Abstract
Age-related decreases in cortical thickness observed during adolescence may be related to fluctuations in sex and stress hormones. We examine this possibility by relating inter-regional variations in age-related cortical thinning (data from the Saguenay Youth Study) to inter-regional variations in expression levels of relevant genes (data from the Allen Human Brain Atlas); we focus on genes coding for glucocorticoid receptor (NR3C1), androgen receptor (AR), progesterone receptor (PGR), and estrogen receptors (ESR1 and ESR2). Across 34 cortical regions (Desikan-Killiany parcellation), age-related cortical thinning varied as a function of mRNA expression levels of NR3C1 in males (R2 = 0.46) and females (R2 = 0.30) and AR in males only (R2 = 0.25). Cortical thinning did not vary as a function of expression levels of PGR, ESR1, or ESR2 in either sex; this might be due to the observed low consistency of expression profiles of these 3 genes across donors. Inter-regional levels of the NR3C1 and AR expression interacted with each other vis-à-vis cortical thinning: age-related cortical thinning varied as a function of NR3C1 mRNA expression in brain regions with low (males: R2 = 0.64; females: R2 = 0.58) but not high (males: R2 = 0.0045; females: R2 = 0.15) levels of AR mRNA expression. These results suggest that glucocorticoid and androgen receptors contribute to cortical maturation during adolescence.
Keywords: gene expression, stress, puberty, hormones, brain maturation, cortical thickness
Introduction
Adolescence is a transitional period that involves a large number of social (e.g., conflicts with peers and adults) and physical (e.g., sexual maturation) events that may contribute to brain maturation (Sisk and Foster 2004; Mills et al., 2014). At a macroscopic level, a number of large-scale magnetic resonance imaging (MRI) studies of typically developing adolescents have identified age-related changes in brain structure, including age-related decreases in cortical thickness (Tamnes et al. 2010; Brown and Jernigan, 2012; Mutlu et al. 2013; Nguyen et al. 2013; Mills et al. 2014; Wierenga et al. 2014; Amlien et al. 2016; Paus et al. 2017).
Cortical thinning during adolescence can be explained by different factors, including stress and sexual maturation. Stress and sex hormones have been found to influence the cellular components (including the number of neuronal cells, dendrites, glial cells, capillaries, etc.) that make up the cortical gray matter in rats (e.g., Gould et al. 1990; Woolley and McEwen 1994; Li et al. 2004; Tata and Anderson 2010) and hamsters (Garelick and Swann 2014), as well as influences on the volume of cortical gray matter and cortical thickness in humans (e.g., Neufang et al. 2009; Peper et al. 2009; Witte et al. 2010; Carrion et al. 2010; Kremen et al. 2010; Paus et al. 2010; Bramen et al. 2011; Nguyen et al. 2013; Savic and Arver 2014; Herting et al. 2015).
In this study, we focus on the hypothalamic–pituitary–adrenal (HPA) and the hypothalamic–pituitary–gonadal (HPG) axes, which have particular relevance to this developmental period. The HPA axis is a key regulator of an individual's response to stress, and its activation results in the release of corticotropin-releasing hormone from the hypothalamus, adrenocorticotropic hormone from the pituitary gland, and cortisol from the adrenal cortex. Cortisol then activates glucocorticoid receptors in the target cells present throughout the body, including the brain. The HPG axis is a key regulator of sexual maturation during puberty; its activation during this developmental period results in the release of gonadotropin-releasing hormone from the hypothalamus, gonadotropins from the pituitary gland, and sex steroids (testosterone, progesterone, and estrogen) from the gonads. Testosterone, progesterone, and estrogen activate androgen receptors (AR), progesterone receptor (PGR), and estrogen receptors (ESR1 and ESR2), respectively.
To understand the mechanism underlying age-related cortical thinning during adolescence, we examined whether inter-regional variations of mRNA expression levels of genes related to the HPA and HPG axes in the human cerebral cortex explain inter-regional variations in age-related cortical thinning. Specifically, we selected the following genes mediating the action of hormones produced by the HPA and HPG axes: glucocorticoid receptor (NR3C1) gene; androgen receptor (AR) gene; progesterone receptor (PGR) gene; and estrogen receptor alpha and beta (ESR1 and ESR2) genes. Using gene-expression data from the Allen Human Brain Atlas (Hawrylycz et al. 2012), we tested whether the expression levels of each of these genes were related to the age-related cortical thinning in a community-based sample of adolescents. As there is also interplay between the HPA and HPG axes (as reviewed by Viau 2002), we also tested for the interaction between the HPA and HPG vis-a-vis age-related cortical thinning.
To ensure that the inter-regional gene-expression profiles across the brain are consistent, we have compared the gene-expression profiles from the Allen Human Brain Atlas with those from the BrainSpan Atlas (www.brainspan.org).
As stress and sex hormones are related to the volume of cortical gray matter and cortical thickness (e.g., Neufang et al. 2009; Peper et al. 2009; Carrion et al. 2010; Kremen et al. 2010; Paus et al. 2010; Witte et al. 2010; Bramen et al. 2011; Nguyen et al. 2013; Savic and Arver 2014; Herting et al. 2015), we hypothesized that inter-regional variations in age-related cortical thinning would be associated with inter-regional variations in gene expression of steroid receptors in the brain.
Materials and Methods
Participants
Data were collected in 1019 adolescents (12 to 18 years of age) recruited in the Saguenay Lac St. Jean (SLSJ) region in Quebec, Canada, as part of the Saguenay Youth Study (SYS; Pausova et al. 2007, 2016). This is a community-based sample of a single ethnicity (French ancestry) recruited in local high schools. Written informed consent from parents and assent from the adolescents were obtained. The participants underwent extensive (15 h) phenotyping, including MRI of the brain. The main exclusion criteria were: (1) positive medical history for meningitis, malignancy, and heart disease requiring heart surgery; (2) treatment for schizophrenia or bipolar disorder; (3) severe mental illness (e.g., autism) or mental retardation (IQ < 70); (4) premature birth (<35 weeks); and (5) MRI contraindications. Additional information about the recruitment, exclusion criteria and all testing procedures are provided in Pausova et al. (2007). Details of procedures relevant for this report are described below.
MRI Acquisition and Analysis
Structural MRI data were collected on a Phillips 1.0-T superconducting magnet (Gyroscan NT; Philips Medical Systems, Best, the Netherlands). T1-weighted images were acquired using the following parameters: 3D RF-spoiled gradient-echo scan with 140–160 slices, 1-mm isotropic resolution, time repetition = 25 ms, time echo = 5 ms, and flip angle = 30°.
We extracted cortical thickness using FreeSurfer (version 5.0.0), a set of automated tools for the reconstruction of the cortical surface (Fischl and Dale 2000). FreeSurfer is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The image processing includes motion correction and averaging of multiple T1-weighted images, removal of nonbrain tissue, registering the volume to the Talairach atlas, segmentation of the subcortical white-matter and deep gray-matter volumetric structures, intensity normalization, tessellation of gray- and white-matter boundaries (i.e., computing meshes with ~160 000 triangles that recover the geometry and the topology of the pial surface and the gray/white interface), automated topology correction, and surface deformation following intensity gradients to place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. A number of deformable procedures were performed, including surface inflation, registration to a spherical atlas which is based on individual cortical folding patterns to match cortical geometry across individuals, and parcellation of the cerebral cortex into units with respect to gyral and sulcal structure (Desikan et al. 2006). Cortical thickness is measured as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface. Regional measures for cortical thickness are obtained by averaging the value of the vertices within each parcellation. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al. 2002; Cardinale et al. 2014; Popescu et al. 2016) and manual measurements (Kuperberg et al. 2003; Salat et al. 2004). Computations were performed on the GPC supercomputer at the SciNet HPC Consortium (Loken et al. 2010). After quality control, a total of 969 (466 males; 503 females) participants were included in the present study.
For each participant, the average cortical thickness was calculated by averaging the left and right hemisphere cortical thickness, as provided by FreeSurfer. For regional cortical thickness, we used thickness values for the 34 Desikan-Killiany regions in each hemisphere, as provided by FreeSurfer.
Allen Human Brain Atlas
Gene-expression data were obtained in postmortem human brains from the Allen Human Brain Atlas that provides comprehensive coverage of the normal adult brain (Allen Institute for Brain Science; Hawrylycz et al. 2012; http://www.brain-map.org). Expression data are available for the left hemispheres of 6 donors of the following sex and age: males (ages 24, 31, 39, 55, and 57 years) and a female (age 49 years). Based on blood samples acquired after death, all donors were free of drugs prescribed for psychiatric disorders.
Using procedures developed by French and Paus (2015), we mapped the gene-expression data from the Allen Human Brain Atlas to the 34 cortical regions defined by the Desikan-Killiany atlas. The expression values of the mapped samples were mean averaged across microarray probes to provide a single expression value for each gene (NR3C1, AR, PGR, ESR1, and ESR2) for a given sample. Median averages were used to summarize expression values within each of the 34 Desikan-Killiany regions in the left hemisphere for a specific donor, then followed by the median average across the 6 donors to provide a single value for each region. The number of donors assayed per Desikan-Killiany region varied slightly; data from all 6 donors were used for 28 of the 34 regions (details, data files, and R script are provided in French and Paus 2015). As described below, consistency of the expression profiles was evaluated in 2 ways: (1) by computing donor-to-median correlations in the Allen Brain Atlas dataset and (2) by comparing average expression profiles based on the Allen Brain Atlas with those extracted from the BrainSpan Atlas. Note that the Allan Human Brain Atlas covers the entire cerebral cortex in a comprehensive manner, thus allowing us to calculate values of gene expression for each of the 34 cortical regions segmented by FreeSurfer. On the other hand, the BrainSpan Atlas provides values of gene expression for 11 of the 34 regions (see below).
BrainSpan Atlas
The BrainSpan Atlas provides gene expression data in the developing human brain (www.brainspan.org). Here, we limited the samples by donor age that is within the age range of adolescents included in the SYS and donors constituting the Allen Human Brain Atlas, namely from 13 to 40 years of age. In this age range, there are 9 individuals of the following sex and age: 5 males (ages 15, 18, 23, 36, and 37 years) and 4 females (ages 13, 21, 30, and 40 years). We then downloaded gene-expression values obtained in 11 cortical regions included in the BrainSpan Atlas that are homologous to those corresponding closely to the Desikan-Killiany parcellation employed in our work with the Allen Human Brain Atlas (Supplementary Table 1).
Comparison of Gene-Expression Profiles in Allen Human Brain Atlas and BrainSpan Datasets
For each gene, the median expression level was calculated across donors for each cortical region. Using correlation analyses, we evaluated the similarity between the Allen Human Brain Atlas and BrainSpan Atlas in the respective expression profiles of the 5 genes.
Sex effects
To determine whether there were any sex differences in the median expression levels (calculated across 11 cortical regions) for the 5 genes, we conducted t-tests to compare the median expression levels between males and females using the BrainSpan Atlas.
Age effects
To determine whether there were any age effects of the brain donor on the median expression levels (calculated across 11 cortical regions for each donor), we conducted correlation analyses between the median expression levels and donor's age for each of the 5 genes.
Results
Sample characteristics are presented in Table 1.
Table 1.
Sample characteristics for male and female adolescents
| Males | Females | P value | |
|---|---|---|---|
| n | 466 | 503 | |
| Age | 179.3 months (14.94 years) | 181.4 months (15.12 years) | 0.14 (ns) |
| Puberty status | 3.37 | 4.09 | 0.001 |
| Average cortical thickness (mm) | 2.55 | 2.58 | 0.001 |
Note: P values of t-tests conducted to examine sex differences for these variables are listed.
Correlation of Cortical Thickness and Age
For each of the 34 Desikan-Killiany regions, we conducted a correlation analysis between cortical thickness and age. To ensure normality of the correlation coefficients, we then conducted a Fisher's Z transformation on each of the 34 correlation coefficients (r). The transformed correlation coefficients and P values of the 34 correlation analyses are provided in Table 2. In the majority of regions, cortical thickness decreased with age in both males and females. Regions that have the greatest age-related cortical thinning during adolescence were in the occipital, parietal, and frontal regions. Cortical thickness in the entorhinal and temporal pole regions did not vary with age in males, but varied positively with age in females. Cortical thickness in the parahippocampal region did vary negatively with age in males, but no relationship was observed in females.
Table 2.
Correlation of cortical thickness and age for each of the 34 Desikan-Killiany region (Fisher Z transformed)
| Brain Region | Males | Females | ||
|---|---|---|---|---|
| (r) Fisher's Z transformed | P value (FDR corrected) | (r) Fisher's Z transformed | P value (FDR corrected) | |
| Entorhinal cortex (T) | 0.06 | 0.19 | 0.15 | <0.001 |
| Temporal pole (T) | 0.03 | 0.49 | 0.15 | 0.001 |
| Rostral anterior cingulate (F) | −0.16 | <0.001 | −0.26 | <0.001 |
| Parahippocampal gyrus (T) | −0.16 | <0.001 | −0.06 | 0.18 |
| Caudal anterior cingulate (F) | −0.21 | <0.001 | −0.23 | <0.001 |
| Pericalcarine cortex (O) | −0.24 | <0.001 | −0.20 | <0.001 |
| Posterior cingulate (P) | −0.29 | <0.001 | −0.35 | <0.001 |
| Isthmus cingulate (P) | −0.30 | <0.001 | −0.33 | <0.001 |
| Frontal pole (F) | −0.31 | <0.001 | −0.18 | <0.001 |
| Medial orbitofrontal cortex (F) | −0.32 | <0.001 | −0.43 | <0.001 |
| Inferior temporal gyrus (T) | −0.32 | <0.001 | −0.15 | <0.001 |
| Superior temporal gyrus (T) | −0.33 | <0.001 | −0.14 | 0.001 |
| Pars triangularis (F) | −0.33 | <0.001 | −0.21 | <0.001 |
| Middle temporal gyrus (T) | −0.34 | <0.001 | −0.14 | 0.002 |
| Transverse temporal cortex (T) | −0.35 | <0.001 | −0.25 | <0.001 |
| Insula | −0.36 | <0.001 | −0.26 | <0.001 |
| Banks of the superior temporal sulcus (T) | −0.37 | <0.001 | −0.27 | <0.001 |
| Cuneus cortex (O) | −0.37 | <0.001 | −0.28 | <0.001 |
| Pars orbitalis (F) | −0.37 | <0.001 | −0.16 | <0.001 |
| Precentral gyrus (F) | −0.37 | <0.001 | −0.17 | <0.001 |
| Pars opercularis (F) | −0.37 | <0.001 | −0.28 | <0.001 |
| Postcentral gyrus (P) | −0.37 | <0.001 | −0.21 | <0.001 |
| Supramarginal gyrus (P) | −0.38 | <0.001 | −0.11 | 0.015 |
| Lingual gyrus (O) | −0.39 | <0.001 | −0.28 | <0.001 |
| Fusiform gyrus (T) | −0.39 | <0.001 | −0.22 | <0.001 |
| Lateral orbitofrontal cortex (F) | −0.40 | <0.001 | −0.31 | <0.001 |
| Lateral occipital cortex (O) | −0.46 | <0.001 | −0.17 | <0.001 |
| Caudal middle frontal gyrus (F) | −0.48 | <0.001 | −0.30 | <0.001 |
| Paracentral lobule (F) | −0.51 | <0.001 | −0.35 | <0.001 |
| Superior parietal cortex (P) | −0.51 | <0.001 | −0.29 | <0.001 |
| Superior frontal gyrus (F) | −0.52 | <0.001 | −0.33 | <0.001 |
| Rostral middle frontal gyrus (F) | −0.58 | <0.001 | −0.31 | <0.001 |
| Inferior parietal cortex (P) | −0.58 | <0.001 | −0.26 | <0.001 |
| Precuneus cortex (O) | −0.60 | <0.001 | −0.42 | <0.001 |
Note: The FDR-corrected P values are listed. F, T, P, and O indicate frontal, temporal, parietal, and occipital lobe, respectively.
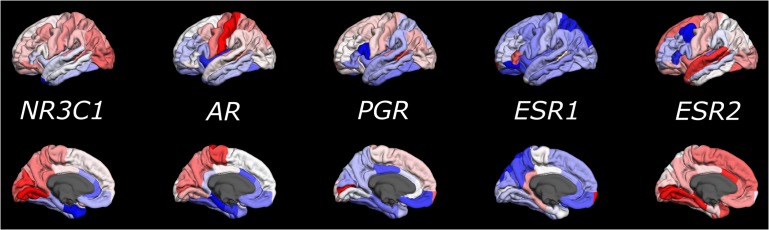
Regional Cortical Gene-Expression Values
For each of the 34 Desikan-Killiany regions, the median mRNA expression levels of the 5 genes (NR3C1, AR, PGR, ESR1, and ESR2) are visualized in Figure 1. To assess consistency of expression values across the 6 donor brains, the donor-to-median profile correlation coefficients of the 6 donors were calculated and averaged. A donor-to-median profile correlation coefficient was calculated by correlating a donor's gene-expression profile (34 gene-expression values, one value per cortical region) with the median gene-expression profile (34 median gene-expression values from the 6 donors). These donor-to-median profile correlation coefficients were calculated for each of the 6 donors before they were averaged. The average donor-to-median profile correlation coefficients for NR3C1, AR, PGR, ESR1, and ESR2 are 0.84, 0.79, 0.46, 0.38, and 0.35, respectively. As recommended in French and Paus (2015), the average donor-to-median profile provides a good approximation across the donors for genes that have a Spearman's ρ > 0.446 (corresponding to one-side P < 0.05 derived from random simulations of donor expression profiles). Thus, there is good approximation across the donors for NR3C1 and AR mRNA expression levels, marginal approximation across the donors for PGR mRNA expression levels, and weak approximation across the donors for ESR1 and ESR2 mRNA expression levels. As reported next, this corresponds to the degree of similarity in the expression profiles obtained in the Allen Human Brain Atlas and the BrainSpan Atlas.
Figure 1.
The mRNA expression levels for the NR3C1, AR, PGR, ESR1, and ESR2 genes. Note: Relative to each specific gene, red regions represent regions with the highest expression levels and blue regions represent regions with the lowest expression levels.
Inter-Regional Profiles in Gene Expression: Comparison of the Allen Human Brain Atlas and the BrainSpan Atlas
For NR3C1, we observed a positive correlation between its mRNA expression levels from the BrainSpan Atlas and the Allen Human Brain Atlas across the 11 cortical regions (r = 0.82, P = 0.002). For AR, we also observed a positive correlation between its mRNA expression levels from the BrainSpan Atlas and the Allen Human Brain Atlas (r = 0.86, P < 0.001). The figures are provided in Supplementary Materials (Supplementary Figure 1A). The correlation analyses between median expression levels of PGR, ESR1, and ESR2 from the BrainSpan Atlas and the Allen Human Brain Atlas were not significant (Ps > 0.11), consistent with the low values of median-to-donor correlations reported above.
To examine possible sex differences in the above inter-regional correlations in the 2 atlases, we have also evaluated the similarity of the median gene-expression levels in males (n = 5) and females (n = 4) from the BrainSpan Atlas with those obtained in the Allen Human Brain Atlas (median gene-expression levels from all 6 donors). We observed positive correlations between mRNA expression levels from the “male” subsample of the BrainSpan Atlas and the Allen Human Brain Atlas for both NR3C1 (r = 0.90, P < 0.001) and AR (r = 0.79, P = 0.004). The figures are provided in Supplementary Materials (Supplementary Figure 1B).
For the “female” subsample of the BrainSpan Atlas, we observed a marginal positive correlation with the single-female profile from Allen Human Brain Atlas for NR3C1 mRNA expression levels (r = 0.58, P = 0.059), and a positive correlation for AR mRNA expression levels (r = 0.76, P = 0.006). The figures are provided in Supplementary Materials (Supplementary Figure 1C).
Sex and Gene Expression in the BrainSpan Atlas
There were no sex differences in the median expression levels for the 5 genes (Ps > 0.31).
Age and Gene Expression in the BrainSpan Atlas
Except for AR mRNA expression levels, there were no relationships between expression levels of NR3C1, PGR, ESR1, and ESR2 with age (Ps > 0.23). For AR, mRNA expression levels decreased as a function of age, R2 = 0.47, F(1,7) = 6.24, P = 0.04 (Supplementary Figure 2).
Similarities in Gene-Expression Profiles in the BrainSpan Atlas by Age and Sex
Across the 11 cortical regions, we observed that expression levels are similar in youth (ages, 13, 15, 18, 21, and 23) and adults (ages 30, 36, 37, and 40) for NR3C1 (r = 0.64, P = 0.035) but not for AR (r = 0.35, P = 0.30). Similarly, we observed that expression levels are similar in males (n = 5) and females (n = 4) for NR3C1 (r = 0.6, P = 0.05) but not for AR (r = 0.45, P = 0.17). One should be cautious, however, in interpreting these findings given the small number of donors in each (age, sex) group used in these comparisons.
Cortical Thickness and Age as a Function of Gene Expression
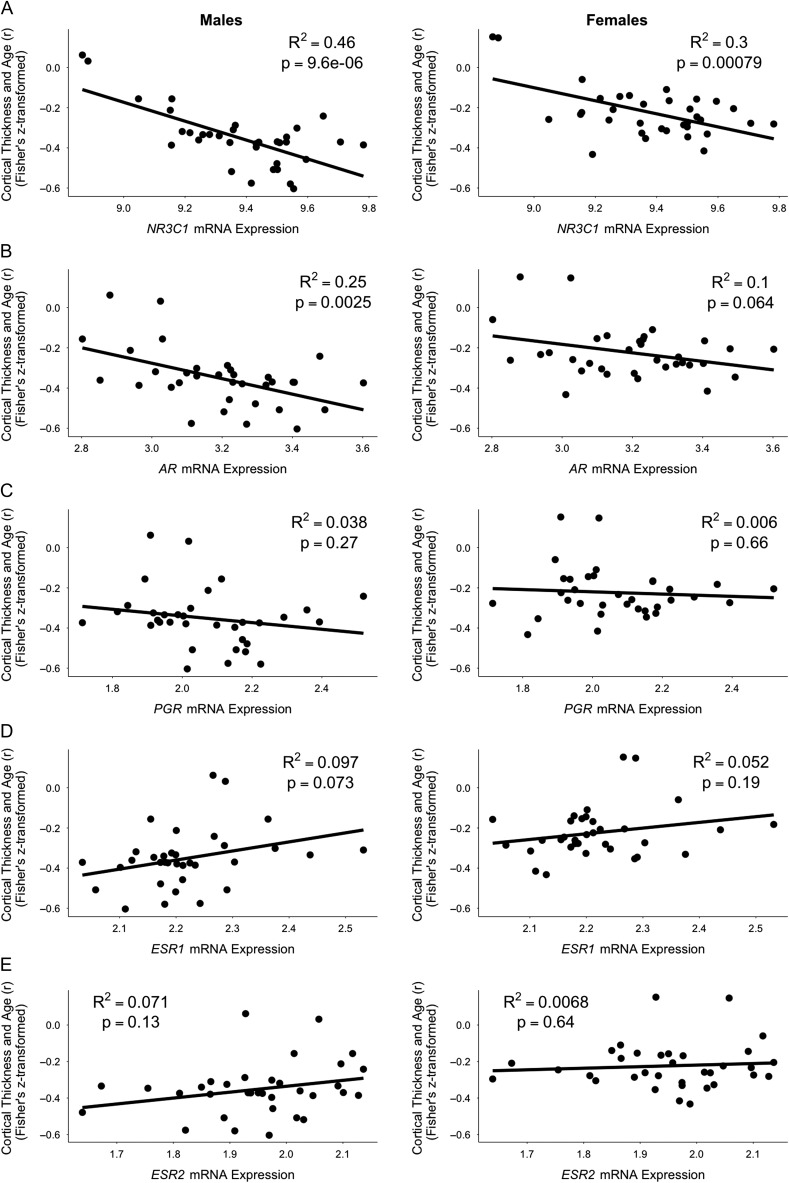
To test whether inter-regional variations in the age-related cortical thinning are related to inter-regional variations in the mRNA expression levels of the 5 genes of interest, we conducted a separate regression analysis for each gene relating its mRNA expression with the Fisher's Z transformed correlation coefficient capturing the age–thickness relationship across the 34 cortical regions.
The Fisher's Z varied negatively as a function of NR3C1 mRNA expression levels in both males R2 = 0.46, F(1,32) = 27.54, P < 0.001, and females, R2 = 0.30, F(1,32) = 13.76, P < 0.001 (Fig. 2A); thus, greater age-related thinning was associated with higher expression levels. The Fisher's Z varied negatively as a function of AR mRNA expression levels in males, R2 = 0.25, F(1,32) = 10.78, P = 0.002, and marginally (also negatively) in females, R2 = 0.10, F(1,32) = 3.69, P = 0.064 (Fig. 2B). The Fisher's Z did not vary as a function of PGR, ESR1, or ESR2 mRNA expression levels in neither males nor females (Fig. 2C, D, and E, respectively). The statistics are provided in Table 3. Since we conducted a total of 10 independent tests (5 genes in each sex), we applied a Bonferroni correction for multiple testing (corrected P value of 0.005). The results remained the same with this corrected cut off.
Figure 2.
Inter-regional age-related cortical thinning as a function of inter-regional mRNA expression levels of NR3C1, AR, PGR, ESR1, and ESR2 genes. The relationship between cortical thickness and age varied negatively as a function of NR3C1 mRNA expression levels in both males and females (A). The relationship between cortical thickness and age varied negatively as a function of AR mRNA expression levels in males only (B). The relationship between cortical thickness and age did not vary as a function of PGR, ESR1, nor ESR2 mRNA expression levels (C, D, and E, respectively). Note: within each subfigure, each point represents 1 of the 34 cortical regions.
Table 3.
Regression analyses between cortical thickness and age correlation coefficients (Fisher's Z transformed) as a function of mRNA expression levels of genes of interest (analyzed independently): NR3C1, AR, PGR, ESR1, and ESR2
| R2 | B | SE B | Beta | Df1 | Df2 | F | P value | |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| NR3C1 | 0.46 | −0.47 | 0.09 | −0.68 | 1 | 32 | 27.54 | <0.001 |
| AR | 0.25 | −0.38 | 0.12 | −0.50 | 1 | 32 | 10.78 | 0.002 |
| PGR | 0.04 | −0.17 | 0.15 | −0.20 | 1 | 32 | 1.27 | 0.27 |
| ESR1 | 0.10 | 0.45 | 0.24 | 0.31 | 1 | 32 | 3.44 | 0.07 |
| ESR2 | 0.07 | 0.32 | 0.21 | 0.27 | 1 | 32 | 2.44 | 0.13 |
| Females | ||||||||
| NR3C1 | 0.30 | −0.33 | 0.09 | −0.55 | 1 | 32 | 13.76 | <0.001 |
| AR | 0.10 | −0.21 | 0.11 | −0.32 | 1 | 32 | 3.69 | 0.06 |
| PGR | 0.01 | −0.06 | 0.13 | −0.78 | 1 | 32 | 0.19 | 0.66 |
| ESR1 | 0.05 | 0.28 | 0.21 | 0.23 | 1 | 32 | 1.76 | 0.19 |
| ESR2 | 0.09 | 0.09 | 0.18 | 0.08 | 1 | 32 | 0.22 | 0.64 |
NR3C1 and AR Interaction
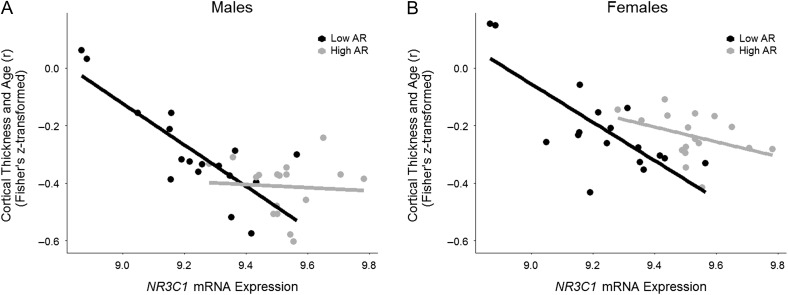
We observed an interaction between NR3C1 and AR expression (vis-à-vis Fisher's Z) in both males (P = 0.001) and females (P = 0.033). When correcting for the 2 tests, this interaction remains significant for males (P = 0.002) but not females (P = 0.066). We examined this interaction further by testing the relationship between Fisher's Z of age-related cortical thinning and NR3C1 mRNA expression level separately for 2 groups of cortical regions, namely those with “low” and “high” levels of AR expression (based on the median split). In the low AR mRNA expression group, Fisher's Z varied negatively as a function of NR3C1 mRNA expression levels in both males (R2 = 0.64, F(1,15) = 26.72, P < 0.001) and females (R2 = 0.58, F(1,15) = 20.42, P < 0.001), see Figure 3. In the high AR mRNA expression group, this relationship was not observed in neither males (R2 = 0.0045, F(1,15) = 0.068, P = 0.80) nor females (R2 = 0.15, F(1,15) = 2.63, P = 0.13), see Table 4.
Figure 3.
The interaction of NR3C1 and AR mRNA expression levels. There was a significant NR3C1 and AR interaction in males and in females. In both males and females, inter-regional age-related cortical thinning varied as a function of inter-regional NR3C1 mRNA expression levels in the low AR mRNA expression group, but not the high AR mRNA expression group. Note: within each subfigure, each point represents 1 of the 34 cortical regions.
Table 4.
The relationship between cortical thickness and age correlation coefficient (Fisher's Z transformed) as a function of NR3C1 mRNA expression levels at low or high AR mRNA expression levels
| R2 | B | SE B | Beta | Df1 | Df2 | F | P value | |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| Low AR | 0.64 | −0.72 | 0.14 | −0.8 | 1 | 15 | 26.72 | <0.001 |
| High AR | 0.0045 | −0.05 | 0.21 | −0.067 | 1 | 15 | 0.068 | 0.80 |
| Females | ||||||||
| Low AR | 0.58 | −0.66 | 0.15 | −0.76 | 1 | 15 | 20.42 | <0.001 |
| High AR | 0.15 | −0.26 | 0.16 | −0.39 | 1 | 15 | 2.63 | 0.13 |
Discussion
As hypothesized, inter-regional variations in age-related cortical thinning observed during adolescence were associated with inter-regional variations in mRNA expression levels of genes related to the HPA and HPG axes. Specifically, cortical thinning varied as a function of NR3C1 mRNA expression levels in both males and females; there is more thinning in brain regions with higher NR3C1 mRNA expression levels. Regions with the highest NR3C1 mRNA expression levels are in the occipital, parietal, and frontal lobe regions; these regions show the strongest age-related cortical thinning. Regions with the lowest NR3C1 mRNA expression levels are found in the temporal lobe; these regions show the least age-related cortical thinning. HPA-axis activation and its subsequent cortisol release is more likely to influence regional brain development in regions with high glucocorticoid receptors levels. In 2 previous studies of fronto-cortical gray matter found an association between cortisol levels and lower gray-matter volumes in the left ventral and inferior prefrontal regions in a sample of youth with posttraumatic stress disorder symptoms (Carrion et al. 2010), and an association between cortisol levels and lower cortical thickness in left dorsolateral and ventrolateral prefrontal regions, and right dorsolateral and medial orbital frontal cortex in healthy middle-aged male twins (Kremen et al. 2010). Since these 2 studies had chosen a priori regions of interests, it is possible that similar relationships would be observed in other regions with high NR3C1 mRNA expression levels.
In our study, inter-regional variations in age-related cortical thinning also varied as a function of inter-regional variations in AR mRNA expression levels; this was the case in males only. Similar to NR3C1 expression, we observed more age-related cortical thinning in brain regions with higher AR mRNA expression. Regions with the highest AR mRNA expression levels, similar to NR3C1 mRNA expression, are also in the occipital, parietal, and frontal lobe regions, where there is the strongest age-related cortical thinning and lowest in the temporal lobe regions, where there is the least age-related cortical thinning. The null finding in females can be due to lower bioavailable testosterone levels in females than in males. During male puberty, rising testosterone levels bind to the androgen receptors in the brain and, in turn, are more likely to influence regional brain maturation during adolescence. Indeed, previous studies of adolescents found that testosterone was associated negatively with gray-matter volumes, particularly in the left frontal and parietal lobes (Neufang et al. 2009; Peper et al. 2009; Paus et al. 2010; Witte et al. 2010; Bramen et al. 2011). In a longitudinal study of adolescents, Nguyen et al. (2013) found that bioavailable testosterone was associated negatively with cortical thickness in several regions in the frontal, parietal, and occipital lobes. Similarly, Savic and Arver (2014) found that bioavailable testosterone was related to parietal and occipital cortical thinning in men.
Consistent with literature on the cross talk of the HPA and HPG axes (as reviewed by Viau 2002), we observed an interaction between inter-regional NR3C1 and AR mRNA expression levels in explaining the inter-regional variations in age-related cortical thinning during adolescence in both males and females. We found that inter-regional age-related cortical thinning varied as a function of NR3C1 mRNA expression levels in brain regions with low AR mRNA expression levels but not in the regions with high AR mRNA expression levels. Regions that tend to have high AR mRNA expression levels also tend to have high NR3C1 mRNA expression levels and display strong age-related cortical thinning. This interaction suggests that during adolescence there might be a floor effect of age-related cortical thinning in brain regions with high NR3C1 and AR mRNA expression levels. This floor effect may be due to the interaction of the glucocorticoid and androgen receptors. In line with this possibility, Hartig et al. (2012) found that corticosteroids upregulate AR expression while decreasing AR activity and altering androgen effects on adipogenesis. Furthermore, the interplay between the HPA and HPG axes is illustrated by the inhibitory effects of stress on reproductive behavior (Rivier and Rivest 1991; Tilbrook et al. 2000), and the inhibitory effect of testosterone on HPA axis response to stress (Viau and Meaney 1996).
Contrary to our hypotheses, inter-regional age-related cortical thinning did not vary as a function of PGR, ESR1, or ESR2 mRNA expression levels. This null finding may be due to the lower consistency of the inter-regional PGR, ESR1, and ESR2 mRNA expression levels across the donor brains (i.e., lower average donor-to-median profile correlations, 0.46, 0.38, and 0.35, respectively), when compared with the high consistency found in NR3C1 and AR mRNA expression profiles (0.84 and 0.79, respectively). The lower consistency of the PGR, ESR1, and ESR2 mRNA expression profiles across the donor brains suggest that there is more individual variability in the mRNA expression levels across different brain regions making the use of median mRNA expressions of these genes less reliable. It is still possible, however, that inter-regional PGR, ESR1, and ESR2 mRNA expression levels for a given individual can be related to the rate of cortical thinning for the same individual during their brain development. This null finding can also be due to the low statistical power we have as we are working with 34 cortical regions as data points in our regression analyses. Finally, the null effect of the PGR mRNA expression on age-related cortical thinning in females can be due to the sexual dimorphic expression of the receptor (Guerra-Araiza et al. 2002); only 1 of the 6 donor brains was a female brain. Nonetheless, we did not observe any sex differences in the expression levels of PGR in the BrainSpan dataset. There is less sexual dimorphism in the distribution of ESR1 and ESR2; the distribution is similar in males and females throughout the brain, with the exception of the hypothalamus (Gillies and McArthur 2010).
Across the cerebral cortex, the NR3C1 mRNA expression levels from the Allen Human Brain Atlas were strongly related to the NR3C1 mRNA expression levels from the BrainSpan Atlas. The same was true also about the AR mRNA expression levels. This cross-atlas consistency in inter-regional gene-expression profiles of the 2 genes provides additional confidence in their generalizability. There were, however, no relationships between expression profiles of PGR, ESR1, and ESR2 from the BrainSpan Atlas and the Allen Human Brain Atlas. This is consistent with low median-to-donor correlations of inter-regional profiles of these 3 genes in the Allen Human Brain Atlas; median-to-donor correlations lower than r = 0.446 suggest low reliability of the profiles (French and Paus 2015).
We used the BrainSpan dataset to examine whether there were sex and age effects on expression levels of NR3C1, AR, PGR, ESR1, and ESR2. We found that there were no sex differences in the expression levels of the 5 genes, and no age effects for 4 of the 5 genes (NR3C1, PGR, ESR1, and ESR2). The only age effect was observed for AR, such that the median AR expression levels decreased with age. It is important to note that this age-related decrease in the overall AR mRNA expression levels is unlikely to influence our findings given that inter-regional variations in AR expression are very similar between the Allen Human Brain Atlas and BrainSpan datasets, despite differences in the donor age.
It should be noted that circulating levels of stress and sex hormones might influence gene expression of their receptors in the brain in a complex manner. For example, rats exposed to acute stress (vs. controls) had lower levels of NR3C1 mRNA expression levels in dentate gyrus of the hippocampus (Mifsud et al. 2016). On the other hand, castrated male rats had higher levels of AR mRNA expression in the hippocampus 4 days after castration, when compared with intact rats (Kerr et al. 1995). The same authors observed lower levels of AR expression in the hippocampus of young adult rats (5 months of age), when compared with old (2 months of age) rats (Kerr et al. 1995). The latter finding suggests that—over an extended time period—lower levels of androgens are associated with up-regulation of AR expression. It is possible that our observation of age-related decreases in AR expression in the human cerebral cortex is related to overall increases in androgen levels between early puberty and young adulthood, as seen in male donors of the subsample of the BrainSpan dataset evaluated here (13–37 years of age).
Entorhinal cortex and temporal pole did not show any age-related cortical thinning during adolescence, and these are regions with the lowest NR3C1 mRNA expression levels (as shown in Fig. 2A and in Fig. 3). Similar to our findings, entorhinal cortex and temporal pole also did not show age-related cortical thinning in a sample of 168 adolescents and young adults ages 8 to 30 from Norway (Tamnes et al. 2010); and in the same ongoing cohort from Norway with 331 participants ages 4 to 30 (Amlien et al. 2016). It is important to note that entorhinal cortex and temporal pole showed age-related cortical thinning during aging in 883 participants ages 18 to 94 when 6 samples recruited from Norway, Sweden, and USA were pooled together (Fjell et al. 2009). Examining the 6 samples independently, entorhinal cortex displayed age-related cortical thinning in 5 of the 6 samples, and temporal pole displayed age-related cortical thinning in all 6 samples. From these studies, the lack of age-related cortical thinning of entorhinal cortex and temporal pole seems to be present in only the samples of adolescent and young adults, but not in the sample of participants from the entire life span. This suggests that the lack of age-related cortical thinning of entorhinal cortex and temporal pole is specific to this developmental period rather than a technical error.
We have shown here that inter-regional variations in cortical thinning during adolescence relate to variations in the expression of glucocorticoid and androgen receptor genes across the same cortical regions. The key limitation inherent in our approach is the fact that the 2 datasets, namely in vivo estimates of cortical thickness and ex vivo estimates of gene expression, come from different individuals. Thus, the thickness–expression relationship is tested at a group level, across 34 cortical regions. This requires high confidence in the group-based profiles of the 2 sets of variables. In case of cortical thickness and its correlation with age, the profiles are based on data obtained in 466 males and 503 females. As we showed elsewhere, the male and female inter-regional profiles in cortical thinning are very similar (Paus et al. 2017), thus supporting their validity. In case of gene expression, we showed that the inter-regional profiles are similar between 2 independent samples of “postmortem” brains (Allen Human Brain Atlas and BrainSpan) for NR3C1 and AR but not for PGR, ESR1, and ESR2. This pattern is consistent with the varied degree of interindividual similarity of these profiles among the 6 donors included in the Allen Human Brain Atlas (French and Paus 2015). It is likely that increasing the number of donors would enhance the number of genes with reliable estimates of inter-regional profiles. Nonetheless, it is remarkable that such profiles of gene expression can be estimated reliably with data obtained in as few as 6 donors for some genes (e.g., NR3C1 and AR). Note also that, in this study, we have made use of gene-expression profiles in a directed rather than exploratory manner, focusing on a specific set of molecules known to be involved in brain maturation during adolescence, namely stress and sex hormones. Future studies could explore such relationships in a multivariate manner or employ other candidate genes selected for testing specific hypotheses. As shown in Supplementary Figure 3, there are many other genes that show similar relationship between the inter-regional profile of age-related cortical thinning and their expression across the 34 cortical regions.
In conclusion, both glucocorticoid and androgen receptors appear to play a role in cortical maturation during adolescence. We found that inter-regional variations in age-related cortical thinning were associated with inter-regional variations in NR3C1 and AR mRNA expression levels in the cerebral cortex. Examining the 2 genes together, the relationship between inter-regional age-related cortical thinning and inter-regional NR3C1 mRNA expression levels was present in cortical regions with low AR mRNA expression levels but not in cortical regions with high AR mRNA expression levels. The observed NR3C1 and AR interaction on cortical maturation during adolescence extends the existing literature on the interplay of the HPA and HPG axes. Future studies can examine how physiological and behavioral measures (e.g., steroid hormones, cognition, stress exposure, and mental health) relate to different cortical regions implicated in this study. These studies will allow us to understand further how stress and sex hormones shape individual differences in cognition and mental health during adolescence.
Supplementary Material
Notes
Conflict of Interest: None declared.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
The SYS project is funded by the Canadian Institutes of Health Research (T.P., Z.P.), Heart and Stroke Foundation of Quebec (Z.P.), and the Canadian Foundation for Innovation (Z.P.). We thank the following individuals for their contributions in acquiring data: Manon Bernard (database architect, The Hospital for Sick Children), Jacynthe Tremblay and her team of research nurses (Saguenay Hospital), Helene Simard and her team of research assistants (Cégep de Jonquière) and Dr Rosanne Aleong (Manager, Rotman Research Institute). T.P. is the Tanenbaum Chair in Population Neuroscience at the Rotman Research Institute, University of Toronto and the Dr John and Consuela Phelan Scholar at Child Mind Institute, New York. Computations were performed on the GPC supercomputer at the SciNet HPC Consortium. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada, the Government of Ontario, Ontario Research Fund – Research Excellence, and the University of Toronto.
References
- Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, Rosa MGP, Walhovd KB. 2016. Organizing principles of human cortical development – thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy. Cereb Cortex. 26:257–267. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. 2011. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 21:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Jernigan TL. 2012. Brain development during the preschool years. Neuropsychol Rev. 22:313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, Lo Russo G, Castana L, Colombo N, Caborni C, et al. 2014. Validation of freesurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 12:535–542. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL. 2010. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biol Psychiatry. 68:491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French L, Paus T. 2015. A FreeSurfer view of the cortical transcriptome generated from the Allen Human Brain Atlas. Front Neurosci. 9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick T, Swann J. 2014. Testosterone regulates the density of dendritic spines in the male preoptic area. Horm Behav. 65:249–253. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 62:155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. 2002. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 59:105–109. [DOI] [PubMed] [Google Scholar]
- Hartig SM, He B, Newberg JY, Ochsner SA, Loose DS, Lanz RB, McKenna NJ, Buehrer BM, McGuire SE, Marcelli M, et al. 2012. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol. 19:1126–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA. 2012. An anatomically comprehnsive atlas of the adult human brain transcriptome. Nature. 489:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. 2015. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. 1995. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 136:3213–3221. [DOI] [PubMed] [Google Scholar]
- Kremen WS, O'Brien RC, Panizzon MS, Prom-Wormley E, Eaves LJ, Eisen SA, Eyler L, Hauger RL, Fennema-Notestine C, Fischl B, et al. 2010. Salivary cortisol and prefrontal cortical thickness in middle-aged men: a twin study. Neuroimage. 53:1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW, et al. 2003. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 60:878–888. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, et al. 2004. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci. 101:2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken C, Gruner D, Groer L, Peltier R, Bunn N, Craig M, Henriques T, Dempsey J, Yu C-H, Chen J, et al. 2010. SciNet:lessons learned from building a power-efficient top-20 system and data centre . J Phys Conf Ser. 256:1–35. [Google Scholar]
- Mifsud KR, Saunderson EA, Spiers H, Carter SD, Trollope AF, Mill J, Reul JM. 2016. Rapid down-regulation of glucocorticoid gene expression in the dentate gyrus after acute stress in vivo: role of DNA methylation and microRNA activity. Neuroendocrinology. 10.1159/000445875. [DOI] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore SJ. 2014. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc Cogn Affect Neurosci. 9:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbane M, Badoud D, Eliez S, Schaer M. 2013. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 82:200–207. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, Konrad K. 2009. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 19:464–473. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. 2013. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 23:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z. 2010. Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 57:63–75. [DOI] [PubMed] [Google Scholar]
- Paus T, Wong AP, Syme C, Pausova Z. 2017. Sex differences in the adolescent brain and body: findings from the Saguenay Youth Study. J Neurosci Res. 95:362–370. [DOI] [PubMed] [Google Scholar]
- Pausova Z, Paus T, Abrahamowicz M, Almerigi J, Arbour N, Bernard M, Gaudet D, Hanzalek P, Hamet P, Evans AC, et al. 2007. Genes, maternal smoking, and the offspring brain and body during adolescence: design of the Saguenay Youth Study. Hum Brain Mapp. 28:502–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, Peron M, Pike GB, Richer L, Séguin JR, et al. 2016. Cohort profile: the Saguenay Youth Study (SYS). Int J Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2009. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 34:332–342. [DOI] [PubMed] [Google Scholar]
- Popescu V, Klaver R, Versteeg A, Voorn P, Twisk JWR, Barkhof F, Geurts JJG, Vrenken H. 2016. Postmortem validation of MRI cortical volume measurements in MS. Hum Brain Mapp. 37:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivest S. 1991. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 45:523–532. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. 2002. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 58:695–701. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2014. Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb Cortex. 24:3246–3257. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. 2004. The neural basis of puberty and adolescence. Nat Neurosci. 7:1040–1047. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. 2010. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20:534–548. [DOI] [PubMed] [Google Scholar]
- Tata DA, Anderson BJ. 2010. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav. 99:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ. 2000. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 5:105–113. [DOI] [PubMed] [Google Scholar]
- Viau V. 2002. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 14:506–513. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. 1996. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 16:1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. 2010. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage. 49:1205–1212. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1994. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 14:7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.