Abstract
Background
Germline genetic testing with hereditary cancer gene panels can identify women at increased risk of breast cancer. However, those at increased risk of triple-negative (estrogen receptor–negative, progesterone receptor–negative, human epidermal growth factor receptor–negative) breast cancer (TNBC) cannot be identified because predisposition genes for TNBC, other than BRCA1, have not been established. The aim of this study was to define the cancer panel genes associated with increased risk of TNBC.
Methods
Multigene panel testing for 21 genes in 8753 TNBC patients was performed by a clinical testing laboratory, and testing for 17 genes in 2148 patients was conducted by a Triple Negative Breast Cancer Consortium (TNBCC) of research studies. Associations between deleterious mutations in cancer predisposition genes and TNBC were evaluated using results from TNBC patients and reference controls.
Results
Germline pathogenic variants in BARD1, BRCA1, BRCA2, PALB2, and RAD51D were associated with high risk (odds ratio > 5.0) of TNBC and greater than 20% lifetime risk for overall breast cancer among Caucasians. Pathogenic variants in BRIP1, RAD51C, and TP53 were associated with moderate risk (odds ratio > 2) of TNBC. Similar trends were observed for the African American population. Pathogenic variants in these TNBC genes were detected in 12.0% (3.7% non-BRCA1/2) of all participants.
Conclusions
Multigene hereditary cancer panel testing can identify women with elevated risk of TNBC due to mutations in BARD1, BRCA1, BRCA2, PALB2, and RAD51D. These women can potentially benefit from improved screening, risk management, and cancer prevention strategies. Patients with mutations may also benefit from specific targeted therapeutic strategies.
Triple-negative breast cancer (TNBC) is an aggressive subtype defined by absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression (1). TNBC accounts for an estimated 15% of breast cancer in the Caucasian population and 35% in the African American population (2). TNBC is associated with advanced disease stage and higher-grade tumors at diagnosis (1) and is associated with an increased recurrence risk and poor five-year survival rates relative to other breast cancers (3). Germline genetic testing of TNBC patients using hereditary cancer gene panels is now common practice because of the high frequency of BRCA1 and BRCA2 mutations and the identification of non–BRCA1/2 predisposition gene mutations in 5% of TNBC patients unselected for age of diagnosis or family history (4), and in 4.5% of TNBC patients receiving clinical genetic testing (5).
National Comprehensive Cancer Network (NCCN) guidelines recommend BRCA1/2 testing for patients meeting hereditary breast and ovarian cancer testing criteria or with a TNBC diagnosis at age 60 years or younger. However, recommendations for testing of other genes are not fully established because the risks of TNBC associated with mutations in cancer predisposition genes have not been established. Thus, a better understanding of gene-specific risks for TNBC is needed to identify the genes that should be tested in the setting of TNBC. This should lead to improved clinical management of individuals at risk for or diagnosed with this aggressive form of breast cancer. We present germline genetic testing results from two cohorts of TNBC patients and provide estimated TNBC risk associated with pathogenic variants in 21 cancer predisposition genes.
Methods
Study Population
This study involved 10 901 TNBC patients, including 8753 from a cohort of 140 449 individuals subjected to clinical germline cancer panel testing between March 2012 and June 2016 at a clinical testing laboratory (Ambry Genetics, Aliso Viejo, CA). Demographic, clinical, and family history information, as well as TNBC status based on histopathology markers, was provided by the ordering physician (Supplementary Methods and Supplementary Figure 1, available online) (6). The clinical testing study was approved by the Western Institutional Review Board. In addition, results from a previous study of 2148 TNBC patients by a Triple-Negative Breast Cancer Consortium (TNBCC) using a 17-gene panel (4) were included (Supplementary Methods, available online). All TNBCC patients provided informed consent for institutional review board–approved studies.
Multigene Panel Testing
Germline genetic testing results for 21 genes (BRCA1, BRCA2, PALB2, BARD1, BRIP1, NF1, MSH2, MSH6, PMS2, CDKN2A, RAD51C, RAD51D, RAD50, NBN, MRE11A, ATM, CHEK2, TP53, PTEN, STK11, CDH1) from the clinically tested cohort of TNBC patients were evaluated. Testing was performed by targeted custom capture and sequencing and chromosomal rearrangement analysis (Supplementary Methods, available online) (6). A five-tier classification system was applied to all alterations (6), and variants from Ambry Genetics were subsequently submitted to ClinVar. Analysis of 17 predisposition genes (BRCA1, BRCA2, PALB2, BARD1, BRIP1, RAD51C, RAD51D, RAD50, NBN, MRE11A, XRCC2, ATM, CHEK2, TP53, PTEN, STK11, CDH1) in 2148 TNBCC patients, unselected for age of diagnosis, family history, or ethnicity, was performed by custom capture panel and sequencing (Supplementary Methods, available online) (4).
Comparison of TNBC Patient and ExAC Reference Control Subjects
The combined frequencies of pathogenic variants and likely pathogenic variants (PVs) in each of 21 genes in TNBC patients from the clinical cohort and 17 genes in TNBC patients from the TNBCC study were compared with PV frequency in more than 26 000 non-Finnish European population (NFE) Exome Aggregation Consortium (ExAC) reference control subjects (7), excluding The Cancer Genome Atlas (TCGA) exomes (Supplementary Methods, available online) (8,9). Both PASS and non-PASS variants in ExAC (GATK Variant Quality Score Recalibration [VQSR] sensitivity of 99.6% and 95%, respectively) were utilized because many non-PASS variants from ExAC were observed as PVs in TNBC patients. All loss of function and missense variants with consensus definitions as “pathogenic” by clinical laboratories in ClinVar were included. The frequencies of PVs by gene in African American TNBC patients from the clinical cohort were compared with frequencies in Genome Aggregation Database (gnomAD) African and African American reference control subjects (gnomAD_AFR) (Supplementary Methods, available online).
Statistical Analysis
Associations between combined PVs in each gene and phenotypic characteristics of TNBC patients and age of diagnosis were assessed using the Fisher exact test and the Kolmogorov-Smirnov test, respectively. Association studies compared summed PV frequency in Caucasian and African American TNBC patients for each gene with ExAC-NFE non-TCGA and gnomAD AFR, respectively. A P value of less than .05 was considered statistically significant. Odds ratios (ORs) and 95% confidence intervals (CIs) were based on the Fisher exact test. Genes were categorized as high risk (OR > 5.0), moderate risk (OR = 2.0–5.0), or low clinical relevance (OR < 2.0). Enrichment of mutations by gene in TNBC relative to all non-TNBC breast cancers was estimated by logistic regression, with adjustment for age of diagnosis and family history of breast cancer. Lifetime absolute risk for TNBC and overall breast cancer were estimated by combining the odds ratio estimates with age-adjusted subtype-specific incidence rates from the Surveillance, Epidemiology, and End Results (SEER) registry in the competing risk component of the absolute risk equation (Supplementary Methods, available online). False discovery rate–adjusted P values were calculated as previously described (10). All statistical tests were two-sided.
Results
Characteristics of Study Population
Among the 8753 TNBC patients from the clinical cohort, 5498 (62.8%) were Caucasian and 1271 (14.5%) were African American (Table 1; Supplementary Table 1, available online). In contrast, 2095 (97.5%) from the TNBCC cohort were Caucasian (Table 1). The mean age (range) at diagnosis was 49.8 (18–90) years for the clinical cohort and 50.8 (20–93) years for the TNBCC cohort (Table 1). In addition, 51.9% of the clinical cohort but only 21.9% of the 2148 TNBCC patients reported a first- or second-degree relative with breast cancer (Table 1).
Table 1.
Study population characteristics
| Characteristics | Clinical TNBC cohort |
TNBCC |
||||
|---|---|---|---|---|---|---|
| No. (%) | % variant | No. (%) | % variant | |||
| Total patients | 8753 (100) | 14.4 | 2148 (100) | 14.51 | ||
| Sex | ||||||
| Female | 8743 (99.9) | 14.4 | 2148 (100.0) | 14.51 | ||
| Male | 9 (0.1) | 0 | 0 (0.0) | 0 | ||
| Unknown | 1 (0.0) | 0 | 0 (0.0) | 0 | ||
| Race/ethnicity | ||||||
| African American | 1271 (14.5) | 14.6 | 34 (1.6) | ND | ||
| Ashkenazi Jewish | 292 (3.3) | 15.1 | 0 (0.0) | ND | ||
| Asian | 334 (3.8) | 16.9 | 10 (0.5) | ND | ||
| Caucasian | 5206 (59.5) | 14.0 | 2095 (97.5) | 14.3 | ||
| Hispanic | 580 (6.6) | 16.2 | 7 (0.3) | ND | ||
| Other/unknown | 1070 (12.2) | 13.8 | 2 (0.1) | ND | ||
| Personal history of cancer | ||||||
| Age at diagnosis, y | ||||||
| ≤36 | 1178 (13.5) | 19.8 | 277 (12.9) | 22.4 | ||
| ≤45 | 3106 (35.5) | 17.8 | 791 (36.8) | 19.1 | ||
| ≤50 | 4532 (51.8) | 16.7 | 1039 (48.4) | 18.4 | ||
| ≤60 | 7287 (83.3) | 15.0 | 1582 (73.6) | 16.0 | ||
| >60 | 1431 (16.3) | 11.4 | 501 (23.3) | 6.9 | ||
| Unknown | 35 (0.4) | ND | 65 (3.0) | ND | ||
| Multiple breast cancer | 1309 (15.0) | 19.5 | NA | ND | ||
| Ovarian | 101 (1.2) | 37.7 | NA | ND | ||
| Colorectal | 86 (1.0) | 18.1 | NA | ND | ||
| Pancreatic | 24 (0.3) | ND | NA | ND | ||
| Family history of cancer* | ||||||
| Breast (no ovarian) | 3983 (45.5) | 15.4 | 470 (21.9) | 16.4 | ||
| Breast and ovarian | 560 (6.4) | 23.9 | NA | ND | ||
| Ovarian (no breast) | 403 (4.6) | 25.8 | 64 (3.0) | ND | ||
| Colorectal | 1862 (21.3) | 13.8 | NA | ND | ||
| Pancreatic | 753 (8.6) | 16.5 | NA | ND | ||
| No breast, ovarian, colorectal, or pancreatic† | 2223 (25.4) | 10.0 | 1612 (75.0) | 12.0 | ||
| Age at TNBC diagnosis, mean ± SD (range), y | 49.8 ± 11.3 | (18–90) | 50.8 ± 12.7 | (20–93) | ||
First- and second-degree relatives. % variant = percentage with inactivating variants in all panel genes; NA = not available; ND = not determined; TNBCC = Triple Negative Breast Cancer Consortium.
No family history of breast or ovarian cancer in TNBCC patients.
Pathogenic Variants Identified by Panel Testing
From the clinical cohort, PVs in all 21 genes were detected in 14.4% (8.4% BRCA1/2, 6.0% non-BRCA) of TNBC patients of any race or ethnicity, 14.0% (7.8% BRCA1/2, 6.2% non-BRCA) of Caucasians, and 14.6% (9.0% BRCA1/2, 5.6% non-BRCA) of African Americans (Tables 1 and 2; Supplementary Tables 1 and 2, available online). Of the 2148 TNBCC patients, 14.5% (10.4% BRCA1/2, 4.0% non-BRCA) had PVs in 17 genes (Supplementary Table 2, available online). PALB2 (1.0%–1.6%) and BARD1 (0.5%–0.7%) were the most commonly mutated non-BRCA1/2 genes in the clinical cohort and TNBCC study (Table 2; Supplementary Table 2, available online). Characteristics of TNBC patients with PVs are shown in Table 1 and Supplementary Tables 1 and 3 (available online).
Table 2.
Gene-based frequency of mutated alleles in TNBC patients of all races/ethnicities*
| Genes | Clinical cohort |
TNBCC cohort |
||||
|---|---|---|---|---|---|---|
| PV | No. cases | Frequency, % | PV | No. cases | Frequency, % | |
| Established breast cancer predisposition genes | ||||||
| ATM | 17 | 6652 | 0.26 | 4 | 2148 | 0.19 |
| BARD1 | 48 | 6464 | 0.74 | 10 | 2148 | 0.47 |
| BRCA1 | 513 | 8537 | 6.01 | 166 | 2148 | 7.73 |
| BRCA2 | 201 | 8537 | 2.35 | 58 | 2148 | 2.70 |
| CHEK2 | 22 | 6639 | 0.33 | 2 | 2148 | 0.09 |
| PALB2 | 111 | 6980 | 1.59 | 22 | 2148 | 1.02 |
| PTEN | 4 | 8719 | 0.05 | 1 | 2148 | 0.05 |
| RAD51D | 16 | 6095 | 0.26 | 8 | 2148 | 0.37 |
| TP53 | 14 | 8741 | 0.16 | 2 | 2148 | 0.09 |
| Total frequency | 11.75 | 12.71 | ||||
| Other cancer predisposition genes | ||||||
| BRIP1 | 27 | 6464 | 0.42 | 10 | 2148 | 0.47 |
| CDH1 | 5 | 8505 | 0.06 | NA | NA | NA |
| CDKN2A | 5 | 1790 | 0.28 | NA | NA | NA |
| MLH1 | 5 | 3497 | 0.14 | NA | NA | NA |
| MRE11A | 6 | 6464 | 0.09 | 4 | 2148 | 0.19 |
| MSH2 | 3 | 3497 | 0.09 | 0 | 372 | ND |
| MSH6 | 9 | 3497 | 0.26 | 1 | 372 | 0.27 |
| NBN | 12 | 6464 | 0.19 | 2 | 2148 | 0.09 |
| NF1 | 9 | 6097 | 0.15 | NA | NA | NA |
| PMS2 | 9 | 3497 | 0.26 | NA | NA | NA |
| RAD50 | 14 | 6464 | 0.22 | 6 | 2148 | 0.28 |
| RAD51C | 31 | 6464 | 0.48 | 8 | 2148 | 0.37 |
| XRCC2 | NA | NA | NA | 2 | 2148 | 0.09 |
| Total frequency | 2.62 | 1.76 | ||||
Frequency = pathogenic variant frequency; NA = not genotyped; PV = pathogenic variant; TNBCC = Triple Negative Breast Cancer Consortium.
Associations Between PVs and TNBC
Comparison of Caucasian TNBC patients from the clinical cohort and the TNBCC study with ExAC NFE non-TCGA reference control subjects showed that BRCA1 PVs were associated with high risk of TNBC, consistent with previous studies (Table 3). Similarly, BRCA2 PVs were associated with high risk (OR > 5.0) of TNBC in both the clinical cohort (OR = 5.42, 95% CI = 4.13 to 7.05, P < 2.2×10-16) and the TNBCC study (OR = 6.33, 95% CI = 4.48 to 8.92, P < 2.2×10-16) (Table 3). Likewise, PVs in PALB2 (OR = 14.41, 95% CI = 9.27 to 22.60, P < 2.2×10-16), BARD1 (OR = 5.92, 95% CI = 3.36 to 10.27, P = 2.2×10-9), and RAD51D (OR = 6.97, 95% CI = 2.60 to 18.66, P = 3.1×10-4), were shown to be statistically significantly associated with high risk of TNBC in the clinical cohort (Table 3). PVs in BRIP1 and RAD51C, both of which were previously excluded as breast cancer predisposition genes (6,11,12), were associated with moderate risk (OR > 2) of TNBC. PVs in NF1 were not statistically significantly associated with moderate risk of TNBC in the clinical cohort after adjustment for multiple testing (Supplementary Table 4, available online). PVs in MSH6 were associated with moderate risk of TNBC in this study, consistent with a twofold increased risk of overall breast cancer (standardized incidence ratio = 2.11, 95% CI = 1.56 to 2.86) for MSH6 PVs in a recent study (13), but the TNBC association was not statistically significant after adjustment for multiple testing (Supplementary Table 4, available online). PVs in ATM, CHEK2, NBN, and RAD50 yielded no clinically relevant risks (OR > 2) of TNBC (Supplementary Table 4, available online). Similar associations with TNBC for all of these genes were observed in the TNBCC study (Table 3; Supplementary Table 4, available online).
Table 3.
Estimated risks of TNBC in Caucasian patients associated with mutations in hereditary cancer panel genes
| TNBC-associated genes | Clinical cohort |
TNBCC cohort |
ExAC controls |
Clinical TNBC risk |
TNBCC TNBC risk |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PV | No. cases | PV | No. cases | PV | No. controls | OR (95% CI) | P* | FDR-adjusted P | OR (95% CI) | P* | FDR-adjusted P | |
| BARD1 | 25 | 4090 | 9 | 2003 | 27 | 26 079 | 5.92 (3.36 to 10.27) | 2.20 × 10-9 | 1.26 × 10-8 | 4.35 (2.02 to 9.30) | 7.60 × 10-4 | .002 |
| BRCA1 | 255 | 5265 | 158 | 2003 | 82 | 26 911 | 16.27 (12.65 to 20.95) | <2.2 × 10-16 | <2.2 × 10-16 | 26.90 (20.51 to 35.51) | <2.2 × 10-16 | <2.2 × 10-16 |
| BRCA2 | 115 | 5265 | 51 | 2003 | 109 | 26 791 | 5.42 (4.13 to 7.05) | <2.2 × 10-16 | <2.2 × 10-16 | 6.33 (4.48 to 8.92) | <2.2 × 10-16 | <2.2 × 10-16 |
| BRIP1 | 17 | 4090 | 9 | 2003 | 49 | 26 841 | 2.28 (1.30 to 4.00) | .006 | .01 | 2.46 (1.18 to 5.08) | .02 | .04 |
| PALB2 | 70 | 4383 | 17 | 2003 | 30 | 26 869 | 14.41 (9.27 to 22.60) | <2.2 × 10-16 | <2.2 × 10-16 | 7.63 (4.05 to 14.08) | 7.05 × 10-9 | 4.00 × 10-8 |
| RAD51C | 15 | 4090 | 8 | 2003 | 37 | 26 647 | 2.64 (1.44 to 4.80) | 3.09 × 10-3 | .01 | 2.88 (1.21 to 6.29) | .01 | .03 |
| RAD51D | 8 | 3814 | 7 | 2003 | 8 | 26 555 | 6.97 (2.60 to 18.66) | 3.10 × 10-4 | .001 | 11.62 (3.79 to 31.91) | 3.23 × 10-5 | 1.10 × 10-4 |
| TP53 | 10 | 5423 | 2 | 2003 | 18 | 26 790 | 2.75 (1.18 to 6.16) | .02 | .04 | 1.49 (0.25 to 6.30) | .65 | .73 |
| TP53† | 6 | 1055 | 2 | 504 | 18 | 26 790 | 8.49 (3.30 to 21.53) | 2.19 × 10-4 | 8.40 × 10-4 | 5.92 (0.98 to 25.16) | .05 | .10 |
Statistical significance of case–control associations was estimated using the Fisher exact test. All tests were two-sided. CI = confidence interval; FDR = false discovery rate; NA = not genotyped; OR = odds ratio from case–control analysis comparing PV frequencies in clinical cohort cases or TNBCC cohort cases with frequencies in ExAC controls; PV = pathogenic variant; TNBCC = Triple Negative Breast Cancer Consortium.
Age at diagnosis of 40 years or younger.
Risk estimates for most genes were not substantially altered 1) when using gnomAD Caucasian control subjects (Supplementary Table 5, available online); 2) when using ExAC PASS controls (Supplementary Table 6, available online); 3) when restricting to patients with TNBC as the first cancer (Supplementary Table 7, available online); and 4) when considering all races and ethnicities (Supplementary Table 8, available online). Exclusion of TNBC patients with personal or family history of ovarian or colorectal cancer had little impact (Supplementary Tables 9 and 10, available online), except for a slightly attenuated association between RAD51C and TNBC (OR = 1.96, 95% CI = 0.93 to 4.07, P = .09) when excluding ovarian cancer (Supplementary Table 9, available online). Furthermore, PVs in BRCA1, RAD51C, BARD1, and RAD51D were enriched more than threefold in TNBC patients relative to all non-TNBC patients, suggesting that these are predominantly TNBC predisposition genes (Table 4). PALB2 and BRCA2 also displayed statistically significant enrichment (Table 4).
Table 4.
Enrichment of pathogenic variants in TNBC genes in Caucasian TNBC relative to non-TNBC breast cancer patients
| Gene* | TNBC |
Non-TNBC |
TNBC/non-TNBC associations |
||||
|---|---|---|---|---|---|---|---|
| Mutated alleles | TNBC BC† | Mutated alleles | Non-TNBC BC† | OR (95% CI) | P‡ | FDR-adjusted P | |
| BARD1 | 27 | 4032 | 62 | 34 437 | 3.73 (2.30 to 5.95) | 2.35 × 10-7 | 6.27 × 10-7 |
| BRCA1 | 292 | 5208 | 443 | 44 503 | 5.77 (4.96 to 6.71) | <2.2 × 10-16 | <2.2 × 10-16 |
| BRCA2 | 116 | 5208 | 695 | 44 503 | 1.43 (1.17 to 1.75) | 6.57 × 10-4 | .001 |
| BRIP1 | 18 | 4032 | 109 | 34 437 | 1.41 (0.84 to 2.35) | .19 | .23 |
| PALB2 | 74 | 4324 | 298 | 36 714 | 2.12 (1.63 to 2.74) | 9.83 × 10-8 | 3.15 × 10-7 |
| RAD51C | 21 | 4032 | 47 | 34 437 | 3.82 (2.23 to 6.39) | 3.62 × 10-6 | 8.27 × 10-6 |
| RAD51D | 10 | 3766 | 27 | 31 818 | 3.13 (1.42 to 6.43) | .004 | .007 |
| TP53 | 10 | 5362 | 95 | 46 065 | 0.90 (0.46 to 1.71) | .87 | .93 |
Only genes with four or more pathogenic variants in TNBC or non-TNBC BCs are displayed. BC = breast cancer; CI = confidence interval; FDR = false discovery rate; OR = odds ratio; TNBC = triple-negative breast cancer.
Breast cancers from the clinical breast cancer cohort.
Significance of associations were estimated using the Fisher exact test. All tests were two-sided.
PV Frequency in African American TNBC Patients
PVs in TNBC genes were identified in 12.7% of 1271 African American TNBC patients, including 3.7% in non-BRCA genes (Supplementary Table 2, available online). No major differences in the PV frequencies in genes between African American and Caucasian TNBC patients were observed (Supplementary Table 2, available online). An exploratory case–control analysis of African American TNBC patients from the clinical cohort and gnomAD-AFR suggested that PVs in BRCA1, BRCA2, BARD1, and PALB2 were statistically significantly associated with high or moderate risk of TNBC (Supplementary Table 11, available online). Similarly, RAD51C PVs were statistically significantly associated with very high risk of TNBC, although these findings will need to be replicated because of limited numbers of PVs in TNBC patients and control subjects. Small numbers of PVs in other genes yielded unstable estimates.
Frequency of PVs Based on Age of Onset and Family History
PVs in moderate-risk and high-risk TNBC genes were observed in 12.0% of TNBC patients from the clinical cohort and 13.2% from TNBCC, including 3.7% in non-BRCA genes in both cohorts (Table 2; Supplementary Table 2, available online). PV frequencies varied based on age at diagnosis and total number of relatives with breast or ovarian cancer among Caucasian and African American TNBC patients (Table 5; and Supplementary Table 12, available online, respectively). For example, PV frequencies ranged from 5% in patients diagnosed older than age 60 years with no family history of breast or ovarian cancer to 35.3% in patients diagnosed younger than age 35 years with a family history of ovarian cancer (Table 5). BRCA1 mutations accounted for a larger proportion of early-onset TNBC whereas, other TNBC genes accounted for a larger proportion of later-onset TNBC. In addition, PVs in TNBC genes were identified in 12.4% of TNBC patients from the clinical cohort meeting NCCN BRCA1/2 testing criteria and in 4.3% of TNBC patients not meeting criteria. When expanding to other breast cancer predisposition genes, overall detection of PVs increased to 13.1% and 4.6%, respectively.
Table 5.
Frequency of mutations by age at TNBC diagnosis and family history of breast and ovarian cancer among Caucasian TNBC patients in the clinical cohort
| Age at TNBC diagnosis, y |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <35 |
35–39 |
40–49 |
50–60 |
>60 |
||||||
| Family cancer history | PV carriers | % PV | PV carriers | % PV | PV carriers | % PV | PV carriers | % PV | PV carriers | % PV |
| No breast, no ovarian | ||||||||||
| BRCA1 | 18 | 9.2 | 12 | 5.9 | 29 | 5.2 | 20 | 2.8 | 2 | 0.9 |
| Other TNBC genes* | 6 | 3.1 | 7 | 4.43 | 23 | 4.9 | 28 | 4.7 | 7 | 4.1 |
| All TNBC genes total | 24 | 12.3 | 19 | 10.31 | 52 | 10.1 | 48 | 7.5 | 9 | 5.0 |
| Other breast cancer† | 2 | 1.3 | 0 | 0.0 | 6 | 1.4 | 2 | 0.4 | 0 | 0.0 |
| All breast cancer genes‡ | 26 | 13.6 | 19 | 10.3 | 58 | 11.5 | 50 | 7.9 | 9 | 5.0 |
| One relative with breast, no ovarian | ||||||||||
| BRCA1 | 15 | 12.6 | 14 | 10.6 | 23 | 5.3 | 22 | 3.6 | 8 | 2.4 |
| Other TNBC genes* | 8 | 6.9 | 4 | 3.1 | 29 | 7.6 | 36 | 6.7 | 16 | 5.4 |
| All TNBC genes total | 23 | 19.6 | 18 | 13.7 | 52 | 12.9 | 58 | 10.3 | 24 | 7.8 |
| Other breast cancer† | 0 | 0.0 | 1 | 0.8 | 2 | 0.6 | 4 | 0.7 | 2 | 0.8 |
| All breast cancer genes‡ | 23 | 19.6 | 19 | 14.6 | 54 | 13.4 | 62 | 11.0 | 26 | 8.5 |
| ≥2 relatives with breast, no ovarian | ||||||||||
| BRCA1 | 13 | 28.9 | 3 | 6.0 | 16 | 8.2 | 16 | 5.2 | 5 | 2.0 |
| Other TNBC genes* | 2 | 5.2 | 6 | 12.4 | 15 | 8.2 | 18 | 6.5 | 15 | 6.3 |
| All TNBC genes total | 15 | 34.1 | 9 | 18.4 | 31 | 16.4 | 34 | 11.7 | 20 | 8.3 |
| Other breast cancer† | 1 | 2.6 | 1 | 1.8 | 2 | 1.1 | 3 | 1.1 | 3 | 1.5 |
| All breast cancer genes‡ | 16 | 36.7 | 10 | 20.2 | 33 | 17.5 | 37 | 12.8 | 23 | 9.8 |
| Any relative with ovarian | ||||||||||
| BRCA1 | 14 | 32.6 | 7 | 13.7 | 18 | 11.8 | 15 | 6.3 | 11 | 7.2 |
| Other TNBC genes* | 1 | 2.8 | 3 | 6.1 | 10 | 7.6 | 21 | 9.4 | 17 | 12.5 |
| All TNBC genes total | 15 | 35.3 | 10 | 19.9 | 28 | 19.4 | 36 | 15.7 | 28 | 19.6 |
| Other breast cancer† | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | 3 | 1.5 | 3 | 2.4 |
| All breast cancer genes‡ | 15 | 35.3 | 10 | 19.9 | 29 | 20.1 | 39 | 17.2 | 31 | 22.0 |
Other TNBC genes: BARD1, BRCA2, BRIP1, PALB2, RAD51C, RAD51D, TP53. PV = pathogenic variant; % PV = % cases with pathogenic variants; TNBC = triple-negative breast cancer.
Other breast cancer genes: ATM, CHEK2, CDH1, PTEN.
All breast cancer genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, PALB2, PTEN, RAD51C, RAD51D, TP53.
Absolute Risk Estimates for Breast Cancer and TNBC
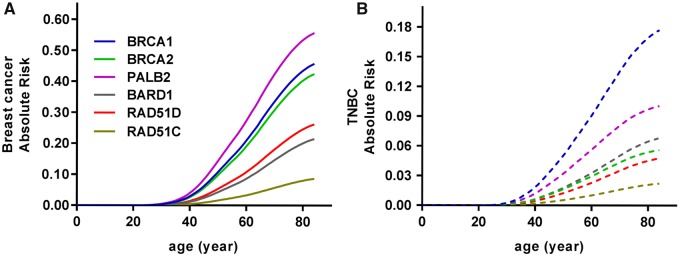
When combining OR estimates for Caucasian TNBC patients from the clinical cohort with age-adjusted subtype-specific incidence rates from the SEER registry, BRCA1 and PALB2 PVs were associated with 18% and 10% lifetime risks of TNBC, respectively, followed by BARD1 (7%), BRCA2 (6%), and RAD51D (5%) (Figure 1). Estimated TNBC risks contributed substantially to the overall breast cancer risks for genes known to confer greater than 20% lifetime risk for breast cancer (PALB2 56%, BRCA1 46%, BRCA2 42%) (14,15) and to estimated absolute risks of 26% for RAD51D and 21% in BARD1 (Figure 1). Similar exploratory studies based on the African American population within the clinical cohort suggested even greater contributions of TNBC risks to greater than 20% lifetime risk estimates for overall breast cancer for several genes (BRCA1 81%, BRCA2 62%, PALB2 41%, BARD1 39%) (Supplementary Figure 2, available online).
Figure 1.
Absolute risk estimates to age 85 for overall breast cancer and triple-negative breast cancer. A) Age-related risk curves for overall breast cancer for six genes are shown as colored lines. B) Age-related risk curves for TNBC for six genes are shown as colored dashed lines. TNBC = triple-negative breast cancer.
Discussion
This study of 10 901 TNBC patients from a cohort of TNBC patients receiving clinical genetic testing and a separate series of TNBC patients unselected for age of diagnosis or family history of breast or ovarian cancer is the first to establish which cancer predisposition genes on multigene hereditary cancer panels are associated with increased risk of TNBC. Consistent with findings from an independent study of TNBC patients (5), 14.4% of TNBC patients in the clinical cohort had PVs in cancer predisposition genes, whereas 12.0% had PVs in the eight TNBC genes.
Among these TNBC predisposition genes, only BRCA1 has been well characterized as a TNBC susceptibility gene, although BARD1, BRIP1, PALB2, and RAD51C PVs have been reported as enriched in TNBC relative to other breast cancer subtypes (5,16,17). Results from this study further characterize the role of these genes in TNBC and also introduce a novel association between RAD51D and risk for TNBC. Importantly, BRIP1, RAD51C, and RAD51D have been primarily characterized by others as ovarian rather than breast cancer susceptibility genes (18,19); however, results from the present study strongly support an association of these genes with TNBC. It is likely that this association was undetected in some previous studies due to the rarity of PVs in these genes among breast and breast/ovarian cancer families, paired with the lower prevalence of TNBC relative to other breast cancer subtypes.
Despite higher general population TNBC risk in African Americans relative to Caucasians (1.91% vs 1.19%) (20), risk estimates conferred by PVs in predisposition genes have not been reported. Thus, although the analysis was limited by cohort size, an exploratory association study using African American TNBC patients and gnomAD AFR control subjects was undertaken. Similar to the Caucasian population, statistically significant associations between BRCA1, BRCA2, BARD1, and PALB2 PVs and TNBC were observed. Importantly, breast cancer absolute risk estimates for patients with BRCA1, BRCA2, PALB2, and BARD1 PVs ranged from 40% to 80%, which may impact breast cancer risk management decisions. However, larger studies of African American patients will be needed to develop more precise risk estimates. Interestingly, RAD51C PVs were associated with high risk of TNBC in the African American population but only moderate risk among Caucasians. Whether RAD51C is a specific and important driver of TNBC in the African American population, as suggested by high rates of epigenetic silencing of the RAD51C promoter in African American basal tumors (21), remains to be determined.
NCCN guidelines currently recommend BRCA1 and BRCA2 testing for individuals with TNBC diagnosed at age 60 years or younger or who meet criteria based on personal and family cancer history. However, guidelines for non-BRCA predisposition gene testing have not been established. In this study, PVs in TNBC genes were identified in 4.3% of TNBC patients from the clinical cohort not meeting NCCN BRCA1/2 testing criteria. When considering all breast cancer predisposition genes, PVs were detected in 4.6% of patients. As most of these variants are considered clinically actionable, these findings suggest that testing criteria for TNBC patients should be expanded to include testing of all breast cancer predisposition genes regardless of age of diagnosis or family history of cancer.
Although absolute risks for TNBC in the general population are low (1.19% in the Caucasian population; https://www.seer.cancer.gov), PVs in TNBC predisposition genes substantially increased breast cancer risk. Absolute risk models accounting for risks of different subtypes of breast cancer identified five TNBC predisposition genes (PALB2, BRCA1, BRCA2, RAD51D, and BARD1) with greater than 20% lifetime risks for overall breast cancer. Thus, the risk assessment and management of patients with PVs in these genes should include some consideration of the increased risk for TNBC. Although NCCN guidelines already recommend additional breast cancer screening via magnetic resonance imaging for women with PALB2, BRCA1, and BRCA2 PVs, the results from the current study provide evidence to support similar screening for patients with PVs in RAD51D and BARD1. Continued study of gene-specific risks for breast cancer subtypes may lead to tailored medical management recommendations for PV carriers. Consistent with this hypothesis, initial studies evaluating intensified screening in high-risk women have suggested that a decrease in mortality from TNBC can be achieved (22).
Although panel-based testing can identify unaffected women at increased risk of TNBC and other cancers who can benefit from risk management strategies, genetic testing can also identify individuals who may benefit from targeted therapeutic strategies. In particular, results from the OlympiAD randomized trial of the olaparib PARP inhibitor in HER2-negative metastatic breast cancer patients suggested that BRCA1 and BRCA2 carriers with TNBC derived the greatest benefit from the PARP inhibitor in combination with chemotherapy (hazard ratio = 0.43, 95% CI = 0.29 to 0.63) (23). Moreover, a recent analysis of TNBC patients from the Geparsixto randomized clinical trial showed that BRCA1 and BRCA2 carriers exposed to anthracycline, taxane, and bevacizumab with or without carboplatin had high pathological complete response (pCR) rates of 65%–67% compared with patients without mutations (pCR rates of 35%–55%) (24). Whether TNBC patients with other predisposition gene mutations will also benefit from these and other targeted therapies remains to be determined, but future clinical trials involving TNBC patients should consider multigene germline panel testing to provide insight into a putative relationship between PVs and targeted TNBC treatment responses.
There are several limitations of this study. As noted above, although this study identifies TNBC-associated predisposition genes, larger case–control studies and family-based segregation studies will be needed to refine risks of TNBC and other breast cancer subtypes associated with mutations in these genes. In addition, sequencing results from ExAC and gnomAD public control subjects were used for the case–control association studies reported here. Although these control subjects likely provide reasonable approximations of population-based allele frequencies (7), the lack of study level matching of the TNBC patients and control subjects and the derivation of sequencing data using different sequencing platforms and allele-calling algorithms may have resulted in some inaccuracies in gene-level PV frequencies and associations with TNBC. However, extensive data cleaning and filtering was used to normalize TNBC patient and control subjects, and the ExAC control subjects have been used successfully in multiple studies to evaluate cancer risk (25).
Overall, this study identifies several genes that predispose to TNBC and are associated with high lifetime risks of TNBC and overall breast cancer. The results suggest that all TNBC patients should undergo multigene panel testing, regardless of age at diagnosis or family history of cancer, for improved cancer risk assessment and because of the ongoing development of targeted therapeutic approaches for TNBC patients with mutations in predisposition genes.
Funding
This study was supported in part by National Institutes of Health (NIH) grants CA176785 and CA192393, an NIH Specialized Program of Research Excellence (SPORE) in Breast Cancer grant (CA116201), and the Breast Cancer Research Foundation. The study was sponsored by Ambry Genetics Inc.
Notes
Affiliations of authors: Department of Laboratory Medicine and Pathology (HS, CH, MA, FJC) and Department of Health Sciences Research (SNH, JN, AT, RMM, JL, ECP, FJC), Mayo Clinic, Rochester, MN; Ambry Genetics, Aliso Viejo, CA (HL, BTD, TP, JSD); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany (HB); University of Tübingen, Tübingen, Germany (HB); German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ), Heidelberg, Germany (HB); Department of Oncology and Metabolism, Sheffield Institute for Nucleic Acids, University of Sheffield, Sheffield, UK (AC); Faculty of Medicine, University of Southampton, Southampton, UK (DME); Division of Human Cancer Genetics, Departments of Cancer Biology and Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuremberg, Germany (PAF); Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF); Division Hematology/Oncology, Department of Medicine, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA (PAF); Molecular Diagnostics Laboratory INRASTES, National Centre for Scientific Research “Demokritos,” Athens, Greece (FF, IK, DY); Center for Cancer Genetics and Prevention, Dana Farber Cancer Institute, Boston, MA (JG); Department of Pathology and Laboratory Medicine, University of Kansas Medical Center, Kansas City, KS (AKG, PS); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland (HN); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Department of Dermatology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT (BJF, DEG).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Boyle P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann Oncol. 2012;23(suppl 6):vi7–v12. [DOI] [PubMed] [Google Scholar]
- 2. Plasilova ML, Hayse B, Killelea BK, et al. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine (Baltimore). 2016;9535:e4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;1612:279–287. [DOI] [PubMed] [Google Scholar]
- 4. Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;334:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buys SS, Sandbach JF, Gammon A, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;12310:1721–1730. [DOI] [PubMed] [Google Scholar]
- 6. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;39:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;5367616:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;24:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;3755:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Storey JD. A direct approach to false discovery rates. J R Stat Soc B Stat Methodol. 2002;64:479–498. [Google Scholar]
- 11. Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;37223:2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Easton DF, Lesueur F, Decker B, et al. No evidence that protein truncating variants in BRIP1 are associated with breast cancer risk: Implications for gene panel testing. J Med Genet. 2016;535:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts ME, Jackson SA, Susswein LR, et al. MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med. 2018 Jan 18. doi: 10.1038/gim.2017.254. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;10511:812–822. [DOI] [PubMed] [Google Scholar]
- 15. Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;3716:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heikkinen T, Karkkainen H, Aaltonen K, et al. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin Cancer Res. 2009;159:3214–322. [DOI] [PubMed] [Google Scholar]
- 17. Cybulski C, Kluzniak W, Huzarski T, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: A prospective cohort analysis. Lancet Oncol. 2015;166:638–644. [DOI] [PubMed] [Google Scholar]
- 18. Loveday C, Turnbull C, Ramsay E, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;439:879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loveday C, Turnbull C, Ruark E, et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;445:475–476; author reply 476. [DOI] [PubMed] [Google Scholar]
- 20. Kurian AW, Fish K, Shema SJ, et al. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;126:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polak P, Kim J, Braunstein LZ, et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;4910:1476–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Podo F, Santoro F, Di Leo G, et al. Triple-negative versus non-triple-negative breast cancers in high-risk women: Phenotype features and survival from the HIBCRIT-1 MRI-Including Screening Study. Clin Cancer Res. 2016;224:895–904. [DOI] [PubMed] [Google Scholar]
- 23. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;3776:523–533. [DOI] [PubMed] [Google Scholar]
- 24. Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;310:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walsh R, Thomson KL, Ware JS, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;192:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.