Background:
Lifestyle factors may be associated with chemotherapy-induced peripheral neuropathy (CIPN). We examined associations between body mass index (BMI) and lifestyle factors with CIPN in the Pathways Study, a prospective cohort of women with invasive breast cancer.
Methods: Analyses included 1237 women who received taxane treatment and provided data on neurotoxicity symptoms. Baseline interviews assessed BMI (normal: <25 kg/m2; overweight: 25–29.9 kg/m2; obese: ≥30 kg/m2), moderate-to-vigorous physical activity (MVPA) (low: <2.5; medium: 2.5–5; high: >5 hours/week) and fruit/vegetable intake (low: <35 servings/week; high: ≥35 servings/week). Baseline and six-month interviews assessed antioxidant supplement use (nonuser, discontinued, continued user, initiator). CIPN was assessed at baseline, six months, and 24 months using the Functional Assessment of Cancer Therapy–Taxane Neurotoxicity (FACT-NTX); a 10% decrease was considered clinically meaningful.
Results: At baseline, 65.6% of patients in the sample were overweight or obese, 29.9% had low MVPA, 57.5% had low fruit/vegetable intake, and 9.5% reported antioxidant supplement use during treatment. In multivariable analyses, increased CIPN was more likely to occur in overweight (odds ratio [OR] = 2.37, 95% confidence interval [CI] = 1.19 to 4.88) and obese patients (OR = 3.21, 95% CI = 1.52 to 7.02) compared with normal weight patients at 24 months and less likely to occur in patients with high MVPA compared with those with low MVPA at six (OR = 0.56, 95% CI = 0.34 to 0.94) and 24 months (OR = 0.43, 95% CI = 0.21 to 0.87). Compared with nonusers, patients who initiated antioxidant use during treatment were more likely to report increased CIPN at six months (OR = 3.81, 95% CI = 1.82 to 8.04).
Conclusions: Obesity and low MVPA were associated with CIPN in breast cancer patients who received taxane treatment.
Taxane-based chemotherapy causes chemotherapy-induced peripheral neuropathy (CIPN) (1,2), which can limit chemotherapy dose, decrease quality of life, and cause long-term side effects. Grade 2–3 CIPN occurs in 15% to 23% of breast cancer patients who receive taxanes (3), and an estimated 67% of patients have persistent symptoms up to 12 months after chemotherapy (3). Currently no effective methods prevent CIPN, and duloxetine is the only agent showing any benefit in reducing painful neuropathy (2). CIPN symptoms are associated with drug type, dose received, age, comorbidities, and genetic factors (4–7), but many factors associated with CIPN onset and persistence are understudied (2).
A growing body of literature suggests that lifestyle factors, such as body mass index (BMI) (8–10), physical activity (11,12), and use of dietary supplements (13–15), may affect the onset and severity of CIPN in a variety of cancer patient populations. Physical activity (11,16,17) and diet (18–20) have been associated with diabetic neuropathy, increasing the plausibility that lifestyle factors may be associated with CIPN. To date, the effects of multiple lifestyle factors have not been simultaneously examined in a prospective cohort of breast cancer patients.
The Pathways Study is a prospective cohort study of 4505 women with a first diagnosis of invasive breast cancer recruited from Kaiser Permanente Northern California (KPNC) (21). We used data from Pathways Study participants who received taxane treatment to prospectively assess associations of baseline BMI, physical activity, fruit/vegetable intake, and antioxidant supplement use during chemotherapy with CIPN symptoms.
Methods
Study Design and Population
Pathways Study subjects were recruited from KPNC between January 2006 and April 2013. Women were eligible if they were age 21 years or older; current KPNC members; recently diagnosed with invasive breast cancer; had no prior history of malignant cancer; spoke English, Spanish, Cantonese, or Mandarin; and lived within a 65-mile radius of a field interviewer. To enroll women prior to adjuvant therapy initiation, the KPNC electronic health record (EHR) was used to rapidly ascertain all new patients diagnosed with malignant breast cancer. Breast cancer diagnosis and patient notification of diagnosis were manually verified through electronic record review. Patients were contacted for recruitment with passive physician consent and provided written consent at the baseline interview. All study procedures were approved by the institutional review boards at Columbia University, KPNC, University of California-San Francisco and Roswell Park Cancer Institute.
Data Collection
Baseline sociodemographics, diet, physical activity, smoking, established breast cancer risk factors, health history, and use of vitamin/mineral supplements were assessed via interviewer and self-administered questionnaires at enrollment, an average of two months postdiagnosis. Baseline anthropometric measures were obtained from the EHR and questionnaires. At six and 24 months, follow-up materials soliciting the same information collected at baseline were mailed to participants, with interviewer assistance offered if needed. Clinical and tumor characteristics were obtained from the KPNC Cancer Registry approximately four months postdiagnosis.
BMI
Baseline BMI was calculated using weight and height data obtained from the EHR in the six months prior to and two months following breast cancer diagnosis. Baseline self-reported weight and height were used to calculate BMI for participants who did not have EHR data (n = 28). BMI was categorized as normal (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2) (22).
Physical Activity
Baseline physical activity data were collected using the Arizona Activity Frequency Questionnaire (23), which assesses frequency and duration of daily household, recreational, transportation, and sedentary activities. Each activity is assigned a standard metabolic equivalent value (MET) (24). Moderate-to-vigorous physical activity (MVPA) was defined as activities of three or more METs (25). MVPA was categorized as low (<2.5 hours/week), medium (2.5–5 hours/week), and high (>5 hours/week) (26).
Fruit and Vegetable Intake
A modified version of the Block 2005 food frequency questionnaire (FFQ; NutritionQuest, Berkeley, CA) assessed baseline diet via 139 food items with foods representative of Hispanic and Asian diets. Fruit and vegetable (F/V) intake was dichotomized as low (<35 servings/week) and high (≥35 servings/week) (27).
Antioxidant Dietary Supplement Use
Antioxidant dietary supplement use and dose were ascertained via self-report on baseline and six-month questionnaires, including beta-carotene, selenium, vitamin C, vitamin E, and zinc. Participants who reported use of any of the above antioxidants at six months and reported use during chemotherapy treatment were coded as "yes" for antioxidant use during chemotherapy.
A variable was created to indicate whether a participant’s use of antioxidants changed from the time prior to breast cancer diagnosis to the time of chemotherapy. Using baseline and six-month data, participants were categorized as initiators (any antioxidant use during chemotherapy but not prior to diagnosis), discontinuers (antioxidant use before diagnosis but not during chemotherapy), continuous users (any antioxidant before diagnosis and during chemotherapy), and nonusers of antioxidants.
Chemotherapy-Induced Peripheral Neuropathy
At baseline, six months, and 24 months, the 11-item neurotoxicity component of the Functional Assessment of Cancer Therapy-Taxane (FACT-NTX) scale assessed CIPN symptoms (28,29). For each item, self-reported symptoms were assessed in the prior seven days using a 0–4 scale (0 = very much; 1 = quite a bit; 2 = somewhat; 3 = a little bit; 4 = not at all). The total FACT-NTX score sums responses for each item (range = 0–44), with lower scores indicating worse neuropathy. A 10% or greater decrease in the FACT-NTX score was considered a clinically meaningful increase in CIPN (14,30). As taxanes primarily affect the sensory component of patient-reported neuropathy (29), self-reported sensory neuropathy was assessed using the four-item sensory subscale of the FACT-NTX, which assesses numbness, tingling, and discomfort in hands and feet (range = 0–16).
Covariates and Potential Confounders
Sociodemographic, clinical, and behavioral characteristics were examined as potential confounders. Sociodemographic characteristics included age, race, education, and household income. Breast cancer and other clinical characteristics included family history of breast cancer, American Joint Committee on Cancer (AJCC) stage (31,32), number of positive lymph nodes, tumor estrogen/progesterone receptor (ER/PR) positivity, human epidermal growth factor-receptor 2 (HER2) positivity, taxane drug, taxane schedule, breast cancer surgery type, other treatments received (ie, chemotherapy, hormonal therapy, radiation therapy), and Charlson comorbidity score (33). Behavioral characteristics included self-reported smoking history.
Statistical Methods
The primary analytic outcomes were between group differences in mean and clinically meaningful changes in FACT-NTX scores from baseline to six and 24 months. Secondary analyses examined between-group differences in the FACT-NTX sensory neuropathy subscale. Unadjusted and adjusted linear regression models tested differences between groups in absolute change from baseline. Fisher’s exact test and multivariable logistic regression evaluated associations between BMI, MVPA, F/V intake, and antioxidant supplement use with a 10% or greater increase in CIPN at six and 24 months. Covariates identified as a priori potential confounders to include in multivariable models were baseline FACT-NTX, age, race, and smoking. Additional covariates were selected if they were associated with the exposure or outcome, or if they modified the unadjusted associations by more than 10%. The final model included baseline FACT-NTX, age, race, education, income, tumor stage, number of positive nodes, taxane drug, taxane schedule, prior taxane treatment, and number of comorbidities.
Sensitivity analyses examined whether study findings held when participants were limited to women who did not initiate taxane prior to baseline.
All tests were two-sided with α value at .05. All analyses were conducted utilizing R statistical software (version 3.2.2, https://cran.r-project.org/).
Results
Cohort Characteristics
A total of 4505 women enrolled in the Pathways Study cohort. Nearly one-third (n = 1237) received taxane chemotherapy and provided FACT-NTX baseline data. For the 1237 participants included in these analyses, the mean (SD) age at diagnosis was 54 (±10.6) years, and the mean (SD) time from diagnosis to enrollment was 2.2 (±0.7) months (range = 0.8–5.4). A large majority of the women (78.2%) initiated taxane chemotherapy following the baseline interview. Table 1 summarizes participant characteristics. At baseline, 65.6% of patients were overweight or obese, 29.9% had low MVPA, 57.5% had low F/V intake, 31.8% reported using antioxidants prior to breast cancer diagnosis, and 9.5% specifically reported concurrent antioxidant use during chemotherapy. Among the 1237 participants, 63.3% were nonusers of antioxidants, 27.2% were discontinuers, 5.2% were continuous users, and 4.3% were initiators. The most commonly used antioxidant supplement during chemotherapy was vitamin C (6.8%), followed by vitamin E (2.4%), zinc (2.7%), selenium (0.8%), and beta-carotene (0.5%; data not shown). Age and race were the demographic factors with the strongest associations with behavioral factors (Supplementary Table 1, available online). A total of 771 (62.3%) women completed the FACT-NTX questionnaire at six months, and 544 (44.8%) at 24 months. Compared with those who provided data at six and 24 months, women who did not provide data were more likely to be younger, to be African American, to have lower education attainment, to be obese, to eat fewer fruit/vegetables, to spend less time in MVPA, and to be nonusers of antioxidants (Supplementary Table 2, available online).
Table 1.
Mean change in FACT-NTX score by demographic, clinical, and behavioral characteristics
| FACT-NTX and sensory subscale scores/Characteristics | Baseline (N = 1237) | 6 mo (n = 771) | 24 mo (n = 544) | ||
|---|---|---|---|---|---|
| FACT-NTX score (11-item) | |||||
| Mean (SD) | 39.5 (5.2) | 33.8 (8.3) | 35.5 (7.7) | ||
| Mean absolute change (SD) | – | -6.1 (8.0) | -4.7 (6.9) | ||
| Mean percent change (SD) | – | -14.9 (21.6) | -11.4 (18.3) | ||
| Participants who reported any increase in CIPN symptoms, no. (%) | – | 573 (74.3) | 397 (73.0) | ||
| Participants who reported ≥10% increase in CIPN symptoms, no. (%) | – | 217 (28.1) | 111 (20.4) | ||
| FACT-NTX sensory subscale (4-item) | |||||
| Mean (SD) | 14.5 (2.6) | 10.7 (4.8) | 11.9 (4.3) | ||
| Mean absolute change (SD) | – | -4.0 (4.8) | -3.0 (4.1) | ||
| Mean percent change (SD) | – | -26.1 (36.4) | -19.7 (29.6) | ||
| Participants who reported any increase in sensory neuropathy symptoms, no. (%) | – | 555 (72.0) | 349 (64.2) | ||
| Participants who reported ≥10% increase in sensory neuropathy symptoms, no. (%) | – | 492 (63.8) | 300 (55.1) | ||
| Absolute change in 11-item FACT-NTX scores from baseline | |||||
| No. (%) | Mean | P* | Mean | P* | |
| Demographic characteristics | |||||
| Age, y | |||||
| <50 | 435 (35.2) | −5.1 | Ref | −3.1 | Ref |
| 50–59 | 411 (33.2) | −6.8 | .02 | −5.5 | .002 |
| 60+ | 391 (31.6) | −6.4 | .07 | −5.1 | .007 |
| Race | |||||
| Non-Hispanic white | 705 (58.8) | −5.7 | Ref | −4.8 | Ref |
| African American | 123 (10.3) | −8.9 | .007 | −7.5 | .05 |
| Asian | 201 (16.8) | −7.6 | .02 | −3.2 | .05 |
| Hispanic | 170 (14.2) | −5.6 | .95 | −4.6 | .79 |
| Education | |||||
| High school or less | 178 (14.4) | −5.5 | Ref | −5.2 | Ref |
| Some college | 381 (30.8) | −5.2 | .77 | −3.8 | .17 |
| College graduate | 382 (30.9) | −6.6 | .26 | −4.5 | .47 |
| Postgraduate | 296 (23.9) | −6.9 | .15 | −5.6 | .67 |
| Annual household Income | |||||
| ≤$25 000 | 80 (7.2) | −4.8 | Ref | −3.9 | Ref |
| $25 000-49 999 | 201 (18.1) | −5.3 | .74 | −5.0 | .44 |
| $50 000-89 999 | 368 (33.1) | −6.3 | .25 | −4.2 | .81 |
| $90 000+ | 464 (41.7) | −6.7 | .12 | −5.2 | .31 |
| Clinical characteristics | |||||
| Stage at diagnosis | |||||
| I | 299 (24.8) | −4.6 | Ref | −3.6 | Ref |
| II | 649 (53.8) | −6.6 | .004 | −4.7 | .12 |
| III | 258 (21.4) | −6.9 | .006 | −6.1 | .004 |
| No. of positive nodes | |||||
| None | 505 (40.8) | −5.4 | Ref | −3.7 | Ref |
| 1–3 | 435 (35.2) | −6.3 | .17 | −5.4 | .01 |
| ≥4 | 297 (24.0) | −7.3 | .007 | −5.5 | .02 |
| Taxane drug | |||||
| Docetaxel | 567 (45.8) | −4.2 | Ref | −3.4 | Ref |
| Paclitaxel | 670 (54.2) | −7.7 | <.001 | −5.8 | <.001 |
| Taxane schedule | |||||
| Every 1 wk | 366 (30.4) | −8.3 | Ref | −7.0 | Ref |
| Every 2 wks | 713 (59.3) | −5.1 | <.001 | −3.8 | <.001 |
| Every 3+ wks | 124 (10.3) | −6.2 | .047 | −3.3 | .001 |
| Started taxane before baseline | |||||
| No | 967 (78.2) | −7.0 | Ref | −5.3 | Ref |
| Yes | 270 (21.8) | −2.6 | <.001 | −2.2 | <.001 |
| Charlson comorbidity index | |||||
| 0 | 1114 (90.1) | −6.1 | Ref | −4.5 | Ref |
| 1 | 57 (4.6) | −7.9 | .19 | −8.8 | .006 |
| 2+ | 66 (5.3) | −5.1 | .45 | −5.5 | .46 |
| Behavioral characteristics | |||||
| Body mass index | |||||
| <25 kg/m2 (normal) | 417 (33.7) | −5.6 | Ref | −3.2 | Ref |
| 25–29.9 kg/m2 (overweight) | 395 (31.9) | −6.8 | .09 | −5.0 | .01 |
| ≥30 kg/m2 (obese) | 425 (34.4) | −6.2 | .41 | −6.2 | <.001 |
| Moderate-to-vigorous physical activity | |||||
| <2.5 h/wk | 369 (29.9) | −7.2 | Ref | −5.6 | Ref |
| 2.5–5 h/wk | 275 (22.3) | −6.3 | .29 | −4.3 | .13 |
| >5 h/wk | 591 (47.9) | −5.5 | .02 | −4.4 | .10 |
| Fruit and vegetable intake | |||||
| <35 servings/wk | 574 (57.5) | −6.2 | Ref | −4.6 | Ref |
| ≥35 servings/wk | 425 (42.5) | −6.2 | .99 | −4.6 | .99 |
| Antioxidant use during chemotherapy† | |||||
| Nonuser | 783 (63.3) | −5.9 | Ref | −4.4 | Ref |
| Discontinued | 337 (27.2) | −6.3 | .64 | −5.7 | .06 |
| Continuous | 64 (5.2) | −6.6 | .54 | −3.3 | .30 |
| Initiator | 53 (4.3) | −6.9 | .39 | −4.6 | .90 |
Linear regression was used to test the null hypothesis that the change in Functional Assessment of Cancer Therapy–Taxane Neurotoxicity scores did not differ by demographic and clinical characteristics. All statistical tests were two-sided. FACT-NTX = Functional Assessment of Cancer Therapy–Taxane Neurotoxicity.
Antioxidant use groups: nonuser = women who reported no antioxidant use at baseline or six months; discontinuous user = women who reported antioxidant use at baseline but not at six months; continuous user = women who reported antioxidant use at baseline and six months; initiator = women who reported no antioxidant use at baseline but reported use at six months.
Mean Change in FACT-NTX from Baseline by BMI and Lifestyle Factors
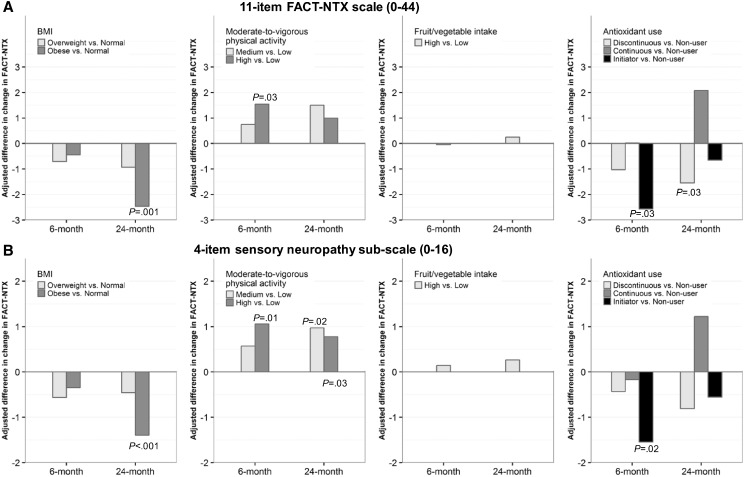
The large majority of patients reported increases in CIPN symptoms at six and 24 months compared with baseline as assessed by the full FACT-NTX scale and the sensory neuropathy subscale (Table 1). The decline in FACT-NTX (increased CIPN) was greater in participants who were older, who were African American or Asian, who were obese, who reported lower levels of MVPA, and who had more comorbidities (Table 1). In multivariable linear regression analyses of between-group differences in change in FACT-NTX, the decrease in FACT-NTX was statistically significantly greater in obese patients compared with normal-weight patients at 24 months (difference = -2.5, 95% CI = -4.0 to -0.9, P = .001) and was statistically significantly lower in patients with high MVPA compared with those with low MVPA at six months (difference = 1.5, 95% CI = 0.2 to 2.9, P = .03) (Figure 1A). There was no difference in FACT-NTX based on F/V intake. Unexpectedly, initiators of antioxidant supplements had a greater decrease in FACT-NTX compared with nonusers at six months (difference = -2.6, 95% CI = -4.8 to -0.3, P = .03) and discontinuers had a greater decrease in FACT-NTX compared with nonusers at 24 months (difference = -1.5, 95% CI = -2.9 to -0.1, P = .03). Results were very similar when analyses were restricted to the sensory neuropathy subscale (Figure 1B).
Figure 1.
Multivariable-adjusted differences in changes in Functional Assessment of Cancer Therapy–Taxane Neurotoxicity (FACT-NTX) scores from baseline to six and 24 months by baseline body mass index, moderate-to-vigorous physical activity, fruit/vegetable intake, and antioxidant use. A) Results are shown for the 11-item FACT-NTX scale (0–44) and for B) the four-item sensory neuropathy subscale (0–16). A larger decrease in FACT-NTX means a greater increase in chemotherapy-induced peripheral neuropathy symptoms. We tested the differences in changes in FACT-NTX using linear regression models adjusted for baseline score, age, race, smoking status, tumor stage, number of positive nodes, estrogen receptor/progesterone receptor, human epidermal growth factor receptor 2, taxane drug, taxane schedule, and prior taxane use. All P values were two-sided. BMI = body mass index; FACT-NTX = Functional Assessment of Cancer Therapy–Taxane Neurotoxicity.
Associations of BMI and Lifestyle Factors with Clinically Meaningful Increase in CIPN
In multivariable logistic regression analyses, an increase in CIPN of 10% or greater as assessed by FACT-NTX was more likely to occur in overweight and obese patients compared with normal-weight patients at 24 months (overweight vs normal weight: OR = 2.37, 95% CI = 1.19 to 4.88, P = .02; obese vs normal weight: OR = 3.21, 95% CI = 1.52 to 7.02, P = .003) and was less likely to occur in patients with high MVPA compared with those with low MVPA at six months (OR = 0.56, 95% CI = 0.34 to 0.94, P = .03) and 24 months (OR = 0.43, 95% CI = 0.21 to 0.87, P = .02) (Table 2). There was no statistically significant association between F/V intake and increased CIPN. Patients who initiated antioxidant use during treatment were more likely to have a 10% or greater increase in CIPN compared with nonusers at six months (OR = 3.81, 95% CI = 1.82 to 8.04, P < .001) (Table 2). When analyses were restricted to the sensory neuropathy subscale, a 10% or greater worsening of symptoms was only observed at 24 months among overweight compared with normal-weight patients (OR = 2.49, 95% CI = 1.43 to 4.39, P = .001) (data not shown).
Table 2.
Univariate and multivariable logistic regression analyses of clinically meaningful worsening CIPN on the 11-item FACT-NTX score with lifestyle behaviors*
| Lifestyle behaviors | Clinically meaningful worsening* at 6 mo |
Clinically meaningful worsening* at 24 mo |
||||||
|---|---|---|---|---|---|---|---|---|
| Yes |
No |
OR (95% CI)† | P† | Yes |
No |
OR (95% CI)† | P† | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||||
| Body mass index | ||||||||
| <25 kg/m2 | 69 (24.2) | 216 (75.8) | Ref | – | 21 (10.7) | 175 (89.3) | Ref | – |
| 25–29.9 kg/m2 | 76 (31.0) | 169 (69.0) | 1.34 (0.83 to 2.16) | .23 | 43 (23.6) | 139 (76.4) | 2.37 (1.19 to 4.88) | .02 |
| ≥30 kg/m2 | 72 (29.9) | 169 (70.1) | 1.20 (0.71 to 2.04) | .49 | 47 (28.3) | 119 (71.7) | 3.21 (1.52 to 7.02) | .003 |
| Moderate-to-vigorous physical activity | ||||||||
| <2.5 h/wk | 72 (34.4) | 137 (65.6) | Ref | – | 43 (29.3) | 104 (70.7) | Ref | – |
| 2.5–5 h/wk | 56 (30.9) | 125 (69.1) | 0.89 (0.51 to 1.55) | .69 | 23 (18.9) | 99 (81.1) | 0.52 (0.24 to 1.10) | .09 |
| >5 h/wk | 89 (23.4) | 292 (76.6) | 0.56 (0.34 to 0.94) | .03 | 45 (16.4) | 230 (83.6) | 0.43 (0.21 to 0.87) | .02 |
| Fruit and vegetable Intake | ||||||||
| <35 servings/wk | 115 (29.6) | 274 (70.4) | Ref | – | 60 (21.7) | 216 (78.3) | Ref | – |
| ≥35 servings/wk | 86 (26.5) | 238 (73.5) | 0.88 (0.58 to 1.33) | .54 | 42 (17.9) | 193 (82.1) | 0.78 (0.43 to 1.41) | .42 |
| Antioxidant supplement use‡ | ||||||||
| Nonuser | 118 (25.9) | 338 (74.1) | Ref | – | 60 (18.7) | 261 (81.3) | Ref | – |
| Discontinuous | 57 (28.8) | 141 (71.2) | 1.31 (0.82 to 2.10) | .26 | 37 (24.8) | 112 (75.2) | 1.81 (0.97 to 3.40) | .06 |
| Continuous | 20 (31.2) | 44 (68.8) | 1.11 (0.53 to 2.23) | .78 | 5 (11.9) | 37 (88.1) | 0.58 (0.18 to 1.61) | .33 |
| Initiator | 22 (41.5) | 31 (58.5) | 3.81 (1.82 to 8.04) | <.001 | 9 (28.1) | 23 (71.9) | 2.74 (0.95 to 7.56) | .05 |
Clinically worse CIPN defined as a 10% or greater decrease in Functional Assessment of Cancer Therapy–Taxane Neurotoxicity score from baseline. CI = confidence interval; CIPN = chemotherapy induced peripheral neuropathy; FACT-NTX = Functional Assessment of Cancer Therapy–Taxane Neurotoxicity; OR = odds ratio.
OR, 95% CI, and P value were estimated from logistic regression model with clinically meaningful change as the dependent variable, and age, race, education, income, BMI, fruit/vegetable intake, moderate-to-vigorous physical activity, antioxidant use, tumor stage, number of positive nodes, taxane drug, taxane schedule, prior taxane treatment, comorbidities, and baseline FACT-NTX score as independent variables. All statistical tests were two-sided.
Antioxidant use groups: nonuser = women who reported no antioxidant use at baseline or six months; discontinuous user = women who reported antioxidant use at baseline but not at six months; continuous user = women who reported antioxidant use at baseline and six months; initiator = women who reported no antioxidant use at baseline but reported use at six months. The grouping rule applies to any and specific antioxidant supplements.
Exploratory analyses of associations of vitamin C, vitamin E, and selenium with CIPN as assessed by FACT-TAX showed similar findings as with general use of antioxidants (Supplementary Table 3, available online).
Sensitivity Analyses
Analyses restricting the population to women who did not initiate taxane chemotherapy before baseline showed similar associations compared with the primary analyses (Supplementary Table 4, available online).
Discussion
In a large prospective cohort of women with newly diagnosed invasive breast cancer who received taxane chemotherapy treatment, we identified lifestyle factors strongly associated with the development and persistence of CIPN symptoms. After adjusting for clinical and demographic factors, at 24 months, obese patients had a more than two-fold increased risk of increased CIPN compared with normal-weight patients, patients who spent more than five hours/week on moderate-to-vigorous MVPA were 60% less likely to have increased CIPN, and women who changed their use of antioxidant dietary supplements near the time of receiving chemotherapy experienced a two- to three-fold increased risk of increased CIPN. These findings suggest that modifiable lifestyle factors may lead to differences in CIPN symptoms for breast cancer patients undergoing taxane treatment.
Previous studies showed smaller and statistically insignificant protective effects of low BMI on CIPN. In a cohort study (n = 4554) of clinical outcomes associated with CIPN in breast cancer patients, obese patients who received weekly paclitaxel were 23% more likely to develop grade 2–4 neuropathy compared with nonobese patients, although the association did not reach statistical significance (8). Similarly, in a study of chemotherapy treatment modification attributed to CIPN (n = 488), obese patients had a higher likelihood of having modified chemotherapy treatment because of CIPN than nonobese patients (OR = 1.17, 95% CI = 0.59 to 2.35) (10). However, a retrospective cohort study (n = 229) of breast cancer patients showed no association between BMI and the development or severity of CIPN (9). The difference in CIPN assessment (clinically diagnosed vs self-reported) and the difference in BMI categorization (obese/nonobese vs obese/overweight/normal weight) may explain some of the differences between our findings and previous findings.
For the association between MVPA and CIPN, our results were more consistent with previous findings. In a Dutch population-based cohort study of colorectal cancer survivors, patients who were treated with chemotherapy (n = 506) and who spent less than 2.5 hours/week in MVPA reported more severe aching/burning pain in toes or feet, trouble handling small objects, and worse sensory and motor scores (12). A randomized controlled trial (n = 61) evaluating the effectiveness of an exercise intervention among lymphoma patients undergoing chemotherapy showed that twice weekly sensorimotor, endurance, and strength training was associated with statistically significant improvements in CIPN (11). Similar results have been reported in diabetic patient populations (11,16,17).
We did not observe an association between F/V intake and CIPN, and we identified no other studies on diet and CIPN with which to compare our results. We unexpectedly observed that a change in antioxidant supplement use near the time of chemotherapy initiation was associated with worse CIPN symptoms at 24 months. The use of vitamin and mineral supplements, including antioxidant supplements, following a cancer diagnosis is often high (34) and has been shown to increase following a breast cancer diagnosis in particular (35). Our findings are particularly provocative because they support the findings of a large multisite randomized controlled trial (n = 409) conducted by our group testing the use of an antioxidant dietary supplement, acetyl-L-carnitine, to prevent CIPN (36). Acetyl-L-carnitine has previously been shown to treat/prevent neuropathy in patients with diabetes and HIV and in patients receiving CIPN-inducing chemotherapy (13,37–39). We hypothesized that acetyl-L-carnitine would prevent CIPN. However, our results showed the exact opposite. Patients randomly assigned to the acetyl-L-carnitine group reported more CIPN symptoms and were more likely to have increased CIPN as measured by FACT-NTX at 24 weeks of follow-up, strongly suggesting that acetyl-L-carnitine should not be used for CIPN prevention. A large-scale trial (n = 207) of vitamin E for CIPN prevention was also ineffective (15).
The mechanisms by which obesity, low MVPA, and antioxidant supplements may interact with taxane chemotherapy to increase CIPN are poorly understood. CIPN occurs primarily as a result of damages to the terminal branches of peripheral nerves by neurotoxic chemotherapeutic agents, such as taxanes. This damage affects fibers responsible for translating pain and temperature, and fibers related to reflexes and muscle function (40). Studies of diabetic neuropathy suggest that exercise, especially strength and endurance training, may reduce diabetic neuropathy by improving glycemic control, insulin sensitivity, and lipid abnormality, which are the primary drivers of diabetic neuropathy (11). Exercise may also improve nerve perfusion and function (41), as well as improve supraspinal reorganization and regeneration of neuromuscular structures after neuropathic injury (11). Antioxidants have been hypothesized to be protective against CIPN by multiple mechanisms, including the oxidative breakdown of cellular DNA and cell membranes (42) and regulation of manganese superoxide dismutase (43).
The primary strengths of this analysis include comprehensive measurement of lifestyle factors at baseline, objective assessment of BMI, and use of patient-reported outcomes to prospectively assess CIPN symptoms. Receipt of taxane therapy is based on systematic EHR abstraction, therefore reducing the likelihood of misclassification. We used novel methods to distinguish antioxidant supplement user groups, which allowed us to observe differences in associations between groups. Continuous and dichotomous outcomes were used to examine associations between behaviors and CIPN; findings were largely consistent using the two outcome definitions, suggesting the observed associations are robust to how the outcome was defined.
Limitations include the potential response bias because participants who provided data at 24 months were relatively more health conscious. The use of self-reported lifestyle factors is subject to misclassification and recall bias. As change in antioxidant use was determined based on baseline and six-month data, we cannot conclude if change in antioxidant use is a cause or a result of CIPN. Furthermore, interpretations of our findings regarding antioxidant use are limited by the small sample size for continuous users and initiators of antioxidant supplements. In addition, we do not have information on clinician recommendations for lifestyle modification during treatment. Many clinicians and institutions dissuade patients from using supplements during treatment, but this is not systematic and many patients do not adhere to recommendations. These findings are hypothesis generating and can set a future research agenda for a side effect that is poorly understood.
In summary, in a prospective observational cohort among women who received taxane chemotherapy treatment, we observed that obesity, low MVPA, and change in use of antioxidant dietary supplements, including both initiation and discontinuation, were associated with more severe and sustained CIPN symptoms. Our results suggest that there are modifiable risk factors for CIPN among breast cancer patients who receive taxane treatment. Currently, management of CIPN is symptom oriented and relies largely on prescribed medications. There are no lifestyle recommendations for CIPN prevention, and patients are not counseled on the benefits and hazards associated with weight management, exercise, and dietary supplement before and during taxane treatment. Future studies should examine how obesity, exercise, and dietary supplements affect CIPN and provide effective lifestyle recommendations for breast cancer patients who receive taxanes and are at high risk of developing CIPN.
Funding
This work was supported by the National Cancer Institute (NCI; NCI K23CA141052 to HG; NCI R01CA105274 to LHK; and U24CA171524 to LHK) and the American Society of Clinical Oncology (ACRA Award to DLH).
Notes
The funders had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
An abstract based on an earlier version of this analysis was presented in an oral presentation at the American Society of Clinical Oncology 2016 Annual Meeting.
The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Schneider BP, Hershman DL, Loprinzi C. Symptoms: Chemotherapy-induced peripheral neuropathy. Adv Exp Med Biol. 2015;862:77–87. [DOI] [PubMed] [Google Scholar]
- 2. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy- induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 3. Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–774. [DOI] [PubMed] [Google Scholar]
- 4. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. [DOI] [PubMed] [Google Scholar]
- 5. Gogas H, Shapiro F, Aghajanian C, et al. The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gynecol Oncol. 1996;61:22–26. [DOI] [PubMed] [Google Scholar]
- 6. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowinsky EK, Eisenhauer EA, Chaudhry V, et al. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 8. Schneider BP, Zhao F, Wang M, et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J Clin Oncol. 2012;30:3051–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Candelario N, Wongrakpanich S, Morginstin MS. Predictors of chemotherapy - induced peripheral neuropathy among breast cancer patients treated with taxanes . J Clin Oncol. 2015;33:abstr 90. [Google Scholar]
- 10. Speck R, Sammel M, Farrar J, et al. Abstract P4-13-01: Racial disparities in the incidence of dose-limiting chemotherapy induced peripheral neuropathy. Cancer Res. 2012;72(24 supplement):P4-13-01–P4-13-01. [Google Scholar]
- 11. Streckmann F, Kneis S, Leifert Ja, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25:493–499. [DOI] [PubMed] [Google Scholar]
- 12. Mols F, Beijers AJM, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015;9(3):512–522. [DOI] [PubMed] [Google Scholar]
- 13. Bianchi G, Vitali G, Caraceni A, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41(1746–1750). [DOI] [PubMed] [Google Scholar]
- 14. Dubois D, Dhawan R, van de Velde H, et al. Descriptive and prognostic value of patient-reported outcomes: The bortezomib experience in relapsed and refractory multiple myeloma. J Clin Oncol. 2006;24(6):976–982. [DOI] [PubMed] [Google Scholar]
- 15. Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support Care Cancer. 2011:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixit S, Maiya AG, Shastry Ba. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: A single blind, parallel group randomized controlled trial. J Diabetes Complications. 2014;28:332–339. [DOI] [PubMed] [Google Scholar]
- 18. Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–1299. [DOI] [PubMed] [Google Scholar]
- 19. Crane MG, Sample C. Regression of diabetic neuropathy with total vegetarian (vegan) diet. J Nutr Med. 1994;4(4):431–439. [Google Scholar]
- 20. Bunner AE, Wells CL, Gonzales J, et al. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr Diabetes. 2015;5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwan ML, Ambrosone CB, Lee MM, et al. The Pathways Study: A prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation In: World Health Organ Tech Rep Ser. Geneva, Switzerland; 2000:i–xii,1–253. [PubMed] [Google Scholar]
- 23. Staten LK, Taren DL, Howell WH, et al. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33(11):1959–1967. [DOI] [PubMed] [Google Scholar]
- 24. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Department of Health and Human Services. Physical activity guidelines for americans - fact sheet for health professionals on physical activity guidelines for adults In: Division of Nutrition PA, and Obesity Physical Activity Guidelines for Adults. Centers for Disease Control and Prevention; Washington, DC; 2008: vii. [Google Scholar]
- 26. Pate R. R., Pratt M., Blair S. N., et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. [DOI] [PubMed] [Google Scholar]
- 27. US Department of Health and Human Services, US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed Washington, DC: US Dept of Health and Human Services; 2015. [Google Scholar]
- 28. Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: The functional assesment of cancer therapy-taxane (FACT-taxane). Cancer. 2003;98(4):822–831. [DOI] [PubMed] [Google Scholar]
- 29. Cella D, Huang H, Homesley HD, et al. Patient-reported peripheral neuropathy of doxorubicin and cisplatin with and without paclitaxel in the treatment of advanced endometrial cancer: Results from GOG 184. Gynecol Oncol. 2010;119(3):538–542. [DOI] [PubMed] [Google Scholar]
- 30. Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31:2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frederick L, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Springer Science & Business Media. New York; 2002. [Google Scholar]
- 32. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 34. Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J Clin Oncol. 2008;26(4):665–673. [DOI] [PubMed] [Google Scholar]
- 35. Greenlee H, Kwan ML, Ergas IJ, et al. Changes in vitamin and mineral supplement use after breast cancer diagnosis in the Pathways Study: A prospective cohort study. BMC Cancer. 2014;14:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31(20):2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3(4):223–231. [DOI] [PubMed] [Google Scholar]
- 38. Di Giulio AM, Gorio A, Bertelli A, et al. Acetyl-L-carnitine prevents substance P loss in the sciatic nerve and lumbar spinal cord of diabetic animals. Int J Clin Pharmacol Res. 1992;12(5-6):243–246. [PubMed] [Google Scholar]
- 39. Famularo G, Moretti S, Marcellini S, et al. Acetyl-carnitine deficiency in AIDS patients with neurotoxicity on treatment with antiretroviral nucleoside analogues. Aids.1997;11(2):185–190. [DOI] [PubMed] [Google Scholar]
- 40. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. [DOI] [PubMed] [Google Scholar]
- 41. Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. [DOI] [PubMed] [Google Scholar]
- 42. Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 43. Janes K, Doyle T, Bryant L, et al. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain. 2013;154(11):2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.