Abstract
This article comments on:
Bossinger G, Spokevicius AV. 2018. Sector analysis reveals patterns of cambium differentiation in poplar stems. Journal of Experimental Botany 69, 4339–4348.
Keywords: Cambium initials, cell fate, poplar, stem cells, wood formation, xylogenesis
Poplar is an important model for gene discovery and characterization. These trees undergo extensive secondary growth to produce wood from the vascular cambium, but it has been challenging to visualize the earliest stages of development. Bossinger and Spokevicius (2018) have used a technique known as sector analysis to visualize cell fate, addressing a long-standing debate on the origins of xylem and phloem in tree cells and providing an unprecedented look at initial wood formation.
Populus spp., commonly called cottonwoods, aspens or poplars, inhabit much of the northern hemisphere yielding up to 19 Mg ha–1 year–1 of biomass in Canada and the United States alone (Sannigrahi et al., 2010). Having a sequenced genome (Tuskan et al., 2006), numerous studies of global gene expression (e.g. Schrader et al., 2004) and collections of activation- (reviewed by Busov et al., 2010) and transposon-tagged (Fladung, 2011) mutants, they are an important model for gene discovery and characterization in angiosperm trees. Unlike other model plants such as Arabidopsis, Populus spp. are perennial trees that undergo extensive secondary growth to produce wood involving the vascular cambium (a lateral meristem). Bossinger and Spokevicius (2018) have used a technique known as sector analysis to visualize cell fate in the vascular cambium and provide an unprecedented look at the early stages of wood development.
Populus wood and the importance of secondary growth
Populus wood is an important feedstock for the pulp and paper industry (Christersson, 2008), and having a heating value of 19 MJ kg–1 for hybrid species, it plays an important role in bioenergy production through thermochemical conversion (e.g. gasification, pyrolysis, hydrothermal liquefaction) (reviewed by Sannigrahi et al., 2010; Slopiecka et al., 2012; Chen et al., 2016; Tekin et al., 2016). Additionally, Populus wood offers an excellent platform for biorefinery development, since the biomass is rich in sugars that can be extracted and used to produce fermentation products such as bioethanol and polyhydroxybutyrates (Dai and McDonald, 2014; Demartini et al., 2015). Thus, key components of secondary xylem, mainly cellulose, hemicellulose and lignin, have become the focus of many studies, but our knowledge of the early stages of wood development remains limited.
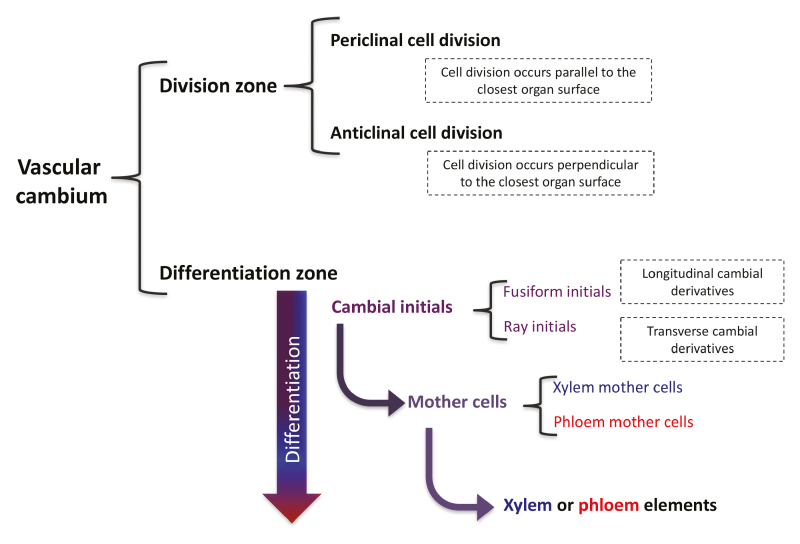
The vascular cambium is a cylindrical meristem that gives rise to xylem (wood) and phloem during secondary growth. While there is plenty of knowledge on the function and differentiation of primary growth meristems (apical and root meristems) based on studies in annual plants such as Arabidopsis, differentiation of the vascular cambium during secondary growth is still poorly understood despite the important role of this meristem in producing secondary xylem and phloem. One of the challenges of studying cells in the cambial zone is the difficulty in morphologically distinguishing cambial initials from mother cells and young xylem and phloem, making it hard to develop a model to describe division and differentiation patterns. What we know of vascular cambium differentiation is that cambial initials have the capacity to give rise to both xylem and phloem mother cells, which in turn give rise to xylem and phloem elements (Box 1). However, there has been a long-standing debate as to whether mother cells are derived from a single layer of cambial initials, or whether multiple layers of cambial initials exist across several radial files (starting with Sanio, 1873, and Raatz, 1892).
Box 1. Structure of the cambial zone
There are two zones that make up the vascular cambium: the division zone and the differentiation zone. The division zone is where the cambial initials undergo cell division, which can occur parallel to, or perpendicular to, the surface of the closest organ (periclinal or anticlinal cell division, respectively). After cambial initials divide, they enter the differentiation zone, forming either xylem or phloem mother cells and, in turn, xylem or phloem. Bossinger and Spokevicius (2018) used sector analysis to determine that cambial initials originate from a single layer of cells, adding compelling evidence to a 120-year debate.
Along with the growing number of genomic and proteomic resources available for Populus has come a growing list of genes responsible for wood development and secondary growth with a major focus on cambial meristem activity, and wood quality based on cell wall properties (e.g. Obudulu et al., 2016; Kucukoglu et al., 2017; Li et al., 2017). Despite this, there is a need for a robust way to visualize cell differentiation in a mature stem to follow xylem and phloem development from the cambial initials and mother cells on to mature tissue.
Sector analysis reveals cell fate in the cambial zone
Bossinger and Spokevicius have developed a system called sector analysis which uses the β-glucuronidase (GUS) reporter gene to follow cell fate in the tree stem. Cells are transformed in the cambial zone of a living tree and, after several months of subsequent growth, the cell and any of its derivatives are revealed by histochemical GUS staining. After creating hundreds of independent transformation events of cells in the cambial zone, this study provides a high-throughput and unprecedented look at the differentiation of cambial initials and mother cells into xylem and phloem. Their evidence provides strong support for three major findings: (i) the vascular cambium is made up of a single layer of cambial initials, (ii) the differentiation of both phloem and xylem mother cells is controlled independently, and (iii) that on average four xylem cells are produced for every one phloem cell (ranging from 2:1 to 6:1). Unlike previous studies that proposed models for the differentiation of the vascular cambium based on anatomical analysis of past cell divisions, sector analysis labels a single cell and follows the fate of its derivatives throughout the stages of xylem and phloem development. This opens up the opportunity to analyse gene function in the cambial zone at a much higher resolution than previously possible.
Future directions
The research by Bossinger and Spokevicius (2018) not only answers basic questions about cambial meristem cell fate and differentiation in Populus, but also provides a standard framework of wild-type cell differentiation in the tree stem. In our own research using activation tagged Populus (Harrison et al., 2007), we have identified several mutants with alterations in wood development including the ratio of xylem to phloem, wood biomass (which is increased) and altered wood properties such as lignin content. Testing these mutants with sector analysis would enable a closer look at the impact of these mutations on the vascular cambium and cell differentiation in the stem, and bring us closer to understanding the intricacies of gene function. Similarly, other studies of candidate genes involved in cambium development or wood formation that use either natural variants or transgenic lines for functional analysis could benefit from incorporating sector analysis to resolve the impact of genes on cell differentiation at the level of individual cell lineages as previously shown by Spokevicius et al. (2016). This is certainly a valuable addition to the Populus tool box for functional characterization of wood formation.
References
- Bossinger G,Spokevicius AV.. 2018Sector analysis reveals patterns of cambium differentiation in poplar stems.Journal of Experimental Botany 69, 4339–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busov V,Yordanov Y,Gou J,Meilan R,Ma C,Regan S,Strauss SH.. 2010Activation tagging is an effective gene tagging system in Populus.Tree Genetics & Genomes 7,91–101. [Google Scholar]
- Chen D,Li Y,Cen K,Luo M,Li H,Lu B.. 2016Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature.Bioresource Technology 218,780–788. [DOI] [PubMed] [Google Scholar]
- Christersson L. 2008Poplar plantations for paper and energy in the south of Sweden.Biomass and Bioenergy 32,997–1000. [Google Scholar]
- Dai J,McDonald AG.. 2014Production of fermentable sugars and polyhydroxybutyrate from hybrid poplar: response surface model optimization of a hot-water pretreatment and subsequent enzymatic hydrolysis.Biomass and Bioenergy 71,275–284. [Google Scholar]
- DeMartini JD,Foston M,Meng X,Jung S,Kumar R,Ragauskas AJ,Wyman CE.. 2015How chip size impacts steam pretreatment effectiveness for biological conversion of poplar wood into fermentable sugars.Biotechnology for Biofuels 8,209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladung M. 2011Analysis of re-integrated AC delement positions in the genome of Populus provides a basis for AC/Ds-transposon activation tagging in trees.Trees 25,551–557. [Google Scholar]
- Harrison EJ,Bush M,Plett JM,et al. . 2007Diverse developmental mutants revealed in an activation-tagged population of poplar.Canadian Journal of Botany 85,1071–1081. [Google Scholar]
- Kucukoglu M,Nilsson J,Zheng B,Chaabouni S,Nilsson O.. 2017WUSCHEL-RELATED HOMEOBOX4 (WOX4)-like genes regulate cambial cell division activity and secondary growth in Populus trees.New Phytologist 215,642–657. [DOI] [PubMed] [Google Scholar]
- Li Y,Jin F,Chao Q,Wang BC.. 2017Proteomics analysis reveals the molecular mechanism underlying the transition from primary to secondary growth of poplar.Journal of Plant Physiology 213,1–15. [DOI] [PubMed] [Google Scholar]
- Obudulu O,Bygdell J,Sundberg B,Moritz T,Hvidsten TR,Trygg J,Wingsle G.. 2016Quantitative proteomics reveals protein profiles underlying major transitions in aspen wood development.BMC Genomics 17,119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatz W. 1892Die Stabbildungen im secondären Holzkörper der Bäume und die Initialentheorie.Jahrbücher für Wissenschaftliche Botanik 23,567–636. [Google Scholar]
- Sanio K. 1873Anatomie der gemeinen Kiefer (Pinus sylvestris L.).Jahrbücher für Wissenschaftliche Botanik 9,50–126. [Google Scholar]
- Sannigrahi P,Ragauskas AJ,Tuskan GA.. 2010Poplar as a feedstock for biofuels: A review of compositional characteristics.Biofuels, Bioproducts and Biorefining 4,209–226. [Google Scholar]
- Schrader J,Nilsson J,Mellerowicz E,Berglund A,Nilsson P,Hertzberg M,Sandberg G.. 2004A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity.The Plant Cell 16,2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopiecka K,Bartocci P,Fantozzi F.. 2012Thermogravimetric analysis and kinetic study of poplar wood pyrolysis.Applied Energy 97,491–497. [Google Scholar]
- Spokevicius A,Taylor L,Melder E,Van Beveren K,Tibbits J,Creux N,Bossinger G.. 2016The use of Induced Somatic Sector Analysis (ISSA) for studying genes and promoters involved in wood formation and secondary stem development.Journal of Visualized Experiments 116doi: 10.3791/54553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin K,Akalin MK,Karagöz S.. 2016The effects of water tolerant Lewis acids on the hydrothermal liquefaction of lignocellulosic biomass.Journal of the Energy Institute 89,627–635. [Google Scholar]
- Tuskan GA,Difazio S,Jansson S,et al. . 2006The genome of black cottonwood, Populus trichocarpa (Torr. & Gray).Science 313,1596–1604. [DOI] [PubMed] [Google Scholar]