Auxin may activate several transcription factors via GhARF2 and GhARF18 proteins to regulate cotton seed fibre cell initiation.
Keywords: Auxin, auxin response factor, cotton, gene expression, seed fibre initiation
Abstract
Auxin signalling plays an essential role in regulating plant development. Auxin response factors (ARFs), which are critical components of auxin signalling, modulate the expression of early auxin-responsive genes by binding to auxin response factor elements (AuxREs). However, there has been no comprehensive characterization of this gene family in cotton. Here, we identified 56 GhARF genes in the assembled Gossypium hirsutum genome. This gene family was divided into 17 subfamilies, and 44 members of them were distributed across 21 chromosomes. GhARF6 and GhARF11 subfamily genes were predominantly expressed in vegetative tissues, whereas GhARF2 and GhARF18 subfamily genes were highly expressed during seed fibre cell initiation. GhARF2-1 and GhARF18-1 were exclusively expressed in trichomes, organs similar to cotton seed fibre cells, and overexpression of these genes in Arabidopsis enhances trichome initiation. Comparative transcriptome analysis combined with AuxRE prediction revealed 11 transcription factors as potential target genes of GhARF2 and GhARF18. Six of these genes were significantly expressed during seed fibre cell initiation and were bound by GhARF2-1 and GhARF18-1 in yeast one-hybrid assays. Our results suggest that GhARF2 and GhARF18 genes may be key regulators of cotton seed fibre initiation by regulating the expression of several transcription factor genes. This study deepens our understanding of auxin-mediated initiation of cotton seed fibre cells and helps us in breeding better cotton varieties in the future.
Introduction
The phytohormone auxin plays a pivotal role in regulating many distinct aspects of plant development, including lateral root initiation, shoot elongation, embryogenesis, vascular growth, tropic development, and flowering and tissue architecture (Teale et al., 2006; Domagalska and Leyser, 2011). Auxin response factors (ARFs), which are important components of auxin signalling pathways, regulate the expression of auxin-responsive genes by directly binding to the auxin-responsive element (AuxRE, TGTCTC) in their promoter regions (Guilfoyle and Hagen, 2007). A typical ARF protein consists of three conserved domains: an N-terminal B3-type DNA-binding domain (DBD) that recognizes the AuxRE; a variable middle region (MR) that activates or represses the expression of auxin-responsive genes depending on its amino acid composition; and two C-terminal dimerization domains (CTDs) that are involved in the formation of homo- and heterodimers between ARFs and auxin/indole-3-acetic acid (Aux/IAA) proteins (Gray et al., 2001). The Aux/IAA proteins can act as repressors of ARF-mediated transcription by forming multimers with ARFs and recruit the co-repressor TOPLESS (TPL) and its family proteins (TPRs) through a typical EAR motif to prevent activation of auxin-responsive genes by activating ARFs (Leyser, 2018). It has been shown that ubiquitination of Aux/IAA proteins by the transport inhibitor response 1 (TIR1) subunit of the SCFTIR1/AFB ubiquitin ligase accelerates Aux/IAA protein degradation by the 26S proteasome (Kepinski and Leyser, 2005). Aux/IAA proteins bind to ARFs and inhibit their binding to downstream target genes. Thus, when the inhibition of ARFs by Aux/IAA proteins is released, they modulate the expression of downstream genes.
The functions of plant ARF proteins, which are encoded by a large gene family, have been identified by classical genetic approaches. In Arabidopsis thaliana, the first identified ARF gene was AtARF1. Mutations in AtARF1 were found to enhance the phenotype of the arf2 mutant, and AtARF1 might act with AtARF2 to control aspects of maturation and senescence (Ellis et al., 2005). Arabidopsis plants with loss of function of AtARF3 displayed defects in gynoecium development (Nishimura et al., 2005). Mutation of the AtARF5 gene caused abnormal vascular strand formation and influenced the development of the embryo axis (Hardtke and Berleth, 1998). The arf7 mutant showed normal responses to auxin in the root, but increased sensitivity to blue light in the hypocotyl in Arabidopsis (Harper et al., 2000). Loss of function of AtARF8 was found to disrupt auxin homeostasis and affect fruit development and hypocotyl elongation (Goetz et al., 2006). The AtARF8 gene was reported to act redundantly with AtARF6, which is the target gene of miR167, and arf6/arf8 double mutants had infertile buds and short stamen filaments in Arabidopsis (Nagpal et al., 2005). AtARF16 is involved in root cap cell differentiation, and gene expression is regulated by miR160 (Wang et al., 2005). The arf19 single mutant showed normal auxin responses in the hypocotyl, but reduced sensitivity in the root in Arabidopsis. Interestingly, the arf19/arf7 double mutant displayed strong auxin resistance and impaired hypocotyl and root development (Narise et al., 2010). In a previous study, transgenic rice expressing an OsARF1 antisense transcript displayed low vigour, stunted growth, sterility, and curled leaves, suggesting that the OsARF1 gene is pivotal for reproductive and vegetative development in rice (Attia et al., 2009). Another rice ARF gene, OsARF16, was found to control iron and phosphate deficiency responses by regulating auxin redistribution in rice (Shen et al., 2013). In tomato, the SlARF4 gene is involved in flower development and fruit set, growth, and ripening (Sagar et al., 2013; X. Zhang et al., 2015).
To date, ARFs have been widely characterized in several plant species. There are 19 ARF genes in sweet orange (Citrus sinensis) (S.B. Li et al., 2015), 24 in Medicago truncatula (Shen et al., 2015), 47 in banana (Musa acuminata L.) (Hu et al., 2015), 25 in the medicinal model plant Salvia miltiorrhiza (Xu et al., 2016), 15 in cucumber (Cucumis sativus) (Liu and Hu, 2013), 17 in Eucalyptus grandis (Yu et al., 2014), and 17 in Vitis vinifera (Wan et al., 2014). ARF genes have also characterized in several economically important crops; there are 31 members in maize (Zea mays L.) (Wang et al., 2012a), 25 in rice (Oryza sativa L.) (Wang et al., 2007), and 51 in soybean (Glycine max L.) (Ha et al., 2013). Cotton is the world’s most important natural textile seed fibre and is also an important oilseed crop. About 27 million metric tons of cotton seed fibre is utilized worldwide per year. Each cotton seed fibre is a single, phenomenally elongated epidermal cell of the cotton seed coat and, in upland cotton (Gossypium hirsutum), which accounts for 90% of the world’s cotton production, the seed fibre generally grows up to 30–40 mm in length. The quality and amount of cotton seed fibre depend mainly on two biological processes: seed fibre initiation [occurring about –3 to 3 days post-anthesis (DPA)], which determines the number of seed fibres present on each ovule; and seed fibre elongation, which determines the final length and strength of each seed fibre (Shi et al., 2006). The accumulation of the plant hormone IAA in the epidermis of cotton ovules was found to increase the number of lint seed fibres, resulting in a significant increase in seed fibre yield (Zhang et al., 2011). However, the mechanism linking auxin signalling to seed fibre growth is largely unknown.
In this study, we identified 56 GhARF genes in G. hirsutum and investigated their evolutionary relationships as well as gene structures to gain insight into the role of auxin signalling in seed fibre growth. Analysis of gene expression patterns revealed that genes in different subfamilies tend to have different expression patterns: GhARF2 and GhARF18 subfamily genes were predominantly expressed during cotton seed fibre initiation, whereas GhARF6 and GhARF11 genes were highly expressed in vegetative tissues. We selected one gene from the GhARF2 and GhARF18 subfamilies to study their function in detail. Ectopic expression of GhARF2-1 and GhARF18-1 in Arabidopsis significantly increased trichome initiation. Finally, we identified six potential target genes of GhARF2-1 and GhARF18-1. Our results suggest that GhARF2 and GhARF18 subfamily genes may play an important role in regulating cotton seed fibre initiation.
Materials and methods
Plant materials
Gossypium hirsutum (Xuzhou 142) and the seed fibreless (fl) mutant, discovered in the same cotton field in China (Zhang and Pan, 1992), were grown in a climate-controlled greenhouse with a 16 h light and 8 h dark cycle at 30 °C as previously reported (Zhang et al., 2017). Fresh cotton seed fibres were excised from bolls at various DPA, and leaves, flowers, stems, and roots were harvested at the indicated time points and then immediately frozen in liquid nitrogen.
RNA extraction and quantitative real-time (qRT-PCR) analysis
Plant materials frozen in liquid nitrogen were ground to a fine powder with a mortar and pestle using a previously described method (He et al., 2017). Total RNA was prepared using the PureLink™ RNA mini kit (Invitrogen, Lot no. 1687455) as previously described (Liu et al., 2015), and cDNA was reverse-transcribed from 5 μg of total RNA with the Superscript™ first-strand synthesis system (Invitrogen, Carlsbad, CA, USA). In qRT-PCR experiments, each result was obtained from three experiments using independent RNA samples. The reaction parameters were as follows: 95 °C for 5 min, followed by 38 cycles of 95 °C for 15 s and 55 °C for 25 s. Then a melting curve was generated from 65 °C to 95 °C. One-way ANOVA was performed using SigmaStat software. Primers used for qRT-PCR analysis are listed in Supplementary Table S5 at JXB online. The cotton ubiquitin gene UBQ7 was used as the internal control for each PCR experiment.
Sequence retrieval and phylogenetic analysis
The cotton and Arabidopsis genome sequences were obtained from the CottonGen website (https://www.cottongen.org) and TAIR 10 (http://www.arabidopsis.org/), respectively (Supplementary Table S6). Other plant genome sequences used in this study were downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). BLASTP with default parameters was used to identify further the ARF proteins with AtARF sequences as the queries based on homology search. The selected cotton ARF proteins were used for further identification of ARFs by searching the cotton database again. All the obtained sequences were sorted as unique sequences, and further protein domain searches were performed using InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan). Subsequently, HMMER software with default parameters and conserved ARF domains were used to search for ARF protein sequences. The genomic DNA and cDNA sequences of predicted genes were obtained from the three cotton genomes. Multiple sequence alignment of all identified ARF proteins was performed using ClustalX with default parameters (Thompson et al., 1997). A phylogenetic tree of deduced ARF amino acid or DNA sequences was constructed using the Neighbor–Joining algorithm with default parameters, with 1000 bootstrap replicates in MEGA 5.0 (https://www.megasoftware.net), respectively. The phylogenetic tree was subsequently visualized using TreeView1.6 (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
Gene structure and chromosomal mapping
The Gene Structure Display Server Program (http://gsds.cbi.pku.edu.cn/) was employed to draw the exon–intron structure of GhARF genes based on the full-length genome sequence and the corresponding coding sequences obtained as described above. The MEME program was used to identify conserved domains in GhARF proteins (Bailey et al., 2006). Chromosomal position information for all GhARFs was obtained from annotation files downloaded from the CottonGen website. We used Circos to draw the distribution of GhARF genes along the chromosomes from the top to the bottom (Zou et al., 2013). The MCScanX software was used to determine and analyse ARF gene synteny and collinearity (Wang et al., 2012b).
In vitro ovule culture and treatment with exogenous NAA
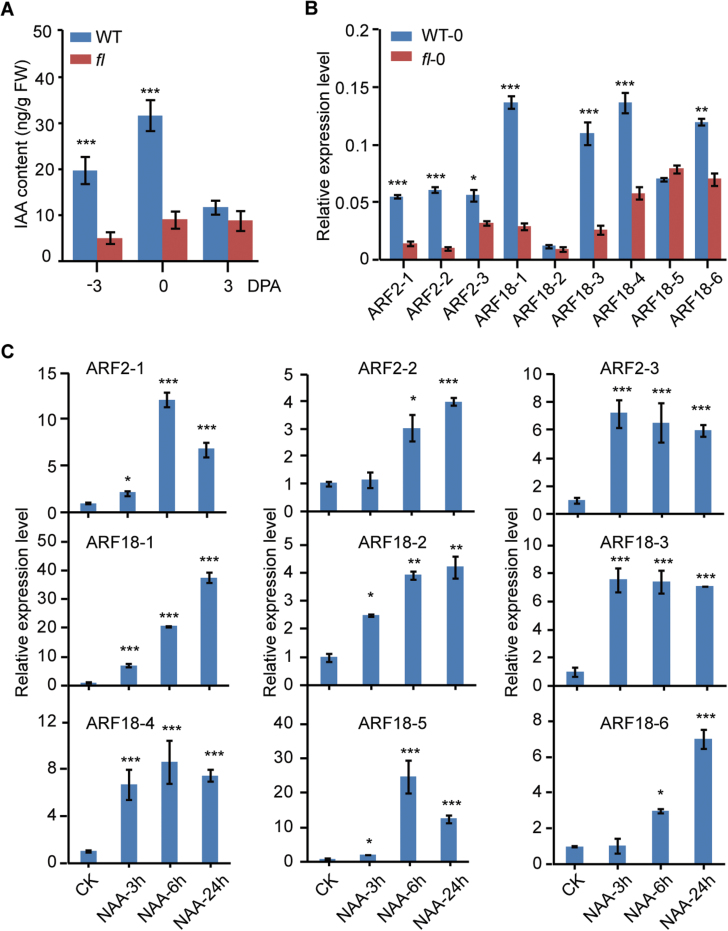
Developing cotton seeds were harvested at 1 DPA, soaked in 10% sodium hypochlorite for surface sterilization, and then rinsed eight times in distilled and deionized water. Ovules were excised from bolls, and 30 ovules were placed in a flask containing 20 ml of liquid ovule culture medium. The ovules were cultured at 30 °C in darkness without agitation as previously described (Xiao et al., 2016). Naphthalene acetic acid (NAA; 5 µM) was added to the culture medium for 3, 6, and 24 h. Finally, the cultured ovules were collected and prepared for the qRT-PCR experiment.
Vector construction, plant transformation, and GUS assay
The full-length GhARF2-1 and GhARF18-1 genes were amplified from cDNA by PCR with gene-specific primers listed in Supplementary Table S5. The PCR products were then cloned into the PQG110 vector driven by the constitutive Cauliflower mosaic virus 35S promoter. These constructs were introduced into Agrobacterium tumefaciens strain GV3101 and subsequently transformed into Arabidopsis plants (Columbia ecotype) using the floral dip method (Y. Zhang et al., 2015). Transgenic plants were selected on solid half-strength Murashige and Skoog (MS) medium plates containing 50 µg ml–1 chloramphenicol. The selected transgenic seedlings were further validated by genomic PCR. Histochemical β-glucuronidase (GUS) assays were performed as previously described (Wang et al., 2004). Samples were stored in 70% ethanol before microscopic observation.
IAA content analysis
IAA extraction was performed as previously described (Zhang et al., 2011). Cotton ovules harvested at –3, 0, and 3 DPA from the wild type (WT) and the fl mutant were immediately frozen and ground into powder in liquid nitrogen. IAA was extracted with 80% (v/v) methanol with 10 ng of [13C6]IAA as the internal standard. The supernatant was evaporated under vacuum, dissolved in water, and further purified on Sep-Pak Plus tC18 cartridges (Waters). The eluant was dried and then stored at −20 °C. IAA was analysed according to a previously described method (Gou et al., 2010).
Scanning electron microscopy
The observation of 0 DPA cotton ovules from the WT and the fl mutant was performed using a previously published method (Zhang et al., 2010). Ovule samples were dehydrated for 30 min each in a series of ethanol solutions (30, 50, 70, 90, and 10%), and then sputter-coated with silver using an iron sputter (Hitachi E-1020). The samples were observed using a scanning electron microscope (Hitachi S-3000N).
Yeast one-hybrid assay
The yeast one-hybrid (Y1H) assay was performed as described (Xu et al., 2017). Briefly, the ORFs of GhARF2-1 and GhARF18-1 were each constructed into the pGADT7 vector. The promoter sequences of 11 transcription factors were each inserted into the pHIS2 vector. Yeast strain (Y187) cells were transformed with a pGADT7 prey vector carrying the ORF of GhARF2-1 or GhARF18-1 and a constructed pHIS2 bait vector described above. The transformed yeast cell suspension was dropped on SD [DDO for –Trp/–Leu, and TDO for –Trp/–Leu/–His with or without 3-amino-1,2,4-triazole (3-AT)] medium plates and cultured at 30 °C for 5 d.
Accession number
The transcriptome data we used are available in the NCBI Sequence Read Archive (SRA) under accession number SRA180756.
Results
Identification of ARF genes in cotton
To identify ARF genes in G. hirsutum, we used 22 ARF protein sequences from Arabidopsis as queries in searches against the G. hirsutum genome database (F. Li et al., 2015; T. Zhang et al., 2015). After that, the screened GhARFs were used as queries to identify further other possible ARF proteins in G. hirsutum. All the candidate GhARF proteins from these step selections were subjected to further selection based on the presence of a conserved ARF domain identified using HMMER software. A total of 56 ARF protein-encoding genes were identified in G. hirsutum, including 27 genes originating from the At subgenome (where ‘t’ stands for tetraploid) and 29 from the Dt subgenome (Supplementary Table S1). The same methods were used to identify ARF genes in the whole-genome sequences of Gossypium arboreum and Gossypium raimondii (K. Wang et al., 2012; Paterson et al., 2012; Li et al., 2014). Each of these diploid cotton species contains 35 ARF genes. Notably, there are <70 ARF genes in allotetraploid G. hirsutum, indicating that gene loss occurred after polyploidization.
Phylogenetic analysis of the ARF gene family
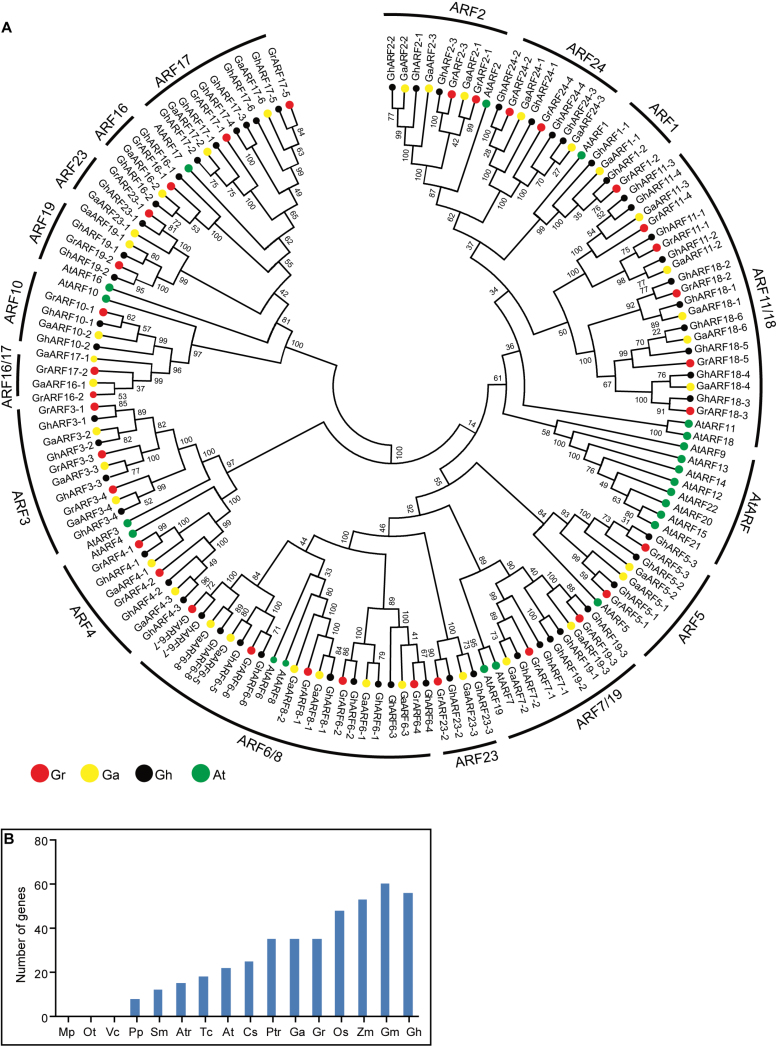
To investigate the evolutionary relationships between ARF genes, the ARF DNA or protein sequences from Arabidopsis, G. arboreum, G. raimondii, and G. hirsutum were used to generate an unrooted phylogenetic tree. ARF genes were classified into 17 subfamilies, and most of the orthologous genes between the allotetraploid and the corresponding diploids were clustered into the same clade based on phylogenetic analysis with ARF DNA sequences (Fig. 1A). According to the phylogenetic tree generated from ARF protein sequences, the protein sequences were divided into 16 subfamilies (Supplementary Fig. S1), which showed a similar result to gene analysis. Subsequently, we explored the evolutionary relationships of ARF genes with the phylogenetic tree generated from DNA sequences. Only one ARF6 and one ARF18 gene were found in Arabidopsis. However, the ARF6 and ARF18 subfamily genes have extensively expanded in G. hirsutum, which contained eight and six members, respectively. Interestingly, ARF9, ARF12, ARF13, ARF14, ARF15, ARF20, ARF21, and ARF22 were present in Arabidopsis but were absent from the three cotton species, indicating that these genes may have been acquired in Arabidopsis after divergence from the common ancestor of Arabidopsis and Gossypium. We searched for ARF genes in 16 species ranging from lower aquatic plants to higher terrestrial plants to determine the origin of the ARF gene. Based on this analysis, an ARF gene is present in Physcomitrella patens, but not in the algae Micromonas pusilla, Ostreococcus tauri, and Volvox carteri (Fig. 1B), suggesting that the ARF gene might have originated in moss. Notably, the number of ARF genes has dramatically increased in important economic crops.
Fig. 1.
Phylogenetic and evolutionary analysis of the ARF gene family in different plant species. (A) An unrooted phylogenetic tree of ARF DNA sequences from Arabidopsis thaliana, Gossypium arboreum, Gossypium raimondii, and Gossypium hirsutum. The phylogenetic tree was constructed using ARF DNA sequences and the Neighbor–Joining (NJ) method in MEGA 5.0 software. (B) Comparisons of ARF gene number across a wide range of plant species. Mp, Micromonas pusilla; Ot, Ostreococcus tauri; Vc, Volvox carteri; Pp, Physcomitrella patens; Sm, Selaginella moellemdorffii; Atr, Amborella trichopoda; Tc, Theobroma cacao; Cs, Cucumis sativus; Ptr, Populus trichocarpa; At, A. thaliana; Os, Oryza sativa Japonica; Gm, Glycine max; Zm, Zea mays; Ga, G. arboretum; Gr, G. raimondii; Gh, G. hirsutum.
Gene structure, conserved motif, and chromosomal location analysis
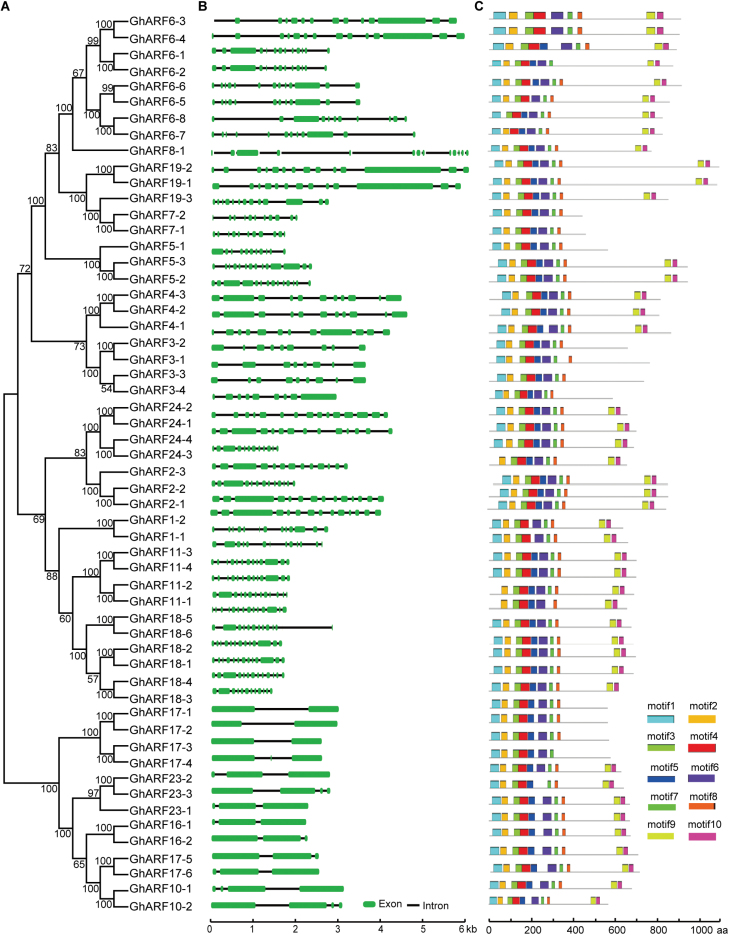
To better understand the evolutionary relationships between different members of the GhARF gene family, we constructed a separate unrooted phylogenetic tree with GhARF DNA sequences (Fig. 2A) and performed a comparative analysis of intron–exon structure. We found that GhARF gene length varied significantly, with GhARF18-1 having the longest (6.1 kb) and GhARF18-4 having the shortest (1.4 kb) genomic sequence. A highly rich distribution of introns was found in the GhARF gene regions. In general, GhARF genes possessed at least two exons and could be divided into two categories based on exon number (Fig. 2B). Forty-three genes had >10 exons, and 13 genes had 2–4 exons. Notably, closely related genes had more similar arrangements of exons and introns, indicating that exon–intron structure and the phylogenetic relationship between GhARF genes are highly correlated. Conserved motifs in the GhARF proteins were identified using MEME software. In total, 10 conserved motifs, named motif 1 to motif 10, were identified in GhARFs, and the number of conserved motifs in each GhARF varied from 7 to 10 (Fig. 2C). Most members had motifs 1, 2, 3, 4, 5, 6, 7, and 8, indicating that they are highly conserved among GhARFs.
Fig. 2.
Phylogenetic, gene structure, and motif analyses of GhARF genes. (A) Phylogenetic relationships between GhARFs. The phylogenetic tree (left panel) was constructed with MEGA 5.0 using the Neighbor–Joining (NJ) method with 1000 bootstrap replicates. (B) Gene structure analysis of GhARF genes. Gene structure maps were drawn with the Gene Structure Display Server 2.0 (Hu et al., 2015). The scale bar is shown at the bottom. (C) Motif analysis of GhARF genes. All motifs were identified by MEME software (http://meme-suite.org/). Lengths of each motif are shown proportionally.
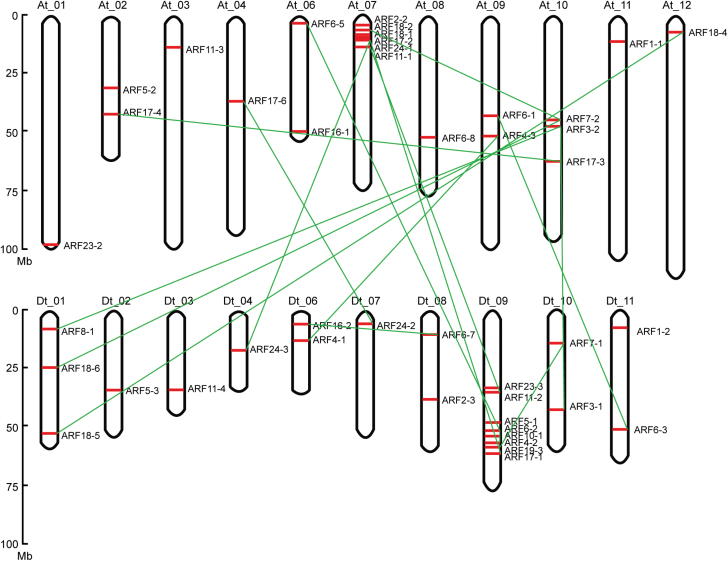
Using the complete G. hirsutum genome sequence (F. Li et al., 2015; T. Zhang et al., 2015), we investigated the chromosomal locations of GhARF genes. A total of 44 genes were distributed throughout the 21 chromosomes comprising 11 located on the At subgenome and 10 located on the Dt subgenome (Fig. 3). The highest numbers of GhARF genes were located on chromosome 7 from the At subgenome (six GhARF genes) and chromosome 9 from the Dt subgenome (eight GhARF genes). The other GhARF genes were found to be located in the scaffolds (Supplementary Table S2). We further investigated the evolutionary history of GhARF genes by conducting genome synteny analysis. Based on syntentic relationships, there are 15 pairs of putative paralogous GhARF genes. Forty-nine GhARF genes were produced by whole-genome duplication (WGD) and seven were produced by singleton duplication (Supplementary Table S3). The high proportion of GhARF genes derived from WGD indicates that these duplication events played a crucial role in GhARF gene expansion in the G. hirsutum genome.
Fig. 3.
Chromosomal distribution and gene duplication of GhARF genes. The genome visualization tool CIRCOS was used to illustrate the chromosomal distribution of GhARF genes. The chromosome number is shown above each chromosome. The chromosomal location of each GhARF is shown from the top to the bottom of the corresponding chromosome. Duplicated gene pairs are linked by lines. The scale bar beside the chromosome indicates the length in megabases (Mb).
GhARF2 and GhARF18 genes are expressed in ovules during fibre cell initiation
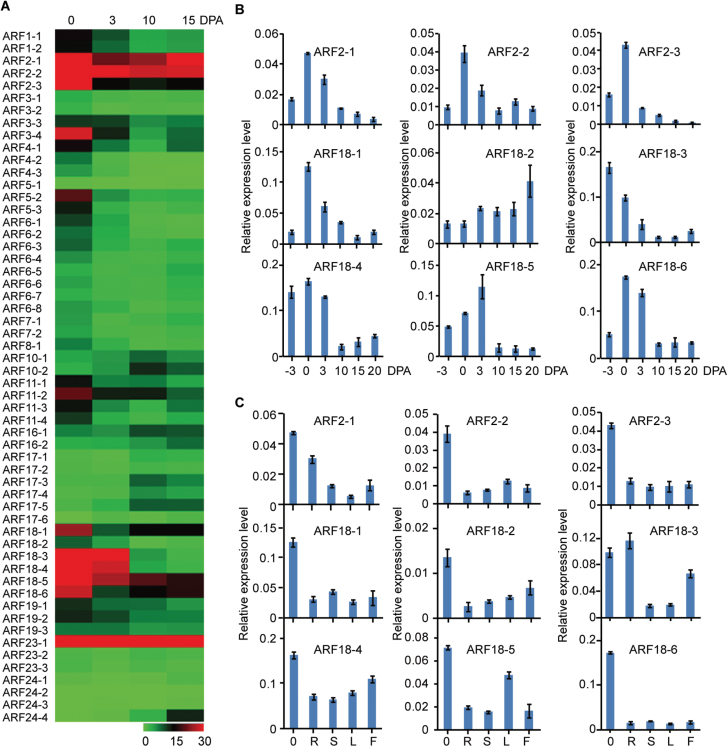
To determine which GhARF genes potentially function in seed fibre development, the expression patterns of individual members were investigated using transcriptome data for different stages of G. hirsutum seed fibre and ovule development. The majority of GhARF genes from the same subfamily had similar expression patterns. Notably, all members of the GhARF2 and GhARF18 subfamilies, except for GhARF18-2, were highly expressed in the 0 DPA ovules (Fig. 4A). In addition, GhARF3-4, GhARF11-2, and GhARF23-1 genes were dramatically expressed in 0 DPA ovules. These results suggest that these genes may function in cotton seed fibre cell initiation. The similar expression patterns of these genes suggest that they may have redundant functions, especially given that within each subfamily genes share a high degree of protein sequence identity. GhARF2-1 shares 98% identity with GhARF2-2 and 84% identity with GhARF2-3, and there is 98% identity between GhARF18-1 and GhARF18-2, 93% identity between GhARF18-3 and GhARF18-4, and 99% identity between GhARF18-5 and GhARF18-6. Genes from the GhARF2 subfamily were highly expressed in 0–15 DPA seed fibre cells, indicating that these genes play an important role in both seed fibre cell initiation and cell elongation. The transcript levels of GhARF2 and GhARF18 in seed fibre cells and vegetative tissues were analysed using qRT-PCR. We found that all GhARF2 and GhARF18 genes, except for GhARF18-2, were predominantly expressed in –3, 0, or 3 DPA cells which corresponds to seed fibre initiation (Fig. 4B, C). GhARF18-3 was highly expressed in both roots and seed fibre cells at the initiation stage (Fig. 4C).
Fig. 4.
Analysis of GhARF expression patterns during seed development and seed fibre initiation. (A) Heat map of the expression level of 56 GhARF genes during different stages of seed fibre growth based on RNA-seq data. (B) Expression profiling of seed and seed fibre initiation-specific GhARF2 and GhARF18 subfamily genes during seed and seed fibre development. Gene expression data were obtained by quantitative real-time PCR with three independent replicates. (C) Expression profiling of GhARF2 and GhARF18 subfamily genes in various tissues. 0, R, S, L, and F represent ovules at 0 DPA, root, stem, leaf and flower, respectively. Gene expression data were obtained by quantitative real-time PCR with three independent replicates.
To assess whether auxin plays an important role in seed fibre initiation, we quantified IAA accumulation and determined the expression levels of the GhARF2 and GhARF18 genes in the WT and the fl mutant. Large numbers of initiated seed fibre cells were observed in WT ovules, whereas no seed fibre cell initiation and differentiation was observed on the surface of fl ovules at 0 DPA (Supplementary Fig. 2). We further analysed the free IAA content in –3, 0, and 3 DPA ovules by LC and MS. A significant increase in IAA level was found in WT ovules relative to fl ovules, especially in –3 and 0 DPA ovules (Fig. 5A). On average, IAA levels in WT ovules were 298% of the level in fl ovules. The expression level of all GhARF2 and GhARF18 subfamily members, except for GhARF18-2 and GhARF18-5, was significantly higher in WT ovules than in fl ovules (Fig. 5B). The transcript levels of GhARF2 and GhARF18 genes increased significantly after the application of 5 µM NAA for 6 h (Fig. 5C).
Fig. 5.
GhARF2 and GhARF18 genes are responsive to auxin in fibre cell initiation. (A) Endogenous IAA levels in wild-type and fl mutant ovules during seed fibre initiation. The bar represents the SD for three samples. (B) The expression level of GhARF2 and GhARF18 subfamily genes in wild-type and fl mutant ovules at 0 DPA. (C) Exogenous NAA promotes the transcription of GhARF2 and GhARF18. Relative expression levels of each gene were determined after normalizing to the expression level in CK ovules, which was set to 1.0. Statistical significance was determined using one-way ANOVA combined with Tukey’s test. *P<0.05; **P<0.01; ***P<0.001.
Taken together, our results suggest that GhARF2 and GhARF18 genes may play important roles in cotton seed development and seed fibre cell initiation. In addition, auxin may enhance the expression of GhARF2 and GhARF18 genes to promote seed fibre cell initiation.
Overexpression of GhARF2-1 and GhARF18-1 stimulates trichome initiation in Arabidopsis
Trichomes in Arabidopsis are organs similar to cotton seed fibres (Zhang et al., 2018). Based on the analysis of RNA expression patterns, GhARF2 and GhARF18 genes are mainly expressed during seed development and seed fibre cell initiation. To investigate further the possible expression region of GhARF2 and GhARF18 genes, we selected GhARF2-1 and GhARF18-1 as examples and detected GUS activities in PGhARF2-1:GUS and PGhARF18-1:GUS Arabidopsis seedlings. GhARF2-1 promoter-driven GUS expression was mainly observed in the base of trichomes in the leaf epidermis, leaf veins, and sepals of flowers (Fig. 6A–D), and GhARF18-1 promoter-driven GUS expression was present exclusively in the base of trichomes in the leaf epidermis and emerging lateral roots (Fig. 6E–G).
Fig. 6.
GUS staining assay of the GhARF2-1 and GhARF18-1 gene promoters. (A–D) GUS staining of 14-day-old Arabidopsis seedlings expressing PGhARF2-1:GUS. GUS staining was observed in the base of the trichome, leaf veins, and sepals of flowers. Scale bars=2.5 mm in (A), 0.5 mm in (B), 0.25 mm in (C), and 1 mm in (D). (E–G) GUS staining of 7-day-old Arabidopsis seedlings expressing PGhARF18-1:GUS. GUS staining was observed in the base of the trichome (E) and emerging lateral roots (F,G). Insert in (E) shows a magnified leaf. Scale bars=5 mm in (E), 1 cm in (F), and 2.5 mm in (G).
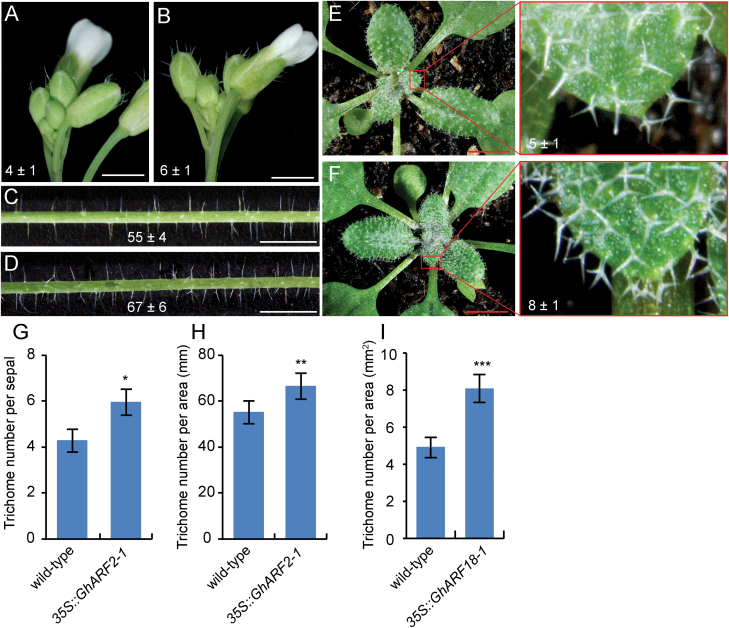
To explore further the functions of GhARF2 and GhARF18, we generated two transgenic Arabidopsis overexpression lines with GhARF2-1 or GhARF18-1 expression driven by the constitutive 35S promoter, and observed the trichomes, which are similar to cotton seed fibres. Compared with WT plants, the transgenic plants overexpressing GhARF2-1 showed an increase in the number of trichomes on the sepals of flowers (Fig. 7A, B) and main inflorescence stems (Fig. 7C, D). The density of trichomes per area in the 35S:GhARF2-1 transgenic lines increased to 141% and 121% of the level in the sepals of flowers and main inflorescence stems of WT plants, respectively (Fig. 7E, F). Moreover, 35S:GhARF18-1 transgenic plants had more trichomes on the leaves than WT plants (Fig. 7G, H). The number of trichomes in the 35S:GhARF18-1 lines increased to 164% of the level in the leaves of WT plants (Fig. 7I). These results indicate that there was some influence of these cotton genes on trichome initiation in the Arabidopsis transgenic plants.
Fig. 7.
Phenotypes of 35S:GhARF2-1 and 35S:GhARF18-1 transgenic Arabidopsis plants. (A) Trichome initiation on the sepals of wild-type flowers. (B) Trichome initiation on the sepals of 35S:GhARF2-1 flowers. (C) Trichomes on the main inflorescence stems of wild-type plants. (D) Trichomes on the main inflorescence stems of 35S:GhARF2-1 transgenic plants. (E, F) Comparison of the densities of trichomes in the sepal (E) and the main inflorescence stems (F) from the wild-type and 35S:GhARF2-1 transgenic plants. Each experiment was performed in three biological replicates, and the error bars represent the mean ±SE. *P<0.05; **P<0.01. (G) Trichomes on the leaves of wild-type plants. (H) Trichomes on leaves of 35S:GhARF18-1 plants. (I) Comparison of the densities of trichomes in the leaf from the wild-type and 35S:GhARF18-1 transgenic plants. Each experiment was performed in three biological replicates, and the error bars represent the mean ±SE. ***P<0.001. Scale bars=2.0 mm.
Identification of potential transcription factors regulated by GhARF
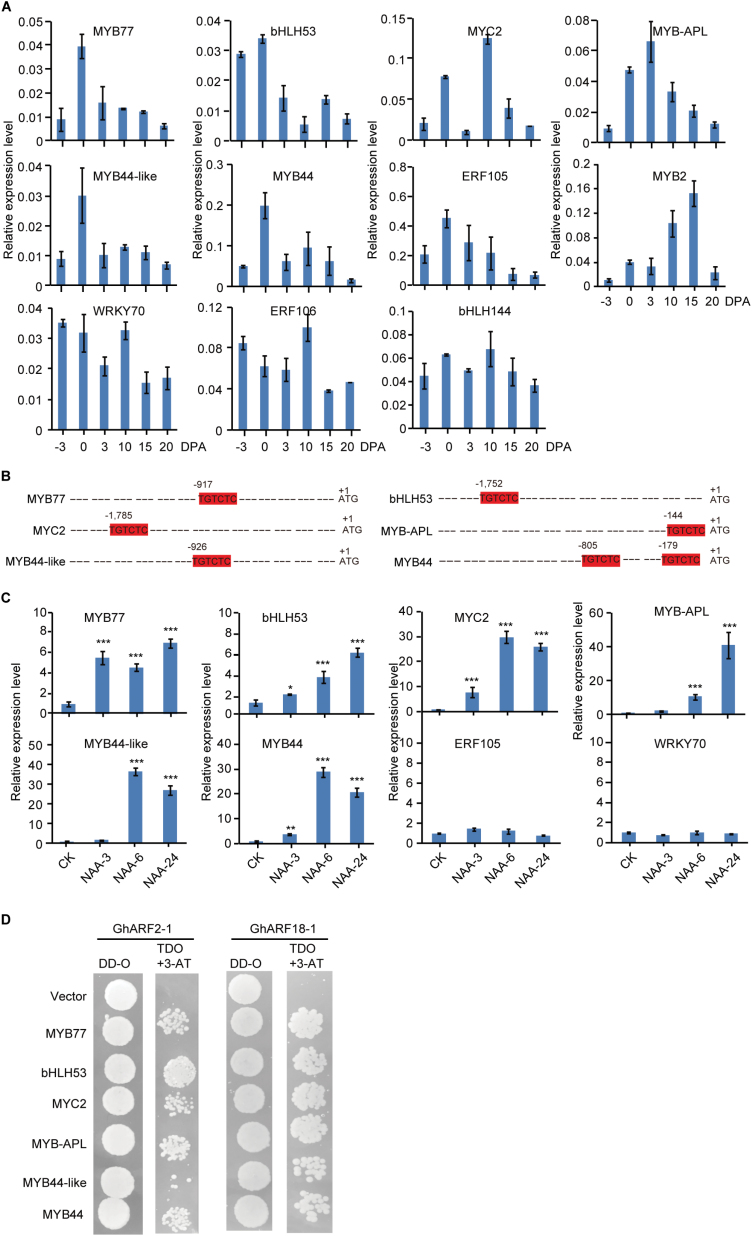
ARFs, which are essential components of auxin signalling pathways, bind specifically to the AuxRE to regulate the expression of target genes (Cho et al., 2014). In order to identify the potential transcription factors directly regulated by GhARF, we screened for transcription factors that were both highly expressed during the seed fibre initial stage and had at least one AuxRE in their promoter. First, we calculated the gene expression level in 0 DPA and 10 DPA samples. We found 3624 genes whose expression level at 0 DPA was over twice that at 10 DPA. We regarded these genes as high expression genes in 0 DPA. Secondly, we investigated the genes which contained at least one AuxRE in their promoter. As a result, we found 21 386 genes in which AuxRE elements were present in their promoter region. Finally, we picked up 11 transcription factors which belong to high expression genes in 0 DPA and contain an AuxRE in their promoter (Supplementary Fig. S3; Supplementary Table S4). qRT-PCR analysis confirmed that six genes, MYC2, MYB44, MYB44-like, MYB-APL, MYB77, and bHLH53, were highly expressed in ovules at the initiation stage (Fig. 8A). MYB2 expression peaked at 15 DPA, when transcript levels were ~15-fold higher than at 0 DPA. There was no obvious difference in ERF105, WRKY70, ERF106, and bHLH144 expression in seed fibre cells at different developmental stages. The promoter regions of each of the six transcription factor genes preferentially expressed during seed fibre initiation were found to contain at least one AuxRE (Fig. 8B). The other transcription factor genes were found to contain one AuxRE in their promoter regions, except for that of ERF106 gene, which contained two AuxREs in the promoter regions (Supplementary Fig. S4). When ovules were incubated for several hours in ovule culture medium containing 5 µM NAA, an analogue of auxin, the transcripts of these genes increased significantly (Fig. 8C). For MYC2, MYB44, and MYB44-like, a >20-fold increase in transcript level was observed after 6 h treatment with NAA. An ~40-fold increase was observed for MYB-APL after NAA treatment for 24 h. NAA also significantly induced the expression of MYB77 and bHLH53. In contrast, application of NAA did not enhance the expression of ERF105 and WRKY70, which do not have an AuxRE in their promoters. Consistent with this, GhARF2-1 and GhARF18-1 could bind to the promoters of these genes in Y1H assays using target gene promoters as baits and GhARF2-1 or GhARF18-1 as prey (Fig. 8D). Interestingly, GhARF2-1 could bind to the MYB2 promoter region and GhARF18-1 could bind to the WRKY70, ERF106, and bHLH144 promoter regions (Supplementary Fig. S5). Taking into consideration the gene expression pattern during cotton seed fibre initiation, we suggest that GhARF2 and GhARF18 may regulate the expression of MYC2, MYB44, MYB44-like, MYB-APL, MYB77, and bHLH53 via binding to the AuxRE element in their promoters during cotton seed fibre initiation.
Fig. 8.
Validation and expression analysis of target transcription factor genes. (A) Expression profiling of 11 target transcription factors during different stages of seed fibre growth. Target genes up-regulated in 0 DPA ovules and containing the auxin-responsive element (AuxRE) in their promoter regions were selected. Gene expression data were obtained by quantitative real-time PCR with three replicates, with each replicate performed using independent materials. (B) Promoter sequence analysis of six transcription factor genes selected based on high expression levels during seed fibre initiation shown in (A). (C) NAA significantly induced the transcription of the six transcription factor genes. RNA samples were prepared from three independent cultures of ovules treated with 5 µM NAA for the indicated time. Relative expression levels of each gene were determined by normalizing to the expression level in CK ovules, which was set to 1.0. Statistical significance was determined using one-way ANOVA combined with Tukey’s test. *P<0.05; **P<0.01; ***P< 0.001. (D) Yeast one-hybrid assay. DD-O, yeast medium lacking leucine and tryptophan. TDO, yeast medium lacking leucine, tryptophan, and histidine. 3-AT, 3-amino-1,2,4-triazole.
Discussion
During the past few years, the whole-genome sequences of three cotton species have been completed (K. Wang et al., 2012; Paterson et al., 2012; F. Li et al., 2014, 2015; T. Zhang et al., 2015), which has greatly promoted the development of cotton science. Cotton seed fibres are single-celled trichomes that differentiate from the ovule epidermis (Basra and Malik, 1984). The development of cotton seed fibres is divided into four well-defined and overlapping stages, namely initiation, elongation, secondary cell wall thickening, and seed fibre maturation (Yoo and Wendel, 2013). Phytohormones, such as auxin, gibberellins, and ethylene, play important roles in seed fibre development (Beasley, 1973; Zhang et al., 2011; Shi et al., 2006). Application of gibberellin (GA3) to the flower buds in the field was found to increase the seed fibre number per ovule greatly (Seagull and Gialvalis, 2004). GhHOX3, a homeodomain transcription factor, interacts with GhSLR1, a cotton DELLA protein, to promote seed fibre cell elongation (Shan et al., 2014). Exogenous application of IAA, the most important natural auxin, to an in vitro ovule culture system significantly increased seed fibre initiation and the number of seed fibre units (Gialvalis and Seagull, 2001). Ectopic expression of the auxin biosynthetic gene iaaM increased IAA levels and the number of lint seed fibres (Zhang et al., 2011). ARFs, key components of auxin signalling pathways, can directly bind to downstream target gene promoters by recognizing TGTCTC AuxREs and regulate gene expression (Cho et al., 2014).
In this work, we simultaneously identified ARF genes in three representative cotton species, upland cotton G. hirsutum and its two diploid ancestors, G. arboreum and G. raimondii. We identified 56 ARF genes in G. hirsutum, and 35 ARF genes each in G. arboreum and G. raimondii. These results indicate that ARF gene loss occurred in G. hirsutum, which is consistent with the higher rate of gene loss in allotetraploid cotton than in both diploid species (F. Li et al., 2015; T. Zhang et al., 2015). ARF6 and ARF18 subfamily genes have extensively expanded in Gossypium, indicating that genes in these two subfamilies may contribute to better agronomic traits in cotton. The role of these genes in agronomically important stages of development is supported by the fact that ARF18 genes are expressed during seed fibre cell initiation. Some ARF genes were only found in Arabidopsis, but lost in Gossypium, suggesting that gene loss has occurred since Arabidosis and Gossypium diverged from their common ancestor. Most GhARF genes possess >10 exons, whereas four GhARF genes each have no more than four exons (Fig. 2B). This indicates that ARF family genes may be regulated by two different mechanisms. There is increasing evidence that introns play important roles in alternative splicing and generation of non-coding RNAs (Rearick et al., 2011). A similar phenomenon was observed for Arabidopsis ARF genes, suggesting that these regulatory mechanisms are relatively conserved in plants.
The expression patterns of ARF genes provided insight into their potential functions. Transcription profiling showed that the GhARF2 and GhARF18 subfamily genes were highly expressed in 0 DPA ovules (Fig. 4). In Arabidopsis, the arf2 loss-of-function mutant displayed many defects including enlarged rosette leaves, reduced fertility, delayed senescence, hypocotyl elongation defects, enlarged seeds, and enlarged cotyledons (Ellis et al., 2005). The functions of the ARF18 and ARF11 genes are still unknown, and loss of function of ARF18 results in no obvious phenotype in Arabidopsis. ARF6 may act redundantly with ARF8 to control stamen elongation and flower maturation in tomato (Liu et al., 2014). MacMillan and his colleagues found that two auxin-responsive-related genes was seed fibre specifically expressed; one of these has moderate expression at 7 DPA and the highest transcript level at 15 DPA and the other was exclusively expressed at 15 DPA, indicating that auxin-responsive-related genes might be involved in secondary cell wall synthesis at the eed fibre elongation (MacMillan et al., 2017). Our results indicate that GhARF2 and GhARF18 genes play important roles in cotton seed fibre initiation; the evidence is as follows. The transcript level of most GhARF2 and GhARF18 genes was significantly higher in WT ovules than in fl ovules (Fig. 4E). GUS activity driven by the GhARF20-1 and GhARF18-1 promoters was present exclusively in trichomes (Fig. 6), and ectopic expression of GhARF2-1 and GhARF18-1 in Arabidopsis resulted in a considerable increase in the number of trichomes (Fig. 7).
As central repressors in the auxin pathway, Aux/IAA proteins can interact with the co-repressor TPL to prevent activation of auxin-responsive genes by activating ARFs (Leyser, 2018). Overexpression of Arabidopsis transcription factors RAV1 and RAV2, which could physically interact with TPL, significantly increased the number of longer fibres in cotton (Mittal et al., 2015). In addition, TPL was reported to mediate brassinosteroid-induced transcriptional repression through interaction with BZR1 (Oh et al., 2014). Brassinosteroid played an important role in seed fibre development, and application of brassinosteroid in vitro significantly promoted cotton seed fibre cell elongation (Zhou et al., 2015). We presumed that TPL may mediate the crosstalk between auxin and brassinosteroid via ARF and BZR1 during cotton seed fibre developemt.
In Arabidopsis, trichome formation is modulated by transcription factors, including members of the bHLH, MYB, and MYC families (Schiefelbein et al., 2014). MYB and homeodomain-leucine zipper (GhHOX3) transcription factors have been reported to be involved in cotton seed fibre development (Shan et al., 2014). We found that bHLH53, MYC2, and four MYB transcription factors are potential target genes of GhARF2 and GhARF18 (Fig. 8). Some of these transcription factors have already been shown to regulate the development of seed fibre cells or other types of epidermal cells. GhMYC2 has previously been shown to interact with GhJAZ2 to regulate seed fibre initiation in cotton (Hu et al., 2016), and AtMYC2 is a positive regulator of lateral root formation in Arabidopsis (Raya-González et al., 2017). apl mutant seedlings display a short, determinate root with only occasional lateral branches (Abe et al., 2015). It has been reported that GhMYB25 regulates early seed fibre and trichome development, and that GhMYB109 and GaMYB2 are required for cotton seed fibre development (Wang et al., 2004; Pu et al., 2008; Machado et al., 2009). In Arabidopsis, MYB77, which interacts with ARF7, regulates the expression of some auxin-responsive genes and is required for lateral root development (Zhao et al., 2014).
In summary, our data have revealed the potentially important role of GhARF2 and GhARF18 subfamily genes in cotton seed fibre cell initiation. Auxin may activate several transcription factors via GhARF2 and GhARF18 proteins to regulate cotton seed fibre cell initiation. In order to understand the molecular mechanisms of seed fibre initiation regulated by auxin, further work will be aimed at characterizing the function of the genes directly targeted by GhARF2 and GhARF18.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Phylogenetic and evolutionary analysis of the ARF gene family in different plant species.
Fig. S2. Scanning electron microscopy images of the surface of wild-type and fl mutant ovules at 0 DPA.
Fig. S3. Venn diagram analysis of potential target transcription factor genes of GhARF. Fig. S4. Promoter sequence analysis of five transcription factor genes.
Fig. S5. Yeast one-hybrid assay.
Table S1. Analysis of G. hirsutum ARF gene family and its orthologues in AA and DD cotton genomes.
Table S2. Location analysis of 12 GhARF genes out of the chromosome.
Table S3. Analysis of duplication events in G. hirsutum ARF genes located in chromosomes.
Table S4. Transcription factor genes up-regulated in 0 DPA ovules and containing an ARF-binding site in their promoter.
Table S5. A list of primers used in this study.
Table S6. ARF gene sequences from the three cottons used in this study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos 31600223 and 31470295), the Fundamental Research Funds for the Central Universities (grant nos GK201603066 and GK201701005), and the State Key Laboratory of Cotton Biology Open Fund (grant no. CB2016A02). We are also grateful to Limin Wang from Ludong University for her kind suggestion.
References
- Abe M, Kaya H, Watanabe-Taneda A, Shibuta M, Yamaguchi A, Sakamoto T, Kurata T, Ausín I, Araki T, Alonso-Blanco C. 2015. FE, a phloem-specific Myb-related protein, promotes flowering through transcriptional activation of FLOWERING LOCUS T and FLOWERING LOCUS T INTERACTING PROTEIN 1. The Plant Journal 83, 1059–1068. [DOI] [PubMed] [Google Scholar]
- Attia KA, Abdelkhalik AF, Ammar MH, Wei C, Yang J, Lightfoot DA, EI-Sayed WM, EI-Shemy HA. 2009. Antisense phenotypes reveal a functional expression of OsARF1, an auxin response factor, in transgenic rice. Current Issues in Molecular Biology 11, 29–34. [PubMed] [Google Scholar]
- Bailey TL, Williams N, Misleh C, Li WW. 2006. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Research 34, W369–W373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra AS, and Malik CP. 1984. Development of the cotton seed fibre. International Review of Cytology 89, 65–113. [Google Scholar]
- Beasley CA. 1973. Hormonal regulation of growth in unfertilized cotton ovules. Science 179, 1003–1005. [DOI] [PubMed] [Google Scholar]
- Cho H, Ryu H, Rho S, et al. 2014. A secreted peptide response during lateral root development. Nature Cell Biology 16, 66–76. [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews. Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guifoyle TJ, Reed JW. 2005. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana.Development 132, 4563–4574. [DOI] [PubMed] [Google Scholar]
- Gialvalis S, Seagull RW. 2001. Plant hormones alter seed fibre initiation in unfertilized, cultured ovules of Gossypium hirsutum.Journal of Cotton Science 5, 252–258. [Google Scholar]
- Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM. 2006. AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. The Plant Cell 18, 1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB. 2010. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. The Plant Cell 22, 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. 2001. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414, 271–276. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ,Hagen G. 2007. Auxin response factor. Current Opinion in Plant Biology 10, 453–460. [DOI] [PubMed] [Google Scholar]
- Ha CV, Le DT, Nishiyama R, et al. 2013. The auxin response factor transcription factor family in soybean: genome-wide identification and expression analyses during development and water stress. DNA Research 20, 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO Journal 17, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. 2000. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. The Plant Cell 12, 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhao P, Wang L, Zhang Y, Wang X, Xiao H, Yu J, Xiao G. 2017. The PIN gene family in cotton (Gossypium hirsutum): genome-wide identification and gene expression analyses during root development and abiotic stress responses. BMC Genomics 18, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, He X, Tu L, Zhu L, Zhu S, Ge Z, Zhang X. 2016. GhJAZ2 negatively regulates cotton seed fibre initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. The Plant Journal 88, 921–935. [DOI] [PubMed] [Google Scholar]
- Hu W, Zuo J, Hou XW, Yan Y, Wei YX, Liu JH, Li MY, Xu BY, Jin ZQ. 2015. The auxin response factor gene family in banana: genome-wide identification and expression analyses during development, ripening, and abiotic stress. Frontiers in Plant Science 6, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451. [DOI] [PubMed] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiology 176, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Fan G, Lu C, et al. 2015. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nature Biotechnology 33, 524–530. [DOI] [PubMed] [Google Scholar]
- Li F, Fan G, Wang K, et al. 2014. Genome sequence of the cultivated cotton Gossypium arboreum. Nature Genetics 46, 567–572. [DOI] [PubMed] [Google Scholar]
- Li SB, OuYang WZ, Hou XJ, Xie LL, Hu CG, Zhang JZ. 2015. Genome-wide identification, isolation and expression analysis of auxin response factor (ARF) gene family in sweet orange (Citrus sinensis). Frontiers in Plant Science 6, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KD, Yuan CC, Li HL, Lin WH, Yang YJ, Shen CJ, Zheng XL. 2015. Genome-wide identification and characterization of auxin response factor (ARF) family genes related to flower and fruit development in papaya (Carica papaya L.). BMC Genomics 16, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Wu S, Van Houten J, Wang Y, Ding B, Fei Z, Clarke TH, Reed JW, van der Knaap E. 2014. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. Journal of Experimental Botany 65, 2507–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Hu LF. 2013. Genome-wide analysis of the auxin response factor gene family in cucumber. Genetics and Molecular Research 12, 4317–4331. [DOI] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. 2009. The MYB transcription factor GhMYB25 regulates early seed fibre and trichome development. The Plant Journal 59, 52–62. [DOI] [PubMed] [Google Scholar]
- MacMillan CP, Birke H, Chuah A, Brill E, Tsuji Y, Ralph J, Dennis ES, Llewellyn D, Pettolino FA. 2017. Tissue and cell-specific transcriptomes in cotton reveal the subtleties of gene regulation underlying the diversity of plant secondary cell walls. BMC Genomics 18, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Jiang Y, Ritchie GL, Burke JJ, Rock CD. 2015. AtRAV1 and AtRAV2 overexpression in cotton increases fiber length differentially under drought stress and delays flowering. Plant Science 241, 78–95. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Ellis CM, Weber H, et al. 2005. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 127, 3877–3888. [DOI] [PubMed] [Google Scholar]
- Narise T, Kobayashi K, Baba S, Shimojima M, Masuda S, Fukaki H, Ohta H. 2010. Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Molecular Biology 72, 533–544. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Wada T, Yamamoto KT, Okada K. 2005. The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. The Plant Cell 17, 2940–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Ryu H, Hwang I, Wang ZY. 2014. TOPLESS mediates brassinosteroid-induced transcriptional repression through interaction with BZR1. Nature Communications 5, 4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AH, Wendel JF, Gundlach H, et al. 2012. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 492, 423–427. [DOI] [PubMed] [Google Scholar]
- Pu L, Li Q, Fan X, Yang W, Xue Y. 2008. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 180, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya-González J, Velázquez-Becerra C, Barrera-Ortiz S, López-Bucio J, Valencia-Cantero E. 2017. N,N-dimethyl hexadecylamine and related amines regulate root morphogenesis via jasmonic acid signaling in Arabidopsis thaliana. Protoplasma 254, 1399–1410. [DOI] [PubMed] [Google Scholar]
- Rearick D, Prakash A, McSweeny A, Shepard SS, Fedorova L, Fedorov A. 2011. Critical association of ncRNA with introns. Nucleic Acids Research 39, 2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M, Chervin C, Mila I, et al. 2013. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiology 161, 1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Huang L, Zheng X. 2014. Regulation of epidermal cell fate in Arabidopsis roots: the importance of multiple feedback loops. Frontiers in Plant Science 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagull RW, Gialvalis S. 2004. Pre- and post-anthesis application of exogenous hormones alters seed fibre production in Gossypium hirsutum L. cultivar Maxxa GTO. Journal of Cotton Science 8, 105–111. [Google Scholar]
- Shan CM, Shangguan XX, Zhao B, et al. 2014. Control of cotton fibre elongation by a homeodomain transcription factor GhHOX3. Nature Communications 5, 5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Wang S, Zhang S, Xu Y, Qian Q, Qi Y, Jiang de A. 2013. OsARF16, a transcription factor, is required for auxin and phosphate starvation response in rice (Oryza sativa L.). Plant, Cell and Environment 36, 607–620. [DOI] [PubMed] [Google Scholar]
- Shen CJ, Yue RQ, Sun T, Zhang L, Xu LQ, Tie SG, Wang HZ, Yang YJ. 2015. Genome-wide identification and expression analysis of auxin response factor gene family in Medicago truncatula. Frontiers in Plant Science 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, Cheng J, Wei LP, Wang ZY, Zhu YX. 2006. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. The Plant Cell 18, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. 2006. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews. Molecular Cell Biology 7, 847–859. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Li W, Zhu Y, Liu Z, Huang W, Zhan J. 2014. Genome-wide identification, characterization and expression analysis of the auxin response factor gene family in Vitis vinifera. Plant Cell Reports 33, 1365–1375. [DOI] [PubMed] [Google Scholar]
- Wang D, Pei K, Fu Y, Sun Z, Li S, Liu H, Tang K, Han B, Tao Y. 2007. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 394, 13–24. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY. 2005. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. The Plant Cell 17, 2204–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang Z, Li F, et al. 2012. The draft genome of a diploid cotton Gossypium raimondii. Nature Genetics 44, 1098–1103. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang JW, Yu N, Li CH, Luo B, Gou JY, Wang LJ, Chen XY. 2004. Control of plant trichome development by a cotton fiber MYB gene. The Plant Cell 16, 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng D, Shi Y, Miao N, Bian Y, Yin Z. 2012a Diversification, phylogeny and evolution of auxin response factor (ARF) family: insights gained from analyzing maize ARF genes. Molecular Biology Reports 39, 2401–2415. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang H, Debarry JD, et al. 2012. b MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Research 40, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Wang K, Huang G, Zhu YX. 2016. Genome-scale analysis of the cotton KCS gene family revealed a binary mode of action for gibberellin A regulated fiber growth. Journal of Integrative Plant Biology 58, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Liao YC, Lv FF, et al. 2017. Transcription factor AsMYC2 controls the jasmonate responsive expression of ASS1 regulating sesquiterpene biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant and Cell Physiology 58, 1924–1933. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ji A, Song J, Chen S. 2016. Genome-wide analysis of auxin response factor gene family members in medicinal model plant Salvia miltiorrhiza. Biology Open 5, 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MJ, Wendel JF. 2013. Comparative evolutionary and developmental dynamics of the cotton (Gossypium hirsutum) seed fibre transcriptome. PLoS Genetics 10, e1004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Soler M, Mila I, et al. 2014. Genome-wide characterization and expression profiling of the AUXIN RESPONSE FACTOR (ARF) gene family in Eucalyptus grandis. PLoS ONE 9, e108906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang T, Guo W. 2010. Effect of H2O2 on fiber initiation using fiber retardation initiation mutants in cotton (Gossypium hirsutum). Journal of Plant Physiology 167, 393–399. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang GQ, Zou D, Yan JQ, Li Y, Hu S, Li XB. 2018. The cotton (Gossypium hirsutum) NAC transcription factor (FSN1) as a positive regulator participates in controlling secondary cell wall biosynthesis and modification of fibers. New Phytologist 217, 625–640. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zheng X, Song S, et al. 2011. Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nature Biotechnology 29, 453–458. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hu Y, Jiang W, et al. 2015. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nature Biotechnology 33, 531–537. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pan J. 1992. Genetic analysis of a fuzzless-lintless mutant in Gossypium hirsutum L. Jiangsu Journal of Agricultural Sciences (China) 7, 13–16. [Google Scholar]
- Zhang X, Yan F, Tang Y, Yuan Y, Deng W, Li Z. 2015. Auxin response gene SlARF3 pays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant and Cell Physiology 56, 2110–2124. [DOI] [PubMed] [Google Scholar]
- Zhang Y, He P, Yang Z, Huang G, Wang L, Pang C, Xiao H, Zhao P, Yu J, Xiao G. 2017. A genome-scale analysis of the PIN gene family reveals its functions in cotton fiber development. Frontiers in Plant Science 8, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiao Y, Liu Z, Zhu YX. 2015. ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nature Communications 6, 6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xing L, Wang X, Hou YJ, Gao J, Wang P, Duan CG, Zhu X, Zhu JK. 2014. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Science Signaling 7, ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang ZT, Li M, Wei XZ, Li XJ, Li BY, Li XB. 2015. Cotton (Gossypium hirsutum) 14-3-3 proteins participate in regulation of fibre initiation and elongation by modulating brassinosteroid signalling. Plant Biotechnology Journal 13, 269–280. [DOI] [PubMed] [Google Scholar]
- Zou C, Lu C, Shang H, Jing X, Cheng H, Zhang Y, Song G. 2013. Genome-wide analysis of the Sus gene family in cotton. Journal of Integrative Plant Biology 55, 643–653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.