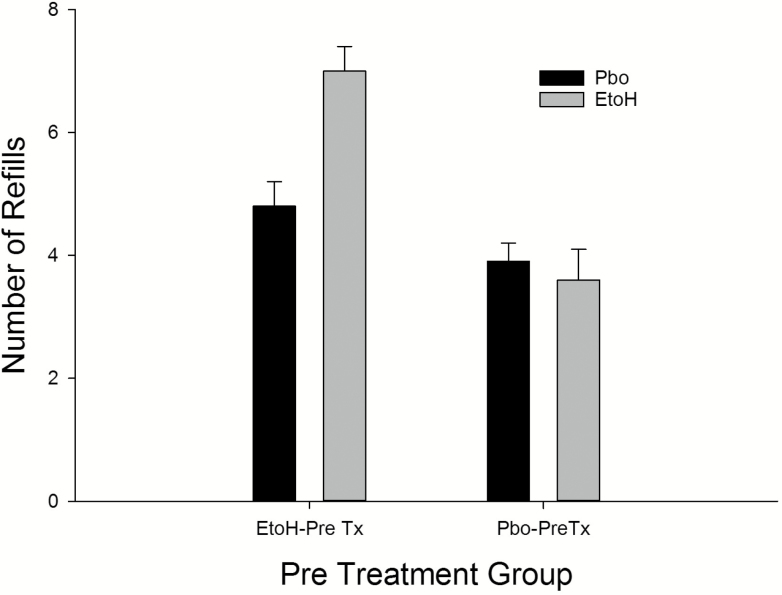
Recall, on choice nights participants could choose either ethanol or placebo based on their previously experienced cup color (i.e. red = placebo, blue = ethanol). The ethanol pretreated group choose ethanol on 54% of nights in comparison to the 37% of ethanol choice nights for the placebo pretreated group (X2 = 2.85, p < .09) (Figure 1). After the beverage choice on a given night 3 refills of their chosen beverage could be requested. Figure 2 presents the number of ethanol and placebo refills as a function of pretreatment group. On the ethanol selected nights, the ethanol pretreated group choose significantly more total ethanol refills on average over the seven nights (7.0 ± 1.9) compared to the 3.57 ± 2.1 ethanol refills of the placebo pretreated group (t = 2.46, p < .03). Given the initial ethanol dose of 0.2 g/kg plus the average nightly addition of one refill, the ethanol pretreated group self-administered a 0.4 g/kg dose before sleep, while the placebo pretreated group took a single refill on approximately one-half of the seven nights. Given ethanol was chosen 53% of nights by the ethanol pretreated group, in comparing groups over the 1–7 choice nights a differential nightly increase in the number of ethanol refills across the seven nights was not seen. Finally, on those nights placebo (both groups chose placebo on some nights) was chosen the ethanol pretreated group choose 4.71 ± 1.7 refills and the placebo pretreated group choose 4.01 ± 1.29 placebo refills (NS).
Figure 1.
The percent of nights ethanol was chosen as a function of pretreatment group. Etoh = ethanol (0.45 g/kg) pretreatment; Pbo = placebo pretreatment.
Figure 2.
Number of ethanol and placebo refills as a function of pretreatment group. Etoh = ethanol pretreatment; Pbo = placebo pretreatment; each refill = 0.2 g/kg per refill.
Discussion
These are the first data to explicitly show the risks associated with the use of alcohol as a “sleep aid” among people with insomnia. Initially the largest ethanol dose (i.e. 0.6 g/kg) of this study improved NPSG sleep efficiency and stage 3–4 sleep in these people with insomnia, but by night 6 tolerance developed to both of these “beneficial” effects. Pretreatment with ethanol for six nights in experiment 2, presumably during which tolerance developed as in experiment 1 (NPSGs were not collected), led to a subsequent between group difference in pre-sleep ethanol self-administration on choice nights. The ethanol pretreated group self-administered significantly more ethanol refills than the placebo pretreated group.
As in our previous study of alcohol effects (0.50 g/kg) in individuals with insomnia, in this study we found the high (0.60 g/kg) ethanol dose was associated with greater minutes stage 3–4 sleep relative to placebo, at least initially on night 2. The literature regarding the effects of ethanol on stage 3–4 sleep in healthy volunteers is equivocal. This study did not include a healthy volunteer comparison group, but in our first study the individuals with insomnia had lower minutes stage 3–4 sleep on placebo than the healthy volunteers and the 0.50 g/kg dose was associated with greater minutes stage 3–4 sleep similar to the level seen in the healthy volunteers (i.e. “normalized” stage 3–4 sleep) [4]. In this study we found a dose-effect of alcohol on minutes stage 3–4 sleep (46 minutes at 0.0 g/kg, 55 minutes at 0.3 g/kg and 74 minutes at 0.6 g/kg).
These effects of ethanol on the sleep of people with insomnia are in contrast to the effects of standard FDA-approved, GABA-acting hypnotics. At clinical doses they reduce (e.g. benzodiazepines) or do not affect stage 3–4 sleep (e.g. non-benzodiazepines), or other sleep stages [9]. As yet the functional significance and clinical relevance of alterations of sleep stages remain equivocal [10]. Ethanol, as in this study and our previous study [4] and all the FDA-approved hypnotic studies initially increase total sleep time [11]. These present data have shown tolerance develops to both the stage 3–4 and the total sleep time promoting effects of ethanol within six consecutive nights of use. In contrast a number of self-report studies of FDA-approved hypnotics have shown an absence of tolerance development over 12 months of nightly hypnotic use [10] and our NPSG study showed an absence of tolerance development over 8 months of nightly use [12].
The critical question then arises, does the use of alcohol as a sleep aid in the population then impact the social use of alcohol during the daytime? These volunteers were all moderate social drinkers.
The results of these studies have to be taken cautiously, as the number of subjects in each study was small and the doses used were low. For ethical reasons we were very conservative in regards to the available ethanol doses and the number of people with insomnia exposed to pre-sleep ethanol use. Along the same line we went to great lengths to meet with the participants at the conclusion of these studies to debrief them and explain to them the problems associated with the use of alcohol as a sleep aid.
In conclusion, these are the first data to explicitly show the risks associated with the use of alcohol as a “sleep aid” among people with insomnia. Initially sleep was “improved,” but tolerance developed rapidly, which was associated with increased pre-sleep self-administration.
Funding
These studies were supported by a National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant (#R01 AA11264) awarded to Dr Roehrs. All authors have seen and approved the manuscript. Paper does not report off-label drug use and does not report the results of a clinical trial.
Notes
Conflict of interest statement. None declared.
References
- 1. Lichstein K, et al. Insomnia: epidemiology and risk factors. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine, 6th ed Philadelphia PA: Elsevier; 2017:761–768. [Google Scholar]
- 2. Ancoli-Israel S, et al. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Sleep Survey. Sleep. 1999;22:S347–S353. [PubMed] [Google Scholar]
- 3. Johnson EO, et al. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–186. [DOI] [PubMed] [Google Scholar]
- 4. Roehrs T, et al. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20:279–286. [DOI] [PubMed] [Google Scholar]
- 5. Rundell JB, et al. Alcohol and sleep in young adults. Psychopharmacology. 1972;26:201–218. [DOI] [PubMed] [Google Scholar]
- 6. Prinz P, et al. Effect of alcohol on sleep and nighttime plasma growth hormone and cortisol concentrations. J Clin Endocrin Metab. 1980;51:759–764. [DOI] [PubMed] [Google Scholar]
- 7. American Academy of Sleep Medicine. ASSM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 8. Rechtschaffen A, et al. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: U.S. Govt Printing Office, USPHS; 1968. [Google Scholar]
- 9. Roehrs T, et al. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. [DOI] [PubMed] [Google Scholar]
- 10. Roehrs T, et al. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth T, et al. Pharmacotherapy of sleep promoting agents. In: Avidan AY, ed. Review of Sleep Medicine. 4th ed Philadelphia, PA: Elsevier; 2017:392–403. [Google Scholar]
- 12. Randall S, et al. Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study. Sleep. 2012;35:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]