Abstract
For almost 20 years, chronic systemic d-galactose, a monosaccharide abundantly present in milk products, fruits, and vegetables, has been used as a tool to achieve models of accelerated aging. Its neurotoxicity, induced by abnormal accumulation of reactive oxygen species and advanced glycation end products, has been widely reported. However, behavioral outcomes are still controversial and little is known about sex-dependent vulnerability. We performed a comprehensive behavioral and multifunctional screening of the chronic effects of low (50 mg/kg) and high (100 mg/kg) doses of d-galactose in 6-month-old male and female gold-standard C57BL/6 mice. Twelve classical tests with convergent validity analyzed sensorimotor, emotional and cognitive domains, indicating the existence of thresholds of response. Distinct vulnerability patterns were found in a selective sex- and dose-dependent manner. In males, d-galactose induced sensorimotor impairment and immunoendocrine senescence, but the low dose resulted in improved learning and memory. Oppositely, d-galactose-treated females exhibited a dose-dependent worse motor and spatial learning, but improved memory. Behavioral outcome items point at distinct neuronal substrates underlying the functional capacity of d-galactose-treated animals to meet task-dependent performance demands. They support that males and females can be regarded as two exceptional natural scenarios to study the functional interplay in the cross talk of homeostatic networks in aging.
Keywords: Animal model, Behavioral screening, Functional screening, Frailty, Corticosterone, Thymus
Male and female sexes can be regarded as two exceptional natural scenarios to study the role of biological, psychological, and social factors; their functional interplay; and their impact on the cross talk of homeostatic networks in health and disease through life cycle. Worse “healthy life expectancy” recorded in mammalian females is in contrast to their longer life expectancy, a phenomenon that was defined as the morbidity and mortality paradox of gender (1). Mechanistic hypothesis of longer life expectancy of mammalian females as compared to males point out to hormones, asymmetric inheritance of sex chromosomes, and mitochondria. Also, we have postulated a better redox state and a higher ability to hamper the derangement of the regulatory systems, namely the nervous, endocrine, and immune systems, that occur during normal and pathological aging (2).
For almost 20 years, d-galactose (d-gal) has been used as a tool to achieve models of accelerated aging (ie, (3–5)). d-Gal is a monosaccharide abundantly present in milk products and other nondairy foodstuffs such as fruits and vegetables (6). However, this physiological nutrient is a reducing sugar that has been shown to induce oxidative stress in vivo, mimicking natural aging and age-related cognitive decline in mice (3–5,7–9). At normal concentrations, d-gal is metabolized into glucose by d-galactokinase and galactose-1-phosphate uridyltransferase in animals (10). However, overdose of d-gal beyond the capacity of those two enzymes allows aldose reductase to catalyze accumulated d-gal in galactitol, which cannot be metabolized but will accumulate in the cells, leading to osmotic stress and generation of reactive oxygen species (11). On the other hand, accumulated d-gal in animal tissues can react with free amino groups of proteins and peptides to form advanced glycation end products, through nonenzymatic glycation (12). Interestingly, it is shown that accumulation of advanced glycation end products accelerates the multisystem functional decline that occurs with aging (13) and it has been linked to the pathogenesis of many age-associated diseases such as diabetes, arteriosclerosis, nephropathy, infection, and Alzheimer’s disease (14). Thus, abnormal accumulation of reactive oxygen species and advanced glycation end products by excess of d-gal could be responsible for neurotoxicity which may lead to behavioral and neurochemical alterations (15,16).
At the translational level, some laboratories have reported that animals treated with d-gal show cognitive deficits (17,18) and/or a decrease in motor skills (19). However, although d-gal-induced biological alterations mimicking those found on aging have been consistently reported, these behavioral results are controversial because not all studies have been able to replicate them. This situation is currently discussed as mainly due to the differing variety of experimental designs and animal strains used which may hinder the convergence of outcomes. For instance, recently, in C57BL/6 mice treated with 100 mg/kg d-gal, factors such as gender, age, and treatment duration have been shown to influence brain oxidative stress and the spatial memory impairment in the Y-maze (20). Discrepancies are also found when studied in rats (21–23). Thus, the most recent scientific literature complains about the lack of consistency of chronic d-gal inducing accelerated-aging changes in behavioral outcomes (24).
In this context, the present study was aimed to characterize the behavioral dose-response of chronic d-gal (low dose, 50 mg/kg; high dose, 100 mg/kg) by means of a comprehensive behavioral and functional screening (25) bearing in mind that it may better unveil the existence of thresholds of responses for d-gal, modeled by sex and dose factor effects. Thus, we used a battery of 12 classical behavioral tests for rodents, which would provide a wide array of variables with convergent validity to assess a triad of physical–emotional–cognitive domains. Both sexes were studied as two plausible biological scenarios because little is known about sex-dependent vulnerability to d-gal. To explore the effects of d-gal dose-response on the cross talk of the neuro-immunoendocrine network and the impact of sexual dimorphism in their functional interplay, the behavioral and functional screening was complemented with the measurement of indicators related to the aging of the immuno-endocrine functions (26). Thus, the involution of thymus, the increase in the size of spleen and adrenal glands, and changes in the plasma levels of glucocorticoids were also analyzed in all the behaviorally assessed animals.
Methods
Animals
Twenty-eight male and 30 female C57BL/6 mice from a breeding program established in our laboratory at Universitat Autònoma de Barcelona were used in this study. Animals were maintained under standard laboratory conditions of food (SAFE A004, Panlab, S.L., Barcelona, Spain) and water ad libitum, 22 ± 2°C, a 12-h dark/light cycle, 50%–60% humidity. Behavioral assessments were performed blind to the experiment, in a counterbalanced manner, under the approval of local policy 2481CEEAH/8700DMAH Generalitat de Catalunya, in accordance with Spanish legislation and the EU Directive (2010/63/UE) on “Protection of Animals Used for Experimental and Other Scientific Purposes.” The study complies with the ARRIVE guidelines developed by the NC3Rs and the aim to reduce the number of animals used.
Chronic d-Gal Administration and Experimental Design
At 4 months of age, male and female mice were injected s.c. for 56 days with saline, 50 or 100 mg/kg of d-gal (d-gal, Sigma, St. Louis, MO) in a mL volume of 10% of their body weight. For each sex, the experimental groups were as follows: S, saline; L, low dose 50 mg/kg; and H, high dose 100 mg/kg (n = 9–10/group).
Physical and Behavioral Assessments
At 6 months of age, the effects of the chronic d-gal treatment were behaviorally assessed in an extensive battery 12 of classical unconditioned tests measuring three main dimensions and functions: sensorimotor, emotional, and cognition.
Day 1. Corner Test and Open-Field Test
Animals were individually placed in the center of a clean standard home cage, filled with wood shave bedding. Neophobia was evaluated for 30 seconds as the number of corners visited, latency to realize the first rearing, and the number of rearings.
Immediately after, mice were placed in the center of an open-field (homemade woodwork, white box, 50 × 50 × 20 cm) and observed for 5 minutes. The sequence of behavioral events was recorded as follows: duration of freezing behavior, latency to leave the central square and that of entering the peripheral ring as well as latency and total duration of self-grooming behavior. Horizontal (crossings of 10 × 10 squares) and vertical (rearings with a wall support) locomotor activities were also measured. Bizarre behaviors observed in this test (stereotyped stretching, stereotyped rearing, backward movements, and jumps) were also measured. During the tests, defecation boli and urination were also recorded.
Day 2. Dark–Light Box Test
The DLB (Panlab, S.L., Barcelona, Spain) consists of a two-compartment box (black and dark, 27 × 18 × 27 cm; white and illuminated 20 W, 27 × 27 × 27 cm) connected by an opening (7 × 7 cm). The mice were placed into the dark compartment and observed for 5 min. Latency to enter into the lit compartment, number of entries, total time spent and distance covered as well as number of rearings and groomings in this compartment were noted. Risk assessment was measured by means of the latency and number of stretch attendances toward the lit area. Defecation boli and urination in each compartment were measured.
Days 3 and 4. T-maze Test
Two different paradigms were carried out in a T-shaped maze (two short arms of 25 × 8 cm and a long arm of 30 × 8 cm).
In the spontaneous alternation test, animals were placed inside the long arm of the maze with its head facing the end wall. The latencies of first movement, start walking, arriving to the intersection of the arms, and completing the exploration of the maze were recorded. Finally, defecation boli and urination were also recorded.
The working memory paradigm was used 24 hour later and consisted of two consecutive trials: one forced choice followed, 90 seconds later by one free choice (recall trial). The arm chosen by the mice and the time spent in each arm during the free choice were recorded. The choice of the already visited arm in the previous trial was considered as an error and the total number was calculated. Also, the time spent to explore the two arms of the maze was recorded to measure the exploratory efficiency. Finally, defecation boli and urination were recorded.
Day 4. Physical Status, Reflexes, Sensorimotor Function, and Motor Learning
Body Weight
Animals were weighted all through the treatment and at the beginning of each behavioral test. The contribution of WAT (intra-abdominal white adipose tissue) to total body weight was calculated as the percentage of the weight of WAT versus the total body weight.
Reflexes and Sensorimotor Tasks
Visual reflex and posterior leg extension reflex were measured three times by holding the animals by the tail and slowly lowering them toward a black surface. Motor coordination and equilibrium were assessed twice (20-second trials) in two consecutive rod tasks of increasing difficulty. The distance covered and the latency to fall off a wooden (1.3 cm wide) and a metal wire (1 cm diameter) rods (both, 1 m long) were recorded. The hanger test was used to measure prehensility and motor coordination by the distance covered, the number of elements of support, and the latency to fall. The animals were allowed to cling with their forepaws from the middle of a horizontal wire (2 mm diameter, 40 cm length, divided into eight 5-cm segments) for two trials of 5 seconds. A third trial of 60 seconds allowed to measure muscle resistance. All the apparatus were suspended 40 cm above a cage with beddings.
Rotarod Test
An automated 4-lane rotarod unit (Panlab, LE8200, Barcelona, Spain) was used to evaluate the motor coordination function. The rotarod unit consists of a rotating spindle (diameter 7.3 cm) and individual compartments (“lanes”) for each animal. The mice were placed on the rod and tested at two different rotation speeds (5 and 10 rpm). The length of time that each animal was able to stay on the rod at a given rotation speed and the total rotations were recorded. In all cases, the number of trial needed by the animals to be able to stay on the rotarod was noted. Once they achieved it, each task was performed twice to measure the distance covered (number of rotations). The results were averaged to obtain a single value for each mouse at a given rotation speed. The first task at a low speed (5 rpm) was performed in all animals and the second one at a faster speed (10 rpm) conducted only in animals that managed to stay on the rotarod in the previous task.
Days 5–10. Morris Water Maze Test
Mice were trained, four trials per day, spaced 30 minutes apart, to locate a platform (7 cm diameter) in a circular pool for mice (Intex Recreation Corp. CA; 91 cm diameter, 40 cm height, 25°C opaque water), located in a completely black test room. Mice were gently released (facing the wall) from one randomly selected starting point (E or W) and allowed to swim until they escaped onto the platform. On the first day a cue task (CUE, DAY 1) assessed the visual perceptual learning and memory of a visible platform, elevated 1 cm above the water level in the N position and indicated by a visible striped flag (5.3 × 8.3 × 15 cm). Extra maze cues were absent in the black painted walls of the room. During the next four consecutive days (PT1–PT4, DAY2–DAY5), the mice searched for a hidden platform which was located in the middle of the S quadrant. White geometric figures hung on each wall of the room were used as external visual clues. In all trials, mice failing to find the platform were placed on it for 10 seconds, the same period as the successful animals. On the last day, 1 hour 30 minutes after the last trial of the place learning task, a probe trial without the platform, was administered during 60 seconds to assess spatial memory for the previously trained platform location.
In all the learning tasks, the variables of time (escape latency), distance, and speed were also recorded by a computerized tracking system (Smart, Panlab S.L., Barcelona, Spain). The number of crossings over the removed platform position (annulus crossings), the time spent, the distance traveled in each quadrant, and the swimming speed were also analyzed.
Immunoendocrine Status
Mice were sacrificed and samples of about 1 mL of whole trunk blood were collected into heparinized tubes and centrifugated immediately at 10,000g for 2 minutes. The plasma obtained was stored at −20°C. Corticosterone content (ng/mL) was analyzed using a commercial kit (Corticosterone EIA Immunodiagnostic Systems Ltd, Boldon, UK) and ELISA EMS Reader MF V.29.-0. The size (weight in mg) and relative size (% vs. body weight) of spleen, thymus, and adrenal glands were recorded.
Statistics
Statistical analyses were performed using SPSS 17.0 software. All data are presented as mean ± SEM or percentage. To evaluate the effects of (S)ex and dose of d-gal (T)reatment, a 2 × 3 S × T factorial analysis design was applied. Differences were studied through multivariate general linear model analysis (mGLM), followed by post hoc Duncan’s test comparisons. Differences between (S)ex and (T)reatment × (t)ime interval interactions in the different Morris Water Maze tasks were analyzed by repeated-measures ANOVA. In all cases, statistical significance was considered at p < .05.
Results
The Dose of 100 mg/kg d-Gal Worsened Motor Performances, Mostly in Males
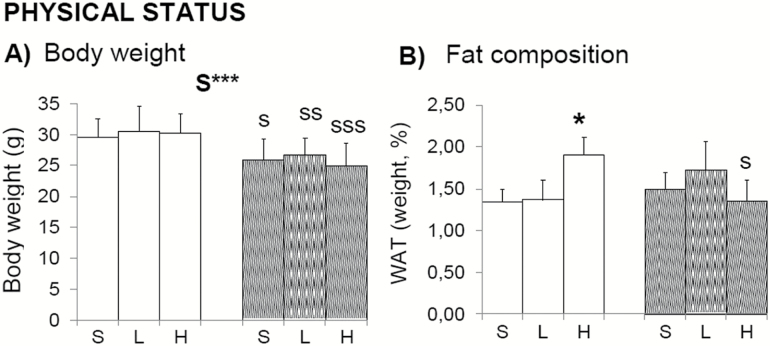
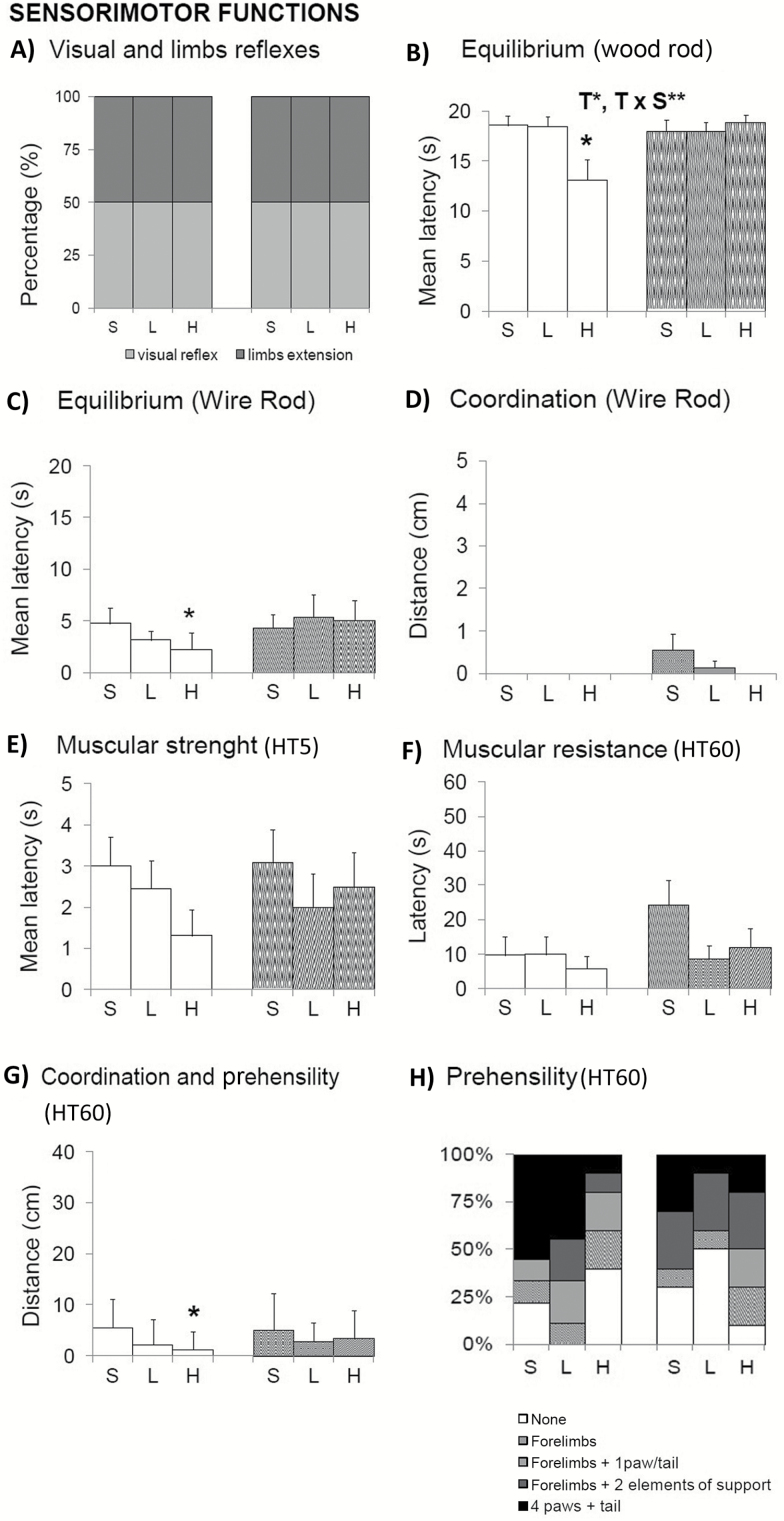
Physical status (Figure 1) and sensorimotor functions (Figure 2) pointed out sex-dependent effects induced by 100 mg/kg d-gal, with reduced equilibrium, muscular strength, and prehensility only in males. Because the total body weight of animals was not modified (Figure 1A), an increase in the percentage of WAT (Figure 1B; Student’s t test, p < .05 vs. controls) may have resulted in reduced muscular body composition (not measured) that could explain these poor performances. That would also explain the observed trend of reduced muscular strength (Figure 2E) and resistance (Figure 2F) in this group. Visual and limb extension reflexes were normal (Figure 2A).
Figure 1.
Effects of chronic d-galactose on the physical status of 6-month-old male and female C57BL/6. Body weight was in agreement with sexual dimorphism and pointed out sex-dependent effects induced by 100 mg/kg d-gal because increased fat composition was only shown in male in spite of being normo-weighted. Values are the mean ± SEM or percentage (%). n = 9–10 per group. Open bars: male animals; stripped bars: female animals. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: ANOVA 2 × 3: S, gender effect; ***p < .001. Post hoc Duncan’s test: sp < .05, ssp < .01. sssp < .001 versus the corresponding male group; *p < .05, versus the corresponding saline group.
Figure 2.
Effects of chronic d-galactose on the sensorimotor functions in 6-month-old male and female C57BL/6. (A–H) Sensorimotor functions pointed out sex-dependent effects induced by 100 mg/kg d-gal, with reduced equilibrium, muscular strength, and prehensility in normo-weight males. Values are the mean ± SEM or percentage (%). n = 9–10 per group. Open bars: male animals; stripped bars: female animals. HT5 and HT60: Hanger test 5-second and 60-second trials, respectively. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: ANOVA 2 × 3: S, gender effect; T, treatment effect; T × S, treatment × gender interaction effects; *p < .05, **p < .01, ***p < .001. Post hoc Duncan’s test: sp < .05, ssp < .01, sssp < .001 versus the corresponding male group; *p < .05, **p < .01 versus the corresponding saline group.
In the rod tests, d-gal-treated male showed a lower mean latency to fall than saline groups in the wood rod (Figure 2B, T, F(2,52) = 4.237; p < .05; T × S, F(2,52) = 5.014; p < .01). When the complexity of the task was increased using the metal wire, equilibrium worsens in all groups (Figure 2C). Among them, the male group treated with the high dose of d-gal showed the worse equilibrium, a difference that was statistically significant as compared with its saline control group (males H vs. males S; T, F(1,17) = 2.451; post hoc p < .05). Males did not cover any distance in the wire rod test, whereas female did it but suggesting dose-dependent lower abilities (Figure 2D). In the hanger test, the male group treated with 100 mg/kg d-gal also showed differences in the distance covered (Figure 2G, males H vs. males S, T, F(1,17) = 2.109; p < .05) as a result of the use of different elements of support (hind limbs and tail) and their motor coordination ability (Figure 2H).
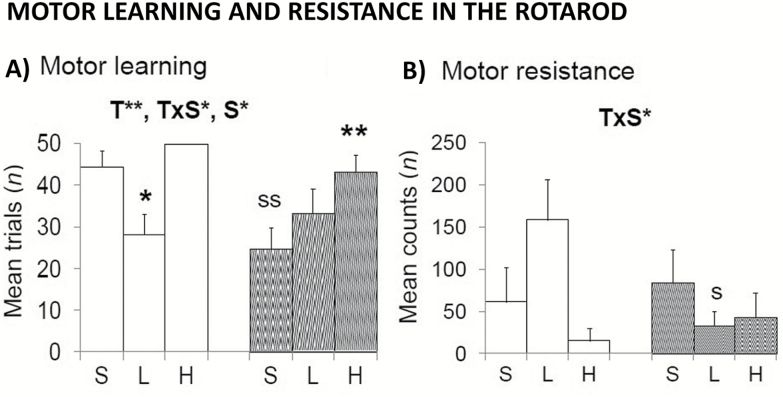
The Dose of 50 mg/kg d-Gal Improved Motor Learning in Males Whereas a Worse and Dose-Dependent Response Was Shown in Treated Females
The effects of d-gal on balance, motor coordination, and learning were also tested on an accelerating rotarod (Figure 3). Sex (S, F(1,52) = 3.959; p < .05), treatment (T, F(2,52) = 7.261; p < .01), and sex × treatment interaction effects (T × S, F(2,52)=3.929; p < .05) were found in the number of training trials needed to learn to walk on the lane (Figure 3A). Similarly, sex × treatment interaction effects were shown as the distance traveled once the task was learned (Figure 3B, T × S, F(2,52) = 3.536; p < .05). Males treated with the low dose showed increased motor abilities as compared to their saline group. Differences in balance and motor coordination were not influenced by body weight because the treatment did not modify it (Figure 1A; T and T × S; F(2,52) < 0.687; p > .508, ns).
Figure 3.
Effects of chronic d-galactose on motor learning in 6-month-old male and female C57BL/6 in the rotarod (A, B). Sex, treatment, and interaction effects in motor learning and resistance were observed. Male-treated 50 mg/kg d-galactose showed improved performances, reaching the level of performance shown by saline-treated female mice. In contrast, females treated with d-galactose required more trials and counts to meet the rotarod performance demands. Values are the mean ± SEM or percentage (%). n = 9–10 per group. Open bars: male animals; stripped bars: female animals. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: ANOVA 2 × 3: S, gender effect; T, treatment effect; T × S, treatment × gender interaction effects; *p < .05, **p < .01. Post hoc Duncan’s test: sp < .05, ssp < .01 versus the corresponding male group; *p < .05, **p < .01 versus the corresponding saline group.
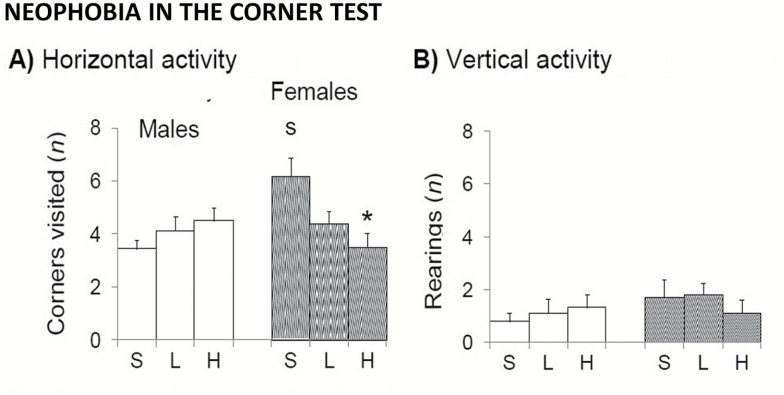
d-Gal Regulated Anxiety-Related Behaviors in a Sex-Dependent Manner
In the corner test for neophobia (Figure 4), saline-treated females visited more number of corners than control males (Figure 4A; t(1,17) = 2.318, p < .05). This sex difference disappeared in animals treated with d-gal. Thus, the treatment reduced the number of visited corners in females in a dose-dependent manner (both t(1,17) < 2.377, p < .05). The poor performances in vertical activity (Figure 4B) did not allow to detect these changes (T, F(2,52) = 0.142; p = .868; ns), or those as compared with males (S, F(1,52) = 1.250, p = .269; ns). The same results were obtained in the variable latency of the first rearing (not shown). In the case of males, all groups, independently of dose, showed the same level of neophobia as measured by horizontal and vertical activities in the corner test (Figure 4A and B).
Figure 4.
Sex-dependent effects of chronic d-galactose treatment in 6-month-old male and female C57BL/6 mice in the corner test. Values are the mean ± SEM. n = 9–10 per group. Open bars: male animals; stripped bars: female animals. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: Student’s t test: sp < .05 versus the corresponding male group; *p < .05 versus the corresponding saline group.
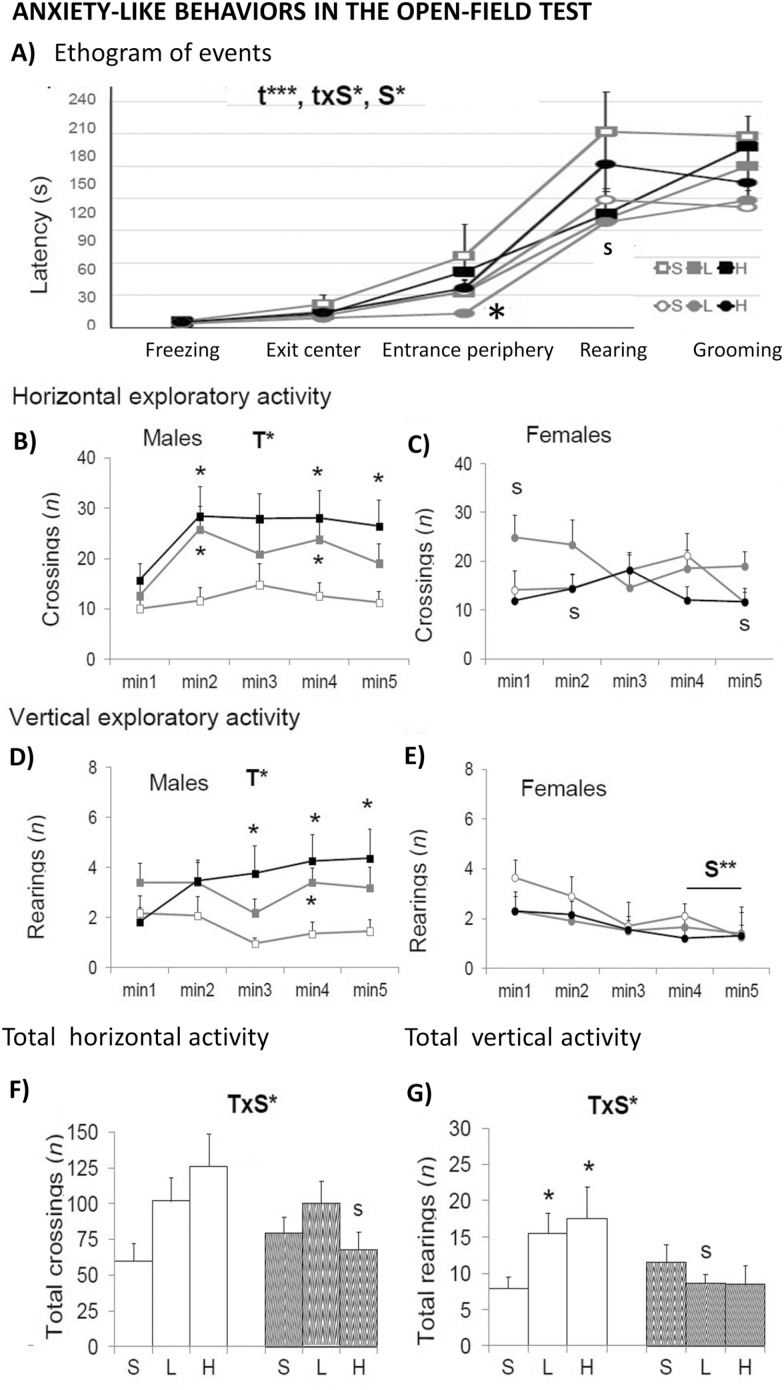
In the open-field test (Figure 5), the presence of bizarre behaviors showed sex differences with males showing a higher incidence than females (Table 1, S, F(1,52) = 22.471; p < .001). Here, the neophobia response exhibited in the first minutes was modified by d-gal in both sexes. In addition, the low dose of d-gal reduced the incidence of most of these bizarre movements in females (Table1; T × S, F(2,52) = 6.753; p < .01). The ethogram or sequence of behavioral events (Figure 5A) showed time, Sex and time × Sex interaction effects, with the females treated with 50 mg/kg, showing shorter times of entrance into the periphery that may explain the reduction of stereotyped behavior and their increase in the number of crossings during the first minute of the test (Figure 5B). Self-grooming behavior was advanced in females (Table 1; S, F(1,52) = 11.202; p < .01), independently of the treatment. Finally, a T × S interaction effect found in males reflected an increase in both the total horizontal (Figure 5F; T × S, F(2,52) = 3.216; p < .05) and vertical activities (Figure 5G; S × T, F(2,52) = 3.233; p < .05).
Figure 5.
Sex-dependent effects of chronic d-galactose treatment in 6-month-old male and female C57BL/6 mice in the open-field test. Values are the mean ± SEM. n = 9–10 per group. Open bars: male animals; stripped bars: female animals. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: ANOVA 2 × 3: S, gender effect; T, treatment effect; T × S, treatment × gender interaction effects; t, time effect, t × S, time × gender interaction effect; *p < .05, **p < .01, ***p < .001. Post hoc Duncan’s test: sp < .05, ssp < .01, sssp < .001 versus the corresponding male group; *p < .05, **p < .01 versus the corresponding saline group.
Table 1.
Effects of Chronic d-Galactose Treatment on Bizarre Behaviors and Emotionality in 6-Month-Old Male and Female C57BL/6 Mice
| Behavioral Variables Recorded at the OF and the DLB Tests | Male C57BL/6 Mice | Female C57BL/6 Mice | |||||
|---|---|---|---|---|---|---|---|
| Saline (n = 9) | d-Galactose 50 mg/kg (n = 9) | d-Galactose 100 mg/kg (n = 10) | Saline (n = 10) | d-Galactose 50 mg/kg (n = 10) | d-Galactose 100 mg/kg (n = 10) | Statistics | |
| Bizarre behaviors at OF | 100 | 100 | 100 | 100 | 50 | 70 | S*** T** SxT** |
| Head streaching (incidence, %) | 77.78 | 77.78 | 40 | 50 | 30 | 60 | ns |
| Backward movements (incidence, %) | 55.56 | 33.33 | 40 | 20 | 10 | 20 | S* |
| Stereotyped rearings (incidence, %) | 100 | 100 | 100 | 90 | 40 | 70 | S*** |
| Stereotyped rearings (n) | 8.7 ± 2.3 | 9.3 ± 2.4 | 3.9 ± 1.2 | 8.0 ± 2.5 | 2.8 ± 1.3 | 4.1 ± 1.7 | T** |
| Jumping (incidence, %) | 11.1 | 22.22 | 0 | 22.22 | 0 | 0 | ns |
| Total bizarre movements (n/maximum) | 22/36 | 21/36 | 18/40 | 18/40 | 8/40 | 15/40 | S*** |
| Self-grooming behavior at OF and DLB | |||||||
| Self-grooming at OF (latency, s) | 177.7 ± 18.7 | 150.1 ± 14.0 | 168.3 ± 12.0 | 111.7 ± 15.6 | 118.2 ± 18.3 | 134.6 ± 16. 6 | S** |
| Self-grooming at OF (s) | 3.7 ± 0.6 | 5.0 ± 1.1 | 2.9 ± 0.3 | 6.6 ± 1.4 | 4.5 ± 0.8 | 4.3 ± 0.5 | ns |
| Self-grooming at DLB (latency, s) | 300.0 ± 0.0 | 259.4 ± 18.5 | 275.7 ± 18.9 | 300.0 ± 0.0 | 300.0 ± 0.0 | 300.0 ± 0.0 | S*** |
| Self-grooming at DLB (s) | 0.0 ± 0.0 | 1.2 ± 0.6 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ns |
| Defecation behavior at OF and DLB | |||||||
| Defecation boli at OF (n) | 2.3 ± 0.5 | 2.0 ± 0.4 | 2.5 ± 0.3 | 1.9 ± 0.3 | 2.5 ± 0.4 | 2.4 ± 0.4 | ns |
| Defecation boli at DLB (n) | 2.1 ± 0.5 | 2.2 ± 0.7 | 1.8 ± 0.4 | 2.4 ± 0.5 | 1.5 ± 0.4 | 2.0 ± 0.4 | ns |
| Defecation boli in the lit area DLB (n) | 0 ± 0 | 0.4 ± 0.2 | 0. 7 ± 0.3 | 1 ± 0.5 | 0.3 ± 0.2 | 0 ± 0 | ns |
| Urination at OF and DLB | |||||||
| Urination at OF (incidence, %) | 55.56 | 22.22 | 20 | 50 | 30 | 30 | ns |
| Urination at DLB (incidence, %) | 22.22 | 22.22 | 10 | 10 | 50 | 40 | ns |
| Urination in the lit area DLB (incidence, %) | 0 | 20 | 0 | 0 | 30 | 0 | ns |
Note: DLB, dark–light box; OF, open field. Data are expressed by mean ± SEM or percentage (%). Statistics: ANOVA 2 x 3: S, sex effect; T, treatment effect; S x T, gender x treatment interaction effect; *p < .05, **p < .01, ***p < .001. Post-hoc Duncan’s test: sp < .05, ssp < .01, sssp < .001 vs. the corresponding male group; *p < .05, ***p < .001, the corresponding saline group.
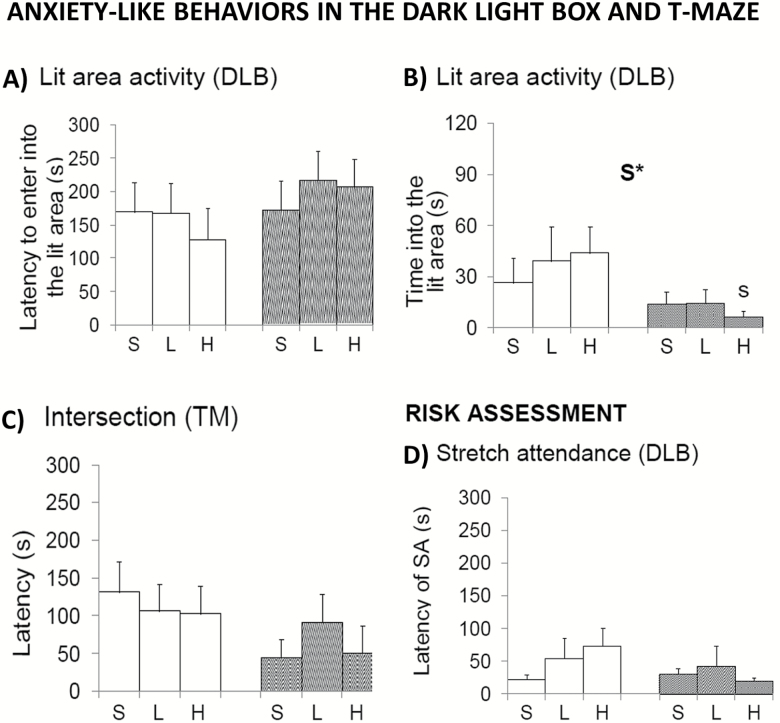
In the dark-light box (Figure 6), females were less active than males in the dark compartment (not shown; S, F(1,52) = 5.962; p < .05). The stretch attendance for risk assessment was similar among all groups (Figure 6D), as well as the latency (Figure 6A) and the number of entries in the lit compartment (not shown). However, females spent less time in the area and lit area (Figure 6B; S, F(1,52) = 6.054; p < .05). There were no significant differences in defecation and urination (Table 1).
Figure 6.
Sex-dependent effects of chronic d-galactose treatment in 6-month-old male and female C57BL/6 mice in the dark-light box test. Values are the mean ± SEM. n = 9–10 per group. Open bars: male animals; stripped bars: female animals. S = saline, L = low dose, 50 mg/kg; H = high dose, 100 mg/kg. Statistics: ANOVA 2 × 3: S, gender effect; *p < .05. Post hoc Duncan’s test: sp < .05 versus the corresponding male group.
In the T-maze (Figure 6C), all animals showed similar latencies in achieving the criterion of the spontaneous alternation task (all F’s (1,52) < 1.692; p > .05). In the working memory test, including the forced trial and the recall trial, all groups spent the same time to reached the criterion of exploration (all F’s(1,52) < 2.665; p > .05, not shown), although not all the animals completed the task. The high percentage of animals that did not complete the test precludes statistical analysis of this paradigm.
Worse Acquisition in Learning and Memory Tasks in Females Treated With d-Gal but Improved Performances in the Recall for Memory
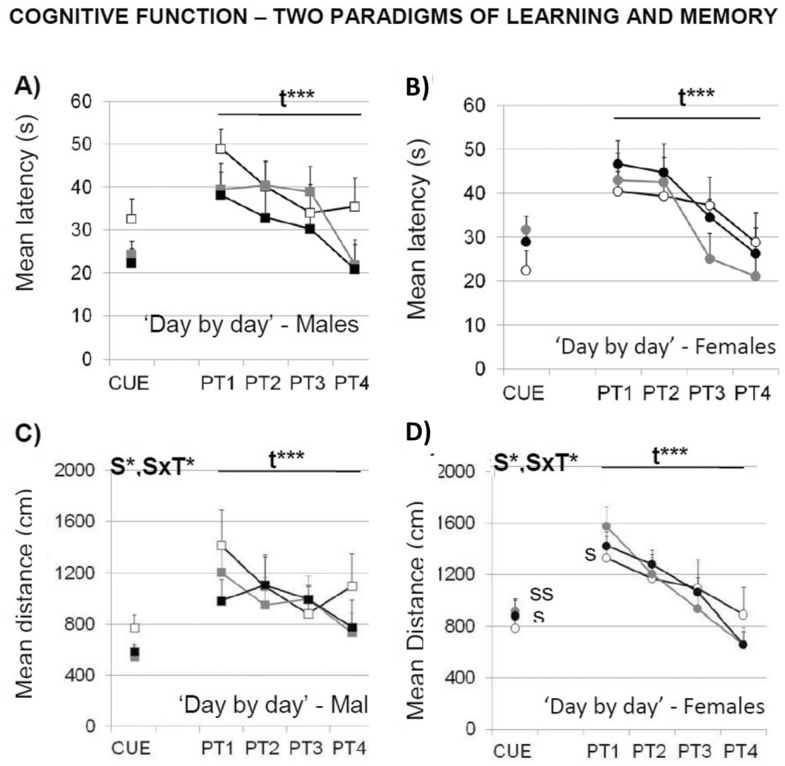
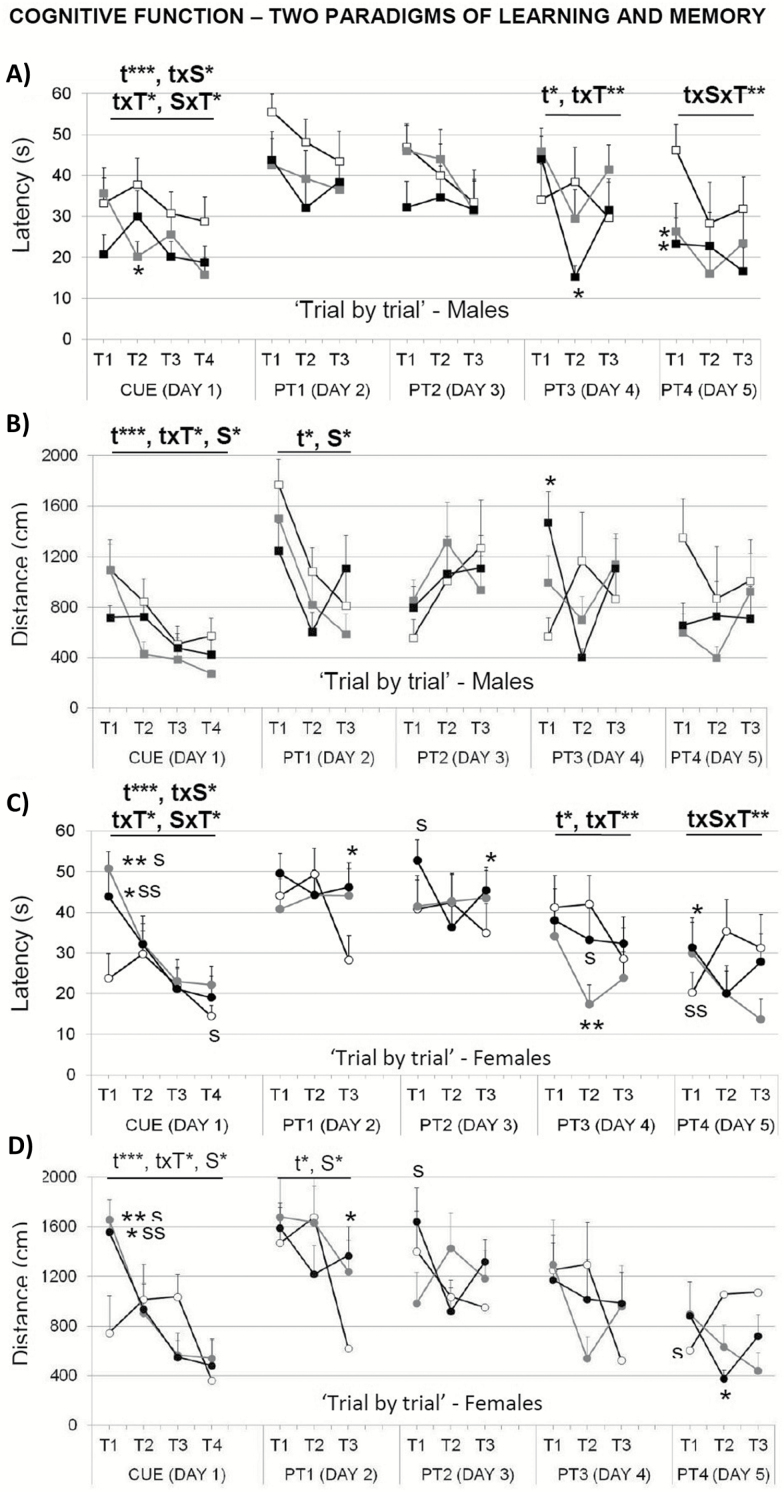
In the cue learning task, the variable distance revealed a sex effect and a “sex × treatment” interaction effect (Figure 7C and D; S, F(1,52) = 4.419; p < .05 and S × T, F(2,52) = 3.397; p < .05) not found using the mean escape latency (Figure 7A and B). A detailed “trial by trial” analysis detected differences in this task (Figure 8). In the first trial, which implies the first experience for the animals in the maze, females spent more time and covered longer distance to find the platform (Figure 8C and D; T1, S, both F’s(1,52) > 13.910; p < .001). This sex effect was mostly due to a treatment effect in females, the difficulty that both groups of d-gal-treated females exhibited to find the platform (Figure 8C and D; T1, T, F(2,52) > 10.469; p < .001).
Figure 7.
Effects of chronic d-galactose treatment in male and female mice at 6 months of age assessed “day by day” for perceptual visual learning and memory (CUE) and spatial reference learning and memory (PT1–PT4) in the Morris water maze. Data are expressed by mean ± SEM. Square: male animals; circle: female animals. White: saline, gray: 50 mg/kg d-galactose; black: 100 mg/kg d-galactose. ANOVA 2 × 3: t, time effect; S, gender effect; t × S, time × gender interaction; S × T, gender × treatment interaction; *p < .05, **p < .01, ***p < .001. Post hoc Duncan’s test: sp < .05, ssp < .01 versus the corresponding male group; *p < .05, **p < .01 versus the corresponding saline group.
Figure 8.
Effects of chronic d-galactose treatment in male and female mice at 6 months of age assessed “trial by trial” for perceptual visual learning and memory (CUE) and spatial reference learning and memory (PT1–PT4) in the Morris water maze. Data are expressed by mean ± SEM. Square: male animals; circle: female animals. White: saline, gray: 50 mg/kg d-galactose; black: 100 mg/kg d-galactose. ANOVA 2 × 3: t, time effect; S, gender effect; T, treatment effect; t × S, time × gender interaction; t × T, time × treatment interaction; S × T, gender × treatment interaction; t × S × T, time × gender × treatment interaction; *p < .05, **p < .01, ***p < .001. Post hoc Duncan’s test: sp < .05, ssp < .01 versus the corresponding male group; *p < .05, **p < .01 versus the corresponding saline group.
In the reversal place learning task, the time effect indicated the presence of a day-by-day acquisition curve for the two variables studied, that is, the mean latency (Figure 7A and B; repeated-measures ANOVA, t, F(3,156) = 26.716, p < .001) and the distance covered (Figure 7C and D; repeated-measures ANOVA, t, F(3,135) = 9.703, p < .001). Only the “trial by trial” analysis (Figure 8) that explores short-term learning and memory, allowed to find sex differences on the first day of the place task. There, females showed longer distance covered (Figure 8D; PT1, S, F(3,42) > 5.548, p < .05). Moreover, a time effect, a “time × treatment” and a “time × sex × treatment” interaction effects were found during the progression of the place task, when using the latency variable (Figure 8A and C; PT3, t, F(2,78) > 3.628, p < .05 and t × T, F(4,78) > 3.425, p < .01; PT4; t × S × T, F(4,78) > 3.681, p < .05). The level of acquisition reached at the end of the task was similar in all groups, independently of sex and treatment administered (Figure 8A and C; latency PT4, T3, all F’s(1,42) < 2.756; p > .05, ns; Figure 8B and D; distance covered, PT4, T3, all F’s(1,42) < 2.270; p > .05, ns). There were no differences in swimming speed, neither in the cue nor in the reversal place learning tasks (to simplify the figure, data are not shown).
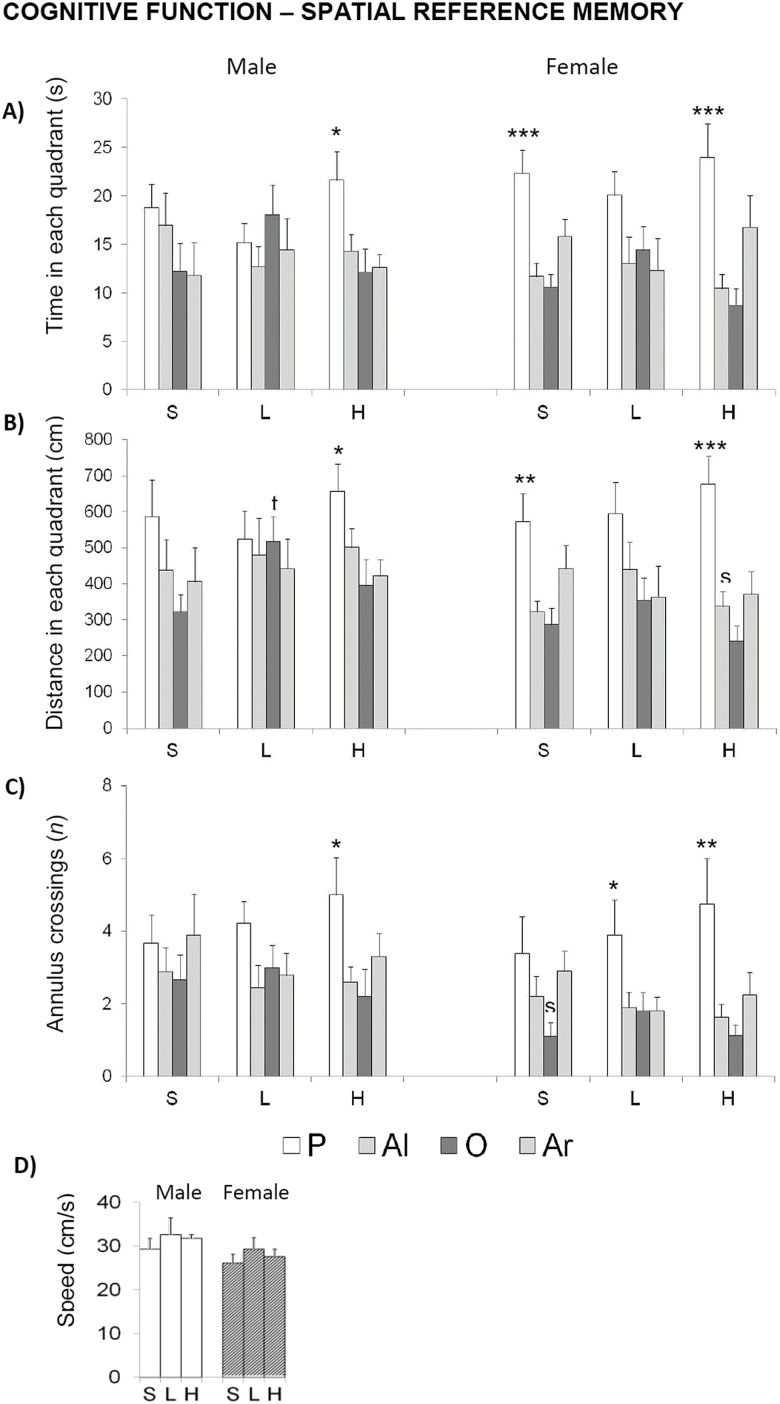
In the last trial, where the platform was removed (Figure 9), both sorts of animals treated with the high dose of d-gal showed a greater preference for the quadrant that contained the platform during the place task (Figure 9A, time and Figure 9B, distance in P vs. other quadrants; ANOVA, F > 4.127; p < .01) and a higher accuracy of the platform location as shown by the number of annulus crossings (Figure 9C; P vs. all other quadrants; ANOVA, F > 2.893; p < .05) compared with their respective saline groups. These differences were not attributable to differences in swimming speed (Figure 9D).
Figure 9.
Effects of chronic d-galactose on spatial reference memory in 6-month-old male and female C57BL/6 mice as assessed in the probe trial in the Morris water maze. Data are expressed by mean ± SEM by time in the Platform (P), Adjacent left (Al), Opposite (O), and Adjacent right (Ar) quadrants. S: saline, L: 50 mg/kg d-galactose; H: 100 mg/kg d-galactose. Statistics: between-group Student’s t test: sp < .05, versus the corresponding male group. Within groups, ANOVA, *p < .05, **p < .01, ***p < .001 P versus all the other quadrants.
d-Gal Induced Involution of Thymus in Males and Increased Corticosterone in Both Sexes
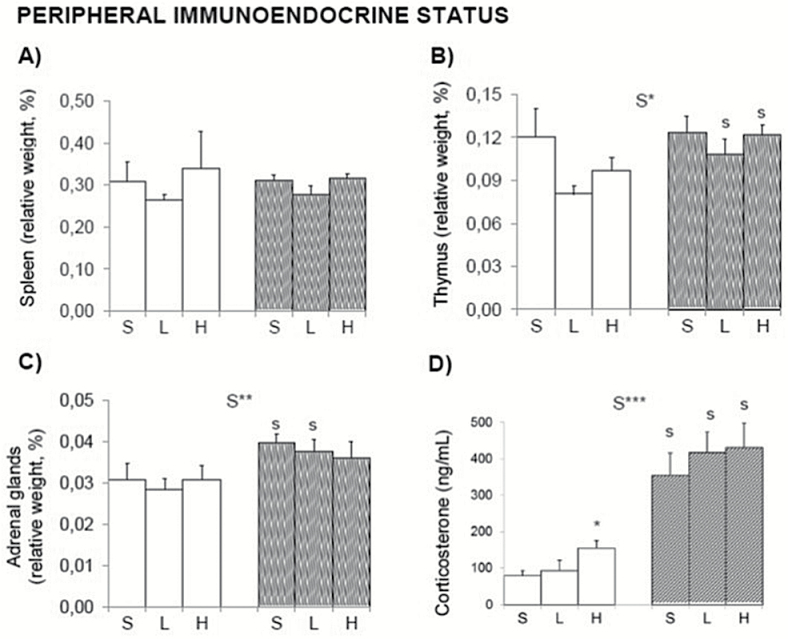
In control animals, sex differences were found in the size of adrenal glands (Figure 10C) and basal levels of corticosterone (Figure 10D). d-Gal substantially increased the corticosterone in males treated with the dose of 100 mg/kg. Both doses of d-gal induced a reduction of relative weight of thymus in males, although the statistical significance was shown as the appearance of sex differences that did not exist (Figure 10B). No differences were observed in the spleen (Figure 10A) although the modulation resembled that observed in the thymus.
Figure 10.
Effect of chronic d-galactose (L, 50 mg/kg; H, 100 mg/kg) on peripheral immune tissues, adrenal glands, and corticosterone content in 6-month-old male and female C57BL/6 mice. Data are expressed by mean ± SEM. (A) No differences were found in the relative weight of spleen, whereas (B) involution of thymus in the males leads to appearance of sex differences. In the endocrine system, the level of corticosterone was increased by 100 mg/kg in males, (D) whereas in females, the increased endocrine status was seen in the corticosterone versus weight of adrenal glands (D/C). Open bars: male animals; stripped bars: female animals. S = saline. ANOVA 2 × 3: sex effect; S*p < .05, S**p < .01. Post hoc Duncan’s test: sp < .05 versus the corresponding male group. Student’s t test, *p < .05 versus saline group.
Discussion
d-Gal-injected rodent models recapitulate many features of brain aging and have been extensively applied to study the mechanisms of brain aging (27,28). d-Gal aging model has been also widely used for antiaging pharmacology research (7,29,30). The administration of d-gal into animals can induce aspects of brain aging similar in many ways to human brain aging, as indicated by increased aging markers such as advanced glycation end product, receptors for advanced glycation end product, aldose reductase, sorbitol dehydrogenase, telomere length shortening, telomerase activity, beta-site amyloid precursor protein cleaving enzyme 1 (BACE-1), amyloid beta protein (Aβ1-42), senescence-associated genes (p16, p21, p53, p19Arf, p21Cip1/Waf1), senescence-associated betagalactosidase (SA-β-gal) staining (31,32), memory deficit, reduced neurogenesis, neurodegeneration, mitochondrial dysfunction and cytochrome C release, caspase-dependent neuronal apoptosis, raised oxidative stress, activation of astrocytes and microglia, BDNF deficiency, decrease in antioxidant enzymes, decreased ATP production, increased mitochondrial DNA mutation, impaired mitochondrial structure, and abnormal control of gene expression in the brain (18,32). In addition, it has been reported in previous studies that chronic administration of d-gal causes neurobehavioral changes including cognition and motor impairment in rodents (27,33). For example, it has been shown that during aging the cognitive functions such as memory are adversely affected, which may be due to the loss of neurons in specific brain regions (30). However, a number of results from neurobehavioral studies are providing evidence of heterogeneous and controversial results. Besides, in spite of relevance of sex on behavioral responses in health and disease, little is known about sex-specific vulnerability to d-gal.
This is the first comprehensive behavioral study that reveals distinct sex- and task-dependent vulnerability/protection threshold effects of a low (50 mg/kg) and high (100 mg/kg) dose of d-gal on the male and female C57BL/6 mice phenotype. It provides new experimental evidence to further discuss the controversial results in the literature regarding the use of d-gal to induce aging-related behavioral phenotypes and neuroimmunoendocrine premature aging.
In the last decade, critical reports have extensively regarded poor behavioral consistency as due to the differing variety of experimental designs, which may hinder the convergence of outcomes. Therefore, the experimental design of the present work chose the most used protocols and experimental conditions for subjects, age, dose, route of administration, and duration of treatment. Thus, C57BL/6 mice were chosen as the gold-standard wild-type strain, which also serves as genetic background of many mutant mice. The age of 6 months was used for adulthood, to avoid the strong influence of onset of reproductive age in young adulthood. The chronic treatment of d-gal described in the literature as a model of accelerated aging is based on the dose of 100 mg/kg. Regarding the duration of the treatment and route of administration, 8 weeks of continuous subcutaneous systemic injection was chosen as the most used protocol, reported to induce an aging model in mice (27). Finally, according to our research approach (25,26), the experimental design was based in a comprehensive screening of several physical, emotional, and cognitive functions successively assessed using a battery of 12 classical tests for these three main behavioral dimensions. Besides, the immunoendocrine status of animals was assessed as a measure of the process of aging (26).
Behavioral assessment was aimed to depict distinct levels of performance. Thus, we used tasks that enabled two levels of difficulty and functional abilities, that is, wood versus metal rod tests, and short versus long hanger tests for sensorimotor functions, as well as visual versus hidden place learning tasks in the water maze. Similarly, the impact of d-gal on the locomotor/exploratory and emotional profile was studied by not only confronting the animals with environments differing in anxiogenic conditions but also providing convergent validity to confirm changes in the anxious-like profile: The black narrow corridor of T-maze resembling natural burrows, the mild neophobia induced by a novel homecage in the corner test, the direct exposure to an open and illuminated field, and the choice between two compartments in the dark–light box test.
Mitochondrial dysfunction induced by chronic d-gal causes an increase in free-radical production in the brain, especially in cerebral cortex, hippocampus, and striatum, areas with high sensitivity to oxidative stress (34). Consequently, d-gal has been reported to cause a progressive decline in memory and learning functions and motor skills (8,27). Therefore, in the present project, cognitive tests involving learning and memory plasticity processes were chosen taking under consideration not only their temporal frames (working, short-term and long-term memories) but also the type of learning and memory processes and brain structures involved. Thus, the prefrontal cortex in the working memory in the T-maze, cerebellar motor learning in the rotarod test, while visual perceptual learning and hippocampal-dependent spatial learning and memory were assessed in the Morris water maze. Overall, the wide array of variables recorded contributed to the assessment of dimensions involving specific functions such as muscle and lower motor neuron function, spinocerebellar function, sensory function, neuropsychiatric function, and autonomic function (35). The work confirms our hypothesis that a comprehensive behavioral and functional screening would be able to point out thresholds of responses for d-gal, modeled by sex and dose factors effects. Also, that behavioral outcome items may reveal to which extent an impaired neuronal substrate allows the animal to meet task-dependent performance demands.
It is known that motor skills and, in particular, the coordination skills on the rotarod, decline with natural aging (36). The present results indicate that the dose of 100 mg/kg of d-gal mimics the effects of aging engines worsening balance and motor coordination of males assessed in the sensorimotor tests. Motor function in females evaluated in the rotarod was also significantly affected with the dose of 100 mg/kg. In addition, the dose of 50 mg/kg also showed a trend in the inability to stay on the rotarod in females. These results agree with previous studies, where it has been observed that chronic treatment of d-gal can accelerate these motor dysfunctions in young rats (8,19).
Current literature suggests a restricted window of aging-induced effects by chronic treatment with d-gal, mostly based on its impact on the motor system. Thus, laboratories using the model to assess neuroprotective effects of new antiaging drugs (ie, (37)) have reported that a standard 125mg/kg/day dose of d-gal (8 weeks) that induced serious impairment in grip strength in mice, left intact the animal’s spatial memory and locomotor coordination. The authors proposed that chronic exposure to d-gal results in specific muscular impairment in mice rather than causing general, premature aging. Other researchers did not identify impairment in motor coordination, open-field activity, or spatial memory in female mice treated during 6 weeks with 100 mg/kg d-gal (23). So, although some laboratories have achieved that animals treated with d-gal show cognitive deficits (16,17) and/or a decrease in motor skills (18), controversy exists because not all studies have been able to replicate them (23,29,37). This is also the case of the present work.
Surprisingly, in the cue learning task for perceptual visual learning, d-gal exerted positive effects in males, independently of the dose. Thus, they needed less time and covered less distance to reach the visible and cued platform with no differences in the navigation speed that could suggest involvement of an anxious-like performance. In contrast, treated females failed to approach the platform for the first time, but afterwards showed a good performance. These results may suggest sex-dependent differences in attention and/or motivation induced by d-gal.
In the place task, d-gal did not induce “day by day” treatment effects as shown by 24-hour acquisition curves for spatial reference learning and memory. However, the “trial by trial” analysis allowed to detect sex-dependent effects. Again, both groups of treated females showed worse performance in specific trials of this place task and treated males did better. Thus, treated females had worse ability to find the hidden platform for the first time and the short-term learning and memory process was poor as compared to controls. However, once the task was learned, d-gal enhanced the memory discrimination accuracy in the probe trial as indicated by the increase in the number of annulus crossings.
In the literature, the paradoxical results on learning and memory shown in d-gal-treated mice, as an aging model, have been attributed to the strain. Most studies reporting cognitive deficits used the Kunming strain (ie, (37–41)), whereas in C57BL/6, some authors have found cognitive deficits (ie, (27,42)), whereas others did not (23,29). Here, sex is also an important factor to take into account. A recent study examined the effects of chronic treatment of d-gal at different ages and in both sexes of C57BL/6 mice strain, showing cognitive deficits depending on the duration of treatment, together with sex and age factors (20). In particular, 2-month-old female mice injected with 100 mg/kg d-gal per day, for 6 weeks, did not show spatial memory impairment or high levels of hydroxyl radical, protein carbonyl, and malondialdehyde in brain homogenates, although brain-reactive oxygen species were increased when compared with saline control mice. In contrast, both age-matched male mice and 6-month-old female mice receiving 100 mg/kg d-gal for 6 weeks, or 2-month-old female mice receiving 100 mg/kg d-gal for 10 weeks showed spatial memory deficits and significant increases in the above oxidative markers, compared with their corresponding controls. However, these arguments do not explain the better performance in males and worse in females. In these cases, it may be wondered which is the role that anxiety levels may play in these performances and if the d-gal treatment could enhance them. In fact, in C57BL/6 mice anxiety level is considered an element that interferes with the execution and the performance in the Morris Water Maze tasks, with mild and high anxiety having opposed effects (43). However, this may not be the case because in these same treated animals the d-gal induced a modulation of neophobia that only was shown in no-escape anxiogenic environments (the corner and the open-field tests) but not in those that offer choices such as the dark-light box test or the T-maze.
We also provide first evidence on the effects of d-gal on bizarre behaviors related to neophobia. The ethogram of events explains that a reduced latency of thigmotaxis may account for the reduction of their expression, at least in females. Thus, it may be important to note distinct effects of d-gal on flight/fight behaviors depending on the sex. In males, the main effect was the dose-dependent increase in horizontal and vertical exploratory activity in the open field, which is opposed to what should be expected in an induced aging (2). A trend of increased total locomotor activity and thigmotaxis (inner zone) of the open field has been recently reported in young male Wistar rats treated with 300 mg/kg d-gal (24). As before, the results obtained with d-gal in the open field may vary depending on the strain used (23) because anxiety levels exhibited in this test are strongly determined by this factor (44). This would also explain why certain studies differ in the capacity to show that d-gal induced changes (ie, (30,38,44–46)) or not (37,47) in exploratory behavior in the open field.
Finally, impaired neuroimmunoendocrine status induced by 100 mg/kg d-gal in males monitored as the involution of thymus and the increase of peripheral levels of corticosterone in this sex would support an accelerated-aging effect at this level (26). Similar sex-dependent neuroimmunoendocrine aging status in males have been reported in our laboratory in an animal model of Alzheimer’s disease (48).
In summary, the dose of 100 mg/kg of d-gal worsened the balance in the rod tests in males and motor learning in the rotarod in both sexes, emulating the effects of aging on the motor system. In males, this worse motor performance, together with the increased endocrine response and involution of thymus, supports aging effects induced by 100 mg/kg d-gal in this sex. The increased exploration in the open field, improvement in cerebellar-depending motor tasks such as those assessed in the rotarod, and the improvement in hippocampal-and striatal-dependent spatial learning in the water maze suggest distinct neuroanatomical effects of not only 100 mg/kg but also 50 mg/kg in males. However, females seemed to be more sensitive to d-gal because worse motor learning in the rotarod and deficits in some specific abilities in spatial learning in the water maze were already observable in the group treated with 50 mg/kg d-gal.
Overall, the comprehensive behavioral analysis points at distinct sex-dependent thresholds of sensitivity in the functional capacity of treated animals to meet task-dependent performance demands. The present work provides evidence of d-gal exerting aging effects at the sensorimotor level and in the immunoendocrine system in males, whereas females show the impaired performances in the motor and spatial learning and memory processes depending on hippocampus and cerebellum. A low dose of 50 mg/kg seems to trigger positive effects in males, whereas low dose of 50 mg/kg follows a behavioral dose-response in females. Neuronal substrates underlying these observations and the neuroimmunoendocrine cross talk are interested to be further studied to elucidate the subjacent mechanisms and to understand the observed differential vulnerability/protection induced by d-gal in male and female adult C57BL/6 mice.
Funding
The work has been supported by Instituto de Salud Carlos III – Subdirección General de Evaluación y Fomento de la Investigación (AES-PI10/00283), co-financed by the European Regional Development Fund (ERDF), 2014-SGR1354 and ArrestAD H2020 Fet-OPEN-1-2016-2017-737390. R.B.C. received a predoctoral grant FI-DGR (2012FI_B1 00198) from Secretaria d’ Universitat i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya.
Conflict of Interest
None reported.
References
- 1. Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Women Health. 1987;12:103–145. doi:10.1300/J013v12n02_07 [DOI] [PubMed] [Google Scholar]
- 2. De la Fuente M, Giménez-Llort L. Models of aging of neuroimmunomodulation: strategies for its improvement. Neuroimmunomodulation. 2010;17:213–216. [DOI] [PubMed] [Google Scholar]
- 3. Li MY, Dicks DW. Enhanced binding of advanced glycation end products (AGE) by the ApoE4 isoform links the mechanism of plaque deposition in Alzheimer’s disease. Neurosci Lett. 1997;226:155–158. [DOI] [PubMed] [Google Scholar]
- 4. Zhang X, Zhang ZB, Yang XP, Li L, Gao WL. Disturbance of operational memory retention and retrieval in rats induced byd-galactose. Chin J Appl Physiol. 1996;12:377–379. [Google Scholar]
- 5. Kaviani E, Rahmani M, Kaeidi A, et al. Protective effect of atorvastatin ond-galactose-induced aging model in mice. Behav Brain Res. 2017;334:55–60. doi:10.1016/j.bbr.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 6. Acosta PB, Gross KC. Hidden sources of galactose in the environment. Eur J Pediatr. 1995;154(7 suppl. 2):S87–S92. [DOI] [PubMed] [Google Scholar]
- 7. Ho SC, Liu JH, Wu RY. Establishment of the mimetic aging effect in mice caused byd-galactose. Biogerontology. 2003;4:15–18. [DOI] [PubMed] [Google Scholar]
- 8. Lei M, Hua X, Xiao M, Ding J, Han Q, Hu G. Impairments of astrocytes are involved in thed-galactose-induced brain aging. Biochem Biophys Res Commun. 2008;369:1082–1087. doi:10.1016/j.bbrc.2008.02.151 [DOI] [PubMed] [Google Scholar]
- 9. Song X, Bao M, Li D, Li YM. Advanced glycation ind-galactose induced mouse aging model. Mech Ageing Dev. 1999;108:239–251. [DOI] [PubMed] [Google Scholar]
- 10. Wang Z. Physiologic and biochemical changes of mimetic aging inducedd-galactose in rats. Lab Anim Sci Admin. 1999;16:23–25. [Google Scholar]
- 11. Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF. Purple sweet potato color repairsd-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem. 2008;90:19–27. doi:10.1016/j.nlm.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 12. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi:10.1111/j.1600-079X.2004.00181.x [DOI] [PubMed] [Google Scholar]
- 13. Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype?J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi:10.1093/gerona/glq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byun K, Yoo Y, Son M, et al. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017;177:44–55. doi:10.1016/j.pharmthera.2017.02.030 [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Li S, Dong HP, Lv S, Tang YY. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci. 2009;85:127–135. doi:10.1016/j.lfs.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 16. Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced byd-galactose in ICR mice. J Neurol Sci. 2009;277:58–64. doi:10.1016/j.jns.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Q, Li X, Cui X, Zuo P. d-Galactose injured neurogenesis in the hippocampus of adult mice. Neurol Res. 2005;27:552–556. doi:10.1179/ 016164105X25126 [DOI] [PubMed] [Google Scholar]
- 18. Cui X, Zuo P, Zhang Q, et al. Chronic systemicd-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res. 2006;84: 647–654. doi:10.1002/jnr.20899 [DOI] [PubMed] [Google Scholar]
- 19. Banji D, Banji OJ, Dasaroju S, Kranthi KC. Curcumin and piperine abrogate lipid and protein oxidation induced byd-galactose in rat brain. Brain Res. 2013;1515:1–11. doi:10.1016/j.brainres.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 20. Hao L, Huang H, Gao J, Marshall C, Chen Y, Xiao M. The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced byd-galactose in mice. Neurosci Lett. 2014;571: 45–49. doi:10.1016/j.neulet.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 21. Salkovic-Petrisic M, Osmanovic-Barilar J, Knezovic A, Hoyer S, Mosetter K, Reutter W. Long-term oral galactose treatment prevents cognitive deficits in male Wistar rats treated intracerebroventricularly with streptozotocin. Neuropharmacology. 2014;77:68–80. doi:10.1016/j.neuropharm.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 22. Tikhonova MA, Yu CH, Kolosova NG, et al. Comparison of behavioral and biochemical deficits in rats with hereditary defined ord-galactose-induced accelerated senescence: evaluating the protective effects of diosgenin. Pharmacol Biochem Behav. 2014;120:7–16. doi:10.1016/j.pbb.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 23. Parameshwaran K, Irwin MH, Steliou K, Pinkert CA. d-Galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res. 2010;13:729–735. doi:10.1089/rej.2010.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardoso A, Magano S, Marrana F, Andrade JP. d-Galactose high-dose administration failed to induce accelerated aging changes in neurogenesis, anxiety and spatial memory on young male Wistar rats. Rejuvenation Res. 2015;18:497–507. doi:10.1089/rej.2015.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giménez-Llort L, García Y, Buccieri K, et al. Gender-specific neuroimmunoendocrine response to treadmill exercise in 3xTg-AD mice. Int J Alzheimers Dis. 2010;2010:128354. doi:10.4061/2010/128354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pérez-Alvarez L, Baeza I, Arranz L, et al. Behavioral, endocrine and immunological characteristics of a murine model of premature aging. Dev Comp Immunol. 2005;29:965–976. [DOI] [PubMed] [Google Scholar]
- 27. Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of thed-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 2006;157:245–251. doi:10.1016/j.bbr.2004. 07.003 [DOI] [PubMed] [Google Scholar]
- 28. Hsieh HM, Wu WM, Hu ML. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated withd-galactose. Food Chem Toxicol. 2009;47:625–632. doi:10.1016/j.fct.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 29. Chang L, Liu X, Liu J, et al. d-Galactose induces a mitochondrial complex I deficiency in mouse skeletal muscle: potential benefits of nutrient combination in ameliorating muscle impairment. J Med Food. 2014;17:357–364. doi:10.1089/jmf.2013.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang XL, An LJ, Bao YM, Wang JY, Jiang B. d-Galactose administration induces memory loss and energy metabolism disturbance in mice: protective effects of catalpol. Food Chem Toxicol. 2008;46:2888–2894. doi:10.1016/j.fct.2008.05.032 [DOI] [PubMed] [Google Scholar]
- 31. Lu J, Wu DM, Zheng YL, et al. Ursolic acid attenuatesd-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb Cortex. 2010;20: 2540–2548. doi:10.1093/cercor/bhq002 [DOI] [PubMed] [Google Scholar]
- 32. Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role ofd-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol. 2018;101:13–36. doi:10.1016/ j.exger.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 33. Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S. Intranasal cerebrolysin attenuates learning and memory impairments ind-galactose-induced senescence in mice. Exp Gerontol. 2017;87(Pt A):16–22. doi:10.1016/j.exger.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 34. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi:10.1152/physrev.1998.78.2.547 [DOI] [PubMed] [Google Scholar]
- 35. Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. [DOI] [PubMed] [Google Scholar]
- 36. Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33:615–624. [DOI] [PubMed] [Google Scholar]
- 37. He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Neuroprotective effects of (−)-epigallocatechin-3-gallate on aging mice induced byd-galactose. Biol Pharm Bull. 2009;32:55–60. [DOI] [PubMed] [Google Scholar]
- 38. Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reversesd-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–260. doi:10.1016/j.bbr.2006.03.043 [DOI] [PubMed] [Google Scholar]
- 39. Luo Y, Niu F, Sun Z, et al. Altered expression of Abeta metabolism-associated molecules fromd-galactose/AlCl(3) induced mouse brain. Mech Ageing Dev. 2009;130:248–252. doi:10.1016/j.mad.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 40. Shan Q, Lu J, Zheng Y, et al. Purple sweet potato color ameliorates cognition deficits and attenuates oxidative damage and inflammation in aging mouse brain induced byd-galactose. J Biomed Biotechnol. 2009;2009:564737. doi:10.1155/2009/564737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiu CS, Deng JS, Hsieh MT, et al. Yam (Dioscorea pseudojaponica Yamamoto) ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced byd-galactose. Am J Chin Med. 2009;37: 889–902. doi:10.1142/S0192415X09007296 [DOI] [PubMed] [Google Scholar]
- 42. Yoo DY, Kim W, Lee CH, et al. Melatonin improvesd-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012;52:21–28. doi:10.1111/j.1600-079X.2011.00912.x [DOI] [PubMed] [Google Scholar]
- 43. Baeta-Corral R, Giménez-Llort L. Persistent hyperactivity and distinctive strategy features in the Morris water maze in 3xTg-AD mice at advanced stages of disease. Behav Neurosci. 2015;129:129–137. [DOI] [PubMed] [Google Scholar]
- 44. Lad HV, Liu L, Paya-Cano JL, et al. Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol Behav. 2010;99:301–316. doi:10.1016/j.physbeh.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 45. Xu XH, Zhao TQ. Effects of puerarin ond-galactose-induced memory deficits in mice. Acta Pharmacol Sin. 2002;23:587–590. [PubMed] [Google Scholar]
- 46. Zhang XL, Jiang B, Li ZB, Hao S, An LJ. Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced byd-galactose. Pharmacol Biochem Behav. 2007;88:64–72. doi:10.1016/j.pbb.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 47. Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced byd-galactose in mice. Food Chem Toxicol. 2010;48:626–632. doi:10.1016/ j.fct.2009.11.043 [DOI] [PubMed] [Google Scholar]
- 48. Giménez-Llort L, Arranz L, Maté I, De la Fuente M. Gender-specific neuroimmunoendocrine aging in a triple-transgenic 3xTg-AD mouse model for Alzheimer’s disease and its relation with longevity. Neuroimmunomodulation. 2008;15:331–343. doi:10.1159/000156475 [DOI] [PubMed] [Google Scholar]