Abstract
Objectives
Evidence in adults suggests that improvements in cognitive performance may follow weight loss resulting from bariatric surgery, and baseline cognitive performance may be associated with weight loss following surgery. This has not been evaluated in adolescents.
Method
Participants were 38 adolescents of age 14–21 years composed of three groups: (1) 12 adolescents with severe obesity who received vertical sleeve gastrectomy during the study (VSG); (2) 14 adolescents with severe obesity who were wait-listed for VSG (WL); and (3) 12 healthy weight controls (HC). Participants completed testing of visual memory, verbal memory, and executive functioning at baseline (T1), which occurred presurgery for the VSG group, and approximately 4 months after baseline (T2). Body mass index (BMI) was assessed at T1, T2, and additionally at 6 months following VSG for the adolescents who received surgery.
Results
Although there was evidence of greater improvement for the VSG as compared with WL and HC groups in visual and verbal memory, group differences did not reach significance and effect sizes were small (η2 < 0.01). There was a significant positive association between indices of baseline executive functioning and excess BMI loss at 6 months postsurgery.
Conclusions
This small pilot study showed no significant differences by group in cognitive performance post-VSG. There was a significant association of baseline cognitive performance with weight loss outcomes. Given the very preliminary nature of these results in a small sample, future research should examine these relationships in a larger sample and evaluate mechanisms of these associations (e.g., insulin resistance, sleep, physical activity).
Keywords: adolescents, bariatric surgery, cognitive performance, obesity
Severe obesity is estimated to affect 9.1% of adolescents in the United States (Ogden et al., 2016), with rates increasing more rapidly than any other category of obesity (Skinner & Skelton, 2014). Consequences of severe obesity include both poor psychosocial and physical outcomes as well as the risk of premature death (Kelly et al., 2013; Ogden, Carroll, Kit, Flegal, 2014). Additionally, severe obesity is associated with cognitive deficits and poorer school performance (Freidl et al., 2013). Bariatric surgery is the treatment option with the most significant and sustained weight loss in youth with severe obesity (Inge et al., 2016; Paulus et al., 2015) and has been demonstrated to significantly reduce weight and improve or eliminate comorbidities including hypertension, dyslipidemia, and type 2 diabetes (Inge et al., 2016). However, weight loss following surgery is highly variable and predictors of outcomes poorly understood (Inge et al., 2016; Nadler, Barefoot, & Qureshi, 2012).
Previous data have established a robust connection between executive functioning and weight status, as well as with behaviors that may contribute to onset or maintenance of obesity (Hayes, Eichen, Barch, & Wilfley, 2018). For example, poor executive functioning may in part contribute to disordered eating behaviors, such as binge eating (Kittel, Schmidt, & Hilbert, 2017). Existing research has identified a transactional association between executive functioning and obesity, such that poorer executive functioning is prospectively associated with weight gain and obesity (Goldschmidt, Hipwell, Stepp, McTigue, & Keenan, 2015) and obesity associated with poorer executive function. For example, a systematic review of 31 studies demonstrated that children and adolescents with obesity perform worse on a variety of tasks of executive function than their healthy weight peers (Reinert, Po'e, & Barkin, 2013). Given the close two-way association between obesity and executive function, Hayes et al. (2018) note the importance of evaluating the effect of intervening on executive function in conjunction with treatment for obesity, as well as the impact of obesity treatment on improvements to executive functioning. Moreover, they note that the directionality of this association should be studied in greater depth. The current study, therefore, aims to fill this gap in the literature through the study of a population of adolescents with severe obesity experiencing rapid weight loss following bariatric surgery.
The role of impulsivity is particularly relevant for adolescents, as executive functioning is still developing during this period. In adolescents with obesity, developmentally normal weaker executive functioning may be compounded by additional deficits as compared with peers in normal weight categories (Braet, Claus, Verbeken, & Van Vlierberghe, 2007; Davis & Fox, 2008; Nederkoorn, Braet, Van Eijs, Tanghe, & Jansen, 2006; Pearce, Mackey, Kietlinski, Nadler, & Vaidya, 2014; Yau, Castro, Tagani, Tsui, & Convit, 2012). These deficits in executive function appear to be associated with maladaptive behaviors (Gettens & Gorin, 2017), such as dysregulated eating behaviors (Galanti, Gluck, & Geliebter, 2007; Gowey et al., 2017; Liang, Matheson, Kaye, & Boutelle, 2014; Nasser, Gluck, & Geliebter, 2004) and less physical activity (Joseph, Alonso-Alonso, Bond, Pascual-Leone, & Blackburn, 2011; Loprinzi, Herod, Cardinal, & Noakes, 2013; Riggs, Chou, Spruijt-Metz, & Pentz, 2010). The evidence of deficits in cognitive performance is supported by imaging studies showing weaker activation in the dorsolateral prefrontal cortex (Carnell et al., 2017; Reinert et al., 2013) and anterior cingulate cortex (Yau, Kang, Javier, & Convit, 2014) of adolescents with obesity, areas of the brain that regulate attention and inhibition.
Memory has also been implicated in obesity. As with executive functioning, this may be a transactional relationship, as poorer memory may be associated with increased caloric intake (Robinson et al., 2013), and insulin resistance, which results from obesity, may be a key mechanism by which memory is affected by obesity (Convit, 2005) because of the impact of insulin on hippocampal processes (McNay et al., 2010). Indeed, memory performance has been found to be poorer in adolescents with obesity than their healthy weight peers, though a systematic review found inconsistency in findings across studies (Liang et al., 2014). Studies of adolescents with severe obesity but without associated comorbidities found no differences in memory functioning compared with healthy peers (Yau et al., 2014), reflecting the importance of examining memory functioning in adolescents with severe obesity who may be more likely to have insulin resistance (Yau et al., 2014) while accounting for the lack of memory differences in adolescents with uncomplicated obesity. Research on adult bariatric surgery patients demonstrates improved memory function as early as 12 weeks and as long as 12 months following surgery relative to control participants with obesity (Alosco, Spitznagel, et al., 2014; Gunstad et al., 2011; Miller et al., 2013). Therefore, it is important to assess differences in memory, both visual and verbal, in adolescents with severe obesity, as well as any potential improvements associated with significant weight loss, which may be accompanied by decreased insulin resistance (Inge et al., 2016).
Evidence in adults indicates that improvements in cognitive performance may result from the significant weight loss following surgery (Alosco, Galioto, et al., 2014; Thiara et al., 2017; Veronese et al., 2017) and that baseline cognitive performance may likewise impact weight loss outcomes (Spitznagel et al., 2014; Spitznagel, Alosco, et al., 2013). However, these associations have not yet been studied in adolescents. Although the adult literature can inform understanding of adolescent cognitive functioning with regard to obesity and bariatric surgery, adolescence is a unique period in cognitive development that requires particular attention. Specifically, adolescent brains are developing rapidly, particularly with regard to executive function and reward sensitivity (Steinberg, 2005). Therefore, changes following surgery may be different from adults in terms of both type and trajectory of change. Existing evidence also suggests unique developmental outcomes of adolescents compared with adults in bariatric surgery, with adolescents receiving bariatric surgery exhibiting more metabolic improvement relative to adults, but fewer hormonal changes (Lawson et al., 2006; Sysko et al., 2012), suggesting processes specific to this developmental group following surgery and the value of studying adolescents independently.
The current study was a small pilot study designed to evaluate preliminary evidence of cognitive changes in adolescents undergoing vertical sleeve gastrectomy (VSG) as compared with adolescents who were eligible for VSG but were not having surgery within the study period, and adolescents of healthy weight matched for age and socioeconomic status (SES) to further control for practice effects and potential baseline differences in cognitive functioning. The current study aimed to: (1) evaluate whether, as hypothesized, there was a greater improvement in cognitive function in the VSG group (VSG) as compared with the wait list (WL) and healthy control (HC) groups between baseline (T1) and 3–4 month follow-up (T2); and (2) evaluate whether, as hypothesized, baseline cognitive function (T1) was associated with weight loss at 3- and 6-month post-VSG (T2 and T3, respectively).
Methods
Procedures
Participants in the VSG and WL groups were enrolled following presurgical psychological evaluations at a large children’s hospital in the Mid-Atlantic. Eligibility for the study was assessed at the time of the evaluation by a licensed clinical psychologist (first author). Criteria for VSG include a BMI ≥ 35 with a medical comorbidity or BMI ≥ 40. Individuals are self-referred, referred by their primary care or specialty physicians, or referred through the lifestyle weight management program at the institution. Patients routinely are seen through the weight management program for 3–6 consecutive months and receive a full psychological evaluation as well as evaluations by other relevant medical specialties (e.g., sleep medicine, endocrinology, cardiology) before being approved for surgery (Nadler Barefoot & Qureshi, 2012 and Pearce et al., 2017). Assignment into the VSG or WL groups was made based on planned timing of surgery and whether surgery would occur during the study period. It is common to experience delays to surgery based on insurance requirements for participation in lifestyle management programs for 3–6 months before surgery or insurance denials for authorization, which created a natural WL group. All patients in the WL group continued with their usual care in the multidisciplinary medical weight management program, as it would be unethical to stop treatment for the duration of the current study. This weight management program, attended by the VSG and WL groups, consists of monthly visits with a medical provider and dietician as well as referrals to other specialists as needed. Healthy controls were recruited through a database maintained by one of the study primary investigators through a large university and medical center in the Mid-Atlantic. Specifically, any participant enrolled in any study through the lab is stored in a database, including their age, gender, ethnicity, and SES and Intelligence Quotient (IQ) from previous study protocols. Participants in this database were initially recruited via flyers in public places, in pediatrician's offices in the metropolitan area, and word-of-mouth from participants. For the current study, participants in the age range and in the same range for IQ and SES as the participants the current study were contacted by trained research assistants to determine interest and eligibility for the current study. Inclusion criteria were (1) the ability to speak and read English to complete study assessments and (2) absence of any significant cognitive impairment (e.g., traumatic brain injury), which would affect their performance on the study tasks. Eligibility was assessed via phone screen by trained research assistants. Demographic/clinical comparisons between those who chose to participate versus those who refused could not be conducted because of characteristics not having been captured for those who refused. Moreover, comparisons between those who were retained versus those who dropped out of the study were not conducted because of small sample size.
All visits were conducted at either the children’s hospital or university at which the study was conducted. Participants could elect which location was most convenient. All batteries were conducted by either the principal investigator or trained research assistants who were currently master’s level or PhD-level psychology students. Assessors were not blind to patient group. At the baseline (T1) visit, participants completed informed consent conducted by either the principal investigator or a trained research assistant, and completed the cognitive performance and self-report battery. This visit occurred approximately 2–4 weeks before surgery for the VSG group. Participants returned around 4 months (T2) following T1 (approximately 3 months postsurgery for the VSG group) to repeat both the cognitive performance tasks. Participants who were on stimulant medications for treatment of ADHD were instructed not to take their medications on the day of testing, resulting in approximately 24 hr without medication before testing. Adherence to this requirement was confirmed on the day of testing for those participants on stimulant medications. For all participants who had VSG, including the WL participants who had surgery following completion of the study, a medical record review was conducted to obtain weight loss at 6 months following VSG (Time 3; T3). All methods were approved by the appropriate institutional review boards. The potential for study related adverse events was monitored by the principal investigator, and none occurred during the course of the study.
Measures
Wechsler Abbreviated Scale of Intelligence—Second Edition. The Wechsler Abbreviated Scale of Intelligence—Second Edition (WASI-II) was administered as a means of estimating IQ to ensure absence of cognitive delay and ensuring similarity in the groups with severe obesity and the healthy controls. The WASI-II is a well-used and validated method of obtaining an estimate of IQ.
Wide Range Assessment of Memory and Learning—Second Edition, Visual Memory Subtests (Sheslow & Adams, 2003). Visual memory was assessed using the Picture Memory and Design Memory subtests, on which participants remember elements of pictures and designs that they have just seen, and the Visual Memory Index Score was used for the current study. The Picture Memory Recognition and Design Memory Recognition subtests, on which participants respond whether they have seen elements of pictures or designs on prior tasks, were used to compute the Visual Recognition Index. The Wide Range Assessment of Memory and Learning—Second Edition (WRAML-II) is a well-established neuropsychology assessment tool for visual memory. There are no alternate forms to use, so the same measure was used for both T1 and T2. Therefore, the changes in score are evaluated in reference to the two control groups rather than any interpretation regarding clinical significance of the scores themselves.
California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 2000). The California Verbal Learning Test (CVLT-II) is a well-established measure that was used as an assessment of short and long-term verbal memory. The Short Delay Standard Score and Long Delay Standard Scores were used to assess verbal memory. The original and alternate forms of the measure were used and were counterbalanced across participants to reduce practice effects. Given that the majority of adolescents receiving bariatric surgery to be recruited into the study are typically >16 years, the adult version of the measure was chosen as the most appropriate for the majority of participants included in the study.
Tasks of Executive Control (Isquith, Roth, & Gioia, 2010). Executive function was assessed using the computerized Tasks of Executive Control (TEC), which is designed for repeated measures using alternate forms, counterbalanced across participants for the current study. The TEC uses n-back procedures as a measure of working memory demand, which overlays a go/no go standard task, to produce indices of functioning on inhibitory control. The TEC is normed for individuals of age <18 years. Because the current sample enrolled participants over this age, in consultation with the developers of the measure, raw scores were used, adjusting for age and gender in all analyses. For the current study, the response time standard deviation (RTSD) was used as an indicator of variability in response time, where higher variability is associated with poorer executive functioning. This measure of executive functioning is supported as a robust indicator of executive functioning in existing literature (Kofler et al., 2013). Scores were collapsed across noninhibit (summing RTSD scores from 0, 1, and 2-back under noninhibit conditions that did not include the go/no go condition) and inhibit (summing RTSD scores from 0, 1, and 2-back under inhibit conditions that included the go/no go paradigm) conditions for indices of inhibitory control and across 0, 1, and 2 n-back for the inhibit and noninhibit conditions (e.g., summing n-back under noninhibit and inhibit conditions) for indices of working memory. Of note is that the TEC experienced a number of technical difficulties (i.e., freezing at the end of administration) resulting in lost data. Therefore, there is a significant amount of missing data in this measure (complete T1 and T2 data were available for 5 VSG participants, 9 WL participants, and 11 HC participants), and only descriptive data are provided. Results should be interpreted with caution and as preliminary data only.
BMI and Weight Loss. Height and weight were obtained at both T1 and T2, and BMI was calculated. BMI, rather than BMI percentile, or z-score was used given the higher levels of BMI among the participants with severe obesity. For all participants who had surgery, including those in the WL group who received surgery following their completion of the study (N = 12), BMI was also calculated from medical records at 6 months postsurgery. As a measure of weight loss following surgery, excess BMI (EBMI) percentage lost was calculated. This is done by assuming a BMI of 25 or lower as within the normal weight category and is a standard calculation for assessing weight loss postsurgery (Deitel & Greenstein, 2003). Specifically, a BMI of 25 is subtracted from T1 BMI to calculate EBMI. BMI at subsequent time points is then used in reference to EBMI to calculate the percentage of EBMI lost. This variable was used in analyses for the two groups with severe obesity only, as this measure is not relevant for youth in a healthy weight range.
Data Analytic Plan. All analyses were done with IBM SPSS Version 24 or SAS 9.4. To assess group differences on cognitive performance, change scores were calculated by subtracting score at T1 from the score at T2. Using change scores on each index as the outcome measure, least squares means estimates from generalized linear models were obtained, and change scores were compared between groups. For the TEC, which used raw scores because the age range of the current sample was beyond the measure’s scope for calculating index scores, gender and age were controlled for in all analyses. For all outcome measures, a Reliable Change Index was also calculated, using published norms for each of the measures (Isquith, Roth, & Gioia, 2010; Sheslow & Adams, 2003; Woods, Delis, Scott, Kramer, & Holdnack, 2006) as a means of additional interpretation of magnitude of change and using a cutoff of 1.96 to indicate clinical significance (Jacobson & Truax, 1991). Eta squared was calculated as an indicator of effect size, with values of 0.01–0.05 indicating a small effect and 0.06–0.13 indicating a moderate effect size. Use of control groups allows for accounting for practice effects on all measures. To assess the association of baseline cognitive performance with prospective weight loss, multiple linear regressions were run by group for EBMI loss at T2 and T3 among participants who had surgery (n = 12), controlling for gender and age on the outcomes based on the TEC only (not visual and verbal memory, given that the index scores were computed based on age and gender) and controlling for T1 BMI in all analyses. Owing to collinearity concerns of the cognitive measures, each outcome was modeled separately. Sample size estimation was based on feasibility of data collection for the current pilot study and power estimates based on the imaging component (Pearce et al., 2017).
Results
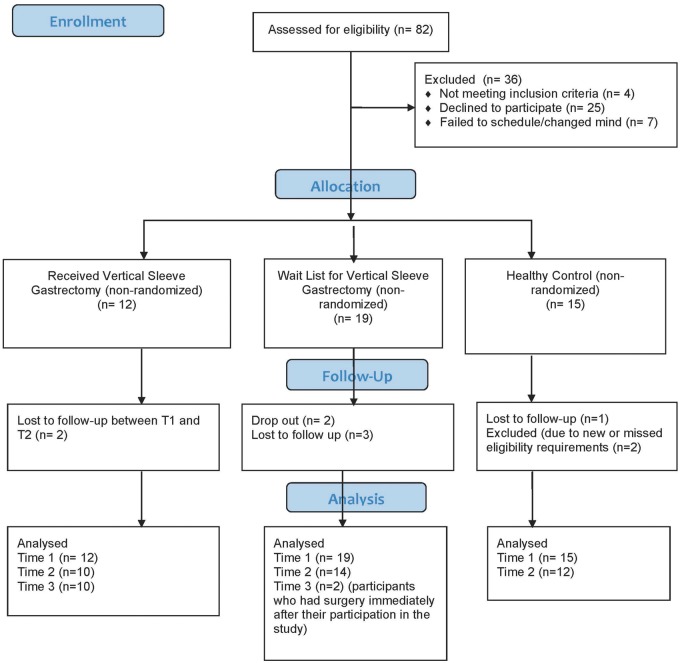
Participants were 36 adolescents of age 14–21 years (mean age = 16.3, SD = 1.5). The majority were female (61%) and from ethnic minority backgrounds (19% Hispanic/Latino; 64% Non-White). The majority of participants (67%) were from households earning <$80,000 annually. Groups did not differ on any of the demographic variables. See Table I for all descriptive information by group, including psychiatric comorbidity and full scale IQ scores from the WASI-II. See Figure 1 for CONSORT table of enrollment. Based on analysis of variances (ANOVAs), groups differed at baseline between the VSG group and the WL group on visual recognition, with the VSG group scoring lower. The VSG group evidenced poorer scores on the inhibit, 1-back, and 2-back RTSD scores as compared with the HC group (see Table I).
Table I.
Descriptive Statistics
| Surgery (VSG) N = 12 |
WL N = 14 |
HC N = 12 |
Baseline diffa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | % | M | SD | Range | % | M | SD | Range | % | M | SD | Range | p-value |
| Age (years) | 17.0 | 1.6 | 15–21 | 16.1 | 1.4 | 14–18 | 15.9 | 1.3 | 14–18 | .15 | |||
| Sex (% female) | 58.3 | 71.4 | 50.0 | .59 | |||||||||
| Ethnicity (% Hispanic/Latino) | 16.7 | 28.6 | 8.3 | ||||||||||
| Race (% Non-White) | 75.0 | 78.6 | 66.7 | ||||||||||
| WASI-II | 92.3 | 17.3 | 79–134 | 93.6 | 11.4 | 74–119 | 97.8 | 9.5 | 89–116 | .56 | |||
| ADHD diagnosis | 25.0 | 14.3 | 8.3 | ||||||||||
| Depression diagnosis | 41.7 | 7.1 | 0.0 | ||||||||||
| Anxiety diagnosis | 8.3 | 21.4 | 0.0 | ||||||||||
| BMI T1 | 48.5 | 7.7 | 38.9–59.8 | 45.5 | 8.2 | 33.5–58.0 | 21.6 | 2.6 | 17.8–26.4 | <.0001 | |||
| BMI T2 | 37.9 | 6.8 | 26.4–48.7 | 46.4 | 8.2 | 33.6–58.4 | 22.1 | 2.9 | 18.4–27.6 | ||||
| Visual Memory T1 | 84.9 | 15.4 | 60–112 | 89.7 | 9.8 | 70–109 | 92.3 | 9.8 | 70–109 | .33 | |||
| Visual Memory T2 | 91.8 | 15.5 | 60–118 | 94.9 | 14.0 | 79–124 | 96.5 | 9.8 | 82–112 | ||||
| Visual Recognition T1 | 88.5 | 11.6 | 71–109 | 101.1 | 9.0 | 90–122 | 93.5 | 17.4 | 58–112 | .06* | |||
| Visual Recognition T2 | 92.8 | 13.8 | 71–109 | 98.7 | 15.3 | 75–131 | 96.9 | 16.7 | 58–112 | ||||
| Verbal Memory Short Delay T1 | −.59 | 0.9 | −2.5 to 0.5 | −0.57 | 1.1 | −2.5 to 1 | −0.75 | 1.1 | −2.5 to 1.5 | .90 | |||
| Verbal Memory Short Delay T2 | 0.0 | 1.4 | −1.5 –to 1.5 | −0.25 | 1.2 | −2.0 to 1.5 | −0.50 | 1.3 | −2.0 to 1.5 | ||||
| Verbal Memory Long Delay T1 | −.55 | 0.9 | −2.0 to 1.0 | −0.57 | 1.0 | −2.0 to 0.5 | −0.63 | 1.2 | −2.5 to 1.5 | .98 | |||
| Verbal Memory Long Delay T2 | −.15 | 1.2 | −2.0 to 1.5 | −0.46 | 1.3 | −2.5 to 1.5 | −0.67 | 1.0 | −2.0 to 1.0 | ||||
| RTSD Noninhibit T1 | 428.7 | 123.5 | 198.2–605.0 | 351.8 | 79.0 | 222.5–457.2 | 327.7 | 115.0 | 221.4–623.4 | .14 | |||
| RTSD Noninhibit T2 | 508.6 | 200.5 | 312.6–837.7 | 371.4 | 136.6 | 194.4–635.0 | 325.1 | 95.2 | 188.8–507.9 | ||||
| RTSD Inhibit T1 | 486.6 | 164.7 | 265.6–693.1 | 386.9 | 99.9 | 270.9–529.6 | 316.0 | 89.2 | 236.5–553.5 | .02† | |||
| RTSD Inhibit T2 | 572.1 | 190.5 | 377.4–811.4 | 409.2 | 165.9 | 165.2–409.2 | 359.0 | 178.6 | 188.4–815.5 | ||||
| RTSD 0-Back T1 | 214.4 | 67.2 | 130.7–308.0 | 185.8 | 59.7 | 121.6–302.7 | 177.5 | 71.7 | 126.3–337.2 | .49 | |||
| RTSD 0-Back T2 | 247.6 | 57.5 | 189.0–339.8 | 187.8 | 89.8 | 92.5–394.9 | 163.0 | 52.1 | 97.9–231.5 | ||||
| RTSD 1-Back T1 | 284.0 | 76.6 | 170.7–410.0 | 228.1 | 49.0 | 163.8–318.9 | 208.3 | 67.4 | 123.8–384.5 | .06† | |||
| RTSD 1-Back T2 | 341.9 | 68.4 | 265.8–441.0 | 249.8 | 56.7 | 123.8–328.4 | 224.7 | 79.7 | 128.0–411.7 | ||||
| RTSD 2-Back T1 | 416.8 | 162.0 | 158.8–657.9 | 324.9 | 94.8 | 215.3–483.9 | 258.0 | 80.5 | 178.8–455.3 | .02† | |||
| RTSD 2-Back T2 | 491.2 | 260.2 | 315.8–947.1 | 343.0 | 151.5 | 131.5–624.4 | 296.4 | 153.6 | 138.0–691.7 | ||||
Note. Legend: ANOVA = analysis of variance; BMI = body mass index; HC = healthy control; RTSD = response time standard deviation; T1 = Time 1; T2 = 3–4 months follow-up; VSG = vertical sleeve gastrectomy; WL = wait list; aOverall p-value between study groups based on ANOVA; *Difference between VSG and WL at p < .05; † Difference between HC and VSG at p < .05; note T2 N’s for the RTSD scores were as follows: VSG N = 5, WL N = 9, HC N = 11.
Figure 1.
CONSORT diagram.
Hypothesis 1: There is a greater change in cognitive functioning in the VSG group.
For visual memory, there were no significant differences on change scores from T2 to T1 between groups; however, the direction of change was in the expected direction for all groups, given practice effects. Though not significant, the VSG group showed a larger improvement as compared with the WL and HC control groups for both visual memory and visual recognition (see Table II). Notably, on average, the WL group evidenced poorer performance on the visual recognition subtest, whereas the other two groups demonstrated modest gains. Findings in verbal memory performance were similar to those found for visual memory, both short term and long term, with no significant differences seen between groups in change between T1 and T2, but with larger change scores in the expected direction for the VSG as compared with the WL and HC groups. Effect sizes of differences between groups were small across outcomes, with the exception of visual recognition that approached moderate size (see Table II). When examining the Reliable Change Index, there were also no significant differences between groups, with most participants regardless of group evidencing stability in their performance across time (see Table III). On the measure of EF, see Table II for response time SD (RTSD) from the TEC by groups.
Hypothesis 2: Baseline cognitive function is associated with weight change.
Table II.
Generalized Linear Models of Mean Change and 95% Confidence Interval in Cognitive Performance by Group
| Scale | Surgery (VSG) | WL | HC | p-value | η2 |
|---|---|---|---|---|---|
| Visual Memory | 6.60 (0.61–12.59) | 5.14 (0.08–10.20) | 4.25 (−1.22 to 9.72) | .85 | 0.009 |
| Visual Recognition | 3.60 (−3.67 to 10.87) | −2.35 (−8.50 to 3.79) | 3.42 (−3.22 to 10.05) | .35 | 0.056 |
| Verbal Memory Short Delay Recall | 0.45 (−0.15 to 1.05) | 0.32 (−0.19 to 0.83) | 0.25 (−0.30 to 0.80) | .89 | 0.006 |
| Verbal Memory Long Delay Recall | 0.25 (−0.30 to 0.80) | 0.11 (−0.35 to 0.57) | −0.04 (−0.54 to 0.46) | .74 | 0.016 |
| RTSD Non-inhibitory* | 50.75 (−58.45 to 159.94) | 19.60 (−61.79 to 100.99) | −2.59 (−76.21 to 71.03) | ||
| RTSD Inhibitory* | 63.48 (−87.94 to 214.89) | 22.26 (−90.60 to 135.12) | 42.96 (−59.12 to 145.04) | ||
| RTSD 0-Back* | 10.89 (−49.37 to 71.14) | 1.98 (−42.93 to 46.89) | −14.45 (−55.08 to 26.17) | ||
| RTSD 1-Back* | 51.86 (−18.13 to 121.85) | 21.75 (−30.42 to 73.92) | 16.39 (−30.80 to 63.57) | ||
| RTSD 2-Back* | 51.47 (−93.24 to 196.19) | 18.13 (−89.74 to 125.99) | 38.44 (−59.13 to 136.00) |
Adjusted for gender and age.
Note that p-values and effect sizes were not calculated for the measure of executive functioning because of the small sample size and missing data.
Note. HC = healthy control; RTSD = response time standard deviation; VSG = vertical sleeve gastrectomy; WL = wait list; Visual and Verbal Memory—higher change scores indicate improved performance; RTSD—higher change scores indicated increased variability in response, indiciative of reduced performance. N’s for analyses are as follows: Visual and verbal memory VSG N = 12, WL N = 14, HC N = 12; RTSD VSG N = 5, WL N = 9, HC N = 11.
Table III.
Reliable Change Index Scores on Outcomes by Group
| Scale | Surgery (VSG) | WL | HC | pa | η2 |
|---|---|---|---|---|---|
| Visual Memory RCI (mean (95% CI)) | 1.32 (0.00–2.64) | 1.15 (0.04–2.27) | 0.72 (−0.48 to 1.93) | .78 | 0.02 |
| Categorical clinical change (n (%)) | .83 | ||||
| Improved | 2 (20.0) | 5 (35.7) | 4 (33.3) | ||
| Stable | 7 (70.0) | 8 (57.1) | 8 (66.7) | ||
| Declined | 1 (10.0) | 1 (7.1) | 0 (0.0) | ||
| Visual recognition RCI (mean (95% CI)) | 0.38 (−0.46 to 1.22) | −0.25 (−0.96 to 0.46) | 0.36 (−0.40 to 1.13) | .39 | 0.06 |
| Categorical clinical change (n (%)) | .84 | ||||
| Improved | 2 (20.0) | 2 (14.3) | 1 (8.3) | ||
| Stable | 8 (80.0) | 12 (85.7) | 11 (91.7) | ||
| Declined | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Verbal Short Delay Recall RCI (mean (95% CI)) | 0.53 (−0.24 to 1.30) | 0.38 (−0.27 to 1.03) | 0.29 (−0.41 to 0.99) | .90 | 0.01 |
| Categorical clinical change (n (%)) | .37 | ||||
| Improved | 0 (0.0) | 2 (14.3) | 0 (0.0) | ||
| Stable | 20 (100.0) | 12 (85.7) | 11 (91.7) | ||
| Declined | 0 (0.0) | 0 (0.0) | 1 (8.3) | ||
| Verbal Long Delay Recall RCI (mean (95% CI)) | 0.31 (−0.43 to 1.06) | 0.13 (−0.49 to 0.76) | −0.05 (−0.73 to 0.63) | .76 | 0.02 |
| Categorical clinical change (n (%)) | 1.00 | ||||
| Improved | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Stable | 10 (100.0) | 13 (92.9) | 12 (100.0) | ||
| Declined | 0 (0.0) | 1 (7.1) | 0 (0.0) | ||
| RTSD Non-inhibitory RCI (mean (95% CI)) | 0.46 (−0.65 to 1.57) | 0.18 (−0.65 to 1.01) | −0.02 (−0.77 to 0.73) | ||
| Categorical clinical change (n (%)) | |||||
| Improved | 0 (0.0) | 0 (0.0) | 1 (9.1) | ||
| Stable | 4 (80.0) | 9 (100.0) | 9 (81.8) | ||
| Declined | 1 (20.0) | 0 (0.0) | 1 (9.1) | ||
| RTSD Inhibitory RCI (mean (95% CI)) | 0.67 (−1.13 to 2.47) | 0.24 (−1.11 to 1.58) | 0.45 (−0.76 to 1.67) | ||
| Categorical clinical change (n (%)) | |||||
| Improved | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Stable | 4 (80.0) | 7 (77.8) | 10 (90.9) | ||
| Declined | 1 (20.0) | 2 (22.2) | 1 (9.1) | ||
| RTSD 0-Back RCI (mean (95% CI)) | 0.26 (−1.36 to 1.88) | 0.05 (−1.16 to 1.26) | −0.35 (−1.44 to 0.75) | ||
| Categorical clinical change (n (%)) | |||||
| Improved | 0 (0.0) | 1 (11.1) | 1 (9.1) | ||
| Stable | 5 (100.0) | 6 (66.7) | 10 (90.9) | ||
| Declined | 0 (0.0) | 2 (22.2) | 0 (0.0) | ||
| RTSD 1-Back RCI (mean (95% CI)) | 1.27 (−0.66 to 3.19) | 0.53 (−0.91 to 1.97) | 0.40 (−0.90 to 1.70) | ||
| Categorical clinical change (n (%)) | |||||
| Improved | 0 (0.0) | 1 (11.1) | 1 (9.1) | ||
| Stable | 4 (80.0) | 7 (77.8) | 8 (72.7) | ||
| Declined | 1 (20.0) | 1 (11.1) | 2 (18.2) | ||
| RTSD 2-Back RCI (mean (95% CI)) | 0.41 (−0.89 to 1.70) | 0.14 (−0.82 to 1.11) | 0.31 (−0.57 to 1.18) | ||
| Categorical clinical change (n (%)) | |||||
| Improved | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Stable | 4 (80.0) | 9 (100.0) | 10 (90.9) | ||
| Declined | 1 (20.0) | 0 (0.0) | 1 (9.1) |
p-value for RCI based on ANOVA between groups and Fisher’s exact test for categorized change.
Note that p-values and effect sizes were not calculated for the measure of executive functioning because of the small sample size and missing data.
Note. ANOVA = analysis of variance; CI = confidence interval; HC = healthy control; RTSD = response time standard deviation; VSG = vertical sleeve gastrectomy; WL = wait list.
Average T2 EBMI loss was 44% (SD = 18%, range 31–90%) for the VSG group indicating significant weight loss and −6% (SD = 10%, range −26 to 7%) and for the WL group, which indicates a relative weight gain and increase from ideal BMI. Average T3 EBMI loss was 46% (SD = 21%, range 14–93%).
For T2, baseline verbal and visual memory was not associated with EBMI loss for either VSG or WL participants. Executive functioning was not associated with EBMI loss for the WL group (see Table III). However, for the VSG group, RTSD under the noninhibit condition demonstrated near significance with T2 EBMI loss, controlling for age, gender, and baseline BMI (F(4, 7) = 5.80, p = .09). RTSD under 2-back conditions, indicating a high working memory load, was also significantly associated with T2 EBMI loss (F(4, 7) = 9.04, p = .05; see Table IV).
Table IV.
Multiple Linear Regressions Predicting Time 2 EBMI Loss
| VSG (N = 8) | WL (N = 9) | |||||
|---|---|---|---|---|---|---|
| Variable | F | β | R2/B 95% CI | F | β | R2/B 95% CI |
| 5.80* | .89 | 0.20 | .17 | |||
| Age | −.31 | −.17 to .07 | .42 | −.09 to .14 | ||
| Gender | −.26 | −.41 to .21 | −.06 | −.01 to .29 | ||
| BMI T1 | .22 | −.02 to .03 | −.02 | −.02 to .02 | ||
| RTSD Non-inhibit | −.78* | −.003 to .00 | −.18 | −.002 to .001 | ||
| 3.83 | .84 | 0.17 | .15 | |||
| Age | −.58 | −.24 to .05 | .41 | −.09 to .14 | ||
| Gender | −.47 | −.55 to .19 | −.02 | −.31 to .30 | ||
| BMI T1 | . 78 | −.03 to .07 | −.04 | −.02 to .02 | ||
| RTSD Inhibit | −1.0 | −.003 to .001 | −.11 | −.001 to .001 | ||
| 3.93 | .84 | 0.59 | .37 | |||
| Age | −.57 | −.24 to .05 | .28 | −.08 to .12 | ||
| Gender | −.43 | −.52 to .20 | .17 | −.24 to .30 | ||
| BMI T1 | .17 | −.03 to .04 | −.17 | −.02 to .02 | ||
| RTSD 0-Back | −.58 | −.004 to .001 | −.54 | −.003 to .001 | ||
| 1.56 | .68 | 0.29 | .23 | |||
| Age | −.36 | −.25 to .14 | .41 | −.09 to .13 | ||
| Gender | −.36 | −.65 to .38 | −.08 | −.30 to .27 | ||
| BMI T1 | .02 | −.05 to .05 | −.14 | −.02 to .02 | ||
| RTSD 1-Back | −.38 | −.005 to .003 | −.33 | −.003 to .002 | ||
| 9.04** | .92 | 0.20 | .17 | |||
| Age | −.41 | −.16 to .03 | .32 | −.10 to .14 | ||
| Gender | −.35 | −.38 to .12 | −.01 | −.30 to .30 | ||
| BMI T1 | .87 | −.009 to .05 | −.03 | −.02 to .02 | ||
| RTSD 2-Back | −1.24** | −.003 to .000 | .19 | −.001 to .002 |
*p ≤. 10, **p ≤ .05; gender coded in reference to females; BMI T1 = body mass index at baseline; CI = confidence interval; EBMI = excess BMI; RTSD = response time standard deviation; VSG = vertical sleeve gastrectomy; WL = wait list; negative beta weights indicate an association of greater response time variability (i.e., poorer executive function) with less EBMI loss, which indicates poorer outcome.
Post-VSG EBMI loss at 6 months (T3) was available for 12 participants. The same regressions were conducted for this group predicting T3 EBMI (see Table V). Regressions indicated that visual recognition at baseline was significantly associated with T3 EBMI loss (F(2, 11) = 15.40, p = .001). Neither of the verbal memory indices predicted T3 EBMI loss. With regard to executive functioning, RTSD under noninhibit conditions (F(4, 8) = 9.26, p = .03) significantly predicted T3 EBMI loss and RTSD under 1-back conditions demonstrated near significance in predicting T3 EBMI loss (F(4, 8) = 4.21, p < .10).
Table V.
Regressions Predicting 6 Months Postsurgery EBMI Loss (N = 12)
| Variable | F | β | B 95% CI | R2 |
|---|---|---|---|---|
| 9.07*** | .67 | |||
| BMI T1 | −.65** | −.03 to −.01 | ||
| Visual Memory | .29 | −.003 to .01 | ||
| 15.40*** | .77 | |||
| BMI T1 | −.65*** | −.03 to −.01 | ||
| Visual Recognition | .44** | .001 to .02 | ||
| 9.26** | .90 | |||
| Age | .18 | −.05 to .10 | ||
| Gender | .24 | −.12 to .30 | ||
| BMI T1 | −.48* | −.03 to .002 | ||
| RTSD Non-inhibit | −.78** | −.002 to .00 | ||
| 2.46 | .71 | |||
| Age | .09 | −.12 to .14 | ||
| Gender | .26 | −.27 to .47 | ||
| BMI T1 | −.52 | −.04 to .01 | ||
| RTSD Inhibit | −.58 | −.002 to .001 | ||
| 3.23 | .76 | |||
| Age | −.04 | −.12 to .10 | ||
| Gender | .16 | −.26 to .38 | ||
| BMI T1 | −.67* | −.04 to .004 | ||
| RTSD 0-Back | −.47 | −.004 to .001 | ||
| 4.21* | .81 | |||
| Age | .23 | −.08 to .15 | ||
| Gender | .28 | −.19 to .41 | ||
| BMI T1 | −.58 | −.04 to .005 | ||
| RTSD 1-Back | −.70* | −.004 to .00 | ||
| 3.11 | .76 | |||
| Age | .17 | −.10 to .15 | ||
| Gender | .28 | −.23 to .45 | ||
| BMI T1 | −.38 | −.04 to .02 | ||
| RTSD 2-Back | −.77 | −.003 to .001 |
*p ≤ .10, **p ≤ .05, ***p ≤ .01; gender coded in reference to females; BMI T1 = body mass index at baseline; CI = confidence interval; EBMI = excess BMI; RTSD = response time standard deviation; negative beta weights indicate an association of greater response time variability (i.e., poorer executive function) with less EBMI loss, which indicates poorer outcome.
Note. N = 12 for visual memory regressions and N = 9 for the executive functioning regressions.
Discussion
The current study adds to the literature on cognitive functioning in adults by examining the cognitive changes occurring following bariatric surgery in adolescents. Additionally, these preliminary results can be interpreted as indication of the need for future research on the association between executive functioning and weight loss via surgery, as well as research on the effects of significant weight loss on executive functioning. Imaging results from the same sample found neural changes following surgery that indicate that weight loss is associated with increased prefrontal activation in the VSG as compared with the WL and HC groups (Pearce et al., 2017). However, the analyses in the current study indicated that there were no such significant differences by group on cognitive performance tasks. Although findings were in the expected direction for visual and verbal memory, such that the VSG group had higher change scores, the VSG did not significantly differ from the control groups. Overall improvements on visual memory scores across groups were likely in part because of practice effects, but the indication of potentially greater improvement in the VSG group as compared with the controls indicates the potential for further study in changes to visual memory following bariatric surgery in adolescents. This is consistent with findings in the adult literature indicating improvements in memory as early as 12 weeks following surgery (Alosco, Spitznagel, et al., 2014; Gunstad et al., 2011). The finding that visual recognition at baseline was associated with weight loss following surgery was novel and may be associated with the fact that visuospatial memory may be linked with food intake, such as that estimation of portion sizes and visual cues for eating inform how much is ingested (Kanoski & Grill, 2017). Therefore, adolescents with better functioning visual memory may be able to regulate their intake following surgery better than those with poorer visual recognition, resulting in less weight loss.
Descriptive findings on the measure of executive functioning are difficult to interpret with the small sample size and technical difficulties. These relationship needs to be assessed in a larger sample to evaluate the potential benefits of bariatric surgery on cognitive functioning comorbidities in severe obesity. It is highly likely that the sample size was too small to detect small to modest improvements that might exist, particularly given the difficulties with the measure of executive functioning. It is also possible that >3–4 months are required postsurgery to see reliable improvements in cognitive function in adolescents, as compared with adults. These results are also different from the imaging results seen in the same sample (Pearce et al., 2017), which may indicate difficulties with the TEC as an indicator of change in executive functioning in this population.
The current study also provided preliminary evidence that supports findings in adult surgery patients (Spitznagel et al., 2014; Spitznagel, Alosco, et al., 2013; Spitznagel, Garcia, et al., 2013), that baseline cognitive functioning, particularly executive functioning, is associated with weight loss outcomes following bariatric surgery in adolescents. However, this same relationship was not observed for those adolescents with severe obesity in lifestyle management programs (i.e., WL group). One hypothesis for the association with weight loss in the VSG group compared with the WL group may be that the significant weight loss following surgery begins a cascade of changes, first seen in neural function (Pearce et al., 2017), then with potential improvement in cognitive performance. Consequently, this may provide an opportunity for those with better executive function to engage in health behaviors that result in increased weight loss with the help of surgery. These findings have potentially significant research and clinical implications if they are replicated in larger samples. One potential avenue of clinical research would be to use experimental methodology to evaluate the association of presurgical executive function with postsurgical health behaviors and weight loss outcomes, for example augmenting executive functioning to determine the effect on postsurgical behavior and weight loss. Moreover, the findings suggest an opportunity for clinical intervention to bolster the presurgical executive function of adolescents who may be at risk of having less weight loss following surgery.
There are a number of limitations with the current study, especially given its original intent to provide preliminary evidence to guide future research. The small sample size limited the ability to detect significant between group differences and control for other potentially important factors and mechanisms such as physical activity, sleep, mood, metabolic factors, or nutritional intake. Future research should use larger samples and assess relevant health behaviors. The measure of executive functioning was chosen based on its generation of a wide range of data and its intent to be used over multiple time points and be sensitive to changes in executive functioning. However, the program encountered a number of technical difficulties, resulting in significant missing data, which is problematic given the already small sample size. Future work should consider alternate tools to assess executive function. The age range also affected the measure of verbal memory, which may have been too difficult for the participants under age 16 years, as the adult version of the measure was used to fit the majority of the study participants. Future research should use alternate measures of verbal memory. The study design was not randomized, as it is not ethical to randomize participants to a surgical or nonsurgical condition that may affect their health, and the assessors were not blind to group membership. Finally, although the participants in the current study were representative of youth in the institution’s bariatric surgery program, there may factors specific to the institution or population that may limit generalizability to other populations of adolescents with severe obesity. For example, these participants had all been referred for bariatric surgery, which is likely a unique subset of adolescents with severe obesity. Therefore, the current findings must be replicated in a larger sample.
Conclusion
The current study evaluated both the association of bariatric surgery with improved cognitive function, as well as the association of baseline cognitive function with postsurgical weight loss outcomes using not only a comparison group of adolescents with severe obesity not receiving bariatric surgery but also a healthy control group. Although this was a small sample size with limited ability to detect significant differences, it lends support to the hypothesis that executive functioning may be an essential factor to consider in the treatment of adolescent severe obesity and that it may play a unique role in the predictors and outcomes of bariatric surgery in adolescents. Future research should examine these associations using experimental methodology to manipulate executive function and larger sample sizes, as well as a longer duration of follow-up to allow for changes to be detected and other types of surgery in addition to VSG.
Acknowledgments
The authors would like to thank Karin Walsh, Psy.D., for her consultation on interpretation of the TEC.
Funding
The work was supported by NIH 1R56DK104644 - 01A1.
Conflicts of interest: None declared.
References
- Alosco M. L., Galioto R., Spitznagel M. B., Strain G., Devlin M., Cohen R., Crosby R. D., Mitchell J. E., Gunstad J. (2014). Cognitive function after bariatric surgery: Evidence for improvement 3 years after surgery. American Journal of Surgery, 207, 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco M. L., Spitznagel M. B., Strain G., Devlin M., Cohen R., Paul R., Crosby R. D., Mitchell J. E., Gunstad J. (2014). Improved memory function two years after bariatric surgery. Obesity, 22, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C., Claus L., Verbeken S., Van Vlierberghe L. (2007). Impulsivity in overweight children. European Child and Adolescent Psychiatry, 16, 473–483. [DOI] [PubMed] [Google Scholar]
- Carnell S., Benson L., Chang K. V., Wang Z., Huo Y., Geliebter A., Peterson B. S. (2017). Neural correlates of familial obesity risk and overweight in adolescence. Neuroimage, 159, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A. (2005). Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiology of Aging, 26, 31–35. [DOI] [PubMed] [Google Scholar]
- Davis C., Fox J. (2008). Sensitivity to reward and body mass index (BMI): Evidence for a non-linear relationship. Appetite, 50, 43–49. [DOI] [PubMed] [Google Scholar]
- Deitel M., Greenstein R. J. (2003). Recommendations for reporting weight loss. Obesity Surgery, 13, 159–160. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. (2000). Manual for the California Verbal Learning Test (CVLT-II). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Freidl E. K., Sysko R., Devlin M. J., Zitsman J. L., Kaplan S. C., Walsh B. T. (2013). School and cognitive functioning problems in adolescent bariatric surgery candidates. Surgery for Obesity and Related Disorders, 9, 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti K., Gluck M. E., Geliebter A. (2007). Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. International Journal of Eating Disorders, 40, 727–732. [DOI] [PubMed] [Google Scholar]
- Gettens K. M., Gorin A. A. (2017). Executive function in weight loss and weight loss maintenance: A conceptual review and novel neuropsychological model of weight control. Journal of Behavioral Medicine, 40:687–701. [DOI] [PubMed] [Google Scholar]
- Goldschmidt A. B., Hipwell A. E., Stepp S. D., McTigue K. M., Keenan K. (2015). Weight gain, executive functioning, and eating behaviors among girls. Pediatrics, 136, e856–e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowey M. A., Lim C. S., Dutton G. R., Silverstein J. H., Dumont-Driscoll M. C., Janicke D. M. (2017). Executive function and dysregulated eating behaviors in pediatric obesity. Journal of Pediatric Psychology, 43, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J., Strain G., Devlin M. J., Wing R., Cohen R. A., Paul R. H., Crosby R. D., Mitchell J. E. (2011). Improved memory function 12 weeks after bariatric surgery. Surgery for Obesity and Related Disorders, 7, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. F., Eichen D. M., Barch D. M., Wilfley D. E. (2018). Executive function in childhood obesity: Promising intervention strategies to optimize treatment outcomes. Appetite, 124, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge T. H., Courcoulas A. P., Jenkins T. M., Michalsky M. P., Helmrath M. A., Brandt M. L., Harmon C. M., Zeller M. H., Chen M. K., Xanthakos S. A., Horlick M., Buncher C. R.; Teen-LABS Consortium. (2016). Weight loss and health status 3 years after bariatric surgery in adolescents. New England Journal of Medicine, 374, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isquith P. K., Roth R. M., Gioia G. A. (2010). Tasks of executive control. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Jacobson N. S., Truax P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59, 12–19. [DOI] [PubMed] [Google Scholar]
- Joseph R. J., Alonso-Alonso M., Bond D. S., Pascual-Leone A., Blackburn G. L. (2011). The neurocognitive connection between physical activity and eating behaviour. Obesity Reviews, 12, 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski S. E., Grill H. J. (2017). Hippocampus contributions to food intake control: Mnemonic, neuroanatomical, and endocrine mechanisms. Biological Psychiatry, 81, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. S., Barlow S. E., Rao G., Inge T. H., Hayman L. L., Steinberger J., Urbina E. M., Ewing L. J., Daniels S. R.; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, and Council on Clinical Cardiology. (2013). Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation, 128, 1689–1712. [DOI] [PubMed] [Google Scholar]
- Kittel R., Schmidt R., Hilbert A. (2017). Executive functions in adolescents with binge-eating disorder and obesity. The International Journal of Eating Disorders, 50, 933–941. [DOI] [PubMed] [Google Scholar]
- Kofler M. J., Rapport M. D., Sarver D. E., Raiker J. S., Orban S. A., Friedman L. M., Kolomeyer E. G. (2013). Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Reviews, 33, 795–811. [DOI] [PubMed] [Google Scholar]
- Lawson M. L., Kirk S., Mitchell T., Chen M. K., Loux T. J., Daniels S. R., Harmon C. M., Clements R. H., Garcia V. F., Inge T. H.; Pediatric Bariatric Study Group. (2006). One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: A multicenter study from the Pediatric Bariatric Study Group. Journal of Pediatric Surgery, 41, 137–143; discussion 137–143. [DOI] [PubMed] [Google Scholar]
- Liang J., Matheson B. E., Kaye W. H., Boutelle K. N. (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity, 38, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi P. D., Herod S. M., Cardinal B. J., Noakes T. (2013). Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Research, 1539, 95–104. [DOI] [PubMed] [Google Scholar]
- McNay E. C., Ong C. T., McCrimmon R. J., Cresswell J., Bogan J. S., Sherwin R. S. (2010). Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of Learning and Memory, 93, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. A., Crosby R. D., Galioto R., Strain G., Devlin M. J., Wing R., Cohen R. A., Paul R. H., Mitchell J. E., Gunstad J. (2013). Bariatric surgery patients exhibit improved memory function 12 months postoperatively. Obesity Surgery, 23, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler E. P., Barefoot L. C., Qureshi F. G. (2012). Early results after laparoscopic sleeve gastrectomy in adolescents with morbid obesity. Surgery, 152, 212–217. [DOI] [PubMed] [Google Scholar]
- Nasser J. A., Gluck M. E., Geliebter A. (2004). Impulsivity and test meal intake in obese binge eating women. Appetite, 43, 303–307. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C., Braet C., Van Eijs Y., Tanghe A., Jansen A. (2006). Why obese children cannot resist food: The role of impulsivity. Eating Behaviors, 7, 315–322. [DOI] [PubMed] [Google Scholar]
- Ogden C., Carroll M., Lawman H., Fryar C., Kruszon-Moran D., Kit B., Flegal K. (2016). Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA, 315, 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C., Carroll M. D., Kit B. K., Flegal K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA, 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus G. F., de Vaan L. E., Verdam F. J., Bouvy N. D., Ambergen T. A., van Heurn L. W. (2015). Bariatric surgery in morbidly obese adolescents: A systematic review and meta-analysis. Obesity Surgery, 25, 860–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A., Mackey E. R., Kietlinski M., Nadler E., Vaidya C. J. (2014). Cognitive functioning and daily life impairement in pediatric obesity. Paper presented at the The Obesity Society Obesity Week, Boston, MA.
- Pearce A. L., Mackey E., Cherry J. B. C., Olson A., You X., Magge S. N., Vaidya C. J. (2017). Effect of Adolescent Bariatric Surgery on the Brain and Cognition: A Pilot Study. Obesity (Silver Spring), 2511, 1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K. R., Po'e E. K., Barkin S. L. (2013). The relationship between executive function and obesity in children and adolescents: A systematic literature review. Journal of Obesity, 2013, 820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs N., Chou C. P., Spruijt-Metz D., Pentz M. A. (2010). Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child Neuropsychology, 16, 279–292. [DOI] [PubMed] [Google Scholar]
- Robinson E., Aveyard P., Daley A., Jolly K., Lewis A., Lycett D., Higgs S. (2013). Eating attentively: A systematic review and meta-analysis of the effect of food intake memory and awareness on eating. American Journal of Clinical Nutrition, 97, 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D., Adams W. (2003). Wide range assessment of memory and learning—2nd Ed administration and technical manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Skinner A. C., Skelton J. A. (2014). Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatrics, 168, 561–566. [DOI] [PubMed] [Google Scholar]
- Spitznagel M. B., Alosco M., Galioto R., Strain G., Devlin M., Sysko R., Crosby R. D., Mitchell J. E., Gunstad J. (2014). The role of cognitive function in postoperative weight loss outcomes: 36-month follow-up. Obesity Surgery, 24, 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel M. B., Alosco M., Strain G., Devlin M., Cohen R., Paul R., Crosby R. D., Mitchell J. E., John Gunstad P. D. (2013). Cognitive function predicts 24-month weight loss success after bariatric surgery. Surgery for Obesity and Related Disorders, 9, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel M. B., Garcia S., Miller L. A., Strain G., Devlin M., Wing R., Cohen R., Paul R., Crosby R., Mitchell J. E., Gunstad J. (2013). Cognitive function predicts weight loss after bariatric surgery. Surgery for Obesity and Related Disorders, 9, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2005). Cognitive and affective development in adolescence. Trends in Cognitive Science, 9, 69–74. [DOI] [PubMed] [Google Scholar]
- Sysko R., Devlin M. J., Hildebrandt T. B., Brewer S. K., Zitsman J. L., Walsh B. T. (2012). Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. Journal of Clinical Psychiatry, 73, 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiara G., Cigliobianco M., Muravsky A., Paoli R. A., Mansur R., Hawa R., McIntyre R. S., Sockalingam S. (2017). Evidence for neurocognitive improvement after bariatric surgery: A systematic review. Psychosomatics, 58, 217–227. [DOI] [PubMed] [Google Scholar]
- Veronese N., Facchini S., Stubbs B., Luchini C., Solmi M., Manzato E., Sergi G., Maggi S., Cosco T., Fontana L. (2017). Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 72, 87–94. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Delis D. C., Scott J. C., Kramer J. H., Holdnack J. A. (2006). The California Verbal Learning Test II: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology, 21, 413–420. [DOI] [PubMed] [Google Scholar]
- Yau P. L., Castro M. G., Tagani A., Tsui W. H., Convit A. (2012). Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics, 130, e856–e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau P. L., Kang E. H., Javier D. C., Convit A. (2014). Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity, 22, 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]