Elevated levels of total EBVgp350 antibody were not protective against future nasopharyngeal carcinoma (NPC) risk in a general population cohort from Taiwan, failing to replicate prior findings among individuals with a strong family history of NPC.
Keywords: Epstein-Barr virus, gp350 antibody, NPC, EBV-related cancer, EBV vaccine
Abstract
Background
We previously reported that higher levels of antibody targeting Epstein-Barr virus (EBV) glycoprotein350 (gp350), an EBV vaccine candidate, were protective against nasopharyngeal carcinoma (NPC) in genetically high-risk families from Taiwan. The current study attempted to extend this association to a general population cohort.
Methods
We compared total and IgA-specific gp350 antibody levels in 35 incident NPC cases and 81 disease-free controls from the Cancer Screening Program in Taiwan (23943 individuals recruited 1991–1992). Luciferase immunoprecipitation assays quantified gp350 antibody.
Results
Total EBVgp350 antibody levels were not higher in individuals who remained disease free compared to those who developed NPC (P = .11). This lack of a protective gp350 association persisted for cases diagnosed ≥5 years (odds ratio [OR] = 1.05; P = .91) and <5 years (OR = 1.85; P = .40) after blood draw. IgA-specific gp350 antibody levels were higher in cases than controls (OR = 7.03; P = .001). This increased risk was most pronounced for cases diagnosed <5 years after blood draw (OR = 11.7; P = .004).
Conclusion
Unlike our prior findings in those with a strong family history of NPC, total gp350 antibody levels were not protective against NPC development in this general population setting.
Nasopharyngeal carcinoma (NPC) is an epithelial tumor attributable to Epstein-Barr virus (EBV) [1, 2]. This ubiquitous herpesvirus latently infects B cells in more than 90% of individuals by adulthood and can lead to certain cancers or lymphoproliferative disorders in a subset of those infected individuals [3, 4]. However, no effective vaccine is licensed for EBV. Targeting viral surface proteins to neutralize cell entry has proven effective in the development of vaccines against other oncogenic infections such as human papillomavirus [5, 6]. Early vaccines targeting glycoproteins that facilitate EBV B-cell entry (glycoprotein350 [gp350]) proved efficacious against EBV-related lymphomas in nonhuman primates [7, 8]. In humans, a phase 2 trial of recombinant gp350 vaccine demonstrated efficacy against clinically acute infectious mononucleosis, but prevention of primary EBV infection was not achieved [9]. Recent efforts to optimize immunogenicity using gp350 nanoparticles have been promising [10], raising the prospect of an effective EBV vaccine that elicits a strong neutralizing response against the virus. Additional EBV glycoproteins, such as gH/gL [11], may also be important for development of an effective vaccine [12]. Recent data have detailed an interaction between this glycoprotein complex and ephrin receptor 2A, which facilitates EBV fusion and entry into epithelial cells [13, 14].
However, because of the long latency period between primary EBV infection and most EBV-associated cancers, vaccine trials designed to evaluate malignancies as a primary outcome will be very challenging to perform. In this situation, longer-term observational cohorts can play a unique role by providing important data evaluating whether immune responses to vaccine targets (eg, EBVgp350 antibody) are protective against EBV-associated chronic disease. We previously reported that higher levels of gp350 antibody were associated with a reduced risk of NPC in a Taiwanese cohort of high-risk individuals with a strong family history of the cancer [15]. We also demonstrated that measuring gp350 antibody using a luciferase immunoprecipitation (LIPS) assay correlated well with data from more intensive assays that measure direct neutralization of EBV B-cell infection [16], suggesting that the gp350 LIPS assay is a good surrogate of neutralization potential for use in epidemiological studies.
To follow up on these findings, we used the LIPS assay to explore the association between gp350 antibody and NPC risk in a prospective, general population cohort from Taiwan. Our earlier work focused on total gp350 antibody, likely reflecting the most abundant antibody in circulation—IgG. Given the fact that NPC is an epithelial tumor arising in the nasopharynx, and the understanding that IgA reflects pathogen exposure along mucosal epithelium, we expanded the current investigation to include IgA-specific gp350 antibody measurements at multiple time points during follow-up.
METHODS
The Cancer Screening Program enrolled 23943 residents ages 30–65 years from the general population in Taiwan (12026 males and 11917 females) between 1991 and 1992 [17]. All individuals were disease free at baseline and provided enrollment blood samples. New cases of NPC that developed during follow-up (incident disease) were ascertained by computerized linkage with the National Cancer Registry in Taiwan through 31 October 2002. A total of 40 incident NPC cases were identified; blood from 35 of these cases was available for EBVgp350 antibody testing. For each NPC case, enrollment blood samples from 2 or 3 individuals who remained disease free during follow-up were matched to cases on age (within 5 years), sex, date of enrollment (within 90 days), and residential township; 81 disease-free controls were included in this analysis.
We also measured antibodies collected from repeat sampling in a subset of participants to better understand temporal patterns of gp350 antibody response preceding NPC diagnosis. We tested 37 samples collected longitudinally from 16 incident cases (average of 1.5 years between samples) and 47 samples collected from 17 disease-free controls. The Cancer Screening Program was approved by the appropriate ethics review boards in Taiwan and at the US National Cancer Institute.
EBVgp350 Assay
The interaction between EBVgp350 and CD21 on B cells is crucial for initiating EBV B-cell entry [18]. We tested enrollment blood samples for antibodies specific to EBVgp350 using previously described LIPS assays. In brief, cell lysate containing EBVgp350-Renilla luciferase fusion protein was probed with a single dilution of study participant serum, immunoprecipitated with protein A/G beads (capturing IgA and IgG antibody), and incubated with coelenterazine substrate. Light units (LU) were quantified using a luminometer to obtain a quantitative measure of the antibody level in each sample. Tests were conducted in triplicate, and statistical analyses were performed using a standardized value—the average of the triplicate tests, divided by a plate-specific cutoff value defined as the mean plus 2 standard deviations of the readings from 4 EBV-negative sera. In addition to total gp350 antibody, we tested serum samples against beads specific to IgA (captured only IgA antibody).
LIPS assays proved to be highly reproducible. Readings from duplicate samples for 17 individuals, tested by laboratory personnel blinded to sample identity, indicated significant across-sample correlation for total gp350 (Pearson = 0.92; Spearman = 0.82; P < .001) and IgA-specific gp350 (Pearson = 0.91; Spearman = 0.75; P ≤ .01).
EBV B-Cell Neutralization Assay
To confirm that the EBVgp350 LIPS assay results were a surrogate for the ability to neutralize EBV B-cell entry in this study population, we performed neutralization assays on 56 participants (27 NPC cases and 29 controls). Serum from study participants was serially diluted and incubated with green fluorescence protein (GFP)-expressing EBV. This mixture was added to Raji B cells and incubated for 3 days at 37°C. Cells were suspended in phosphate-buffered saline (PBS), fixed in 2% paraformaldehyde in PBS, and GFP-positive B cells were quantified using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) and BD CSampler software. The dilution of participant serum that neutralized EBV infectivity by 50% (IC50), based on the reduction of the number of GFP-positive B cells, was calculated by nonlinear regression using GraphPad PRISM.
Statistical Analyses
We measured baseline (enrollment) differences in mean total gp350, IgA-specific gp350 antibody, and EBV neutralizing titers between cohort members who did versus did not develop NPC during follow-up. Differences were evaluated using an unpaired, Welch-Satterthwaite t test that did not assume equal variance, with alpha <0.05 considered to be statistically significant. Analyses were performed overall and stratified by time between baseline sampling and NPC diagnosis (<5 vs ≥5 years).
The change in gp350 antibody response over time (kinetics) was assessed by subtracting the antibody level at time point N from the level at time point (N-1). This between-sample change was calculated for 16 NPC cases, stratified by time between sampling and NPC diagnosis, and 17 disease-free controls. For NPC cases, the timing of between-sample change was expressed relative to diagnosis. For example, a case diagnosed in the year 1999 with samples collected at baseline (1991) and again in 1993 and 1995 would have the following between-sample measurements: (1) 1993 minus 1991 (measured 6 years before NPC) and (2) 1995 minus 1993 (measured 4 years before NPC). The between-sample changes in antibody level among disease-free controls were not plotted because of the lack of an anchor point (ie, no date of NPC diagnosis).
Among the 81 disease-free controls, we also characterized the relatedness of different antibody responses by calculating the Spearman correlation coefficient between total gp350 antibody and: (1) IgA-specific gp350 antibody and (2) EBV neutralizing titers. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) and GraphPad PRISM.
RESULTS
We evaluated blood samples from 35 incident NPC cases and 81 age, sex, date, and region-matched controls to determine if higher EBVgp350 antibody levels were protective against NPC (ie, higher in controls compared to cases). Blood samples were obtained an average of 5 years (range: 1–8 years) before NPC diagnosis. In disease-free participants, we observed a more than 10-fold difference in the mean total gp350 antibody level compared to the IgA-specific gp350 antibody response (148.2 versus 10.9 LIPS assay LU; P < .001). The correlation between total and IgA-specific anti-gp350 antibody was statistically significant but moderate (Spearman = 0.51, P < .001).
Individuals Who Developed NPC Did Not Have Lower Levels of Total or IgA-Specific EBV gp350 Antibody Compared to Disease-Free Controls
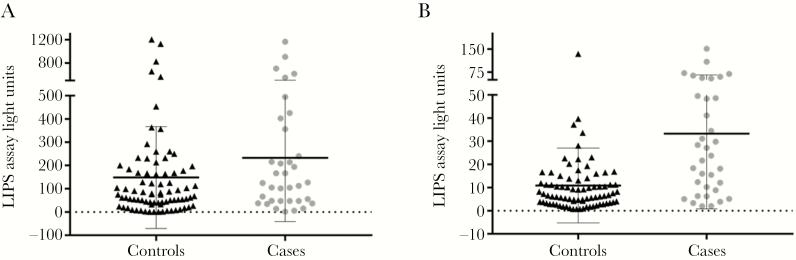
Mean total gp350 antibody levels measured at baseline were nonsignificantly higher (P = .11) in individuals who developed NPC (233.1 LU) compared to controls who remained disease free (148.2 LU; Figure 1A). When antibody levels were categorized into quartiles based on the distribution among controls, we observed no association between total gp350 and NPC after adjustment for age and sex (P = .42; Table 1). IgA-specific gp350 antibody levels were significantly higher in persons who developed NPC compared to disease-free controls (33.3 vs 10.9 LU, P < .001; Figure 1B). Compared to individuals in the lowest IgA-specific gp350 quartile, those with levels in the highest quartile had a 7-fold increased risk of NPC (odds ratio [OR] = 7.03; 95% confidence interval [CI], 1.75–28.2; P = .001; Table 1).
Figure 1.
A, Mean total Epstein-Barr virus (EBV) gp350 antibody level by nasopharyngeal carcinoma (NPC) status. B, Mean IgA-specific EBVgp350 antibody level by NPC status. Abbreviation: LIPS, luciferase immunoprecipitation.
Table 1.
Epstein-Barr Virus (EBV) gp350 Antibody and Nasopharyngeal Carcinoma (NPC) Risk in a General Population Cohort From Taiwan, According to Lag Between Antibody Measurement and NPC
| EBV Antibody Measure (LU) | Overall | Restricted to NPC Cases With Sampling <5 Years Before Diagnosis (N = 17 Cases) | Restricted to NPC Cases With Sampling ≥5 Years Before Diagnosis (N = 13 Cases) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, N (%) | Controls, N (%) | ORa | 95% CI | Cases, N (%) | OR | 95% CI | Cases, N (%) | OR | 95% CI | |
| Total gp350 | ||||||||||
| Quartile 1b (<40.15) | 7 (20) | 21 (25.9) | 1.0 | 3 (17.7) | 1.0 | 3 (23.1) | 1.0 | |||
| Quartiles 2/3 (40.15–168.1) | 14 (40) | 40 (49.4) | 0.98 | 0.32–3.06 | 7 (41.2) | 1.16 | 0.25–5.31 | 6 (46.2) | 0.73 | 0.16–3.40 |
| Quartile 4 (≥168.1) | 14 (40) | 20 (24.7) | 1.55 | 0.47–5.15 | 7 (41.2) | 1.85 | 0.39–7.78 | 4 (30.8) | 1.05 | 0.20–5.49 |
| P trend = .42 | P trend = .40 | P trend = .91 | ||||||||
| IgA-specific gp350 | ||||||||||
| Quartile 1 (<3.48) | 3 (8.6) | 21 (25.9) | 1.0 | 1 (5.9) | 1.0 | 2 (15.4) | 1.0 | |||
| Quartiles 2/3 (3.48–12.41) | 9 (25.7) | 40 (49.4) | 1.40 | 0.33–5.98 | 4 (23.5) | 2.24 | 0.23–22.2 | 4 (30.8) | 1.00 | 0.16–6.08 |
| Quartile 4 (≥12.41) | 23 (65.7) | 20 (24.7) | 7.03 | 1.75–28.2 | 12 (70.6) | 11.7 | 1.32–103 | 7 (53.9) | 3.87 | 0.68–21.9 |
| P trend = .001 | P trend = .004 | P trend = .07 | ||||||||
Abbreviations: CI, confidence interval; LU, luciferase immunoprecipitation assay light units.
aOdds ratios (OR) calculated using logistic regression models adjusted for age and sex.
bQuartiles calculated among disease-free individuals from the cohort.
The Ability to Neutralize EBV B-Cell Infection Did Not Differ Between NPC Cases and Disease-Free Controls
To ensure that the lack of a protective effect observed for total gp350 antibody levels was not explained by the gp350 LIPS assay being a poor surrogate for EBV B-cell neutralizing titers in this population, we evaluated both the correlation between total gp350 levels and neutralizing activity and the association between EBV neutralizing titers and NPC risk. We observed significant correlation between total gp350 antibody levels and the ability of participant serum to neutralize EBV B-cell infection (Spearman = 0.65; P < .0001). In addition, consistent with our findings using the gp350 LIPS assay, EBV neutralizing titers were nonsignificantly elevated in serum from NPC cases (IC50 = 227.6) compared to disease-free controls (IC50 = 140.5; P = .24).
IgA-Specific EBV gp350 Antibody Levels Were Elevated in NPC Cases Close to the Time of Diagnosis
Next, we evaluated cases stratified by time between baseline blood draw and diagnosis (<5 years vs ≥5 years) to explore whether a protective association between total gp350 antibody and future NPC risk was evident in cases whose blood was collected at least 5 years before disease. This would avoid any potential reverse causality resulting from higher EBV immune responses in those with existing but undiagnosed cancer (ie, higher levels in blood collected close to NPC diagnosis). However, we observed no association between total gp350 antibody level and NPC risk in this stratum (OR = 1.05highest vs lowest quartile; 95% CI, 0.20–5.49; P = .91; Table 1). Individuals in the highest quartile for IgA-specific gp350 had a suggestive but nonsignificantly higher risk for NPC diagnosed at least 5 years after blood draw (OR = 3.87; 95% CI, 0.68–21.9; P = .07). In contrast to what we observed for antibodies measured at least 5 years before NPC diagnosis, elevated IgA-specific gp350 antibody levels were associated with a significant, more than 10-fold increased risk for NPC diagnosed within 5 years of blood draw (OR = 11.7highest vs lowest quartile; 95% CI, 1.32–103; P = .004).
Kinetics of EBV gp350 Antibody Response Over Time
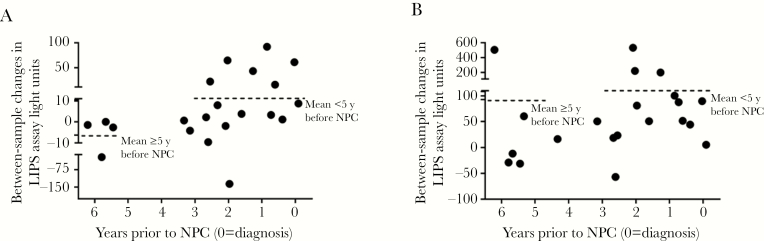
To further evaluate the patterns of IgA-specific gp350 antibody response relative to NPC diagnosis, we subtracted the antibody level at time point N from the level at time point N-1 for a set of 16 cases with available longitudinal data (see Methods for details). Although our modest sample size resulted in wide confidence intervals that precluded definitive statements, the between-sample increase in IgA-specific gp350 antibody appeared higher when sample pairs were collected within 5 years of NPC diagnosis (+10.4 LU; 95% CI, −14.0 to 34.7) relative to collection more than 5 years prior to disease (−6.3 LU; 95% CI, −22.1 to 9.6; Figure 2A). This mean between-sample increase reached 32.3 LU (95% CI, 0.24–64.3) when we restricted assessment to the 7 sample pairs where the most recent blood collection occurred within 2 years of NPC diagnosis, much higher than the background level of change over time observed in disease-free controls (−4.8 LU; 95% CI, −10.8 to 1.3).
Figure 2.
A, IgA-specific gp350 antibody dynamics in nasopharyngeal carcinoma (NPC) cases relative to time of diagnosis. B, Epstein-Barr virus (EBV) total gp350 antibody dynamics in NPC cases relative to time of diagnosis. Abbreviation: LIPS, luciferase immunoprecipitation.
The plotted values for total gp350 antibody revealed significantly positive between-sample changes for case pairs collected within 5 years of NPC diagnosis (+92.7 LU; 95% CI, 25.0–160.4; Figure 2B). Between-sample changes for cases pairs collected ≥5 years before NPC diagnosis and controls were 108.3 LU (95% CI, −313.3 to 530) and 28.2 LU (95% CI, −26.1 to 82.4), respectively.
DISCUSSION
We did not replicate our previous finding that higher levels of total antibody against EBVgp350 were associated with lower NPC risk. This lack of a protective association was also observed using a more-intensive B-cell neutralization assay, confirming that the null effect reported for total gp350 antibody was not explained by gp350 being a poor surrogate for EBV neutralization. In contrast to the lack of association between total gp350 antibody and NPC, IgA-specific gp350 antibody levels were elevated in NPC cases, particularly when measured close to the time of cancer diagnosis.
The participants for this study were drawn from the general population in Taiwan; this differed from our earlier work conducted in multiplex families with a likely genetic predisposition for disease (≥2 first or second-degree family members affected by NPC) [15]. We previously reported that mucosal immune responses targeting other EBV proteins in these multiplex family members differed from the general Taiwanese population (eg, higher Epstein‐Barr nuclear antigen‐1 IgA antibody levels) [19]. It is possible that population-specific characteristics of the immune response to gp350, such as uniquely low EBVgp350 antibody production in NPC-prone multiplex family members, could partially explain the discrepant findings. The lack of replication should also prompt additional investigations into nonneutralizing mechanisms that could account for an association between lower total gp350 antibody and higher NPC risk in multiplex family members. Early gp350 vaccine studies posited that the protection observed in nonhuman primates was not entirely mediated through EBV neutralization, suggesting that additional immune responses to gp350 may be important for protection from disease [20, 21]. Finally, although we confirmed that LIPS gp350 antibody results correlated with those generated using B-cell neutralization assays in both our previous and current work, we cannot rule out the possibility that one of the studies represented a chance finding.
Adapting the LIPS assay for measurement of IgA antibody targeting EBV gp350 was a unique feature of this study. Prior work reported that levels of IgA-specific gp350 antibody were elevated in NPC cases in a manner similar to IgA against other EBV proteins such as the viral capsid antigen (VCA) [22]. However, that work was conducted among prevalent cases (ie, blood tests conducted after NPC diagnosis) and was therefore susceptible to a disease effect—the potential for immune responses to be elevated in patients with existing disease. To avoid such an effect, we measured gp350 antibodies in a cohort of disease-free individuals, ensuring that any associations observed would be meaningful years prior to disease. Ultimately, the IgA-specific gp350 patterns we observed in this prospective dataset mirrored those from the prevalent disease setting (ie, elevated in those who developed NPC).
Strengths of our study included the prospective collection of data to assess whether longer-term gp350 antibody levels were protective against future EBV-related disease, conduct of the study in a high-risk region for NPC (Southeast Asia), adaptation of the LIPS assay to measure IgA targeting gp350, use of the B-cell neutralization assay to validate the LIPS assay findings, and characterizing the kinetics of gp350-directed immune responses over time in EBV-infected adults. However, this study was not without limitations, including the small number of NPC cases available for stratified analyses and the inability to assess immune responses to gp350 other than B-cell neutralizing antibody.
Elevated levels of total EBVgp350 antibody were not protective against future NPC risk in this cohort from the general population in Taiwan. IgA-specific gp350 antibody also did not confer protection against NPC. Instead, IgA-specific gp350 behaved more similarly to high-risk biomarkers such as VCA IgA, which are elevated close to the time of NPC diagnosis. These data failed to replicate prior findings from a study conducted among high-risk individuals with a strong family history of NPC and suggest that future work is warranted to understand how and whether naturally-occurring gp350 antibody relates to protection against EBV-related cancers over time. Neutralizing antibody to gp350 peaks late after infection, implying that antibody maturation continues long after initial infection and may reflect in large part ongoing viral replication or exposure [23]. More study is also needed to understand whether antibody targeting non-gp350 viral glycoproteins responsible for EBV entry [11], or T-cell reactivity to proteins expressed during viral latency [24], correlates with protection from EBV-positive cancers and could be important for an effective EBV vaccine.
Presented in part. 17th International Symposium on EBV and Associated Diseases, Zurich, Switzerland, July 2016.
Notes
Financial support. This research was supported by the Intramural Research Program of the National Cancer Institute and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Henle W, Henle G, Ho HC et al. Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst 1970; 44:225–31. [PubMed] [Google Scholar]
- 2. zur Hausen H, Schulte-Holthausen H, Klein G et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970; 228:1056–8. [DOI] [PubMed] [Google Scholar]
- 3. Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000; 343:481–92. [DOI] [PubMed] [Google Scholar]
- 4. Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4:757–68. [DOI] [PubMed] [Google Scholar]
- 5. Kreimer AR, González P, Katki HA et al. ; CVT Vaccine Group Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica vaccine trial. Lancet Oncol 2011; 12:862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehtinen M, Paavonen J, Wheeler CM et al. ; HPV PATRICIA Study Group Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89–99. [DOI] [PubMed] [Google Scholar]
- 7. Epstein MA, Morgan AJ, Finerty S, Randle BJ, Kirkwood JK. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature 1985; 318:287–9. [DOI] [PubMed] [Google Scholar]
- 8. Ragot T, Finerty S, Watkins PE, Perricaudet M, Morgan AJ. Replication-defective recombinant adenovirus expressing the Epstein-Barr virus (EBV) envelope glycoprotein gp340/220 induces protective immunity against EBV-induced lymphomas in the cottontop tamarin. J Gen Virol 1993; 74(Pt 3):501–7. [DOI] [PubMed] [Google Scholar]
- 9. Sokal EM, Hoppenbrouwers K, Vandermeulen C et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis 2007; 196:1749–53. [DOI] [PubMed] [Google Scholar]
- 10. Kanekiyo M, Bu W, Joyce MG et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell 2015; 162:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sathiyamoorthy K, Jiang J, Möhl BS et al. Inhibition of EBV-mediated membrane fusion by anti-gHgL antibodies. Proc Natl Acad Sci U S A 2017; 114:E8703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JI. Epstein-Barr virus vaccines. Clin Transl Immunology 2015; 4:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Sathiyamoorthy K, Zhang X et al. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat Microbiol 2018; 3:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Li Y, Wang HB et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol 2018; 3:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Coghill AE, Bu W, Nguyen H et al. High levels of antibody that neutralize B-cell infection of Epstein-Barr virus and that bind EBV gp350 are associated with a lower risk of nasopharyngeal carcinoma. Clin Cancer Res 2016; 22:3451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sashihara J, Burbelo PD, Savoldo B, Pierson TC, Cohen JI. Human antibody titers to Epstein-Barr virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology 2009; 391:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CJ, Yang HI, Su J et al. ; REVEAL-HBV Study Group Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73. [DOI] [PubMed] [Google Scholar]
- 18. Longenecker R, Kieff E, Cohen JI. Epstein-Barr virus. In: Knipe DM, Howley P, Cohen JI. et al. , eds. Fields virology. 6th ed Philadelphia: Lippincott Williams & Wilkins, 2013:1898–959. [Google Scholar]
- 19. Pickard A, Chen CJ, Diehl SR et al. Epstein-Barr virus seroreactivity among unaffected individuals within high-risk nasopharyngeal carcinoma families in Taiwan. Int J Cancer 2004; 111:117–23. [DOI] [PubMed] [Google Scholar]
- 20. Khyatti M, Patel PC, Stefanescu I, Menezes J. Epstein-Barr virus (EBV) glycoprotein gp350 expressed on transfected cells resistant to natural killer cell activity serves as a target antigen for EBV-specific antibody-dependent cellular cytotoxicity. J Virol 1991; 65:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss ER, Alter G, Ogembo JG et al. High Epstein-Barr virus load and genomic diversity are associated with generation of gp350-specific neutralizing antibodies following acute infectious mononucleosis. J Virol 2017; 91:pii:e01562-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao QY, Rowe M, Morgan AJ et al. Salivary and serum IgA antibodies to the Epstein-Barr virus glycoprotein gp340: incidence and potential for virus neutralization. Int J Cancer 1991; 48:45–50. [DOI] [PubMed] [Google Scholar]
- 23. Bu W, Hayes GM, Liu H et al. Kinetics of Epstein-Barr virus (EBV) neutralizing and virus-specific antibodies after primary infection with EBV. Clin Vaccine Immunol 2016; 23:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor GS, Steven NM. Therapeutic vaccination strategies to treat nasopharyngeal carcinoma. Chin Clin Oncol 2016; 5:23. [DOI] [PubMed] [Google Scholar]