Abstract
Introduction
Tobacco use among persons living with HIV represents an important risk factor for poor treatment outcomes, morbidity, and mortality. Thus, efforts designed to inform the development of appropriate smoking cessation programs for this population remains a public health priority. To address this need, a study was conducted to longitudinally assess the relationship between intention to quit smoking and cessation over the 12-month period following initiation of HIV care.
Methods
Patients initiating HIV care at a large inner city safety net clinic were enrolled (n = 378) in a 12-month prospective study. Audio computer-assisted self-interviews were conducted at baseline, and at 3, 6, 9, and 12 months post-enrollment, and HIV-related clinical data were collected from participants’ electronic medical records. Variables of interest included intention to quit smoking, 7-day point prevalence smoking abstinence (biochemically verified), and stage of HIV. Data were collected in Houston, Texas from 2009 to 2015.
Results
The sample was 75% male and 62% Black. Findings indicated that intention to quit smoking increased between baseline and 3 months, and subsequently trended downward from 3 to 12 months. Results from linear and generalized linear mixed models indicated that participants with advanced HIV disease (vs. not advanced) reported significantly (p < .05) higher intention to quit smoking at 3, 6, and 12 months post-study enrollment. A similar though nonsignificant pattern was observed in the smoking abstinence outcome.
Conclusions
HIV treatment initiation appears to be associated with increases in intention to quit smoking thus serves as a potential teachable moment for smoking cessation intervention.
Implications
This study documents significant increases in intention to quit smoking in the 3-month period following HIV care initiation. Moreover, quit intention trended downward following the 3-month follow-up until the 12-month follow-up. In addition, a marked effect for HIV disease stage was observed, whereby participants with advanced HIV disease (vs. those without) experienced a greater increase in intention to quit. HIV treatment initiation appears to be associated with increases in intention to quit smoking, thus serves as a crucial teachable moment for smoking cessation intervention for people living with HIV.
Introduction
The current era of antiretroviral therapy has brought about significantly increased life expectancies for persons living with HIV/AIDS (PLWH).1,2 However, this era has also seen increasing rates of cardiovascular disease and non-AIDS-associated malignancies.3 Thus, efforts to reduce the occurrence of these diseases represent an important public health priority. Perhaps the most effective way to increase life expectancies of PLWH would be to reduce the prevalence of cigarette smoking in the HIV-positive population.4 Existing estimates indicate that the prevalence of cigarette smoking in PLWH is approximately double the prevalence in the general population.5,6 Moreover, the rates of various oral diseases, pulmonary complications, coronary heart disease, and various cancers are significantly higher for HIV-positive smokers than HIV-positive nonsmokers.7–9 Cigarette smoking among PLWH is also associated with higher rates of both antiretroviral treatment failure and overall mortality.10,11 Despite the significant need, relatively few smoking cessation trials with PLWH appear in the literature. Preliminary efforts suggest that HIV-positive smokers are receptive to both behavioral and pharmacological smoking cessation treatments, and that implementing smoking cessation treatment programs in the HIV clinic setting is feasible.12 However, the published smoking relapse rates are high, thus additional efforts are needed to improve cessation outcomes.13
A potential approach to improve cessation outcomes among PLWH who smoke might consist of promoting cessation treatment within the context of HIV-related events (eg, HIV diagnosis, disease progression, treatment failure, etc.) to optimize/improve cessation outcomes. The literature contains various examples of increased smoking cessation at the time of other health-related events, such as cancer screening, cancer diagnosis, or pregnancy/child birth.14–16 In fact, these health-related events are often described as “teachable moments” for smoking cessation interventions. Briefly stated, a teachable moment is characterized as any event that can prompt individuals to adopt risk-reducing behaviors that will improve their health or reduce their risk of adverse health outcomes.17 While the relationship between health status and smoking cessation has not yet been fully explored in the context of HIV/AIDS, previous efforts have suggested that persons diagnosed with HIV/AIDS may spontaneously adopt healthier lifestyles, including reductions in the number of cigarettes smoked.18 However, the timing of the change in smoking status in relation to HIV diagnosis is less clear, and the observed positive changes may occur years after the HIV diagnosis.19 In summary, the existing literature does suggest that PLWH may be receptive to making positive health behavior changes, including quitting smoking. However, a detailed prospective characterization of the associations between HIV-related events, individual-level variables, and receptiveness to smoking behavior change is needed to inform intervention development.
To address this gap, the primary aim of the current study was to assess the relationship between HIV care initiation, disease stage, and smoking outcomes over the 12-month period following the initiation of HIV care. It was hypothesized that initiation of HIV care and advanced HIV disease would be associated with smoking outcomes (ie, intention to quit smoking and smoking abstinence). Specifically, we hypothesized that the strongest intentions to quit smoking and highest rates of smoking cessation would be observed near the time of HIV care initiation, and trend downward over the follow-up time period. In addition, we hypothesized that participants with advanced HIV disease, as compared to participants without advanced disease, would report greater intention to quit smoking and higher cessation rates.
Methods
Potentially eligible participants were identified by reviewing the daily infectious disease appointment schedules at a large, safety net HIV care center located in Houston, Texas. New center patients were then proactively approached and screened by research staff at the time of appointment check-in. Eligibility criteria included the following: (1) HIV-positive and newly initiating HIV care (as determined by electronic medical record review); (2) currently smoking (defined as self-report of smoking at least 100 lifetime cigarettes and currently smoking every day or most days); and (3) English or Spanish speaking. Infectious disease physicians at the center had the discretion to deem candidates ineligible based on medical or psychiatric concerns. After confirming eligibility, research staff administered the informed consent process. The University of Texas MD Anderson Cancer Center Institutional Review Board and The University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects approved all study-related activities and provided oversight.
Consenting participants were asked to complete a 30- to 45-minute baseline assessment, which was administered in a private location within the HIV care center in the form of an audio computer-assisted self-interview (ACASI) to accommodate varying literacy levels and to optimize privacy. Tablet computers were used to record participant responses directly into a computerized database containing programmed logic checks and skip patterns. Although no cessation treatment was offered as a component of the study, all participants were provided with information about smoking cessation resources, including a program consisting of supportive/educational groups and nicotine replacement therapy (in the form of transdermal nicotine patches) that was available on site at the HIV care center.
Follow-up assessments were conducted at the HIV care center at 3, 6, 9, and 12 months post-baseline. These assessments were scheduled to coincide with routinely scheduled clinic appointments in order to reduce study-related burden. Procedures to increase follow-up completion rates included: (1) reminder phone calls/text messages; (2) offering follow-up assessments on different days/times to accommodate different schedules; and (3) obtaining the names and phone numbers of at least three collaterals (ie, friends or relatives). Follow-up assessments included an ACASI (similar to the baseline ACASI) and an expired carbon monoxide (CO) test. Participants received $25 gift cards for each assessment they completed.
Dependent Variables
The primary outcomes of interest were intention to quit smoking and point prevalence smoking abstinence, biochemically verified by expired CO. Behavioral intention is a well-recognized construct of many effective behavioral models and is among the strongest predictors of future behavior.20,21 Intention to quit smoking was assessed with a single Likert-type scale in which participants were asked the question “Do you plan to quit smoking?”. Responses ranged from 1 (lowest intention to quit) to 7 (highest intention to quit). The determination of point prevalence smoking abstinence was based on the Society for Research on Nicotine and Tobacco recommendations.22 Participants were considered abstinent if they self-reported not smoking (not even a single puff) in the past 7 days and had an expired CO level of less than 7 parts per million.
Independent Variables
The independent variables of interest included time from HIV treatment initiation and HIV disease progression. Time from initiation was estimated by time point of assessment (eg, time of treatment initiation, 3, 6, 9, and 12 months post-initiation). HIV disease stage determination was informed by the Centers for Disease Control and Prevention (CDC) HIV infection stage criteria.23 Specially, at each time point, participants with CD4+ T-lymphocyte counts of less than 200 cells/µL or an HIV-related illness were coded as having advanced HIV disease, while participants not meeting these criteria were coded as not advanced.
Covariates
Demographic variables of interest included age at study entry, gender identity, race/ethnicity, education, work status, route of HIV transmission, and drug/alcohol use. Psychosocial variables included depressive symptoms, affect, social support, and nicotine dependence. Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D)24; the 20-item Positive and Negative Affect Schedule (PANAS) was used to assess affect25; the 12-item Interpersonal Support Evaluation List (ISEL) was used to assess social support26; and the 6-item Fagerström Test for Nicotine Dependence (FTND) was used to assess dependence levels.27
Statistical Plan
Descriptive statistics, including frequencies, proportions, means, and SDs, were generated, as appropriate, to characterize the sample. To assess the associations between HIV disease status (advanced vs. not advanced) and intention to quit smoking at each time point, unadjusted linear regression was used. Similarly, unadjusted logistic regression was used to evaluate the associations between disease status and smoking abstinence (biochemically verified by expired CO) at each follow-up time point.
To examine the relationship between HIV disease status and smoking outcomes across the time points,28 the MIXED procedure in SAS 9.4 (SAS Institute, Cary, NC) was used to model the repeated outcome measures of intention to quit using an R-side variance components covariance structure. The GLIMMIX procedure was used to model the repeated outcome measures of smoking abstinence using an R-side unstructured covariance structure. For both smoking outcomes, we introduced an interaction term to allow the effect of HIV disease status to vary across the time points and obtained parameter estimates via maximum likelihood. These interaction models also accounted for demographic and psychosocial variables identified as follows. Each covariate was assessed in a univariate model and then associated variables (α = .2) were included in a multivariable model which was reduced to the final model using backward selection. This final model included those covariates found to be significantly associated with the smoking outcome (α = .05) or to alter the main relationship of interest by more than 20% when excluded. This model was used to evaluate the associations between disease status and the smoking outcomes by obtaining the difference between the adjusted mean (least squares mean estimate) in those with advanced HIV disease and those without advanced HIV disease at each time point. Since participants had to be current smokers to be eligible for the study, we modeled the point prevalence smoking abstinence using only the follow-up assessments.
Several methods were used to handle missing data, including analysis of available data (ie, complete case analysis), intention to treat (ITT) (in which missing 7-day smoking abstinence values were coded as smoking), and sequential multiple imputation (SMI).29,30 Missing rates ranged from roughly 25% to 53% across the time points for the smoking outcomes, and the proportion of missingness was unbalanced across participants with advanced versus not advanced disease. Such unbalanced missingness has the potential to result in an overestimation of the effect of disease status on the smoking outcomes, when a missing outcome is treated as smoking.31 Therefore, to address the missingness and reduce the nonresponse bias in our statistical analysis, we used SMI as our primary approach.29,30 Before implementing SMI, we identified covariates to include in the imputation of each variable at each time point with missing data. First, for each item with missing data, we assessed models to identify which covariates significantly predicted either the variable or its missingness. These covariates were examined for collinearity and then stepwise regression was used to identify the set of covariates to be included in imputation for each variable. To implement SMI, linear regression models were used to impute the Likert scale variable, intention to quit, with values restricted between 1 and 7 and then the imputed values were rounded to integers. Point prevalence smoking abstinence was imputed using logistic regression models. We generated 40 imputed values for each missing item, then used Rubin’s rule to combine the parameter estimates which takes into account both the within- and between-imputation variance.32
Results
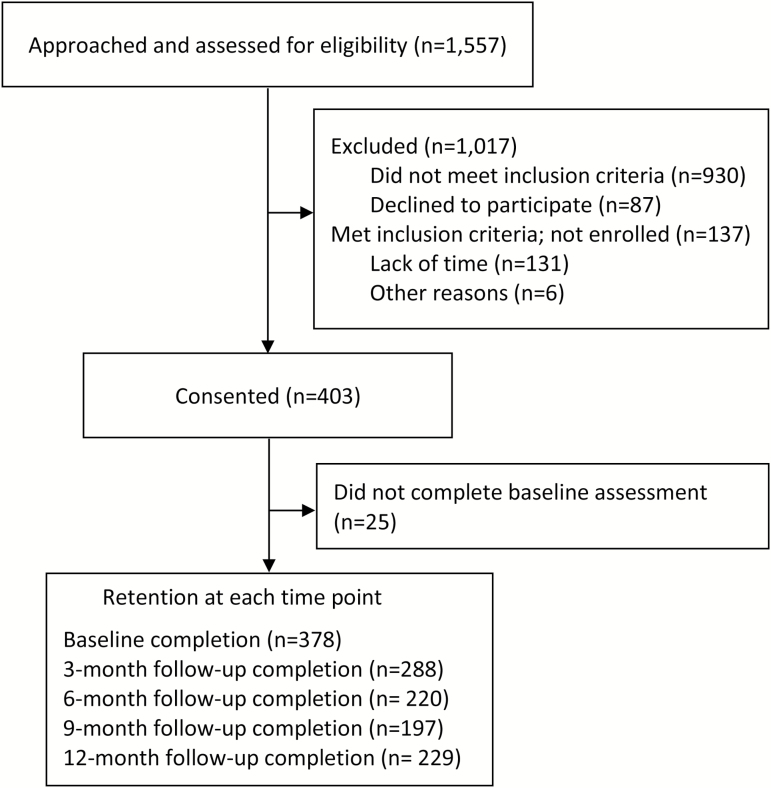
Between the spring of 2009 and the fall of 2014, 1557 patients from the HIV care center were approached and screened for eligibility. Of the 540 eligible individuals identified, 403 (74.6%) consented to participate in the study. The most frequently cited reason for not consenting was lack of available time to complete the baseline assessment. Twenty-five of the consenting participants did not complete the baseline assessment, thus the final sample size available for analysis was 378. Follow-up rates were 76% at 3 months, 58% at 6 months, 52% at 9 months, and 61% at 12 months (see Figure 1).
Figure 1.
Diagram of Project STATE: screening, study enrollment, and retention through 12-month follow-up.
The baseline demographic and psychosocial characteristics of the participants (n = 378) are provided in Table 1. Participants reported a mean (SD) age of 38.7 (10.5) years and were predominately male (73%), African American (64%), single/not living with a significant other (81%), and reported high school education or less (75%). At the time of study enrollment, 40% of participants smoked more than 10 cigarettes per day and 67% reported smoking their first cigarette of the day within 30 minutes of waking. The proportion of participants with advanced HIV disease trended downward over the 12-month follow-up period: 55.3% at baseline, 49.7% at 3 months, 30.4% at 6 months, 24.6% at 9 months, and 26.7% at 12 months.
Table 1.
Demographic, Behavioral, and Psychosocial Characteristics of All Participants at Baseline
| Characteristic | n (%) or mean ± SD |
|---|---|
| Demographic variables | |
| Age, years | 38.7 ± 10.5 |
| Sex | |
| Female | 102 (26.98) |
| Male | 276 (73.02) |
| Race/ethnicity | |
| White | 68 (17.99) |
| Black | 242 (64.02) |
| Hispanic | 54 (14.29) |
| Other | 14 (3.70) |
| Relationship status | |
| Single/not living with significant other | 308 (81.48) |
| Living with significant other | 70 (18.52) |
| Highest level of education | |
| Less than high school | 136 (35.98) |
| High school or equivalent | 147 (38.89) |
| More than high school | 95 (25.13) |
| Work status | |
| Working full or part time | 60 (15.87) |
| Can’t find work | 95 (25.13) |
| Not working—other | 40 (10.58) |
| Not working—poor health | 183 (48.41) |
| Route of HIV infection | |
| Heterosexual | 161 (42.59) |
| MSM | 141 (37.30) |
| Injection drug use | 34 (8.99) |
| Other | 42 (11.11) |
| Illicit drug use in the past 30 days | |
| Yes | 176 (46.56) |
| No | 202 (53.44) |
| Nicotine dependence, FTND score | 4.2 ± 2.5 |
| Cigarettes smoked each day | |
| >10 | 151 (39.95) |
| ≤10 | 227 (60.05) |
| Time to first cigarette after waking | |
| Within 30 min | 254 (67.20) |
| >30 min | 124 (32.80) |
| Alcohol use, AUDIT score | |
| ≥8 | 98 (25.93) |
| <8 | 280 (74.07) |
| Psychosocial variables | |
| Depression, CES-D score | |
| ≥16 | 254 (67.20) |
| <16 | 124 (32.8) |
| Negative Affect, PANAS-N score | 21.3 ± 10.1 |
| Positive Affect, PANAS-P score | 30.8 ± 10.5 |
| Social support, ISEL overall score | 34.7 ± 8.0 |
AUDIT, Alcohol Use Disorders Identification Test; CES-D, Center for Epidemiologic Studies Depression Scale; FTND, Fagerström Test for Nicotine Dependence; ISEL, Interpersonal Support Evaluation List; MSM, men who have sex with men; PANAS, Positive and Negative Affect Schedule.
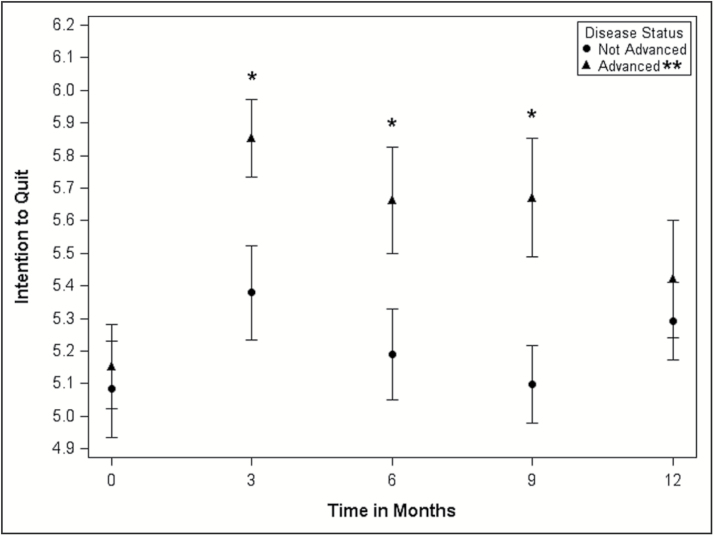
The relationship between advanced HIV disease status and intention to quit smoking over time is depicted in Figure 2. In general, the figure indicates that regardless of disease status, intention to quit increased between baseline and 3 months, and subsequently trended downward from 3 to 12 months. Results from the unadjusted single time point and the adjusted longitudinal analyses for HIV disease status and the intention to quit outcome, using SMI methodology to account for missing data, are provided in Table 2. Unadjusted significant differences in intention to quit by disease status were not observed at the time of study enrollment nor at the 12-month follow-up. However, participants with advanced disease did report significantly (p < .05) higher intention to quit at 3, 6, and 9 months post-study enrollment. Results from the longitudinal adjusted model analyses, accounting for FTND and Positive Affect at baseline, revealed similar trends. The adjusted mean intention to quit was significantly different between those with advanced and without advanced HIV disease at the 3- and 6-month follow-up visits. The adjusted mean intention to quit was roughly 0.4 higher in participants with advanced HIV disease. Analysis of available data, unadjusted and adjusted, also suggested that participants with advanced disease reported higher intention to quit at the 3- and 6-month follow-ups (results not shown).
Figure 2.
Intention to quit smoking at each assessment time point by HIV disease status.*P < .05. **Advanced HIV disease defined as CD4+ T-lymphocyte counts of <200 cells/µL or an HIV-related illness.
Table 2.
Relationship Between HIV Status and Intention to Quit
| Pairwise comparisons between HIV disease status | ||
|---|---|---|
| Time point | Unadjusted mean difference, single time point models (95% CI)a | Adjusted mean difference, longitudinal model (95% CI)b |
| Baseline | 0.0703 (−0.3163, 0.4568) | 0.2253 (−0.0859, 0.5364) |
| 3 months | 0.4740 (0.1116, 0.8363)* | 0.3921 (0.0549, 0.7293)* |
| 6 months | 0.4716 (0.0474, 0.8957)* | 0.4071 (0.0538, 0.7603)* |
| 9 months | 0.5747 (0.1546, 0.9947)* | 0.3046 (−0.0547, 0.6639) |
| 12 months | 0.1303 (−0.2908, 0.5514) | 0.1135 (−0.2323, 0.4593) |
CI, confidence interval.
aEstimated by least squares mean in single variable linear regression model.
bEstimated by least squares mean in multiple variable repeated measures linear regression model, adjusting for Fagerström Test for Nicotine Dependence and Positive Affect at baseline.
*p < .05.
The trends in biochemically verified 7-day smoking abstinence over the 12-month follow-up window indicated that cessation rates were approximately 10% at 3 months, 12% at 6 months, 14% at 9 months, and 8% at 12 months. The unadjusted single time point and the adjusted longitudinal associations between HIV disease status and smoking abstinence, using SMI approach, are provided in Table 3. The unadjusted models did not identify a significant association between HIV disease status and smoking abstinence at any of the follow-up time points. The available data analyses revealed similar results. However, the single time point ITT analyses indicated that individuals with advanced disease (vs. not advanced) were significantly more likely to quit at the 3-month follow-up (odds ratio [OR] = 2.6, 95% confidence interval [CI]: 1.1, 6.0) and at the 6-month follow-up (OR = 2.7, 95% CI: 1.1, 6.2). Results from the longitudinal adjusted SMI models did not reveal any statistically significant differences by advanced disease status. However, age was retained in the final model, indicating that older participants were less likely to quit smoking (OR = 0.96, 95% CI: 0.94, 0.98). Longitudinal models using ITT methodology indicated that participants with advanced disease (vs. not advanced) were more likely to quit at 3 months (OR = 2.4, 95% CI: 1.0, 6.2) and 6 months (OR = 3.1, 95% CI: 1.2, 7.9), though only the comparison at 6 months reached statistical significance. No significant associations were revealed from the available data methodology (results not shown).
Table 3.
Relationship Between HIV Status and Abstinence Status
| Pairwise comparisons between HIV disease status | ||
|---|---|---|
| Time point | Unadjusted odds ratio, single time point models (95% CI)a | Adjusted odds ratio, longitudinal model (95% CI)b |
| 3 months | 1.51 (0.72, 3.15) | 1.41 (0.66, 3.02) |
| 6 months | 1.20 (0.55, 2.62) | 1.43 (0.62, 3.28) |
| 9 months | 0.86 (0.38, 1.94) | 0.91 (0.39, 2.12) |
| 12 months | 1.24 (0.51, 3.03) | 1.18 (0.47, 2.95) |
CI, confidence interval.
aEstimated by least squares mean in single variable logistic regression model.
bEstimated by least squares mean in multiple variable repeated measures logistic regression model, adjusting for age, Fagerström Test for Nicotine Dependence, and Negative Affect at baseline.
Discussion
The findings from this study indicate that time from HIV treatment initiation may be associated with intention to quit smoking. Overall, increases in intention to quit smoking were observed between the time of study enrollment (ie, time of HIV treatment initiation) and the 3-month follow-up period, and quit intention trended downward following the 3-month follow-up until the 12-month follow-up. In addition, a marked effect for HIV disease stage was observed. Specifically, when using the SMI methodology, compared to participants without advanced HIV disease, those who had advanced disease (ie, CD4+ T-lymphocyte counts of <200 cells/µL or an HIV-related illness) experienced a greater increase in intention to quit, which was maintained from month 3 through month 9 in the single time point analyses and from month 3 through month 6 longitudinal analyses. While similar, the trends that were observed when 7-day smoking abstinence was considered were not identical to the trends observed with intention to quit. That is, after initiating HIV care, about 10% of participants achieved smoking abstinence by the 3-month follow-up. However, unlike the intention to quit outcome which dropped after month 3, abstinence rates continued to increase until month 9 (14%), before dropping at month 12. Again, the effects were more pronounced in participants with advanced HIV. In general, there was no significant relationship observed between time from treatment entry, advanced HIV status, and the 7-day abstinence outcome, in the SMI or available data methodologies. Though a significant finding was observed when the ITT methodology was used, its importance is weakened by the unbalanced nature of the missingness in those with and without advanced disease. Thus, the findings from this study suggest that, like other health-related events, the initiation of HIV treatment may serve as a teachable moment for promoting increased intention to quit smoking.14–16
To our knowledge, no other efforts to prospectively evaluate the relationships between smoking outcomes and HIV-related events (eg, diagnosis, progression, treatment initiation) appear in the literature. However, several studies have attempted to examine the effects of markers of HIV disease on cessation outcomes. For example, a recent report from the Multicenter AIDS Cohort Study (MACS) described the association between several HIV-related variables and current smoking status. Unlike the findings from the current study, results from the MACS report suggested that participants with more advanced HIV disease, as indicated by detectable HIV RNA levels in plasma, were more likely to smoke cigarettes.33 Indeed, this finding indicating that cigarette smoking may be associated with poor virologic response to antiretroviral therapy has been previously reported and provides a strong rationale for the need for cessation interventions targeting PLWH.11,34 It should be emphasized, however, that previously reported findings do not account for time of HIV diagnosis or HIV treatment initiation. That is, while findings from the current study indicate that individuals with advanced HIV disease are more likely to intend to quit, and/or achieve abstinence, this relationship is strongest near the time of HIV care initiation and likely does not remain over time. This suggests that, consistent with McBride’s framework, it may be imperative to pair smoking cessation interventions for PLWH at or near the time of HIV diagnosis not only to reduce the risk of longer-term adverse health effects but also to capitalize on a temporary increase in receptivity.17
Findings from this study should be interpreted in the context of several design-related considerations and limitations. Among these was the decision to use the time of HIV care initiation as the entry point for study inclusion rather than the time of HIV diagnosis. That is, participants were recruited upon arrival at the HIV care center for their first visit with an infectious disease physician. While HIV care initiation ideally occurred within 30 days of their HIV diagnosis, some participants may have postponed initiation for a longer duration of time (>30 days). Given the limitations of our available data, we were not able to assess the relationship between time of actual HIV diagnosis and smoking outcomes. Thus, it is possible that the teachable moment effect could have been diminished for some participants. Despite this possibility, targeting recruitment efforts to the time of HIV care initiation provided the team with a feasible way to standardize participant recruitment efforts. Assuming that HIV care initiation represents an important health care system engagement moment for PLWH, pairing future tobacco treatment intervention with this contact would likely be more readily implemented compared to field-based HIV screening programs. Results should also be considered in light of the available cessation program (consisting of supportive/educational groups and free/low cost nicotine replacement therapy) at the study site. It is possible that the availability of this program may have influenced the observed relationships between time from care initiation, HIV disease stage, and smoking-related variables, thus findings may not generalize to other HIV clinic sites with other (or no) cessation programs. It is also possible that the results from this sample may not be generalizable to other populations given some of the unique aspects of this sample. This sample was majority male and Black, recruited from one clinic in Texas, many of whom reported drug use and a high level of addiction to combustible cigarettes. Finally, missing data, particularly at the longer-term (ie, 9- and 12-month) follow-ups, were higher than anticipated. Missing data were not necessarily attributable to high study dropout rates. In fact, for the most part (>90%), participants who missed study follow-up also missed scheduled appointments at the HIV care center. While the SMI analytic approach utilized in the current study provides a robust method to handling missing data,29,30 it remains possible that our observed effects could be biased, due to potential non-ignorable missingness.35
Findings from this study suggest that initiating HIV care may serve as a teachable moment for smoking cessation. Given the limited success of the published tobacco intervention trials for PLWH,13 these findings may provide some direction for future intervention development. For example, directly pairing tobacco cessation treatment with an HIV diagnosis, or at the time that HIV care is initiated, may offer the potential to meaningfully increase cessation rates. Thus, future efforts to develop cessation treatments that are appropriate for implementation at this time point are especially needed. Similarly, efforts to design cessation interventions for PLWH that can be dynamically tailored according to an individual’s current stage of HIV may further increase abstinence. For example, the ideal treatment content, or treatment approach, for an individual with advanced stage HIV may differ from that of an individual with stable disease or early stage disease. Future efforts will also attempt to tease apart the between- and within-subject effect of disease status and examine potential moderators and mediators of the relationships between HIV disease status, smoking cessation outcomes, and demographic covariates such as age, gender, depression, and nicotine dependence. Finally, efforts to more fully evaluate other salient components of this observed effect are planned. Specifically, we are currently evaluating the role(s) of perceived impact of HIV and perceived seriousness of HIV infection. Such knowledge may help to better inform the next generation of tobacco cessation treatments for PLWH, as well as contributing to the understanding of the mechanisms of the teachable moment.
Funding
This work was supported by the National Cancer Institute grant R01CA132636 (PI: DJV) and from the Oklahoma Tobacco Settlement Endowment Trust grant 092-016-0002 (PI: DJV).
Declaration of Interests
None declared.
Acknowledgments
We are grateful to the patients and staff at the Thomas Street Health Center in Houston Texas.
References
- 1. Lloyd-Smith E, Brodkin E, Wood E, et al. . Impact of HAART and injection drug use on life expectancy of two HIV-positive cohorts in British Columbia. AIDS. 2006;20(3):445–450. [DOI] [PubMed] [Google Scholar]
- 2. Palella FJ Jr, Delaney KM, Moorman AC, et al. . Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. [DOI] [PubMed] [Google Scholar]
- 3. Palella FJ Jr, Baker RK, Moorman AC, et al. ; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. [DOI] [PubMed] [Google Scholar]
- 4. Reddy KP, Parker RA, Losina E, et al. . Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: A US-based modeling study. J Infect Dis. 2016;214(11):1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30(2):273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mdodo R, Frazier EL, Dube SR, et al. . Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 7. Rasmussen LD, Helleberg M, May MT, et al. . Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–1423. [DOI] [PubMed] [Google Scholar]
- 8. Helleberg M, Gerstoft J, Afzal S, et al. . Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS. 2014;28(10):1499–1508. [DOI] [PubMed] [Google Scholar]
- 9. Vidrine DJ. Cigarette smoking and HIV/AIDS: health implications, smoker characteristics and cessation strategies. AIDS Educ Prev. 2009;21(Suppl. 3):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helleberg M, May MT, Ingle SM, et al. . Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR; INSIGHT SMART Study Group Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy Clinical Trial. Am J Public Health. 2010;100(10):1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacek LR, Cioe PA. Tobacco use, use disorders, and smoking cessation interventions in persons living with HIV. Curr HIV/AIDS Rep. 2015;12(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst Rev. 2016;6:CD011120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piñeiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review. Lung Cancer. 2016;98:91–98. [DOI] [PubMed] [Google Scholar]
- 15. Westmaas JL, Newton CC, Stevens VL, Flanders WD, Gapstur SM, Jacobs EJ. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J Clin Oncol. 2015;33(15):1647–1652. [DOI] [PubMed] [Google Scholar]
- 16. Winickoff JP, Healey EA, Regan S, et al. . Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics. 2010;125(3):518–525. [DOI] [PubMed] [Google Scholar]
- 17. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. [DOI] [PubMed] [Google Scholar]
- 18. Collins RL, Kanouse DE, Gifford AL, et al. . Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol. 2001;20(5):351–360. [PubMed] [Google Scholar]
- 19. Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7(4):511–522. [DOI] [PubMed] [Google Scholar]
- 20. Fishbein M. A reasoned action approach to health promotion. Med Decis Making. 2008;28(6):834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutton S. Predicting and explaining intentions and behavior: how well are we doing?J Appl Soc Psychol. 1998;28:1317–1338. [Google Scholar]
- 22. SRNT Committee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Revised surveillance case definition for HIV infection—United States, 2014. MMWR Recomm Rep. 2014;63(RR-03):1–10. [PubMed] [Google Scholar]
- 24. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 26. Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, eds. Social Support: Theory, Research, and Applications. The Hague, Holland: Martinus Nijhoff; 1985:73–94. [Google Scholar]
- 27. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 28. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. 2nd ed Hoboken, NJ: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 29. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;24(1):85–95. [Google Scholar]
- 30. Zhu J, Raghunathan TE. Convergence properties of a sequential regression multiple imputation algorithm. J Am Stat Assoc. 2015;110(511):1112–1124. [Google Scholar]
- 31. Blankers M, Smit ES, van der Pol P, de Vries H, Hoving C, van Laar M. The missing=smoking assumption: a fallacy in internet-based smoking cessation trials?Nicotine Tob Res. 2016;18(1):25–33. [DOI] [PubMed] [Google Scholar]
- 32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 33. Akhtar-Khaleel WZ, Cook RL, Shoptaw S, et al. . Trends and predictors of cigarette smoking among HIV seropositive and seronegative men: the Multicenter Aids Cohort Study. AIDS Behav. 2016;20(3):622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feldman JG, Minkoff H, Schneider MF, et al. . Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health. 2006;96(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Little RJA, Rubin DB.. Statistical Analysis With Missing Data. 2nd ed New York: John Wiley & Sons; 2002. [Google Scholar]