Abstract
STUDY QUESTION
Does 27-hydroxycholesterol (27OH) actively facilitate the progression of luteolysis?
SUMMARY ANSWER
There is increased mRNA expression of the enzyme that produces 27OH during luteolysis in vivo in rhesus macaques and sheep, and 27OH reduces progesterone secretion from human luteinized granulosa cells.
WHAT IS KNOWN ALREADY
There is an increase in mRNA expression of liver x receptor (LXR) and a decrease in sterol regulatory element binding protein 2 (SREBP2) target genes during spontaneous luteolysis in primates, which could result in reduced cholesterol availability for steroidogenesis. Concentrations of 27OH are also increased in primate corpora lutea (CL) during luteolysis, and 27OH is a dual LXR agonist and SREBP2 inhibitor.
STUDY DESIGN SIZE, DURATION
This was an in vitro study using primary human luteinized granulosa cells in a control versus treatment(s) design. Analyses of CL from sheep undergoing induced or spontaneous luteolysis were also performed, along with database mining of microarray data from rhesus macaque CL.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Primary luteinizing granulosa cells were obtained from 37 women aged 24–44 who were undergoing oocyte donation or IVF for male factor or idiopathic infertility, and cells were further luteinized in vitro using human chorionic gonadotropin. Three approaches to test the effect of 27OH produced via CYP27A1 (cytochrome p450, family 27, subfamily A, polypeptide 1) on luteinized granulosa cells were used: (i) direct 27OH supplementation, (ii) induction of endogenous CYP27A1 activity via pharmacologic inhibition of steroidogenesis, and (iii) siRNA-mediated knockdown to directly inhibit CYP27A1 as well as cholesterol transport into the mitochondria via the steroidogenic acute regulatory protein (STAR). Endpoints included: progesterone (P4) secretion into culture media determined by enzyme immunoassay, cholesterol efflux and uptake assays using fluorescent lipid analogs, and mRNA expression determined via semi-quantitative real-time PCR (QPCR). An additional experiment involved QPCR analysis of 40 CL collected from ewes undergoing induced or spontaneous luteolysis, as well as database mining of microarray data generated from 16 rhesus macaque CL collected during spontaneous luteolysis and 13 macaque CL collected during a luteinizing hormone ablation and replacement protocol.
MAIN RESULTS AND THE ROLE OF CHANCE
The mRNA expression of CYP27A1 was significantly increased during luteolysis in rhesus macaques and sheep in vivo, and CYP27A1 transcription was suppressed by luteinizing hormone and hCG. There was a significant decrease in hCG-stimulated P4 secretion from human luteinized granulosa cells caused by 27OH treatment, and a significant increase in basal and hCG-stimulated P4 synthesis when endogenous 27OH production was inhibited via CYP27A1 knockdown, indicating that 27OH inhibits steroidogenesis. Pharmacologic inhibition of steroidogenesis by aminoglutethimide significantly induced LXR and inhibited SREBP2 target gene mRNA expression, indicating that increased oxysterol production occurs when steroidogenesis is suppressed. Inhibiting cholesterol delivery into the mitochondria via knockdown of STAR resulted in reduced SREBP2 target gene mRNA expression, indicating that STAR function is necessary to maintain SREBP2-mediated transcription. The effects of 27OH treatment on markers of LXR and SREBP2 activity were moderate, and knockdown of CYP27A1 did not prevent aminoglutethimide-induced changes in LXR and SREBP2 target gene mRNA expression. These observations indicate that 27OH inhibits P4 secretion partially via mechanisms separate from its role as an LXR agonist and SREBP2 inhibitor, and also demonstrate that other oxysterols are involved in modulating LXR and SREBP2-mediated transcription when steroidogenesis is suppressed.
LARGE SCALE DATA
None.
LIMITATIONS REASONS FOR CAUTION
Luteinized granulosa cells may differ from luteal cells, and the effect on luteal function in vivo was not directly tested. The mechanisms that cause the initial rise in CYP27A1 mRNA expression during luteolysis are also not clear.
WIDER IMPLICATIONS OF THE FINDINGS
The factors causing luteolysis in primates have not yet been determined. This study provides functional evidence of a novel mechanism via increased 27OH synthesis during luteolysis, which subsequently represses progesterone secretion. Increased 27OH may also facilitate the progression of luteolysis in domestic animal species.
STUDY FUNDING AND COMPETING INTEREST(S)
The authors have nothing to disclose. Support was provided by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health (NIH), award number R00HD067678 to R.L.B.
Keywords: luteolysis, 27-hydroxycholesterol, CYP27A1, liver x receptor, sterol regulatory element binding protein, STAR, cholesterol metabolism, progesterone, corpus luteum, human luteinized granulosa cells
Introduction
The mechanisms that lead to a loss of progesterone (P4) synthesis and luteolysis of the corpus luteum (CL) during the normal menstrual cycle in primates have not been determined (Stouffer et al., 2013). Previous microarray studies have found that there is increased expression of liver x receptor (LXR) target genes and decreased low density lipoprotein receptor (LDLR) during spontaneous luteolysis in the primate CL (Bogan et al., 2009; Bogan and Hennebold, 2010). The LXR isoforms α (NR1H3) and β (NR1H2) are master regulators of cholesterol efflux by inducing the transcription of numerous genes involved in this process, including the membrane efflux proteins ATP binding cassette subfamily A1 (ABCA1) and G1 (ABCG1) (Lund et al., 2003), the extracellular cholesterol acceptor apolipoprotein E (Laffitte et al., 2001b), and NR1H3 itself (Laffitte et al., 2001a). Extracellular cholesterol uptake from LDL and de novo synthesis of cholesterol are controlled by the sterol regulatory element binding proteins (SREBPs) (Horton et al., 2002). The SREBPs are embedded in the endoplasmic reticulum in their inactive state but when sterol concentrations are low, they are transported to the Golgi apparatus where they undergo two proteolytic cleavage events to release the active transcription factor that travels to the nucleus (Goldstein et al., 2006). There are three SREBPs that arise from two genes: SREBP1a and SREBP1c are encoded by sterol regulatory element binding transcription factor 1 (SREBF1), while SREBP2 is encoded from SREBF2 (Horton et al., 2002). In human tissues SREBP1c is the predominant product of SREBF1 (Shimomura et al., 1997), and SREBP1c preferentially controls transcription of genes involved in fatty acid synthesis (Horton et al., 2002). Conversely, SREBP2 preferentially regulates the mRNA expression of LDLR and the rate-limiting enzyme in cholesterol biosynthesis 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (Horton et al., 2002). Because cholesterol is the substrate for P4 synthesis, increased LXR-mediated efflux of cholesterol and reduced SREBP2-mediated uptake of cholesterol within the primate CL may contribute to luteolysis.
Not only do the LXRs and SREBP2 have reciprocal actions on cholesterol homeostasis, but their transcriptional activity is also differentially regulated by oxysterols (hydroxylated cholesterol) (Goldstein et al., 2006; Hong and Tontonoz, 2014). Enzymatically generated oxysterols include 24S-hydroxycholesterol (24SOH), 25-hydroxycholesterol (25OH) and 27-hydroxycholesterol (27OH) (Russell, 2000). The enzyme producing 24SOH is predominantly expressed in the brain (Russell, 2000). Cholesterol 25-hydroxylase that produces 25OH is in the endoplasmic reticulum membrane (Lund et al., 1998), the same subcellular region as the SREBPs. The enzyme CYP27A1 (cytochrome p450, family 27, subfamily A, polypeptide 1) that produces 27OH is localized to mitochondria (Russell, 2000). These oxysterols have been identified as dual LXR agonists and SREBP inhibitors by preventing proteolytic processing of the SREBPs (Russell, 2000; Fu et al., 2001; Goldstein et al., 2006, Hong and Tontonoz, 2014).
It has been previously reported that luteal 27OH concentrations are increased during spontaneous luteolysis in the rhesus macaque CL (Bogan et al., 2012). Therefore, 27OH is an attractive candidate as the endogenous oxysterol that stimulates the LXRs and reduces SREBP2 activity during luteolysis. Products of steroidogenesis including pregnenolone and P4 inhibit the enzyme activity of CYP27A1 (Rennert et al., 1990). This indicates that during the luteal phase steroidogenesis suppresses 27OH formation. However, during luteolysis the decrease in steroidogenesis may increase CYP27A1 activity, which would promote 27OH synthesis and lead to increased LXR and decreased SREBP2 activity and ultimately a reduction in cholesterol availability for steroidogenesis. Therefore, we hypothesize that 27OH produced via CYP27A1 facilitates the progression of luteolysis by inducing LXR and reducing SREBP2 activities. The first objective of this study is to use a comparative physiology approach to determine how CYP27A1 mRNA expression changes in vivo during spontaneous luteolysis in rhesus macaques, during spontaneous and prostaglandin F2α (PGF2α)-induced luteolysis in sheep, and how the key luteotropins luteinizing hormone (LH) and human chorionic gonadotropin (hCG) regulate CYP27A1 mRNA expression. The second objective is to use three different methods to determine the effects of 27OH produced via CYP27A1 on P4 secretion and markers of LXR and SREBP2 activity in vitro: (i) direct 27OH supplementation, (ii) induction of endogenous CYP27A1 activity via pharmacologic inhibition of steroidogenesis and (iii) siRNA-mediated knockdown to directly inhibit CYP27A1 as well as cholesterol transport into the mitochondria via the steroidogenic acute regulatory protein (STAR). For pharmacologic inhibition of steroidogenesis, aminoglutethimide was used to inhibit the conversion of cholesterol into pregnenolone (Rennert et al., 1990), and trilostane was used to block the conversion of pregnenolone into P4 (Komanicky et al., 1978). A human luteinized granulosa cell model was employed for this objective as it is an in-vitro model that closely recapitulates CL function in vivo (Stewart and Vandevoort, 1997).
Materials and Methods
Ethical approval
The University of Arizona Institutional Review Board approved the study and patients provided informed written consent. Procedures involving sheep were approved by the University of Arizona Institutional Animal Care and Use Committee.
Isolation of human granulosa cells
The follicular aspirates used in this study were from 37 female patients undergoing oocyte donation or IVF for male factor or idiopathic infertility at the Reproductive Health Center, Tucson, AZ. The patients were 24–44 years old at the time of follicle aspiration. Follicular aspirates were centrifuged (500 g for 5 min at 4°C) to recover cells, and cell pellets were suspended in nutrient mixture F10 Ham (Ham’s F10) with 0.1% (w:v) bovine serum albumin. Cells were layered onto a 40% (v:v) Percoll gradient (GE Healthcare, Uppsala, Sweden) in Hanks’ balanced salt solution (Sigma-Aldrich Inc., St. Louis, MO, USA), and centrifuged at 500 g for 15 min at 4°C. The supernatant was collected and diluted 2-fold with Ham’s F10 and centrifuged, and cells were washed once more. The cell pellet was suspended in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F12) with 10% fetal bovine serum (FBS) and 0.02 IU/ml hCG (Sigma-Aldrich), and viable cells were counted by trypan blue. The luteinizing granulosa cells were plated in fibronectin-coated 24-well or 96-well plates at a density of 5 × 104 cells/cm2. Luteinizing granulosa cells were cultured for 5 days in luteinization medium (DMEM/F12 with 10% FBS; 100 units/ml penicillin and 0.1 mg/ml streptomycin or Pen/Strep; and 0.02 IU/ml hCG), and were incubated at 37°C and 5% CO2 in a humidified environment with media changed daily. For experiments involving 27OH, insulin–transferrin–selenium (ITS) supplement (Sigma-Aldrich) was used in luteinization medium instead of FBS.
Cell treatments
To determine the effect of 27OH, a 2 × 2 factorial design was used. Cells were first treated with vehicle (0.1% v:v DMSO) or 27OH (2 μM, Cayman Chemical, Ann Arbor, MI, USA) in treatment medium (DMEM/F12 containing ITS, Pen/Strep, 20 μg/ml LDL and 10 μg/ml HDL cholesterol). After 16 h, fresh treatments with or without 2 IU/ml hCG were added for another 4 h to test hCG responsiveness. Because hCG prevents luteolysis (Stouffer and Hennebold, 2015), and we hypothesize that 27OH promotes luteolysis, a short hCG treatment was selected to minimize the possibility of it masking 27OH effects. To determine the chronic effects of hCG on CYP27A1 mRNA expression, after the 5-day luteinization period cells were switched to treatment medium in the presence or absence of 0.2 IU/ml hCG, and cells were collected every day for 3 days.
To determine the effect of pharmacologic inhibitors of steroidogenesis (Fisher Scientific, Hampton, NH, USA), cells were treated with vehicle (0.1% v:v DMSO), aminoglutethimide (500 μM) or trilostane (10 μM). Also, 0.1 or 1 μM P4 or pregnenolone were added to additional groups in the presence of aminoglutethimide. Cells were treated for 24 h. Media was replaced with fresh treatments, and cells were incubated for an additional 4 h until cell harvest.
Small interfering RNA (siRNA) transfection
After 4 days of luteinization, siRNA transfection was performed. Pre-designed Silencer® Select siRNAs (Fisher Scientific) against human STAR (siRNA ID s13527), CYP27A1 (siRNA ID s3887), or a non-targeting negative control siRNA (catalog 4 390 843) were used. A 100 nM concentration of siRNA was diluted with Opti-MEM medium (Fisher Scientific), mixed with 2 μl per well (24-well plate) of TransIT-siQUEST transfection reagent (Mirus Bio LLC, Madison, WI, USA), and incubated for 30 min at room temperature before being added to cells in luteinization medium for 24 h. Cells were then treated as described previously.
Ovine induced and spontaneous luteolysis model
Procedures for collection of CL from sheep during either PGF2α-induced or spontaneous luteolysis have been described previously (Seto and Bogan, 2015).
Microarray database mining
To determine CYP27A1 mRNA expression during spontaneous luteolysis in rhesus macaques, the raw data files (.CEL) from two publically available microarray databases in rhesus macaque CL (Bogan et al., 2008, 2009) were loaded into the Affymetrix Expression Console version 1.0. Expression files (.CHP) were generated after using the robust multichip average (RMA) algorithm for normalization. Normalized mRNA expression data was then searched for probeset ID MmuSTS.4195.1.S1_at, which corresponds to CYP27A1. To determine the effect of LH on CYP27A1 mRNA expression in rhesus macaques, the raw data files from another publically available microarray database (Bishop et al., 2009) were analyzed in the same manner. In this analysis, only files from monkeys treated with the GnRH antagonist antide, monkeys receiving antide and LH replacement, and control monkeys were used (Bishop et al., 2009).
Progesterone extractions and enzyme immunoassay
Progesterone in culture media was extracted and concentrations were determined by enzymatic immunoassay as previously described (Seto and Bogan, 2015). Assay sensitivity is 2.6 pg/well, the intra-assay coefficient of variation is 6–9% (in the range of 20–80% maximum binding or B/B0), and the inter-assay coefficient of variation is 7–11% (20–80% B/B0). The manufacturer of the P4 antibody used for EIA (Cal Bioreagents, San Mateo, CA, USA, catalog P132) reports that cross-reactivity with most steroids including estradiol is <0.1%. Cells were lysed in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Fisher Scientific), and protein concentrations were determined using a bicinchoninic acid (BCA) protein assay (Fisher Scientific) according to the manufacturer recommendations. Total protein concentration was used to normalize P4 in media.
Cholesterol efflux assay
Bodipy cholesterol (2 μg/ml, Avanti Polar Lipids Inc., Alabaster, AL, USA) was added to cells during the 16-h initial treatment period of the 27OH supplementation experiment. Unlabeled cells were used to control for background fluorescence. Media was aspirated and cells were washed once with PBS, and then incubated 4 h in treatment medium as described earlier. Media were collected and cells were lysed using M-PER reagent (Fisher Scientific). The fluorescence from cells and media was quantified, and cholesterol efflux was calculated by the equation 100 × (media fluorescence)/(cell lysate fluorescence + media fluorescence).
LDL uptake assay
Fluorescent Dil-LDL (10 μg/ml, Fisher Scientific) was substituted for LDL in treatment medium during the final 4-h treatment period of the 27OH supplementation experiment, and cells that did not receive Dil-LDL were used to control for background fluorescence. Cells were washed once with PBS and lysed in RIPA buffer. Fluorescence was quantified in the cell lysate, and a BCA protein assay was then performed. Total protein concentration was used to normalize Dil fluorescence values.
Semi-quantitative real-time PCR
Total RNA was extracted with Trizol reagent and purified over RNeasy columns (Fisher Scientific) according to manufacturer recommendations, and RNA concentration was determined by spectrophotometry. The RNA was DNAse treated and reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Fisher Scientific) following the manufacturer recommendations. Primers and Taqman probe sequences are listed in Table I. Taqman MGB probes or SYBR green were used for detection of amplification. Serial dilutions of cDNA pools were used to determine the linearity and efficiency of amplification, and relative mRNA abundance was determined by extrapolating Ct values from the resultant standard curve. Relative mRNA concentrations were normalized to glucose-6-phosphate dehydrogenase (G6PD), which has previously been reported to be invariant in human luteinized granulosa cells (Nio-Kobayashi et al., 2015). However, trilostane dramatically altered G6PD mRNA expression (data not shown), so in experiments where trilostane was used mitochondrial ribosomal protein S10 (MRPS10) was used as an alternative housekeeping gene (Bogan and Hennebold, 2010). In experiments involving Semi-quantitative real-time PCR (QPCR) of sheep CL, MRPS10 was used as the housekeeping control.
Table I.
Primer and Taqman MGB probe sequences used in QPCR analyses.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | MGB probe (5′–3′) or SYBR |
|---|---|---|---|
| Human sequences | |||
| ABCA1 | TCCAGGCCAGTACGGAATTC | TCCTCGCCAAACCAGTAGGA | CTGGTATTTTCCTTGCACCAA |
| ABCG1 | GACCAGCTTTACGTCCTGAGTCA | GTTCAGACCCAAATCCCTCAAAT | AAAGTCTGCAATCTTGTGCC |
| LDLR | CTGGTCAGATGAACCCATCAAAG | GCCGATCTTAAGGTCATTGCA | CCAACGAATGCTTGGAC |
| NR1H2 | ATCGTGGACTTCGCTAAGCAA | GATCTCGATAGTGGATGCCTTCA | TGCCTGGTTTCCTGC |
| NR1H3 | TCCCCATGACCGACTGATG | CAGACGCAGTGCAAACACTTG | TCCCACGGATGCTAAT |
| MRPS10 | TTCCAAAGGATTTGACCAAACC | TCGTGACCTTTCACCAAAACC | ATCTCTGATGAACCAGACAT |
| CYP27A1 | GGCCAAGTACGGTCCAATGT | CGCATCACTTGCTCCAAGAG | SYBR Green |
| HMGCR | AACACGATGCATAGCCATCCT | AAGGCCAGCAATACCCAAAA | SYBR Green |
| STAR | GTGGGTGCCTTCCAGAAATATAGT | TGACTGGTGCCTATGAAAGCAA | SYBR Green |
| SREBF2 | ATCGCTCCTCCATCAATGACA | TCCTCAGAACGCCAGACTTGT | SYBR Green |
| G6PD | GAAGCCGGGCATGTTCTTC | TAGGCGTCAGGGAGCTTCAC | SYBR Green |
| Ovine sequences | |||
| CYP27A1 | TGGAGTAGACACGACATCCAACA | ACGCCCACCACTTCCTTATG | CCCTGTACCATCTTTCAA |
| MRPS10 | TTGAGCTCTCGAGAAATCACTGAA | CTGGGTGCAAACCTAACAAAAGA | ATTGGTTCCTTCATCCTGT |
Statistical analyses
Each individual treatment was administered to duplicate or triplicate wells. Based on power calculations, each experiment was repeated three times for QPCR outcomes, four times for P4 analysis, and six times for cholesterol efflux and LDL uptake experiments using different preparations of cells. An individual cell preparation (n = 1) may consist of cells from one individual or a pool from multiple individuals depending on the cell yield and needs of the experiment. Statistical analysis was performed with mixed effects regression analysis (Stata Version 14) to account for repeated measures. Treatment was set as the fixed effect with cell replicate representing the random effect to account for variation due to different cell preparations. A Bonferroni multiple testing procedure was used for pairwise comparisons with α = 0.05. Data were log-transformed if necessary to equalize variance.
Results
Expression of CYP27A1 mRNA during luteolysis in vivo in rhesus macaques and sheep, and the effects of LH and hCG
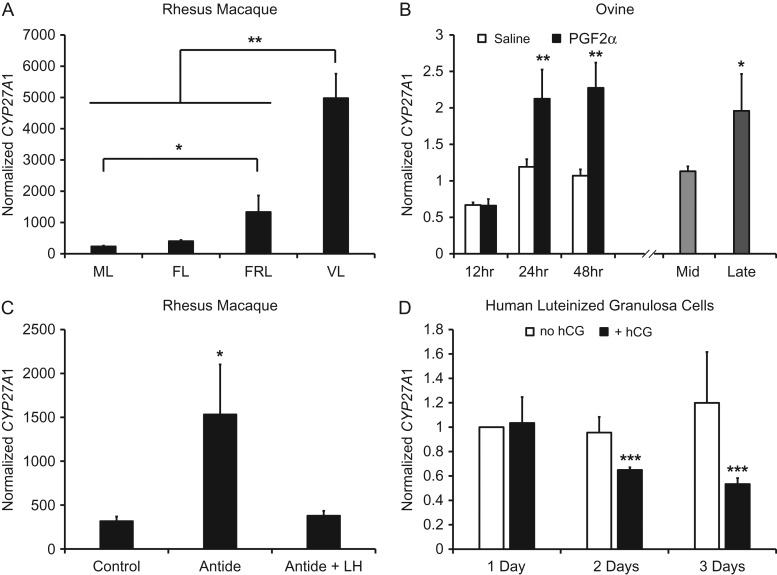
The concentration of CYP27A1 mRNA was evaluated within macaque CL at specific stages during the luteal phase (Fig. 1A). There was a significant increase in CYP27A1 from the mid-late to the functionally regressed late stage, and a further significant increase by the very late stage (menses) (Fig. 1A). During PGF2α-induced luteolysis in sheep, the mRNA expression of CYP27A1 was significantly increased 24 and 48 h after PGF2α-treatment (Fig. 1B). During spontaneous lutelysis in sheep, CYP27A1 was also significantly increased compared to mid-luteal phase CL (Fig. 1B). Ablation of endogenous gonadotropin secretion achieved via antide treatment in macaques caused a significant increase in CYP27A1 mRNA expression, which was returned to control levels with LH replacement (Fig. 1C). Also, treatment of human luteinized granulosa cells with hCG caused a significant reduction in CYP27A1 mRNA after 2 and 3 days of culture (Fig. 1D).
Figure 1.
Expression of CYP27A1 mRNA during luteolysis in rhesus macaques and sheep, and regulation of CYP27A1 mRNA by LH and hCG. Panel (A) shows normalized microarray mRNA expression of CYP27A1 in rhesus macaque CL collected during the luteal phase (ML = mid-late, Days 10–12; FL = functional late, Days 14–16 and P4 > 1.5 ng/ml; FRL = functionally regressed late, Days 14–16 and P4 < 0.5 ng/ml; VL = very late, menses, Days 18–19). Asterisks denote significant differences between groups indicated by brackets. Panel (B) displays CYP27A1 mRNA expression normalized to MRPS10 during PGF2α-induced and spontaneous luteolysis in the ovine CL. The x axis is separated between the induced and spontaneous luteolysis models. Asterisks denote significant differences between saline and PGF2α treatments at the corresponding time point (induced luteolysis), or between mid (Days 9–10) and late (Days 14–16 with serum P4 < 1 ng/ml) luteal phase CL (spontaneous luteolysis). Panel (C) is the effect of LH on CYP27A1 mRNA in rhesus macaques. Antide is a gonadotropin releasing hormone antagonist. The asterisk denotes a significant difference from all other groups. Panel (D) is the effect of hCG on CYP27A1 mRNA in human luteinized granulosa cells. Data are displayed as the fold change relative to the no hCG, 1 Day, group. Asterisks denote a significant difference between treatments on the corresponding day. For all panels, error bars indicate one standard error of the mean (SEM); *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of 27OH on P4 secretion, cholesterol efflux and LDL uptake
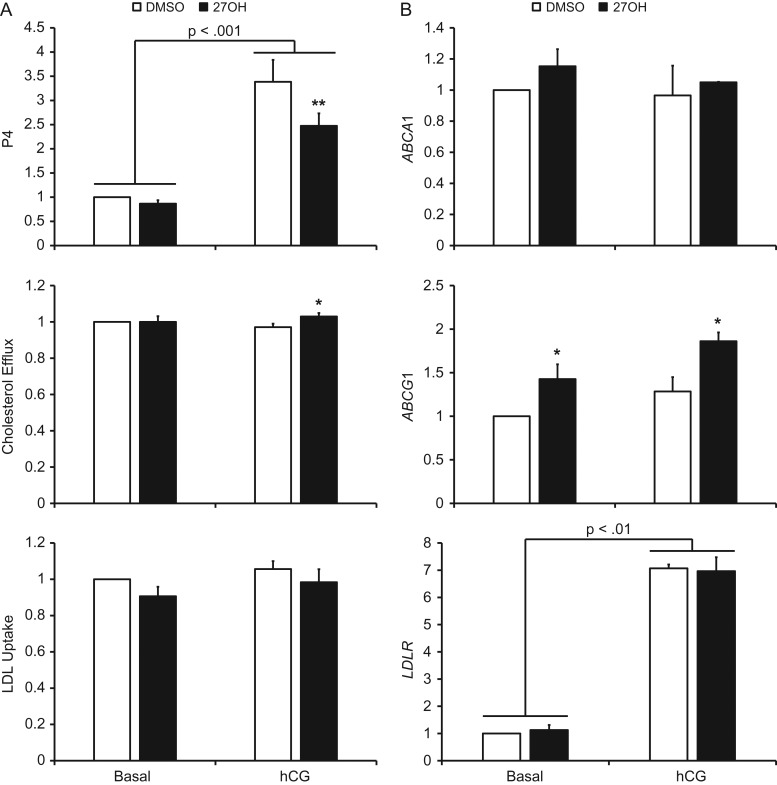
In the presence of hCG, P4 secretion was significantly decreased by 27OH. In the absence of hCG, 27OH did not significantly affect P4 synthesis (Fig. 2A). Cholesterol efflux was significantly increased by 27OH in the presence of hCG, while there was no significant effect of 27OH on LDL uptake (Fig. 2A). There were no significant effects of 27OH on mRNA expression of either LXR isoform, SREBF2 or its target gene HMGCR (data not shown). Furthermore, there were no significant effects of 27OH on ABCA1 or LDLR mRNA expression, however, 27OH significantly increased ABCG1 in the presence and absence of hCG (Fig. 2B).
Figure 2.
The effect of 27OH on P4 secretion and markers of LXR and SREBP2 activity in human luteinized granulosa cells. Panel (A) displays the effect of 27OH on P4 secretion and cholesterol metabolism in the presence and absence of hCG. Panel (B) shows QPCR results of the LXR target genes ABCA1 and ABCG1, as well as the SREBP2 target gene LDLR. For all charts, data are displayed as the fold change relative to DMSO-treated cells in the absence of hCG. Error bars indicate one SEM. Brackets indicate a significant difference between indicated groups due to hCG, and asterisks indicate significant differences due to 27OH within the respective hCG exposure status; *P < 0.05, **P < 0.01.
Effect of pharmacologic inhibition of steroidogenesis on LXR and SREBP2 target gene mRNA expression
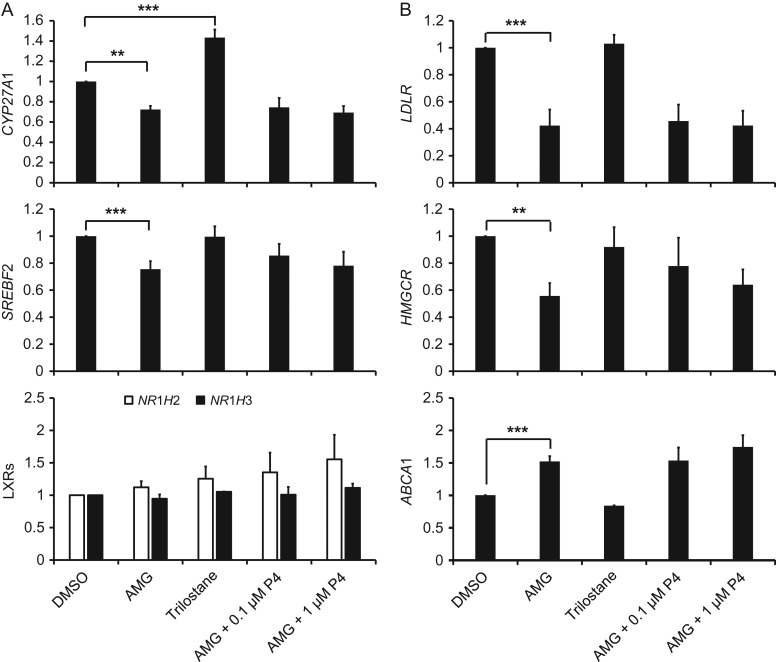
Aminoglutethimide caused a significant decrease in CYP27A1 and SREBF2, but did not affect either LXR isoform (Fig. 3A). Trilostane caused a significant increase in CYP27A1, but did not alter SREBF2 or the LXRs. The SREBP2 target genes LDLR and HMGCR were both significantly reduced by aminoglutethimide, while the LXR target gene ABCA1 was significantly increased (Fig. 3B). Another LXR target gene ABCG1 displayed a similar pattern of mRNA expression as ABCA1, but its differences were not statistically significant (data not shown). Addition of 0.1 or 1 μM P4 (Fig. 3) or pregnenolone (data not shown) to the culture media had no significant effect on aminoglutethimide-induced changes in mRNA expression of any genes. There was no effect of trilostane on LDLR, HMGCR or ABCA1 (Fig. 3B).
Figure 3.
The effects of pharmacologic inhibitors of steroidogenesis and steroid replacement on mRNA expression of genes involved in SREBP2 and LXR-mediated cholesterol metabolism in human luteinized granulosa cells. Panel (A) displays QPCR data for CYP27A1, SREBF2, and both LXR isoforms. Panel (B) displays the SREBP2 target genes LDLR and HMGCR, as well as the LXR target gene ABCA1. For all charts, data are displayed as the fold change relative to DMSO. Error bars indicate one SEM, and asterisks indicate significant differences between groups connected by brackets; **P < 0.01, ***P < 0.001. Neither dose of P4 replacement significantly altered the mRNA expression of any gene relative to the aminoglutethimide-only treatment.
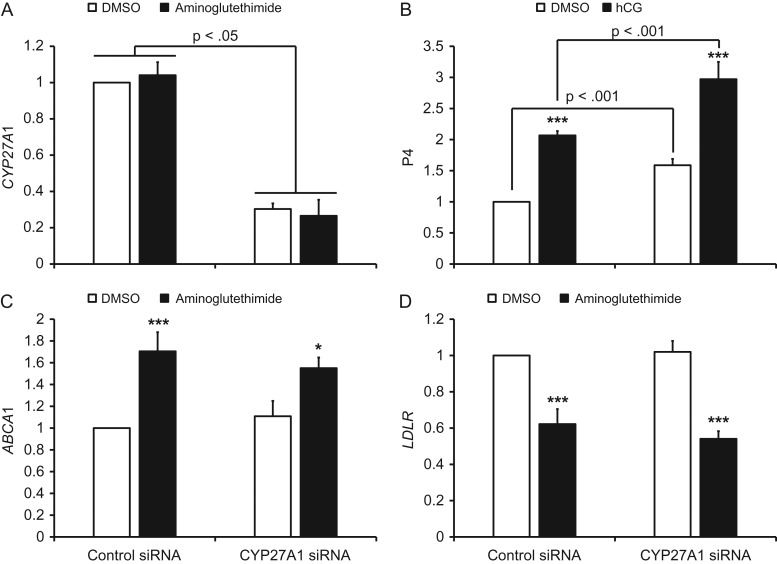
Effect of CYP27A1 knockdown on P4 secretion and aminoglutethimide-induced gene expression
The mRNA expression of CYP27A1 mRNA was significantly decreased approximately 70% by transfection with the CYP27A1 siRNA (Fig. 4A). Secretion of P4 was significantly increased in the presence or absence of hCG in CYP27A1 knockdown cells (Fig. 4B). Aminoglutethimide significantly increased ABCA1 and decreased LDLR mRNA expression in cells transfected with the control siRNA, and the aminoglutethimide effect was not prevented by CYP27A1 knockdown (Fig. 5C and D).
Figure 4.
The effect of CYP27A1 knockdown on P4 secretion and aminoglutethimide-induced changes in LXR and SREBP2 target gene mRNA expression in human luteinized granulosa cells. Panel (A) displays QPCR data for CYP27A1 and panel (B) is P4 secretion in the presence and absence of hCG. Panels (C and D) are ABCA1 and LDLR mRNA expression in response to aminoglutethimide, respectively. For all charts, data are displayed as the fold change relative to DMSO-treated, control siRNA-transfected cells. Error bars indicate one SEM. Brackets indicate significant differences between the indicated groups due to CYP27A1 siRNA transfection, and asterisks denote significant differences due to hCG (Panel B) or aminoglutethimide (Panels C, D) within the respective siRNA transfection group; *P < 0.05, ***P < 0.001.
Figure 5.
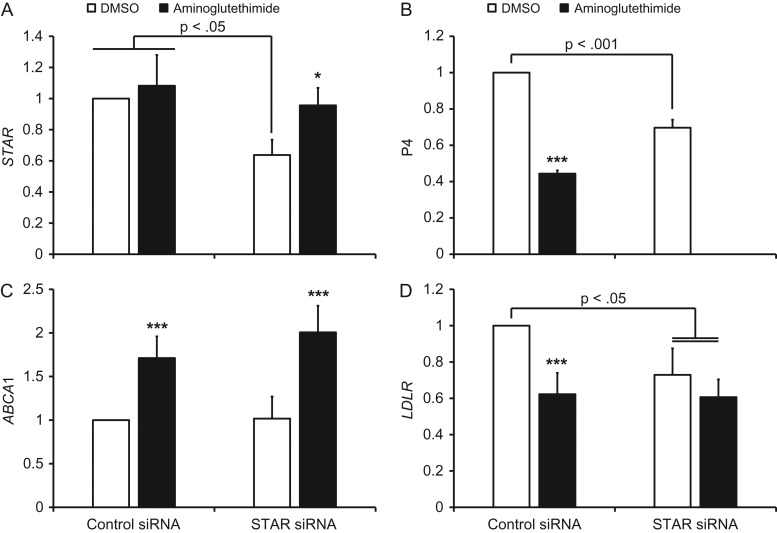
The effect of STAR knockdown on aminoglutethimide-induced changes in LXR and SREBP2 target gene mRNA expression in human luteinized granulosa cells. Panel (A) displays QPCR data for STAR and panel (B) is P4 secretion. Panels (C and D) are ABCA1 and LDLR mRNA expression in response to aminoglutethimide, respectively. For each chart, data are displayed as the fold change relative to DMSO-treated, control siRNA-transfected cells. Error bars indicate one SEM. Brackets indicate significant differences between the indicated groups due to STAR siRNA transfection, and asterisks denote significant differences due to aminoglutethimide within the respective siRNA transfection group; *P < 0.05, ***P < 0.001.
Effect of STAR knockdown on aminoglutethimide-induced gene expression
The mRNA expression of STAR was significantly decreased ~35–40% by transfection with the STAR siRNA in the absence of aminoglutethimide compared to cells transfected with control siRNA (Fig. 5A). In the presence of aminoglutethimide there was no significant knockdown of STAR, which was due to a single replicate that had ~25% higher STAR mRNA expression in the knockdown compared to the control cells. Synthesis of P4 was significantly decreased in STAR knockdown cells compared to the control siRNA in the absence of aminoglutethimide (Fig. 5B), and aminoglutethimide significantly decreased P4 in cells treated with control siRNA. The LXR target gene ABCA1 was significantly increased by aminoglutethimide, while the STAR siRNA did not prevent this effect on ABCA1 transcription (Fig. 5C). The LDLR was significantly decreased by aminoglutethimide in control siRNA-transfected cells. Knockdown of STAR significantly decreased LDLR mRNA expression relative to the control siRNA in the absence of aminoglutethimide, and was not different from aminoglutethimide treatment (Fig. 5D).
Discussion
A summary of the data and our proposed interpretations are provided in Fig. 6. The mRNA expression of CYP27A1 is significantly increased in both rhesus and ovine CL during luteolysis in vivo (Fig. 1). This is consistent with a previous report that luteal 27OH concentrations are increased during spontaneous luteolysis in the rhesus macaque CL (Bogan et al., 2012). However, it is not clear whether an increase in CYP27A1 mRNA expression and activity is a cause or effect of luteolysis. In this study, 27OH itself significantly decreased P4 secretion (Fig. 2A), while CYP27A1 knockdown significantly increased P4 synthesis (Fig. 4B). This indicates that 27OH produced via CYP27A1 inhibits P4 secretion, and thus could actively facilitate the progression of luteolysis.
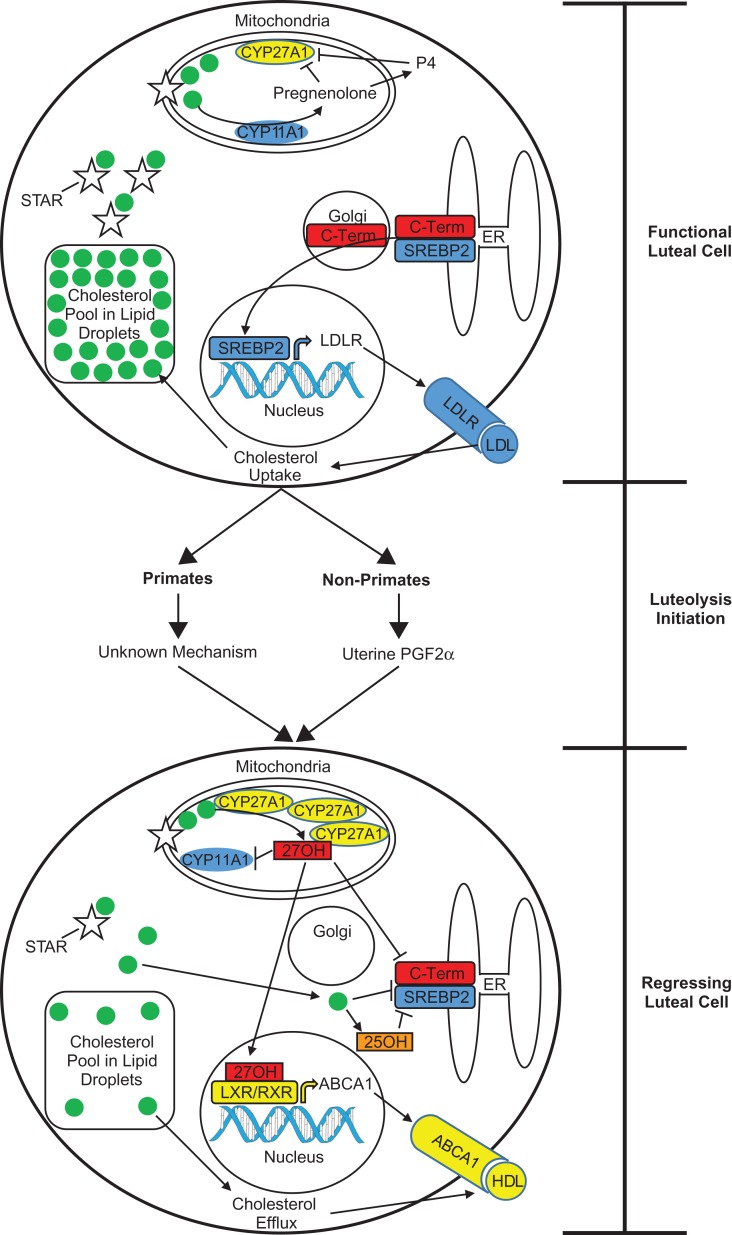
Figure 6.
Proposed model of luteolysis in primate and non-primate species. During the luteal phase, functional luteal cells have a large reserve of cholesterol stored in lipid droplets. Free cholesterol is transported into the mitochondria via STAR, and converted into pregnenolone by CYP11A1 (aka, p450 side-chain-cleavage). Pregnenolone leaves the mitochondria and is converted to P4, and both steroids inhibit the enzyme activity of CYP27A1. Because cholesterol is effectively sequestered by lipid droplets and STAR, SREBP2 is transported from the endoplasmic reticulum (ER) to the Golgi where the c-terminal regulatory region is cleaved. The active transcription factor travels to the nucleus and stimulates LDLR transcription, ultimately resulting in increased LDL uptake and maintenance of cholesterol reserves. Luteolysis is initiated by uterine-derived PGF2α in non-primate species, whereas the mechanism initiating luteolyis in primates is not known. Regardless of the differences in the initiation of luteolysis, the mechanisms mediating the regression of luteal cells may be similar. Elevated CYP27A1 protein concentrations in mitochondria cause increased conversion of cholesterol into 27OH, which subsequently inhibits SREBP2 proteolytic processing and is an agonist for LXR/RXR (retinoid x receptor) heterodimers. This results in increased transcription of LXR target genes including ABCA1. There may also be direct inhibition of CYP11A1 enzymatic activity and steroidogenesis by mitochondrial 27OH. Additionally, decreased STAR expression contributes to reduced cholesterol sequestration and increased association of free cholesterol with the ER. This prevents SREBP2 proteolytic processing, possibly due to cholesterol binding to SCAP and/or increased 25OH synthesis from the ER-localized cholesterol 25-hydroxylase enzyme. The net result of impaired LDL uptake and enhanced cholesterol efflux is a depletion of cholesterol reserves and a gradual loss in steroidogenic capacity.
Several factors may contribute to the increase in CYP27A1 mRNA expression during luteolysis. In primates, luteolysis is associated with a decreased sensitivity to LH and a more potent LH receptor ligand (i.e. hCG) is needed to prevent luteolysis (Stouffer et al., 2013). Inhibiting endogenous LH release in rhesus macaques caused a significant increase in CYP27A1 while LH replacement reversed this effect (Fig. 1C), and hCG significantly reduced CYP27A1 mRNA expression over time in human luteinized granulosa cells (Fig. 1D). These observations indicate that LH and hCG suppress CYP27A1 mRNA expression, and thus a loss in LH sensitivity may underlie the observed increase during luteolysis. Because CYP27A1 mRNA increased in both rhesus macaques and sheep during luteolysis in vivo, this indicates that there may be a common pathway controlling CYP27A1 transcription in both species. It is known that LH and hCG activate protein kinase A (PKA) (Ascoli et al., 2002), and PGF2α decreases PKA activity in the ovine CL (Agudo et al., 1984). Thus, decreased PKA activity may be a common mechanism that results in increased CYP27A1 mRNA expression during luteolysis in primate and non-primate species. Finally, changes in cellular composition of the CL during luteolysis may contribute to increased CYP27A1 mRNA concentrations. It is known that macrophages invade the CL during luteolysis in many different species (Pate and Landis Keyes, 2001), and macrophages express CYP27A1 (Fu et al., 2001).
The steroids pregnenolone and P4 may also directly regulate CYP27A1 transcription and enzymatic activity. To test this, inhibitors of steroidogenesis were used. Aminoglutethimide blocks the conversion of cholesterol to pregnenolone by inhibiting the cholesterol side-chain cleavage enzyme CYP11A1 (cytochrome P450 family 11 subfamily A member 1) (Rennert et al., 1990). Trilostane inhibits P4 production by blocking the conversion of pregnenolone into P4 by the 3β-hydroxysteroid dehydrogenase enzyme system (Komanicky et al., 1978). Aminoglutethimide significantly decreased but trilostane significantly increased CYP27A1 mRNA (Fig. 3A). Thus, data regarding the effect of pregnenolone and P4 on CYP27A1 transcription are conflicting, but changes in mRNA expression do not necessarily correspond with enzymatic activity. It has previously been demonstrated that aminoglutethimide increases 27OH formation while pregnenolone and P4 replacement both inhibit the enzyme activity of CYP27A1 in isolated rat ovarian mitochondria (Rennert et al., 1990). Because trilostane only blocks P4 production and does not affect pregnenolone synthesis, it would not be expected to result in increased CYP27A1 enzyme activity. Consistent with this, inhibiting P4 production by trilostane did not affect the mRNA expression of ABCA1 and LDLR even though it significantly increased CYP27A1 mRNA (Fig. 3). In contrast, ABCA1 was significantly increased while LDLR was significantly decreased by aminoglutethimide (Fig. 3B), consistent with an increase in endogenous oxysterol concentrations. This is not consistent with the aminoglutethimide effect on CYP27A1 mRNA expression, but is consistent with the previous report that aminoglutethimide increases CYP27A1 enzymatic activity (Rennert et al., 1990). Furthermore, endogenous concentrations of other oxysterols likely increase during aminoglutethimide treatment. For example, CYP27A1 knockdown did not prevent the effect of aminoglutethimide on ABCA1 and LDLR mRNA expression (Fig. 4C and D), indicating that oxysterols other than 27OH may be involved. Indeed, 22R-hydroxycholesterol is an LXR agonist that is an intermediate product in the conversion of cholesterol to pregnenolone by CYP11A1 (Wojcicka et al., 2007), so aminoglutethimide treatment may result in increased escape of 22R-hydroxycholesterol from the mitochondria. Also, more 25OH may be produced as cholesterol accumulates during aminoglutethimide treatment, and 25OH is the most potent SREBP inhibitor (Russell, 2000; Goldstein et al., 2006). Replacing P4 or pregnenolone did not reverse the effects of aminoglutethimide. This may be because the steroids were added to the culture media and were not able to enter the mitochondria in sufficient quantities to inhibit CYP27A1 enzymatic activity. Also, because of the possibility that multiple oxysterols mediate the aminoglutethimide effect, steroid replacement may not prevent the formation of these additional oxysterols. Due to the limited availability of luteinized granulosa cells, we were not able to directly quantify 27OH production or concentrations of other oxysterols. Collectively, these data indicate that steroidogenesis in functional luteal cells may suppress 27OH formation by reducing CYP27A1 enzymatic activity (Fig. 6), as well as by potentially inhibiting generation of additional oxysterols.
We also wanted to determine how interrupting cholesterol transport into the mitochondria would affect LXR and SREBP activity. There was a modest 35–40% knockdown of STAR in human luteinized granulosa cells (Fig. 5A). However, the knockdown of STAR was secondarily validated by the significant decrease in P4 concentrations (Fig. 5B). Knockdown of STAR significantly decreased LDLR mRNA expression (Fig. 5D), indicating it inhibited SREBP processing. The STAR protein plays a critical role in the PKA-induced transport of cholesterol across the mitochondrial membrane, the rate-limiting step in steroidogenesis (Manna et al., 2016). While knockdown of STAR would prevent cholesterol transport into the mitochondria where CYP27A1 resides, it would also result in accumulation of free cholesterol outside the mitochondria. This may increase cholesterol binding to the sterol-sensing domain of the SREBP chaperone protein known as SCAP (SREBP cleavage activating protein), which would prevent shuttling of SREBPs to the Golgi (Goldstein et al., 2006). Also, 25OH concentrations may increase because cholesterol 25-hydroxylase is localized to the endoplasmic reticulum membrane (Lund et al., 1998), and 25OH would prevent SREBP processing (Russell, 2000; Goldstein et al., 2006). Collectively, this indicates that inhibiting STAR function during luteolysis may result in reduced SREBP activity due to the accumulation of extra-mitochondrial free cholesterol and/or 25OH (Fig. 6).
We hypothesized that a luteolytic effect of 27OH produced via CYP27A1 would be due to its activation of the LXRs (Fu et al., 2001) and inhibition of SREBP processing (Russell, 2000). However, the effects of 27OH on cholesterol efflux, uptake and LXR and SREBP2 target gene mRNA expression were modest and did not appear sufficient to explain its effect on P4 secretion. One possible explanation for this observation is that the dose of 27OH used in the current study (2 μM) is well within the physiologic range previously detected in the macaque CL during spontaneous luteolysis (up to 5 μM) (Bogan et al., 2012), and a 5 μM dose of 27OH significantly inhibited LDLR mRNA and protein while a 1 μM dose did not in primary macaque luteal cells (Bogan et al., 2012). Thus, a higher physiologically relevant dose of 27OH than that used in the current study would likely result in more dramatic effects on cholesterol efflux and LDL uptake. Because some oxysterols will serve as substrates for P4 synthesis (Wiltbank et al., 1989; Belfiore et al., 1994), a dose of 27OH approximating the average of physiologic concentrations was selected in this study to limit the possibility of it being converted to P4. Nevertheless, it appears that 27OH suppresses P4 synthesis by other mechanisms as well. This could include direct effects of 27OH on the steroidogenic machinery and the mitochondrial environment. In support of this, 25OH treatment of rat leydig cells has been shown to dramatically suppress CYP11A1 enzymatic activity (Georgiou et al., 1987), and thus it is likely that 27OH produced within the mitochondria would similarly repress CYP11A1 activity (Fig. 6).
There are obvious differences between luteolysis in sheep and primates as it has been clearly demonstrated that uterine-derived PGF2α causes luteolysis in sheep, while in primates the uterus is not involved in initiating luteolysis and the role of PGF2α, if any, is uncertain (Stouffer et al., 2013; Stouffer and Hennebold, 2015). However, while the initiation of luteolysis may differ, the mediation of luteolysis via increased CYP27A1 activity may be a common feature in both species. In the current study, the mRNA expression of CYP27A1 was significantly increased in vivo during luteolysis in both rhesus macaques and sheep. Additionally, the effects of aminoglutethimide on ABCA1 and LDLR mRNA expression in the current study are very similar to the magnitude of changes in these genes observed during PGF2α-induced and spontaneous luteolysis in sheep (Seto and Bogan, 2015). It has been demonstrated in cattle that multiple pulses of PGF2α are necessary to cause luteolysis with initial pulses causing transient declines in P4 secretion followed by a rebound in P4 production (Ginther et al., 2010a, 2010b; Ginther and Beg, 2012). This rebound capability is gradually lost with exposure to repeated pulses of PGF2α (Ginther et al., 2010a, 2010b; Ginther and Beg, 2012). Findings from the current study support the possibility that CYP27A1 may mediate this loss in the capability of the CL to resume P4 secretion following repeated PGF2α pulses in cattle. Therefore, although the mechanism initiating luteolysis differs between primates and domestic species such as cattle and sheep, increased mRNA expression and activity of CYP27A1 may be a common feature that ensures sustained suppression of P4 secretion (Fig. 6).
Overall, these data support the hypothesis that CYP27A1-derived 27OH may contribute to the P4 decrease during luteolysis, partially by activating the LXRs and inhibiting SREBP2. However, other oxysterols are also likely involved. The roles of other endogenous oxysterols in mediating cholesterol metabolism and P4 production during luteolysis awaits further clarification. Also, the mechanisms that lead to the initial rise in CYP27A1 mRNA expression and 27OH production during luteolysis need further elucidation.
Authors’ roles
The overall study concept and design of the experiments was performed by R.L.B. Follicular aspirates were obtained by J.J.H. and S.M.H. Data acquisition was performed by Y.X., and analysis and interpretation of data was performed by Y.X. and R.L.B. The article was drafted and edited by Y.X. and R.L.B., with additional editing by J.J.H. and S.M.H. All authors approved the final article.
Funding
Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Award number R00HD067678) to R.L.B.
Conflicts of interest
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank the patients and staff at the Reproductive Health Center in Tucson for follicular aspirates. The authors would also like to thank Nickie L. Seto who performed QPCR of CYP27A1 in sheep CL.
References
- Agudo LS, Zahler WL, Smith MF. Effect of prostaglandin F2 alpha on the adenylate cyclase and phosphodiesterase activity of ovine corpora lutea. J Anim Sci 1984;58:955–962. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 2002;23:141–174. [DOI] [PubMed] [Google Scholar]
- Belfiore CJ, Hawkins DE, Wiltbank MC, Niswender GD. Regulation of cytochrome P450scc synthesis and activity in the ovine corpus luteum. J Steroid Biochem Mol Biol 1994;51:283–290. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Hennebold JD, Stouffer RL. The effects of luteinizing hormone ablation/replacement versus steroid ablation/replacement on gene expression in the primate corpus luteum. Mol Hum Reprod 2009;15:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Debarber AE, Hennebold JD. Liver x receptor modulation of gene expression leading to proluteolytic effects in primate luteal cells. Biol Reprod 2012;86:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Hennebold JD. The reverse cholesterol transport system as a potential mediator of luteolysis in the primate corpus luteum. Reproduction 2010;139:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Hennebold JD. Dynamic changes in gene expression that occur during the period of spontaneous functional regression in the rhesus macaque corpus luteum. Endocrinology 2009;150:1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol 2008;22:1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 2001;276:38378–38387. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Perkins LM, Payne AH. Steroid synthesis-dependent, oxygen-mediated damage of mitochondrial and microsomal cytochrome P-450 enzymes in rat Leydig cell cultures. Endocrinology 1987;121:1390–1399. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Beg MA. Dynamics of circulating progesterone concentrations before and during luteolysis: a comparison between cattle and horses. Biol Reprod 2012;86:170. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Shrestha HK, Fuenzalida MJ, Shahiduzzaman AK, Beg MA. Characteristics of pulses of 13,14-dihydro-15-keto-prostaglandin f2alpha before, during, and after spontaneous luteolysis and temporal intrapulse relationships with progesterone concentrations in cattle. Biol Reprod 2010. a;82:1049–1056. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Shrestha HK, Fuenzalida MJ, Shahiduzzaman AK, Hannan MA, Beg MA. Intrapulse temporality between pulses of a metabolite of prostaglandin F 2alpha and circulating concentrations of progesterone before, during, and after spontaneous luteolysis in heifers. Theriogenology 2010. b;74:1179–1186. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 2006;124:35–46. [DOI] [PubMed] [Google Scholar]
- Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 2014;13:433–444. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komanicky P, Spark RF, Melby JC. Treatment of Cushing’s syndrome with trilostane (WIN 24,540), an inhibitor of adrenal steroid biosynthesis. J Clin Endocrinol Metab 1978;47:1042–1051. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Joseph SB, Walczak R, Pei L, Wilpitz DC, Collins JL, Tontonoz P. Autoregulation of the human liver X receptor alpha promoter. Mol Cell Biol 2001. a;21:7558–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 2001. b;98:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, Kerr TA, Sakai J, Li WP, Russell DW. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J Biol Chem 1998;273:34316–34327. [DOI] [PubMed] [Google Scholar]
- Lund EG, Menke JG, Sparrow CP. Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:1169–1177. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016;51:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Trendell J, Giakoumelou S, Boswell L, Nicol L, Kudo M, Sakuragi N, Iwanaga T, Duncan WC. Bone morphogenetic proteins are mediators of luteolysis in the human corpus luteum. Endocrinology 2015;156:1494–1503. [DOI] [PubMed] [Google Scholar]
- Pate JL, Landis Keyes P. Immune cells in the corpus luteum: friends or foes? Reproduction 2001;122:665–676. [DOI] [PubMed] [Google Scholar]
- Rennert H, Fischer RT, Alvarez JG, Trzaskos JM, Strauss JF 3rd. Generation of regulatory oxysterols: 26-hydroxylation of cholesterol by ovarian mitochondria. Endocrinology 1990;127:738–746. [DOI] [PubMed] [Google Scholar]
- Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta 2000;1529:126–135. [DOI] [PubMed] [Google Scholar]
- Seto NL, Bogan RL. Decreased cholesterol uptake and increased liver X receptor-mediated cholesterol efflux pathways during prostaglandin F2 alpha-induced and spontaneous luteolysis in sheep. Biol Reprod 2015;92:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 1997;99:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Vandevoort CA. Simulation of human luteal endocrine function with granulosa lutein cell culture. J Clin Endocrinol Metab 1997;82:3078–3083. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Bishop CV, Bogan RL, Xu F, Hennebold JD. Endocrine and local control of the primate corpus luteum. Reprod Biol 2013;13:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer RL, Hennebold JD. Structure, function, and regulation of the corpus luteum In: Plant TM, Zeleznik AJ (eds). . Knobil and Neill’s Physiology of Reproduction. San Diego: Academic Press, 2015:1023–1076. [Google Scholar]
- Wiltbank MC, Knickerbocker JJ, Niswender GD. Regulation of the corpus luteum by protein kinase C. I. Phosphorylation activity and steroidogenic action in large and small ovine luteal cells. Biol Reprod 1989;40:1194–1200. [DOI] [PubMed] [Google Scholar]
- Wojcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online) 2007;61:736–759. [PubMed] [Google Scholar]