Human immunodeficiency virus (HIV)–infected Ugandan women receiving suppressive therapy have higher levels of biomarkers of inflammation, immune activation, and gut permeability than HIV-uninfected women and treated HIV-infected men. Sex-specific effects of HIV on long-term disease complications require further evaluation.
Keywords: HIV, inflammation, gut permeability, Uganda, sub-Saharan Africa, sex, women
Abstract
In a cohort of human immunodeficiency virus (HIV)–infected individuals and age- and sex-matched HIV-uninfected comparators, we assessed soluble CD14 (sCD14), sCD163, interleukin 6, intestinal fatty acid binding protein (IFAPB), and high-sensitivity C-reactive protein (hs-CRP) levels. The median age was 51 years. Among HIV-positive individuals, the median antiretroviral therapy (ART) duration was 7 years, the median CD4+ T-cell count was 433 cells/μL, and 86% had an undetectable viral load. Although HIV-positive individuals had higher sCD14, IFABP, and hs-CRP levels, we found evidence of interaction by sex, such that HIV-positive women had greater differences from HIV-negative women, compared with differences between HIV-positive men and HIV-negative men. In models restricted to HIV-positive individuals, women had higher levels of all 5 biomarkers than men.
Although antiretroviral (ART)–induced virologic suppression reduces levels of biomarkers of inflammation and immune activation, levels remain elevated among people living with human immunodeficiency virus (HIV; PLWH), compared with those among HIV-uninfected individuals, even after early ART initiation [1–5]. Elevated levels of these biomarkers have been independently associated with non-AIDS morbidity and all-cause mortality [6–8]. Discerning the pathways through which nonreplicating HIV induces persistent inflammation [9] and identifying the interventions to mitigate its downstream consequences [10, 11] are leading HIV research priorities.
Recently emerged data suggest that sex might modify the effect of HIV on chronic inflammation. Women appear to experience smaller reductions in levels of markers of inflammation after initiation of ART, despite having lower pretreatment viral load and greater improvements in CD4+ T-cell counts than men [12]. Moreover, multiple studies have suggested that the increased risk of cardiovascular events attributable to HIV infection might be more pronounced in women [13–15].
Relationships between HIV, inflammation, and sex have not been thoroughly investigated in sub-Saharan Africa, home to >25 million HIV-infected individuals and where the prevalence of HIV is nearly twice as high in women than men. Yet, numerous factors that may alter inflammatory pathways are distinct in sub-Saharan Africa, including traditional cardiovascular risk factors, coinfections, and exposures to environmental toxins, such as cooking-related air pollution [16]. To respond to this lack of data, we measured markers of immune activation and gut permeability in a mixed cohort of HIV-negative and antiretroviral-treated PLWH in Uganda. We aimed to compare levels of biomarkers of immune activation on the basis of HIV serostatus and to test for effect modification by sex in this population.
METHODS
Study Setting, Participants, and Laboratory Methods
The Ugandan Non-Communicable Diseases and Aging Cohort Study (UGANDAC; clinical trials registration NCT02445079) is a prospective observational cohort study and has been described in detail previously (Supplementary Methods) [17, 18]. Briefly, the study comprises ambulatory ART-experienced PLWH and HIV-uninfected individuals enrolled from a cluster of villages approximately 20 km from the clinic. Participants are seen annually to complete questionnaires and for blood specimen collection [19].
Laboratory Methods
We tested cryopreserved specimens for 5 biomarkers: serum levels of high-sensitivity C-reactive protein (hs-CRP), using latex immunoturbidimetry (LabCorp, Burlington, NC), and plasma levels of interleukin 6 (IL-6; MesoScale Discovery, Rockville, MD), soluble CD14 (sCD14; R&D Systems, Minneapolis, MN), sCD163 (R&D Systems), and intestinal fatty acid binding protein (IFABP; R&D Systems). Inflammatory markers were log transformed and divided by their interquartile range (IQR), such that each 1-unit increase in the coefficient represents an increase in biomarker IQR [20], with the exception of hs-CRP, which was modeled categorically as <1, 1–3, and >3 mg/dL to comply with recommended clinical guidelines for hs-CRP risk stratification [21]. Latex immunoturbidimetry was performed at LabCorp, and all other tests were performed at the Laboratory for Clinical Biochemistry Research at the University of Vermont. HIV-1 RNA loads and CD4+ T-cell counts were abstracted from electronic records at the Mbarara Regional Referral Hospital HIV Clinic.
Statistical Methods
We first compared demographic and clinical characteristics, by HIV serostatus and sex. We then fit 3 sets of regression models to examine relationships between HIV serostatus and inflammation over time, sex-specific differences in inflammation, by HIV serostatus, and sex-specific differences in inflammation restricted to PLWH (Supplementary Appendix). All regression models were adjusted for potential confounding by age, body mass index, and smoking status and included a random intercept and/or slope to account for within individual clustering.
Ethics Considerations
Study participants gave written informed consent, and the protocol was approved by the institutional review boards of Partners Healthcare and the Mbarara University of Science and Technology.
RESULTS
Participant Characteristics
A total of 308 individuals were enrolled in the study and provided blood specimens for inflammatory marker testing. The analytic sample was evenly divided by HIV serostatus (50% PLWH [155 of 308]) and sex (49% female [150 of 308]). The median age at enrollment was 51 years (IQR, 48–55 years), with no difference between subgroups (P = .91; Supplementary Table 1). PLWH were less likely to be current smokers than HIV-uninfected individuals (P < .001). The median body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) was 21.8, without differences by HIV serostatus (P = .23), but women had a higher BMI then men, regardless of HIV serostatus (P = .001). At enrollment, the median CD4+ T-cell count for PLWH was 433 cells/μL (IQR, 336–559 cells/μL), and 86% (133 of 155) had an undetectable viral load, while the remainder largely had low-level viremia (median, 39 copies/mL; IQR, 27–100 copies/mL). The median ART duration at baseline was 7.2 years (IQR, 6.4–8.0 years). The majority (>90%) were taking an efavirenz- or nevirapine-based regimen.
Comparisons Between PLWH and HIV-Uninfected Subgroups
In models adjusted for age, sex, smoking status, and body mass index, PLWH had significantly higher levels of sCD14, IFABP, and hs-CRP (Supplementary Figure 1). We found no significant differences, by HIV serostatus, in levels of either sCD163 or IL-6. Levels of biomarkers remained consistent over time, with no evidence for a change over time, by HIV serostatus (Supplementary Table 2). In models with sCD14 and sCD163 levels specified as the outcomes of interest and the IFABP level included to estimate the potential mediating effect of gut barrier dysfunction on monocyte activation, we found that the IFABP level was independently correlated with both the sCD14 level and the sCD163 level (P < .01; Supplementary Figure 2). However, the IFABP level did not significantly change the estimated associations between HIV serostatus and the level of either monocyte activation marker (adjusted β for HIV serostatus changed from 0.087 to 0.084 with inclusion of IFABP in the sCD14 outcome model and from −0.027 to −0.033 for sCD163 with inclusion of IFABP in the sCD163 outcome model).
Effect Modification by Sex
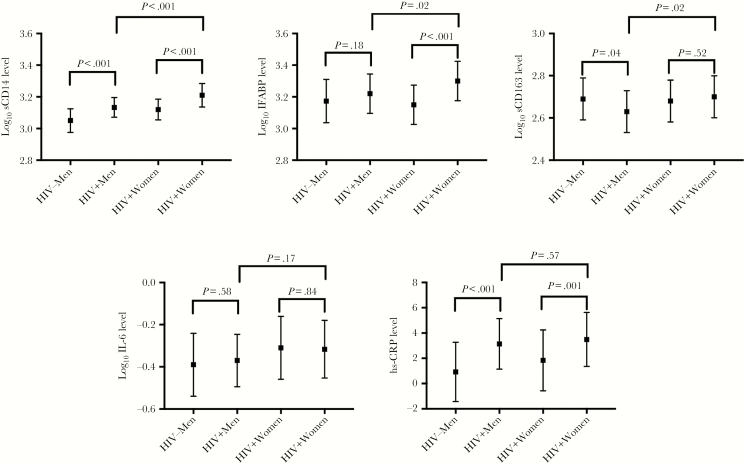
When comparing biomarkers by HIV serostatus and sex, men with HIV infection had significantly higher levels of sCD14 and hs-CRP than their HIV-uninfected counterparts after adjustment for age, BMI, and smoking status (Supplementary Table 3 and Figure 1). Men with HIV infection had a paradoxically decreased level of sCD163 as compared to men without HIV infection. By contrast, women with HIV infection had statistically significant elevations in sCD14, IFABP, and hs-CRP levels as compared to those without HIV infection and similar levels of sCD163 as compared to women without HIV infection. We found evidence of interaction by sex and HIV serostatus, such that women with HIV infection had a greater difference than men in IFABP and sCD163 levels. When we restricted estimations to PLWH and included HIV disease characteristics, women had higher levels of all 5 inflammatory biomarkers (P < .10 for all comparisons; Table 1). Results were similar when restricted to visits with an undetectable viral load.
Figure 1.
Distribution of levels of biomarkers of inflammation, systematic inflammation, and gut permeability, by sex and human immunodeficiency virus (HIV) serostatus. Estimates are means and 95% confidence intervals derived from postestimation margins from mixed-effects regression models with the level of each inflammatory marker as the outcome of interest and sex, HIV serostatus, smoking status, and body mass index as covariables in each model. IFABP, intestinal fatty acid binding protein; IL-6: interleukin 6; hs-CRP, high-sensitivity C-reactive protein; sCD14, soluble CD14; sCD163, soluble CD163; −, negative; +, positive.
Table 1.
Multivariable Adjusted Regression Models of Markers of Inflammation and Gut Permeability, by Sex, Among People Living With Human Immunodeficiency Virus (HIV)
| Biomarkera | Female vs Male HIV-Seropositive Patientsb | |||
|---|---|---|---|---|
| Detectable Viral Load (n = 152) |
Undetectable Viral Load (n = 147) |
|||
| β (95% CI) | P | β (95% CI) | P | |
| sCD14 level | 0.40 (.19–.60) | <.001 | 0.44 (.23–.65) | <.001 |
| IFABP level | 0.21 (−.04–.46) | .10 | 0.21 (.06–.47) | .13 |
| sCD163 level | 0.23 (−.01–.48) | .06 | 0.29 (.05–.54) | .02 |
| IL-6 level | 0.28 (−.01–.58) | .06 | 0.28 (−.03–.58) | .06 |
| AOR (95% CI) | P | AOR (95% CI) | ||
| hs-CRP levelc | 1.17 (.41–2.13) | .004 | 1.26 (.34–2.18) | .007 |
Models had random intercepts and were adjusted for age, smoking status, body mass index, CD4+ T-cell count, viral load (detectable vs undetectable), and antiretroviral therapy duration.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IFABP, intestinal fatty acid binding protein; IL-6: interleukin 6; sCD14, soluble CD14; sCD163, soluble CD163.
aUnless otherwise indicated, levels of inflammatory markers were log transformed and divided by interquartile range.
bUnless otherwise indicated, data are from 349 observations and for 152 individuals with a detectable HIV load and from 307 observations and for 147 with an undetectable HIV load.
cThe high-sensitivity C-reactive protein (hs-CRP) level (735 observations from 308 individuals) was modeled with ordered mixed-effects logistic regression categorizing the level as <1, 1–3, and >3 mg/L.
DISCUSSION
In a longitudinal cohort comprising PLWH receiving suppressive ART and community-based, HIV-uninfected individuals in Uganda, we found persistent elevations in HIV-associated inflammation and gut membrane permeability and evidence of sex-based disparities in these associations. Men with HIV infection had persistently higher levels of sCD14 and hs-CRP, but lower sCD163 than HIV-uninfected men. Women also had higher sCD14 and hs-CRP, but by contrast, also had higher IFABP and similar sCD163 compared to HIV uninfected women. Moreover, in models restricted to people living with HIV, we found that women had higher levels of all 5 biomarkers than men. The relationships identified were independent of traditional correlates of inflammation and of HIV-specific mediators, such as HIV-1 RNA viremia and ART duration.
Sex-specific differences in immune responses to HIV before and after ART have also been described in US-based cohorts. In a cohort from Boston with an HIV treatment profile similar to that in our cohort, women with HIV infection had significantly elevated levels of sCD163 and sCD14 as compared to both HIV-uninfected women and HIV-infected men. However, there were few significant differences between groups in levels of other biomarkers, including hs-CRP [22]. In the PEARLS study (AIDS Clinical Trials Group A5174), women had less of a decrease in hs-CRP and sCD14 levels as compared to men after 48 weeks of ART [12]. Yet, in stark contrast to our results, due to higher levels of biomarkers prior to ART in men, the net result was that men and women had similar levels of sCD14 and hs-CRP after 48 weeks of ART.
Our results offer support for a role for gut permeability as a mechanism to explain differential inflammatory profiles in women with HIV infection. We found that the IFABP level in women with HIV infection was greater than that in men with HIV infection and that the IFABP level was associated with both the sCD14 level and the sCD163 level after years of ART. Intriguingly, 2 prior studies have demonstrated increases in the IFABP level after ART initiation [5, 23], but to our knowledge our study is the first to show strong sex-specific differences in this relationship. Downstream effects of these phenomena in sub-Saharan Africa are an important area of further study. In the United States and Europe, women with HIV infection experience an increased rate of cardiovascular disease–associated mortality, compared with men [15, 24–26]. Two studies investigating the impact of HIV on stroke in Malawi did not report effect modification by sex, but these studies either matched by sex or were not powered to detect subgroup differences [27, 28]. Nonetheless, although men have lower rates of linkage to care and present with more-advanced disease in sub-Saharan Africa [29], a large cohort study in South Africa involving HIV-infected men and women aged >60 years demonstrated higher mortality among women [30]. Our results suggest that this phenomenon might be due to increased chronic inflammation among women with HIV infection and that differential gut permeability could play a role as an intervention target [31].
We also observed distinct patterns of biomarker profiles in PLWH receiving suppressive ART in this Ugandan cohort. IFABP, hs-CRP, and sCD14 levels remained persistently elevated as compared to those in HIV-uninfected individuals many years after ART initiation. In contrast, in the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency Health Study (WIHS), levels of a majority of markers of inflammation decreased among PLWH after ART initiation, reaching levels similar to those in HIV-uninfected comparators within 2–3 years [32, 33]. Also unlike prior studies, we did not find a persistently elevated sCD163 level among PLWH [34, 35]. Instead, there was evidence from our cohort that men with HIV infection had a lower sCD163 level than their HIV-uninfected comparators. We have previously demonstrated significant reductions in the sCD163 level among HIV-infected individuals in Uganda from before to 6 months after ART initiation. This suggests that our results represent a normalization of the sCD163 level in the HIV-infected group, rather than a relative increase in the HIV-uninfected group [20]. The mechanisms behind differences in reduction of the sCD163 level after ART initiation should be an active area of investigation, particularly because the sCD163 level is strongly and independently correlated with all-cause mortality in treated HIV infection in Europe [8] but not Uganda [36]. Although the cause for these differing patterns is unknown, differing pathogenic mechanisms between sCD14 and sCD163 may hold clues to this distinction. For example, sCD14 is released from monocytes, neutrophils, and hepatocytes in inflammatory states, whereas sCD163 is cleaved from monocytes only in response to Toll-like receptor activation by pathogenic ligands [37, 38]. Finally, the increased sCD14 level with HIV infection did not appear to be mediated by gut integrity. This could be explained by independent effects of HIV on monocyte activation and gut permeability, by the fact that IFABP is an imperfect marker of gut permeability, or because villous atrophy reverses during ART, increasing the concentration of cells capable of releasing IFABP [39].
Our study should be interpreted with limitations in mind. Results should be generalized to rural sub-Saharan African populations, characterized by subsistence farming and high rates of food insecurity and biomass cooking fuel exposure [40, 41]. ART regimens, which have been associated with differential reductions in systemic inflammation [42], were limited to nonnucleoside reverse transcriptase inhibitors, and we studied a population with low nadir CD4+ T-cell counts. However, these characteristics are representative of disease stage and regimen selection in much of sub-Saharan Africa [43]. Our study was strengthened by high retention in a longitudinal cohort, analysis of multiple observations within individuals, and inflammatory marker testing at the laboratory where the MACS and WIHS cohorts were assessed, enabling cross-cohort comparisons. Overall, our results from sub-Saharan Africa, where HIV infection remains the most common cause of death for women of childbearing age [44], contribute to data demonstrating an undue burden of HIV-related pathology in women. These data should prompt additional attention to the long-term consequences of treated HIV infection among women in the region and a concerted effort to identify effective interventions to mitigate these consequences.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R21 HL124712, P30 AI060354, R24 AG044325, P30 AG024409, K23 MH096620, K23 MH099916, and K43 TW010715), the Massachusetts General Hospital Executive Committee on Research, and Friends of a Healthy Uganda.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hunt PW, Brenchley J, Sinclair E, et al. . Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li JZ, Arnold KB, Lo J, et al. . Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009; 200:1212–5. [DOI] [PubMed] [Google Scholar]
- 4. Cassol E, Malfeld S, Mahasha P, et al. . Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–33. [DOI] [PubMed] [Google Scholar]
- 5. Sereti I, Krebs SJ, Phanuphak N, et al. ; RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 protocol teams Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017; 64:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duprez DA, Neuhaus J, Kuller LH, et al. ; INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knudsen TB, Ertner G, Petersen J, et al. . Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 9. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214(Suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker JV, Huppler Hullsiek K, Prosser R, et al. . Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One 2012; 7:e46894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Funderburg NT, Jiang Y, Debanne SM, et al. . Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 68:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathad JS, Gupte N, Balagopal A, et al. ; New Work Concept Sheet 319 and AIDS Clinical Trials Group A5175 (PEARLS) Study Teams Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 2016; 73:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang S, Mary-Krause M, Simon A, et al. ; French Hospital Database on HIV (FHDH)–ANRS CO4 HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis 2012; 55:600–7. [DOI] [PubMed] [Google Scholar]
- 14. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012; 60:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.