Abstract
Introduction
Smoking likely exacerbates comorbidities which people living with HIV (PLWH) are predisposed. We assessed prevalence and correlates of smoking among PLWH in South Africa, which has 7 million PLWH but inadequate reporting of smoking.
Methods
A cross-sectional survey was conducted among randomly selected adults with HIV infection in Klerksdorp, South Africa. Current smoking was assessed by questionnaire, exhaled carbon monoxide (eCO), and urine cotinine.
Results
Of 1210 enrolled adults, 753 (62%) were women. In total, 409 (34%) self-reported ever smoking: 301 (74%) were current and 108 (26%) were former smokers. Using eCO and urine cotinine tests, 239 (52%) men and 100 (13%) women were defined as current smokers. Nearly all smokers (99%) were receiving ART, and had a median (IQR) CD4 count of 333 cells/μL (181–534), viral load of 31 IU/mL (25–4750), and BMI of 21 kg/m2 (19–24). Adjusted analysis among men showed higher odds of smoking with marijuana use (OR = 7.5, 95% CI = 4.1 to 14.6). Among women, 304 (43%) reported using snuff, compared to only 11 (3%) of men, and snuff use was inversely associated with smoking (OR = 0.1; 95% CI = 0.05 to 0.2). A subset of participants (n = 336) was asked about alcohol use, which was positively associated with smoking for men (OR = 8.1, 95% CI = 2.8 to 25.9) and women (OR = 8.5, 95% CI = 2.9 to 26.8).
Conclusion
Smoking prevalence among PLWH in South Africa is alarmingly high. Prevention and cessation strategies that consider marijuana and alcohol use are needed.
Implications
As long-term HIV care continues to improve, more people living with HIV (PLWH) will die of diseases, including tuberculosis, for which smoking plays an important causal role. The prevalence of smoking is markedly higher among PLWH in high-resource settings, but data for Africa and other low-resource settings that shoulder the brunt of the HIV epidemic has previously not been well documented. We report an alarmingly high prevalence of smoking among PLWH in South Africa, particularly among men, and a strong association between current smoking and use of other substances.
Introduction
Antiretroviral therapy (ART) has dramatically improved the prognosis of individuals with HIV infection, resulting in increased longevity.1,2 Although longevity is improving, people living with HIV (PLWH) and on ART are at greater risk than HIV seronegative peers for cardiovascular disease,3–6 malignancies,7 and pulmonary comorbidities, including tuberculosis (TB).8 Though the effect of smoking on HIV prognosis is not clearly understood, smoking is associated with increased risk for all of these conditions, and likely exacerbates conditions for which PLWH are highly vulnerable.9–11 Importantly, smoking doubles the risk of TB among people with12 and without HIV.13,14 TB has long been the leading cause of death among PLWH,15 and as smoking rates climb, the potential contribution of smoking to TB morbidity and mortality will rise. As long-term HIV care continues to improve, more PLWH will die of non-HIV related illness for which smoking plays an important causal role.
South Africa has the highest rate of HIV in the world, with a prevalence of 19.2% among adults and 380000 new infections occur annually.16 Approximately 3.4 of the 7 million HIV-infected individuals in South Africa are receiving ART, and this number continues to rise as ART coverage continues to increase.16 While the prevalence of smoking is often found to be markedly higher among PLWH than the general population in high-resource settings,17 the prevalence of smoking in those with HIV infection is not well documented in Africa. In preparation for a smoking cessation clinical trial among HIV-infected patients, we assessed the prevalence of and correlates associated with current smoking among adults with HIV infection in South Africa.
Methods
This cross-sectional survey was conducted among randomly selected adults (≥18 years) with HIV infection attending three HIV clinics in Klerksdorp, South Africa between April 2012 and August 2013. The three clinics selected for inclusion were chosen given they were high-volume clinics that provide HIV services, and are representative of the epidemic in the province. Each day a random seed was drawn in each clinic, and the nth individual (as determined by the random seed) referred by the clinic staff was eligible for inclusion in the study. Clinic staff referred adults (age ≥ 18 years) with HIV infection to trained research staff, who determined eligibility based on the day’s random seed, obtained written informed consent, and administered survey questions. All patients were provided with HIV counseling and smokers were provided with brief smoking cessation advice from clinic staff, pamphlets (Make A Fresh Start, Your personal guide to stop smoking) from the National Council Against Smoking (NCAS), and referred to NCAS for further smoking cessation advice.18
To capture self-reported tobacco use, study staff administered a survey adapted from the Centers for Disease Control and Prevention’s Global Adult Tobacco Survey (GATS) with additional questions on alcohol and marijuana consumption. Participants self-reported demographic information and personal smoking history, and smokers were administered the Fagerstrom test for nicotine dependence and reported quit history.19 Surveys were conducted in English or alternate local languages, Setswana, Zulu or Xhosa, based on the participant’s language preference. In addition to self-reported non-daily and daily smoking status, all participants breathed into a handheld exhaled carbon monoxide (CO) monitor (coVita, USA), which measures smoking exposure over the previous 8 hours, and provided a urine sample for point of care cotinine testing, a metabolite of nicotine, using SmokeScreen (GFC Diagnostics, UK). SmokeScreen is a semi-quantitative assay indicating light, medium, and heavy smoking through colorimetric evaluation, with the urine changing in color after addition of the assay chemicals. The urine cotinine test measures tobacco exposure over the past 48–72 hours. A positive screen was defined as values at or above 10 parts per million (ppm) for exhaled CO, and a test result showing light, medium, or heavy smoking through evaluation of urine color was defined as a positive test for the urine sample. For purpose of assessing prevalent smoking, we defined a current smoker using positive test results for CO and urine cotinine tests in addition to self-report with consideration of reported use of snuff and marijuana, and current TB status due to interaction of isoniazid with the urine test resulting discoloration or false positives.20
Ethics approvals were obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) and the Johns Hopkins University Institutional Review Board. Written consent was obtained from all participants prior to enrollment.
Statistical Analysis
Prevalence of current smoking and corresponding normal approximation of the 95% confidence intervals were calculated across study categories of interest. Sample size was calculated to estimate prevalence with strong precision to within 5% for women (10–30% prevalence) and men (30–55% prevalence). The Chi (χ2) squared and Wilcoxon rank-sum tests, as appropriate, were used to compare socio-demographic and clinical characteristics with current smoking status using reported smoking, exhaled breath CO, and urine cotinine tests. Variables significantly associated at the univariate level (p < .20), or those determined of importance a priori, were included in a multivariable logistic regression for current smoking as the outcome of interest. All data were analyzed using R (Version 3.3.1).21
Results
A total of 1210 adults with HIV infection were enrolled in the study; median (IQR) age was 41 years (34–48) and 753 (62%) were women. Nearly all (99%) participants had initiated ART, and the median (IQR) CD4 count was 351 cells/mm3 (199–538) and median (IQR) viral load was 31 IU/mL (25–4750). Women had significantly higher CD4 counts (median 388 cells/mm3, IQR: 243–564) than men (median 292 cells/mm3; IQR 151–458) (p < .0001), as well as lower viral loads (median 25 IU/mL, IQR: 25–2500) than men (median 45 IU/mL, IQR: 25–97760) (p = .047). At the time of interview, 409 (34%) participants self-reported ever smoking, of whom 301 (74%) reported current smoking and 108 (26%) reported smoking only in the past. After participants were classified as current smokers or non-smokers using exhaled breath CO and urine cotinine tests, 239 (52%) males and 100 (13%) females were defined as current smokers. Participants were asked to self-report TB disease, and 123 (10%) reported current and 704 (58%) prior TB disease. Among men, 44% of those with current TB were current smokers.
Among current smokers, 261 (87%) reported daily smoking and 40 (13%) report non-daily smoking. The median (IQR) age of initiation of regular smoking was 19 years (16–23) among all current smokers, and 22 years (18–28) and 18 years (15–22) among non-daily and daily smokers, respectively. The median (IQR) number of years smoking for daily and non-daily smokers was 22 (13–29) and 15 (8–24), respectively. Only 59 men and 35 women reported being former smokers, thus we did not have sufficient power to determine significant differences between former and current smokers in this sample. Overall, smokers reported smoking a median (IQR) of 6 (3–10) cigarettes per day, with daily smokers smoking more cigarettes per day (median 7, IQR 4–10) than non-daily smokers (median 2.5, IQR 2–4). A majority of daily smokers reported having their first cigarette within one hour of waking (n = 198, 84%), as did nearly half of the non-daily smokers (n = 17, 44%). The median (IQR) score on the Fagerstrom test for nicotine addiction was 5 (3, 7) and 3 (1, 4) for daily and non-daily smokers, respectively. Importantly, 80% of both daily and non-daily smokers report wanting to stop smoking within the next month, and 206 (79%) of daily and 36 (90%) of non-daily smokers reported trying to quit smoking within the past year. Little dual use of snuff and smoking was reported, however 304 (43%) women reported current snuff use, while only 11 (3%) of men report using snuff. Given the large proportion of women reporting snuff use, a separate analysis assessing correlates of snuff use is being conducted and will be presented in a separate manuscript. For men and women, respectively, only 12 (3%) and 26 (3%) self-reported non-smoking and were found to be smokers using our study definition.
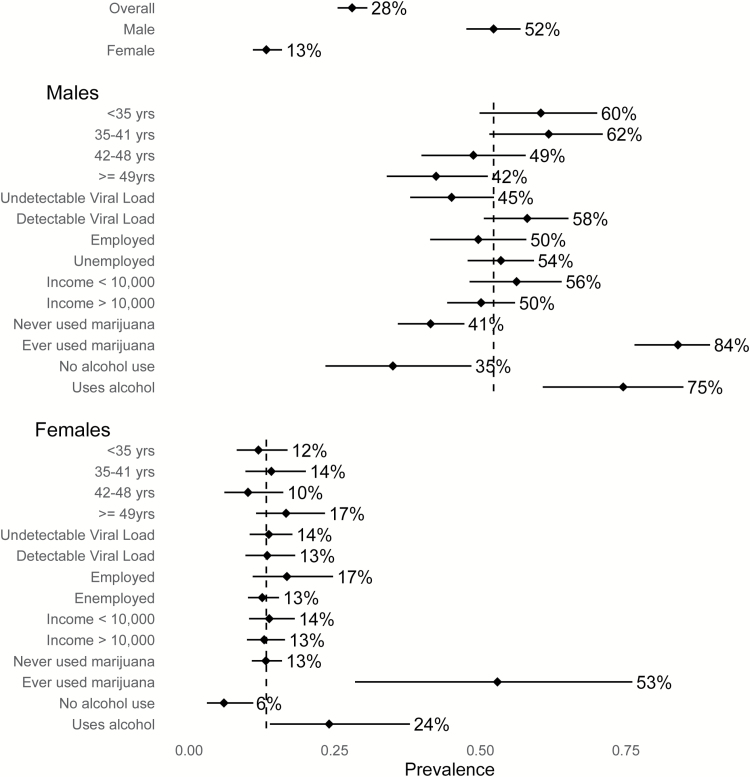
Given the large difference in smoking prevalence between males and females, we stratified our analyses by sex (Figure 1). Male smokers (Table 1) were younger (median 45 years vs. 41 years; p = .0004) and had significantly lower BMI than non-smokers (median (IQR): 19.9 (18.3, 21.8) and 21.5 (19.2, 24.6), respectively; p < .0001). Male smokers also had higher viral load than non-smokers (median (IQR): 110 (25–8173) and 28 (24–5400), respectively; p = .03). Male smokers had fewer rooms in their homes (p = .04) and fewer had a mobile phone (p = .03). Traditional measures of socioeconomic status (SES), including education, employment, and household income, did not vary by smoking status. Overall, 137 (31%) of men reported ever marijuana use, which was more prevalent among smokers than non-smokers (n = 115 (48%) and n = 22 (11%), respectively; p < .001), and 31 (13%) of current smokers self-reported marijuana use at least weekly. Questions on alcohol use were incorporated into the survey after the study began. Of 115 men surveyed, 55 (48%) reported current alcohol use, and prevalence was significantly higher among smokers than non-smokers (n = 41 (66%) and n = 14 (26%), respectively; p < .0001).
Figure 1.
Prevalence of current smoking among men and women across correlates of interest.
Table 1.
Demographic characteristics and correlates of current smoking among of 457 male and 753 female individuals with HIV infection in Klerksdorp, South Africa
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 457) | Non-smoker (n = 218) | Smoker (n = 239) | p* | Total (n = 753) | Non-smoker (n = 653) | Smoker (n = 100) | p* | |
| Age (years), n (%) | ||||||||
| <36 | 96 (21) | 38 (17) | 58 (24) | .01 | 235 (31) | 207 (32) | 28 (28) | .32 |
| 36–43 | 102 (22) | 39 (18) | 63 (26) | 191 (25) | 164 (25) | 27 (27) | ||
| 44–49 | 127 (28) | 65 (30) | 62 (26) | 158 (21) | 142 (22) | 16 (16) | ||
| ≥50 | 132 (29) | 76 (35) | 56 (23) | 168 (22) | 140 (21) | 28 (28) | ||
| Education, n (%) | ||||||||
| <Grade 12 | 371 (81) | 177 (81) | 194 (81) | 1.00 | 617 (82) | 532 (81) | 85 (85) | .49 |
| Grade 12 or higher | 86 (19) | 41 (19) | 45 (19) | 136 (18) | 121 (19) | 15 (15) | ||
| Employment, n (%) | ||||||||
| Employed | 149 (33) | 75 (34) | 74 (31) | .48 | 125 (17) | 104 (16) | 21 (21) | .20 |
| Unemployed | 308 (67) | 143 (66) | 165 (69) | 628 (83) | 549 (84) | 79 (79) | ||
| Household income, n (%) | ||||||||
| <1000 | 160 (35) | 70 (32) | 90 (38) | .24 | 319 (42) | 275 (42) | 44 (44) | .75 |
| >1000 | 297 (65) | 148 (68) | 149 (62) | 434 (58) | 378 (58) | 56 (56) | ||
| Disability grants, n (%) | 81 (18) | 36 (17) | 45 (19) | .54 | 125 (17) | 106 (16) | 19 (19) | .47 |
| Current living situation, n (%) | ||||||||
| House/Flat | 330 (72) | 159 (73) | 171 (72) | .75 | 575 (76) | 505 (77) | 70 (70) | .13 |
| Shack/Homeless | 127 (28) | 59 (27) | 68 (28) | 178 (24) | 148 (23) | 30 (30) | ||
| Number of people in living situation, median (IQR) | 4 (2, 5) | 4 (2, 5) | 3 (2, 5) | .39 | 4 (3, 5) | 4 (3, 5) | 3 (2, 5) | <.001 |
| Number of people in living situation, n (%) | ||||||||
| <3 | 124 (27) | 59 (27) | 65 (27) | .85 | 150 (20) | 118 (18) | 32 (32) | .005 |
| 3–5 | 258 (56) | 121 (56) | 137 (57) | 425 (56) | 374 (57) | 51 (51) | ||
| >5 | 75 (16) | 38 (17) | 37 (15) | 178 (24) | 161 (25) | 17 (17) | ||
| Number of rooms, median (IQR) | 4 (2, 4) | 4 (3, 5) | 4 (2, 4) | .04 | 4 (2, 4) | 4 (2, 4) | 3 (2, 4) | .07 |
| Number of rooms, n (%) | ||||||||
| ≤4 | 359 (79) | 132 (75) | 196 (82) | .07 | 595 (79) | 515 (79) | 80 (80) | .90 |
| >4 | 98 (21) | 55 (25) | 43 (18) | 158 (21) | 138 (21) | 20 (20) | ||
| Crowding (people per room) | 1 (0.7, 1.5) | 1 (0.7, 1.5) | 1 (0.8, 1.5) | .57 | 1 (0.8, 1.7) | 0.8 (1, 1.8) | 0.8 (1, 1.5) | .11 |
| Mobile phone for self, n (%) | 373 (82) | 187 (86) | 186 (78) | .03 | 646 (86) | 558 (85) | 88 (88) | .64 |
| CD4 Count, median (IQR) | 292 (150, 458) | 277 (156, 451) | 313 (146, 481) | .49 | 388 (243, 564) | 389 (243, 564) | 371 (246, 577) | .90 |
| Viral Load, cells/mL, median (IQR) | 45 (25, 6664) | 28 (24, 5400) | 110 (25, 8173) | .03 | 25 (25–2500) | 25 (25–2905) | 25 (25–603) | .87 |
| BMI, median (IQR) | 20.5 (18.7, 23.3) | 21.5 (19.2, 24.6) | 19.9 (18.3, 21.8) | <.0001 | 24.7 (20.9, 29.2) | 24.8 (21.0, 29.5) | 23.4 (10.7, 28.3) | .12 |
| Other substance use | ||||||||
| Ever marijuana use, n (%) | 137 (31) | 22 (11) | 115 (48) | <.0001 | 17 (2) | 8 (1) | 9 (9) | <.0001 |
| Weekly marijuana, n (%) | 34 (8) | 3 (2) | 31 (13) | <.0001 | 3 (.4) | 0 (—) | 3 (3) | .003 |
| Snuff use, n (%) | 11 (3) | 9 (5) | 2 (1) | .03 | 304 (43) | 294 (49) | 10 (10) | <.0001 |
| Alcohol use, n (%) | 55 (48) | 14 (26) | 41 (66) | <.0001 | 54 (24) | 41 (21) | 13 (57) | <.001 |
*Bold values statistically significant at p < .05.
In adjusted analysis among men, odds of current smoking were significantly higher for those ever using marijuana (OR = 7.5, 95% CI = 4.1 to 12.6) and lower among those with higher BMI (OR 0.9, 95% CI = 0.8 to 0.9) (Table 2). When alcohol is included in the model for the subset with available data, odds of current smoking were significantly lower among those with a higher BMI (OR = 0.8, 95% CI = 0.6 to 0.9). Odds of smoking were significantly higher among those who reported ever use of marijuana (OR = 16.9, 95% CI = 3.4 to 129.6) and current alcohol use (OR = 7.8, 95% CI = 2.1 to 35.0). Detectable viral load was marginally significant in the model without alcohol data (OR = 1.6, 95% CI = 0.98 to 2.6), however is not associated with smoking when alcohol was added into the model (OR = 1.7, 95% CI = 0.4 to 7.7).
Table 2.
Univariate and multivariate logistic regression for odds of current smoking among adult males with HIV infection (n = 358) in Klerksdorp, South Africa
| Non-smoker | Smoker | Univariate OR (95% CI) | p* | Multivariate OR (95% CI) | p* | |
|---|---|---|---|---|---|---|
| Age (years), n (%) | ||||||
| <36 | 38 (17) | 58 (24) | REF | REF | ||
| 36–43 | 39 (18) | 63 (26) | 1.1 (0.6 to 1.9) | .85 | 1.5 (0.7 to 3.3) | .32 |
| 44–49 | 65 (30) | 62 (26) | 0.6 (0.4 to 1.1) | .09 | 1.3 (0.6 to 2.7) | .49 |
| ≥50 | 76 (35) | 56 (23) | 0.5 (0.3 to 0.8) | .01 | 0.8 (0.4 to 1.7) | .58 |
| Employment, n(%) | ||||||
| Employed | 75 (34) | 74 (31) | REF | REF | ||
| Unemployed | 143 (66) | 165 (69) | 1.2 (0.8 to 1.7) | .43 | 1.3 (0.8 to 2.3) | .32 |
| Monthly household income, n (%) | ||||||
| <1000 | 70 (32) | 90 (38) | REF | REF | ||
| >1000 | 148 (68) | 149 (62) | 0.8 (0.5 to 1.2) | .22 | 0.8 (0.5 to 1.3) | .35 |
| Number of rooms, median (IQR) | 4 (3, 5) | 4 (2, 4) | 0.9 (0.8 to 1.0) | .04 | 0.9 (0.8 to 1.0) | .17 |
| Ever marijuana use | 22 (11) | 115 (48) | 7.4 (4.5 to 12.6) | <.0001 | 7.5 (4.1 to 14.6) | <.0001 |
| BMI, median (IQR) | 21.5 (19.2, 24.6) | 19.9 (18.3, 21.8) | 0.9 (0.8 to 0.9) | <.0001 | 0.9 (0.8 to 0.9) | <.0001 |
| Detectable viral load, n (%) | 78 (42) | 108 (55) | 1.7 (1.1 to 2.5) | .01 | 1.6 (0.98 to 2.6) | .06 |
| Subset with alcohol data (n = 83) | ||||||
| Age (years), n (%) | ||||||
| < 36 | 8 (15) | 16 (26) | REF | REF | ||
| 36–43 | 8 (15) | 13 (21) | 0.8 (0.2 to 2.8) | .74 | 2.8 (0.3 to 33.5) | .40 |
| 44–49 | 18 (34) | 17 (27) | 0.5 (0.2 to 1.4) | .17 | 2.7 (0.3 to 22.6) | .35 |
| ≥50 | 19 (36) | 16 (26) | 0.4 (0.1 to 1.2) | .12 | 1.2 (0.2 to 9.5) | .86 |
| Employment, n (%) | ||||||
| Employed | 12 (23) | 19 (31) | REF | REF | ||
| Unemployed | 41 (77) | 43 (69) | 0.7 (0.3 to 1.5) | .34 | 0.6 (0.1 to 2.7) | .50 |
| Monthly household income, n (%) | ||||||
| <1000 | 18 (34) | 21 (34) | REF | REF | ||
| >1000 | 35 (66) | 41 (66) | 1.0 (0.5 to 2.2) | 1.0 | 0.4 (0.1 to 1.9) | .25 |
| Number of rooms, median (IQR) | 4 (3, 5) | 3 (2, 4) | 0.8 (0.7 to 1.0) | .04 | 0.7 (0.5 to 1.0) | .08 |
| Ever marijuana use, n (%) | 7 (13) | 30 (48) | 6.2 (2.5 to 16.8) | .0001 | 16.9 (3.4 to 129.6) | .002 |
| BMI, median (IQR) | 22.1 (19.7, 25.7) | 20.2 (18.4, 22.4) | 0.8 (0.7 to 0.9) | .002 | 0.8 (0.6 to 0.9) | .004 |
| Detectable viral load, n (%) | 20 (49) | 22 (52) | 1.2 (0.5 to 2.7) | .74 | 1.7 (0.4 to 7.7) | .46 |
| Alcohol use, n (%) | 14 (26) | 41 (66) | 5.4 (2.5 to 12.5) | <.0001 | 7.8 (2.1 to 35.0) | .004 |
*Bold values statistically significant at p < .05.
Among women, no difference in CD4 count or BMI was found between smokers and non-smokers. Female smokers reported significantly fewer people in their homes (p < .001) as compared to non-smokers, and have a significantly higher prevalence of substances use (marijuana, snuff, and alcohol; p < .001) (Table 1). In adjusted analysis among women, odds of smoking decreased with increasing number of people in the home (OR 0.9, 95% CI = 0.8 to 0.98). Current snuff use was also associated with a lower odds of smoking (OR 0.1; 95% CI = 0.05 to 0.2). In the subset with information on alcohol use, current use of alcohol increased the odds of smoking (OR 8.5, 95% CI = 2.9 to 26.8), and snuff use decreased the odds of smoking (OR 0.1; 95% CI = 0.01 to 0.3) (Table 3).
Table 3.
Univariate and multivariate logistic regression for odds of current smoking among adult females with HIV infection (n = 753) in Klerksdorp, South Africa
| Non-smoker | Smoker | Univariate OR (95% CI) | p* | Multivariate OR (95% CI) | p* | |
|---|---|---|---|---|---|---|
| Age (years), n (%) | ||||||
| <36 | 207 (32) | 28 (28) | REF | REF | ||
| 36–43 | 164 (25) | 27 (27) | 1.2 (0.7 to 2.1) | 0.50 | 1.0 (0.5 to 1.8) | 1.0 |
| 44–49 | 142 (22) | 16 (16) | 0.8 (0.4 to 1.6) | .58 | 0.7 (0.4 to 1.5) | .40 |
| ≥50 | 140 (21) | 28 (28) | 1.5 (0.8 to 2.6) | .18 | 1.6 (0.9 to 3.0) | .14 |
| Employment, n (%) | ||||||
| Employed | 104 (16) | 21 (21) | REF | REF | ||
| Unemployed | 549 (84) | 79 (79) | 0.7 (0.4 to 1.2) | .21 | 0.7 (0.4 to 1.3) | .29 |
| Monthly household income, n (%) | ||||||
| <1000 | 275 (42) | 44 (44) | REF | REF | ||
| >1000 | 378 (58) | 56 (56) | 0.9 (0.6 to 1.4) | .72 | 0.8 (0.5 to 1.3) | .34 |
| Number of rooms, median (IQR) | 4 (2, 4) | 3 (2, 4) | 0.9 (0.8 to 1.0) | .18 | 1.0 (0.8 to 1.1) | .59 |
| Number of people in living situation, median (IQR) | 4 (3, 5) | 3 (2, 5) | 0.9 (0.8 to 1.0) | .008 | 0.9 (0.8 to 0.98) | .03 |
| BMI, median (IQR) | 24.8 (21.0, 29.5) | 23.4 (10.7, 28.3) | 1.0 (1.0 to 1.03) | .37 | 1.0 (1.0 to 1.0) | .27 |
| Snuff use, n (%) | 294 (49) | 10 (10) | 0.1 (0.06 to 0.2) | <.0001 | 0.1 (0.05 to 0.2) | <.0001 |
| Subset with alcohol data (n = 221) | ||||||
| Age (years), n (%) | ||||||
| <36 | 58 (29) | 8 (35) | REF | REF | ||
| 36–43 | 50 (25) | 6 (26) | 0.9 (0.3 to 2.7) | .81 | 0.7 (0.2 to 2.6) | .38 |
| 44–49 | 40 (20) | 1 (4) | 0.2 (0.01 to 1.04) | .11 | 0.1 (0.01, 0.8) | .69 |
| ≥50 | 50 (25) | 8 (35) | 1.2 (0.4 to 3.4) | .78 | 0.95 (0.3 to 3.4) | .52 |
| Employment, n (%) | ||||||
| Employed | 30 (15) | 6 (26) | REF | REF | ||
| Unemployed | 168 (85) | 17 (74) | 0.5 (0.2 to 1.5) | .19 | 0.8 (0.2 to 2.9) | .61 |
| Monthly household income, n (%) | ||||||
| <1000 | 92 (46) | 13 (57) | REF | REF | ||
| >1000 | 106 (54) | 10 (43) | 0.7 (0.3 to 1.6) | .36 | 0.5 (0.2 to 1.5) | .33 |
| Number of rooms, median (IQR) | 4 (2, 4) | 2 (2, 4) | 0.9 (0.7 to 1.2) | .60 | 1.1 (0.8 to 1.6) | .57 |
| Number of people in living situation, median (IQR) | 4 (3, 5) | 3 (2, 4.5) | 0.8 (0.6 to 1.0) | .10 | 0.8 (0.6 to 1.02) | .20 |
| BMI, median (IQR) | 26.0 (21.5, 30.7) | 24.5 (20.8, 30.0) | 1.0 (0.9 to 1.1) | .68 | 0.95 (0.9 to 1.04) | .30 |
| Snuff use, n (%) | 86 (43) | 2 (9) | 0.1 (0.02 to 0.4) | .01 | 0.1 (0.01 to 0.3) | .0004 |
| Alcohol use, n (%) | 41 (21) | 13 (57) | 5.0 (2.0 to 12.5) | <.0001 | 8.5 (2.9 to 26.8) | .001 |
*Bold values statistically significant at p < .05.
Discussion
We report an alarmingly high prevalence of smoking among adults with HIV infection attending HIV clinics in South Africa, particularly among men. Additionally, a large proportion of HIV-infected men with current TB also are current smokers. Snuff is highly prevalent among women, primarily among those who do not smoke, and almost no men report using snuff. There is a strong association between current smoking and use of other substances, specifically marijuana use among men and alcohol use in both men and women. Among those who smoke, most have tried to quit in the past or express willingness to quit in the next month, highlighting the need for appropriate and accessible cessation solutions for this population.
We report 52% current smoking among men and 13% among women, higher than the general population in South Africa. In general, 31.9% of South African males and 7.0% of females are smokers.22 Further, our data suggests a considerably higher prevalence than the sparse existing data on smoking prevalence among PLWH in South Africa. One small cross-sectional study of South African adults with HIV infection has been conducted, which reported 15% self-reported current smoking. In that study, only a subset provided urine for cotinine testing, of which nearly 30% were categorized as smokers.23 Similar to our findings, they report a higher smoking prevalence among men (23%) than women (7.5%).23 Our results also demonstrate a higher prevalence of smoking among adults with HIV infection in South Africa than other countries in the African region. Among HIV-infected adults in West African countries, 15.6% of men and 0.6% of women reported current smoking.24 In Nigeria, a cross-sectional survey among nearly 300 adults with HIV infection, 11% of males and 0 females reported current smoking.25
Substance use emerged as an important correlate of smoking in both men and women in our study. While alcohol use was only collected among a subset of participants, it was highly associated with current smoking. Marijuana use was also increased odds of smoking among men, emphasizing the need for concurrent use of these substances to be taken into consideration when designing smoking cessation interventions. We did not find an association between current smoking and traditional measures of SES, likely due to the relatively homogenous SES of the population from which our sample was drawn. Traditional measures of SES may not adequately distinguish between categories in this generally low-SES population.
Our findings were consistent with prior studies in South Africa and Africa, suggesting that most smokers are interested in quitting, indicating a prime opportunity for intervention. We report 80% of current smokers to be interested in quitting in the next month. In 2011, we found that among self-reported current smokers in South African HIV clinics, 42% reported intent to quit in the next year, and 82% reported attempting a quit in the previous year.26 A small prevalence study of adults with HIV infection in Johannesburg found that nearly all self-reported smokers were interested in quitting, however they were not aware of resources available to aid in a cessation attempt.23 We have also previously shown that HIV-infected smokers prefer cessation counseling and nicotine replacement therapy as cessation aids.26 In general, nicotine replacement therapy has been shown to be well-known by South Africans, but rarely utilized.27
Our study is the first to collect biomarker-confirmed smoking prevalence among a randomly selected sample of adults with HIV in Africa. The use of urine cotinine to confirm smoking status among those self-reporting non-use provides a more accurate estimation of prevalence in this population. We are also able to estimate the prevalence of snuff use in this population, which has not previously been described. Generalizability of our results may be limited as participants were recruited from clinics in one health district, which may not be representative of the diversity of settings in South Africa. Additionally, our participants are most representative of adults in care for HIV infection, as virtually all were receiving ART, thus our results may only be generalizable to similar populations. We are also limited in our assessment of alcohol consumption, as we were only able to collect current, self-reported alcohol use on a subset of participants. Not included in this study were questions pertaining to psychosocial factors and how individual risk-taking and mental health characteristics might influence smoking, which should be explored in future research. Additionally, studies should further assess the impact of smoking on HIV viral suppression and immunological recovery.
There is a clear need for development of effective smoking cessation strategies for men and smokeless tobacco cessation strategies for women in this resource-limited region with a high prevalence of HIV, TB, and tobacco exposure. As ART uptake scales up across South Africa, PLWH will survive longer, putting them at increased risk of smoking related illnesses. Additional research is needed to identify those who are at highest risk in this population, and to provide effective, targeted prevention and cessation services. HIV programs should regularly collect information on tobacco use from patients at every clinic visit, which would provide not only data to inform programmatic planning but also provide an entry point for clinician intervention.
Funding
Research reported in this manuscript was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA030276. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interests
None declared.
Acknowledgments
The authors thank the participants of this study and the staff at the Perinatal HIV Research Unit.
References
- 1. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. Aids. 2014;28(8):1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 3. UN Joint Programme on HIV/AIDS (UNAIDS). Global AIDS Update 2016. Geneva, Switzerland; 2016. [Google Scholar]
- 4. Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8(3):e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farahani M, Mulinder H, Farahani A, et al. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS. 2017;28(7):636–650. [DOI] [PubMed] [Google Scholar]
- 8. Triplette M, Crothers K, Attia EF. Non-infectious pulmonary diseases and HIV. Curr HIV/AIDS Rep. 2016;13(3):140–148. [DOI] [PubMed] [Google Scholar]
- 9. Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9(3):223–230. [DOI] [PubMed] [Google Scholar]
- 10. Calvo M, Laguno M, Martinez M, Martinez E. Effects of tobacco smoking on HIV-infected individuals. AIDS Rev. 2015;17(1):47–55. [PubMed] [Google Scholar]
- 11. Shirley DK, Kaner RJ, Glesby MJ. Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clin Infect Dis. 2013;57(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bronner Murrison L, Martinson N, Moloney RM, et al. Tobacco Smoking and Tuberculosis among Men Living with HIV in Johannesburg, South Africa: A Case-Control Study. PLoS One. 2016;11(11):e0167133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007. ;4(1):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11(10):1049–1061. [PubMed] [Google Scholar]
- 15. World Health Organization. Global Tuberculosis Report 2016.Geneva, Switzerland; 2016. [Google Scholar]
- 16. UNAIDS. AIDSinfo 2016. http://aidsinfo.unaids.org/. Accessed February, 2017.
- 17. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 18. Smoking NCA. Make a Fresh Start, Your personal guide to stop smoking.https://www.health-e.org.za/wp-content/uploads/2013/05/4624863c0cb23ac13ce42ed341d4cec1.pdf. Accessed February, 2017.
- 19. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 20. Nicolau I, Tian L, Menzies D, et al. Point-of-care urine tests for smoking status and isoniazid treatment monitoring in adult patients. PLoS One. 2012;7(9):e45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 22. World Health Organization. WHO Report on the Global Tobacco Epidemic, 2015. Raising taxes on tobacco.Geneva: World Health Organization; 2015. [Google Scholar]
- 23. Waweru P, Anderson R, Steel H, et al. The prevalence of smoking and the knowledge of smoking hazards and smoking cessation strategies among HIV- positive patients in Johannesburg, South Africa. S Afr Med J. 2013;103(11):858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaquet A, Ekouevi DK, Aboubakrine M, et al. Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in West African countries. Int J Tuberc Lung Dis. 2009;13(11):1433–1439. [PMC free article] [PubMed] [Google Scholar]
- 25. Iliyasu Z, Gajida AU, Abubakar IS, et al. Patterns and predictors of cigarette smoking among HIV-infected patients in northern Nigeria. Int J STD AIDS. 2012;23(12):849–852. [DOI] [PubMed] [Google Scholar]
- 26. Shapiro AE, Tshabangu N, Golub JE, et al. Intention to quit smoking among human immunodeficiency virus infected adults in Johannesburg, South Africa. Int J Tuberc Lung Dis. 2011;15(1):140–142. [PMC free article] [PubMed] [Google Scholar]
- 27. Agaku IT, Ayo-Yusuf OA. Awareness of nicotine replacement therapy among South African smokers and their interest in using it for smoking cessation when provided for free. Nicotine Tob Res. 2014; 16(4):500–505. [DOI] [PubMed] [Google Scholar]