Abstract
Rat somatosensory genital cortex contains a large sexually monomorphic representation of the penis in males and the clitoris in females. Genital cortex microstimulation-evoked movements of legs, trunk and genitals, which showed sex-specific differences related to mating behaviors and included thrusting in males and lordosis-like movements in females. Erections/tumescence of penis or clitoris could not be evoked, however. Anterograde tracer injections into penis/clitoris cortex revealed eleven corticocortical and 10 subcortical projection targets, which were qualitatively similar in both sexes. Corticocortical genital-cortex-projections innervated about 3% of the cortical surface and most were analog to other somatosensory projections targeting motor cortex, secondary somatosensory cortex, parietal cortex and perirhinal cortex. Corticocortical projections that differed from other parts of somatosensory cortex targeted male scrotum cortex, female vulva cortex, the somatosensory–ear–auditory-cortex-region and the caudal parietal area. Aligning cytoarchitectonic borders with motor topography, sensory genital responses and corticocortical projections identified a candidate region for genital motor cortex. Most subcortical genital-cortex-projections were analog to other thalamic, tectal or pontine projections of somatosensory cortex. Genital-cortex-specific subcortical projections targeted amygdala and nucleus submedius and accumbens. Microstimulation-effects and projections support a sexual function of genital cortex and suggest that genital cortex is a major hub of sexual sensorimotor processing in rodents.
Keywords: clitoris, microstimulation, penis, somatosensory cortex, tracing
Introduction
Even though the cortical genital representation is relatively large, rat somatosensory genital cortex has only recently been identified (Lenschow et al. 2016). Remarkably this cortical region is sexually monomorphic, despite the marked sexual dimorphism of external genitals in rats. Sexual monomorphism of the cortical genital representation was also observed in other mammals (Lauer et al. 2017). A variety of observations point to a sexual function of rat genital cortex: (1) The genital representation has a phallic appearance, reminiscent of the erect penis. (2) Receptive fields in genital cortex show sexually dimorphic multibody part responses; the response fields appear to match with the contact patterns induced during mating in the respective sex. (3) Genital cortex undergoes a massive expansion during puberty.
Prompted by these observations we wondered if the analysis of functional outputs of genital cortex might offer insights into cortical sexual information processing. While we have relatively detailed information about subcortical structures involved in sexual behaviors (Nomoto and Lima 2015, Hashikawa et al. 2016), we know little about cortical sexual information processing. Most information on cortical sexual information processing stems from human studies. Already Penfield and Rasmussen (1950) identified a genital representation in human somatosensory cortex. This genital representation was displaced below the foot representation. While such a nonsomatotopic genital representation was supported by more recent fMRI imaging work (Komisaruk et al. 2011), other current studies challenged this idea. Both extracellular recordings in monkeys (Rothemund et al. 2002) and fMRI imaging (Kell et al. 2005) provided conclusive evidence for a somatotopic genital representation in primate somatosensory cortex. A conserved somatotopically arranged genital representation in rodents is suggested by a recent comparative study (Lauer et al. 2017). This comparative study also pointed to a role of sexual selection in shaping the size of genital cortex.
Sexual behavior—like most other mammalian behaviors—persists after decortication, although subtle alterations, that is, abnormalities in the execution of movements, are observed (Carter et al. 1982, Whishaw and Colb 1985). Subcortical structures mediating sexual behaviors such as medial preoptic area (MPOA), the bed nucleus of the stria terminalis (BNST) and the ventromedial hypothalamus (VMH) differ in 2 significant aspects from genital cortex. First, these brain structures show sexual dimorphism (Raisman and Field 1971, Matsumoto and Arai 1983, Hines et al. 1992). Second, lesions of such structures strongly affect basic aspects of sexual behavior (Pfaff and Sakuma 1979, Valcourt and Sachs 1979, Paredes et al. 1993, Yang et al. 2013). These differences suggest that subcortical structures might be relevant for basic and sex-specific aspects of sexual information processing.
The functional role of genital cortex is still unclear. A strong hint for the relevance of genital cortex in sexual function came from our developmental work on genital cortex. This developmental work showed that the pubertal expansion of rat genital cortex is under the control of sex hormones and depends on sexual experience. Most interestingly, this work also indicated that rat genital cortex contributes to the hastening of puberty by sexual touch (Lenschow et al. 2017). To understand how the genital cortex exerts such effects, we need to understand the connectivity of this cortical region.
In the current study we used microstimulation to determine motor outputs and tracer injections to determine anatomical outputs of genital cortex. Specifically, we sought to answer the following questions. (1) What types of movements, if any, are evoked by stimulation of genital somatosensory cortex? (2) Do male and female genital cortex generate different motor outputs, despite the monomorphic map? (3) Is the connectivity of genital cortex the same as the connectivity of other parts of somatosensory cortex? (4) Do the subcortical targets of genital cortex provide an insight, as to how genital cortex influences pubertal development?
Materials and Methods
Animal Welfare
All experimental procedures were performed according to German guidelines on animal welfare under the supervision of local ethics committees (animal permit numbers: G0244/16 and G0193/14).
Wistar rats were purchased from Janvier Labs. All animals were kept on a 12 h:12 h normal light/dark cycle with lights off at 10:00 p.m. Rats had ad libitum access to food and water.
Determination of Sexual Receptivity in Females
Since typical sexual movements, that is, lordosis behavior, are only observed during female sexual receptivity (Kow et al. 2007), microstimulation experiments were conducted when females were in their proestrous phase of the reproductive cycle. In order to detect the proestrous state, daily vaginal lavage was conducted in females. The proestrous phase can be reliably separated from the other days of the reproductive cycle by the dominance of nucleated cells in the vaginal smear (Marcondes et al. 2002). Measuring the vaginal conductance confirmed the assignment of the proestrous day which are detected by estrous scores higher than 3 (Ramos et al. 2001).
Microstimulation
We performed acute experiments, in which we microstimulated somatosensory genital cortex and surrounding areas in Wistar rats aged between 6 and 8 weeks. Animals were anesthetized by injection of an initial dose of 100 mg/kg ketamine and 7.5 mg/kg xylazine and fixed by ear bars into a stereotactic frame where they were restrained during the experiment. Respiration, blink and pinch reflex were observed throughout the surgery and, if needed, animals were injected with an extra shot (25%) of ketamine/xylazine mixture or a 25% dose of ketamine alone. Temperature was monitored using a rectal probe and was maintained at 34–36 °C with a heating pad (Stoelting). Lidocaine was locally injected in the scalp, which was then removed. An approximately 4 × 4 mm2 sized craniotomy was made 4 mm posterior to and 4 mm lateral to bregma. After the surgery animals were observed until anesthesia was light. Light anesthesia was maintained by additional alternating doses of 5% of the initial dose in ketamine/xylazine amount or 5% of the corresponding ketamine dose alone, respectively. Motor responses could only be elicited in lightly anaesthetized animals and even though it was tried to maintain the animal in the same anesthetic state throughout the experiment, this could not always be succeeded. Thus, in light animals (with moving their whiskers), movements were elicited with very little current (<20 μA) whereas stimulation thresholds for movements were higher (>50 μA) in animals anesthetized more deeply. Independent of such variations we observed striking sex differences in current thresholds for movements (Fig. S1).
At each microstimulation site, a 5 MΩ tungsten electrode was lowered to layer 5 of genital cortex (~1500 μm), currents ranging from 5 to 300 μA were injected using a stimulus isolator (Model no. A365RC, World Precision Instruments) and possible movements were documented. We used exclusively unipolar currents, since it was shown that they have important advantages compared with bipolar current application. More precisely unipolar current application offers high reliability, high fatigue resistance and less tissue damage (Comte 1982).
In order to test if a possible erection or lordosis posture was elicited, higher currents (>100 μA) were applied at the corresponding recording sites. Elicited movements were recorded using 2 different cameras. An overview of the animal’s body was filmed using a WebCam (Logitech, 30 Hz) and a high magnification video from the genital area was recorded using a Low-Speed Color Camera (Siemens, 25 Hz). Once penis/clitoris or sexual movements (thrusting or lordosis posture) were observed at a recording site, a lesion was placed below layer 4 and the animals were transcardially perfused. The brain was sectioned tangentially as described below and stimulation sites assigned by histology.
Anterograde and Retrograde Neuronal Labeling
Anterograde solutions containing Biotinylated Dextrane Amine (BDA) (10% w/v; 10 000 MW) were injected into the brain of Wistar female and male rats aged approximately 8 weeks. Surgical procedures were the same as described above. The exact position of injection was localized by electrophysiological mapping recordings. Glass electrodes with a tip diameter of 10–20 μm were filled with a 10% BDA solution and lowered into the target region at a depth of 1300 μm below the pia. The tracer was iontophoretically injected using a stimulus isolator (National Instruments; 7 s on/off current pulses of 1–5 mA for 20 min). After the injections, the pipettes were left in place for several minutes and quickly retracted. The craniotomy was closed using silicone (Kwik-Cast) and dental cement (Heraeus). The animals survived for 7 days to allow neuronal transport of BDA. Subsequently the animals were transcardially perfused and the brain histologically processed as described in the following section.
The procedure for retrograde neuronal labeling differed slightly. Solutions containing choleratoxin-B (CTB) (1 mg/ml) coupled with either Alexa 488 or 555 were pressure injected using an injector (Stoelting) in the brain of Wistar female and male rats, aged between 6 and 8 weeks. Targeting of contralateral genital cortex and primary motor cortex was achieved with subsequent mapping recordings.
Histology
At the end of the microstimulation experiments, animals were anaesthetized using a 20% urethane solution and perfused with phosphate buffer followed by a 2% paraformaldehyde solution (PFA). Brains were removed, hemispheres were separated, and cortices were flattened between 2 glass slides separated by clay spacers. Glass slides were weighed down with small ceramic weights for ∼3 h. Afterwards, flattened cortices were stored overnight in 2% PFA and 80 μm sections were cut on a Vibratome (Leica). Sections were stained for cytochrome-oxidase activity using the protocol of Divac et al. (1995). After the staining procedure, sections were mounted on gelatin coated glass slides with Mowiol mounting medium. Subsequently, pictures were taken on a Neurolucida microscope (Olympus) and layer 4 areas of somatosensory cortex were drawn on the brain sections. In order to identify the body region where the lesion was placed in, the obtained body maps were aligned onto every brain section.
For the analysis of the anterograde tracing experiments, animals were deeply anaesthetized after 7 days as described above. Perfusion procedure was the same too. In order to assure that genital cortex was well targeted and to characterize corticocortical connections, the brain hemispheres were separated, cortices were flattened and sectioned using the protocol described above. Cytochrome oxidase activity was labeled using the protocol from Wong-Riley (1979). Subsequently, the neuronal transport of BDA was visualized using the avidin–biotin–peroxidase method. For subcortical projection patterns, brains were processed in the same way, but cut coronally. Brains from retrograde tracing experiments were cut coronally and mounted as described above. The tagged fluorophors (Alexa 488 and 555) allowed the analysis of labeling using a fluorescence microscope (Leica).
Results
Microstimulation-Evoked Movements
As a first assessment of the physiological outputs of somatosensory cortex we applied microstimulation in the genital somatosensory cortex. In rats lightly anesthetized with ketamine/xylazine, we exposed the genital somatosensory cortex and mapped motor responses by applying brief (0.3 ms pulses at 200 Hz for 300 ms, tip negative, 5–300 μA) pulse trains to deep layers.
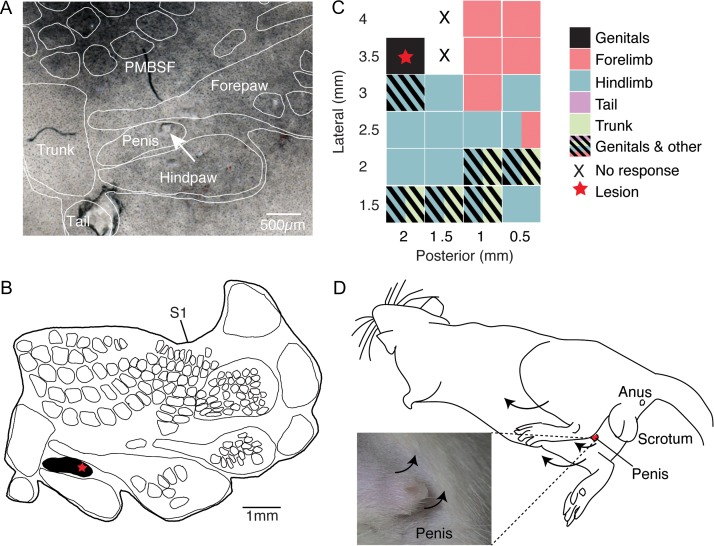
As in other experiments we flattened the cortical hemisphere and stained tangential sections for cytochrome oxidase reactivity to reveal the layer 4 somatosensory body representation. As shown in Figure 1A lesion sites were aligned through serial sections to the layer 4 drawing (Fig. 1B). We mapped several sites in somatosensory cortex in this experiment and observed a variety of movements, which mapped in a systematic fashion on the cortical sheet. Posterior and lateral in the exposure we observed penis movements and at this site we placed an electrolytic lesion (Fig. 1C). Anterior and lateral in the exposure we observed forepaw movements, more medially hindlimb movements were evoked and very far medially we again observed genital movements (Fig. 1C). The comparison of this stimulation map (Fig. 1C) and the body map (Fig. 1B) shows a good correspondence and that genital cortex stimulation indeed evoked penis movements. The stimulation map also indicates the existence of a second more medial movement field for genital movements, which putatively corresponds to genital primary motor cortex and will be discussed below. Movements evoked at the genital cortex stimulation site are shown in Figure 1D. We observed thrusting movements of the lower trunk (Supplemental movie 1) and local penis movements (penis flipping up, Supplemental movie 2) in all 4 males.
Figure 1.
Physiological outputs of male rat genital cortex. (A) Cytochrome oxidase staining of a tangential somatosensory cortex section with the overlaid corresponding histological analysis (white outlines mark the body representation as revealed in B, the annotation of body parts is based on the results of Lenschow et al. (2016), the arrow indicates the lesion, positioned within the penis representation). (B) Outline of the corresponding somatosensory cortex map from the brain of an adult male (8 weeks). The star marks the lesion site, shown in the section in A. (C) A physiological motor map of male posterior somatosensory cortex. Stimulation sites are indicated relative to bregma. Colors indicate movements of different body parts elicited by intracortical microstimulation. Spacing of stimulation penetrations was 0.5 mm. Thus each rectangle represents a stimulation site (0.5 × 0.5 mm2). The lesion was placed (red star) at a site, where local penis and thrusting movements were achieved by microstimulation. (D) Scheme illustrating the observed movements at the lesion site in C. Additionally to thrusting movements of the lower trunk, local penis movements (penis flipping up) were elicited. See also Supplemental movies 1 and 2.
The experiment shown in Figure 1 was in many regards typical for the stimulation effects observed in 4 further experiments in male genital cortex. Thus, we often observed thrusting movements and penis upward movements, 2 movement patterns, which appear in the male rat’s sexual repertoire. We did not observe penile erection as a consequence of stimulation; the absence of penile erection held true for both short (300 ms) and long (several seconds) stimulation trains and for perithreshold (typically 10 μA) and suprathreshold stimulation (typically 300 μA).
We next describe a microstimulation experiment in the somatosensory cortex of an adult female rat. Since typical sexual movements, that is, lordosis behavior, are only observed during female sexual receptivity (Kow et al. 2007), all microstimulation experiments (n = 4) on female rats were performed on animals in proestrous (see Materials and Methods for details on tracking the reproductive cycle in females).
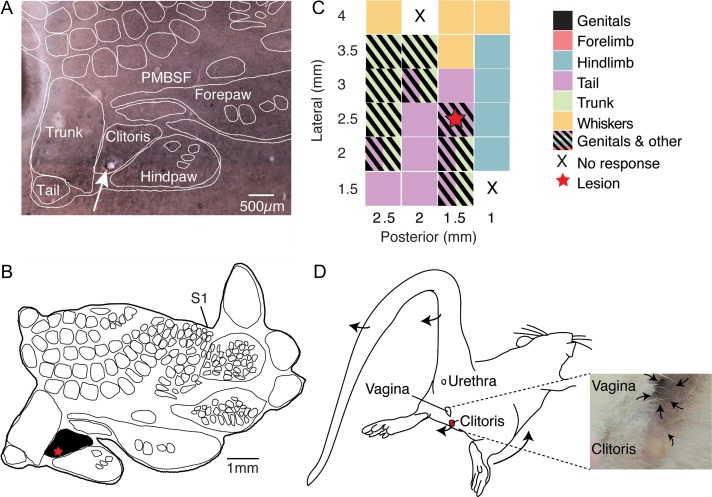
We again obtained a layer 4 body map by cytochrome oxidase reactivity and aligned lesion sites through serial sections to the layer 4 drawing (Fig. 2A, B)). We mapped several sites in somatosensory cortex in this experiment and again observed a variety of movements (Fig. 2C). Stimulating lateral in the exposure elicited whisker movements; posterior and more medially we observed trunk movements and posterior and maximally medial stimulation elicited tail movements. Further anterior we observed hindlimb movements (Fig. 2C). Again, the body map (Fig. 2B) and the stimulation map (Fig. 2C) align very well. The movements evoked by stimulation in female genital cortex were quite different from the movements observed by stimulating male genital cortex. As shown in Figure 2D, lordosis-like movements (lower body part was moved anterior and tail was flipped dorsally; Supplemental movie 3) were observed. In addition, local clitoris and vagina movements were elicited (Supplemental movie 4). Lordosis like movements (with the implication of tail movements) were observed in 2 out of 4 stimulation experiments with females. The other 2 females showed bilateral movements towards anterior and implicated the whole trunk. Clear local clitoris movements were observed only in 1 out of 4 females but vagina movements could be elicited in all 4 animals. Pooling the currents applied in males (Fig. S1A) and females (Fig. S1B), showed that significantly higher currents were needed in females in order to elicit movements (Fig. S1C). This was particularly true for the induction of thrusting/lordosis and clitoris/penis movements (P = 0.004). For a better quantification of the movements elicited by microstimulation, we assigned movement scores to every location tested: a score of 1 stands for low movements, 2 describes moderate movements and a score of 3 stands for high qualitative movements. Whereas hindpaw, forepaw and whisker movements were not qualitatively different (data not shown), the elicited movements in the genitals differed significantly between males and females (Fig. S1D, P = 0.03). Thrusting and lordosis movements were assigned with similar scores for all animals tested (Fig. S1D, P = 0.14).
Figure 2.
Physiological outputs of female rat genital cortex. (A) Cytochrome oxidase stain of a tangential somatosensory cortex section with the overlaid corresponding histological analysis (white outlines mark the body representation as revealed in B, the annotation of body parts is based on the results of Lenschow et al. (2016), the arrow indicates the lesion within the clitoris representation). (B) Outline of the corresponding somatosensory cortex map from the brain of an adult female (8 weeks). The star marks the lesion site, shown in the section in A. (C) A physiological motor map of female posterior somatosensory cortex is plotted. Stimulation sites are indicated relative to bregma. Colors indicate movements of different body parts elicited by intracortical microstimulation. Spacing of stimulation penetrations was 0.5 mm. Thus each rectangle represents a stimulation site. The lesion was placed (red star) at a site, where local clitoris and tail movements were achieved by microstimulation. (D) Scheme illustrating the observed movements at the lesion site in C. Additionally to lordosis-like movements (lower body part was moved anterior and tail was flipped dorsally), local clitoris and vagina movements were elicited. See also Supplemental movies 3 and 4.
Cortical Projection Pattern of Penis Cortex
Next we aimed at determining the anatomical output of somatosensory genital cortex. To this end we exposed somatosensory cortex in anesthetized rats and performed a fast multiunit mapping of extracellular responses to body palpation to determine the center of genital cortex. Then we placed an anterograde tracer (10% BDA) at this location and let the animals recover and survive for 7 days allowing tracer transport. Subsequently animals were killed, brains were removed and processed to visualize the tracer as well as the layer 4 body map, which was visualized by cytochrome oxidase reactivity.
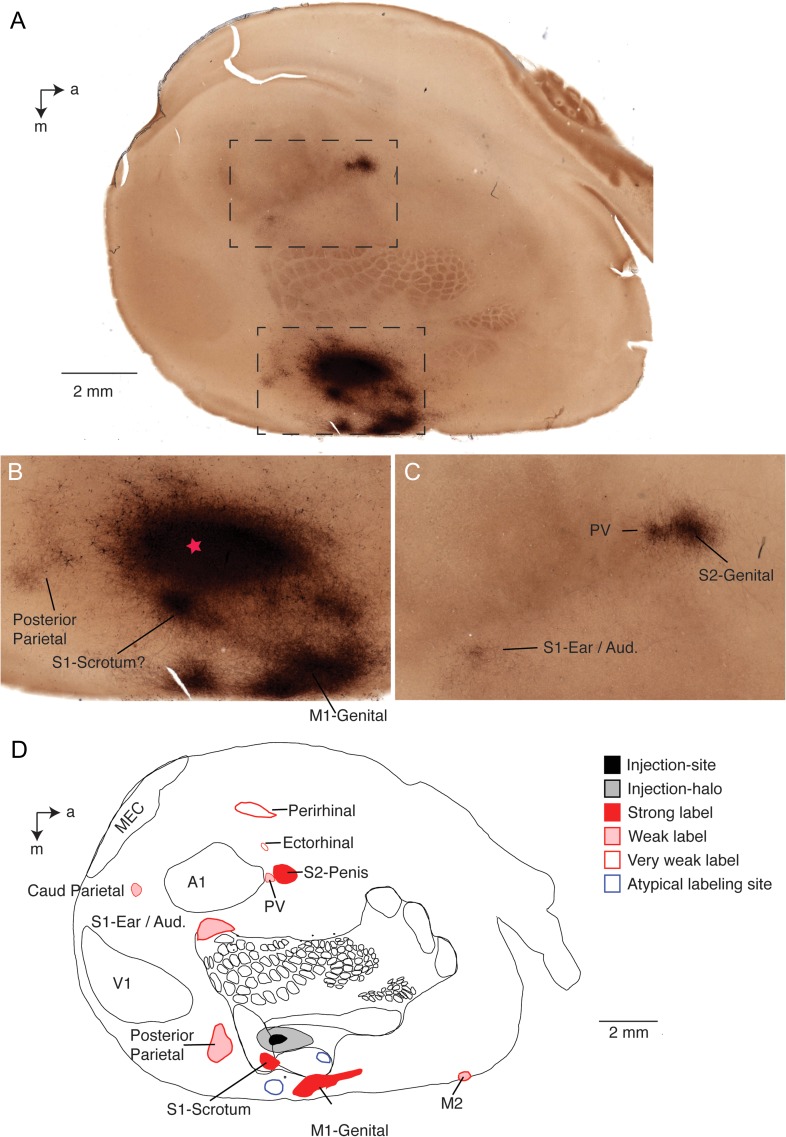
A representative result of such an experiment from a tracer injection into the deep layers of the penis representation of an adult male rat is shown in Figure 3. Here we analyzed corticocortical connections of the somatosensory penis region in a flattened cortical hemisphere. The tangential section (Fig. 3A) stems roughly from layer 4 and reveals tracer label (blackish) and the layer 4 body map (brownish) revealed by the cytochrome oxidase stain. The approximate site of tracer injection was visible in the deep layers by tissue distortions and is marked by a star in Figure 3B. There is a big label “halo” around the injection site; in this and in all other penis cortex injections this halo did not extend radially, but instead was stretched in the anterior–posterior direction resulting in an oval shape; this elongated shape follows the direction, in which the cortical penis representation extends (Lenschow et al. 2016). The blackish injection halo prevented visualizing directly the cortical penis representation by cytochrome oxidase reactivity. Judging from the body map, however, the tracer injection was well centered on the expected location of the cortical penis representation. Nearby, we observed a strongly labeled spot medial and posterior from the injection site (Fig. 3B); this spot appeared strongly labeled in all penis cortex injection experiments and is, where we previously observed scrotum sensory responses (Lenschow et al. 2016). Another strong elongated labeling spot was more medial from somatosensory cortex (Fig. 3B); this labeling site was seen in all penis cortex injection experiments and is the putative genital representation of primary motor cortex as we discuss in detail below. Weak labeling could be observed in putative posterior parietal cortex. As discussed below there were also 2 untypical labeling sites, which were only seen in this experiment. Some of the distant cortical labeling sites are shown in Figure 3C. The most prominent one of these is the putative secondary somatosensory cortex (S2) spot, which came up as a strongly labeled cortical location in all penis cortex injection experiments. The cytochrome oxidase signal provides also some information about the S2 somatotopy (Catania and Kaas 1995) and accordingly we suggest this labeling spot corresponds to the S2-genital location (Fig. 3C). There was a further weakly labeled site posterior from the S2 spot (Fig. 3C), which was seen in all penis cortex injection experiments. This labeling spot may correspond to label in area posterior ventral (PV, a third somatosensory cortical area observed in many mammals, Remple et al. 2003). This interpretation cannot be ascertained however. Another weak labeling site seen in all penis cortex injection experiments was at the border of the primary somatosensory cortex ear region and auditory cortex (Fig. 3C). An overview of all labeling sites observed in this hemisphere along with a drawing of the S1 body map and the location of primary visual cortex (V1), primary auditory cortex (A1) and medial entorhinal cortex (MEC) is given in Figure 3D. In addition to the aforementioned labeling sites it shows 2 further weak labeling sites, which were observed in all penis cortex injection experiments: the caudal parietal area (Caud Parietal) and a putative labeling site in secondary motor cortex (M2).
Figure 3.
Ipsilateral corticocortical connections in males. (A) Cytochrome oxidase activity stained (brownish color) tangential cortical section of a flattened hemisphere with an anterograde tracer (BDA; blackish color) injected into the cortical penis representation of an adult male rat. Note that the cytochrome oxidase activity stain reveals barrels and other parts of the body representation. The injection site is surrounded by a halo and several close and distant labeling sites (blackish color) can be recognized. The dashed boxes refer to image regions shown enlarged in C (lower box) and D (upper box). a = anterior, m = medial. (B) Enlarged view of the injection site (approximate center labeled with a red star) and adjacent labeling sites, 3 close by sites seen in all animals are named with their putative cortical locations; 2 atypical sites not seen in further animals are not named. S1 = primary somatosensory cortex; M1 = primary motor cortex. All labels are putative as indicated by question marks. (C) Enlarged view of 3 distant labeling sites named with their putative cortical locations. S2 = secondary somatosensory cortex; PV = posterior ventral cortical area. Other conventions as in B. (D) Drawing of all labeling sites seen in this section and named with their putative cortical locations. Superimposed is cytochrome oxidase reactivity based drawing of the somatosensory map, primary visual cortex V1, primary auditory cortex A1, caudal parietal area caud parietal, secondary motor cortex M2 and the medial entorhinal cortex MEC. All in all 12 labeling sites were detected here; 3 strong ones (red filled), 5 weak ones (pink), 2 very weak ones (red circles); all of these sites were also seen in other animals. Two atypical labeling sites (i.e., sites not seen in other animals) are labeled by blue circles. Other conventions as in B, C. Note that area assignments are putative.
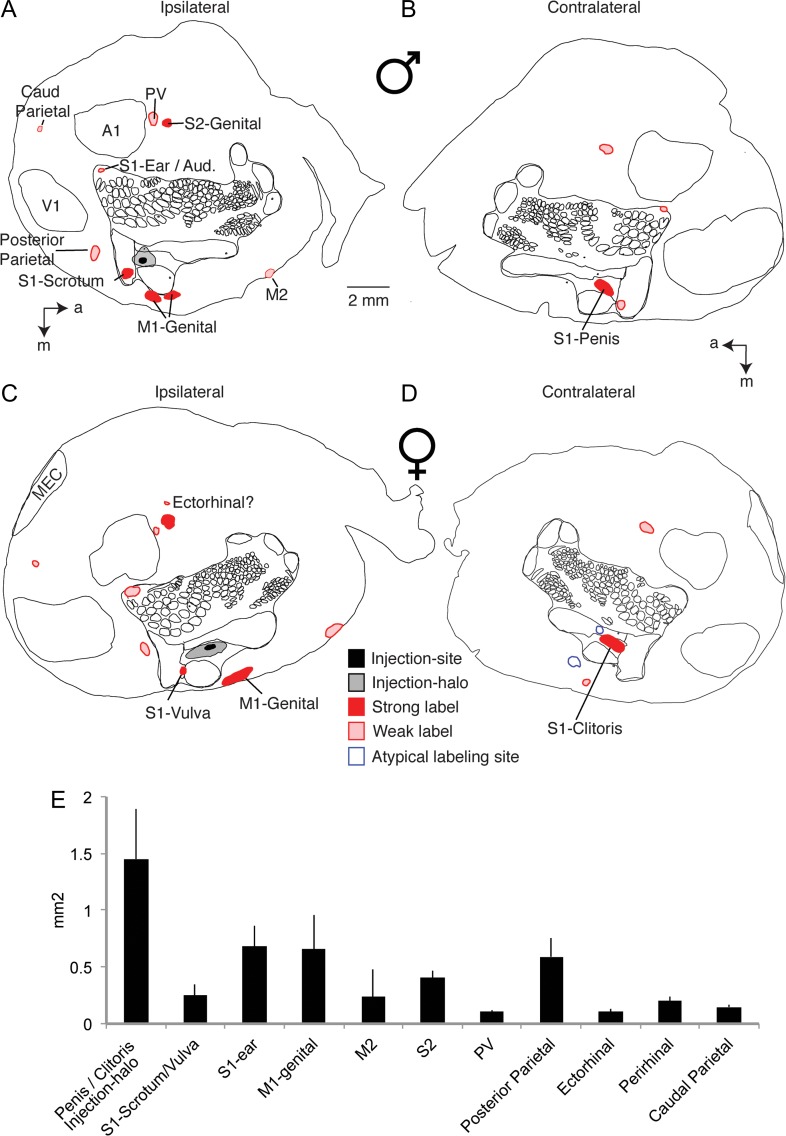
All penis cortex injection experiments (n = 5) lead to very similar results with respect to corticocortical connectivity. This conclusion is enforced by the overview data from another penis cortex injection experiment shown in Figure 4A. In this experiment we also stained the layer 4 body map and areas V1 and A1 by cytochrome oxidase reactivity. As judged from the body map, the tracer injection was again well centered on the expected location of the cortical penis representation. We again observed 3 strong (S1-Scrotum, M1-Genital, S2-Genital) and 5 weak labeling spots, all which were at sites identical to the spots detected in the experiment shown in Figure 3 and summarized in Figure 3D. In Figure 4B we show an overview of labeling in the contralateral hemisphere. In this, as well as in all other cases, contralateral labeling was noticeably weaker than ipsilateral labeling. By far the strongest contralateral labeling was observed at the expected location of the contralateral cortical penis representation (Fig. 4B); in fact, the blackish tracer label overshadowed any cytochrome oxidase reactivity signal at this location. Three other weak contralateral labeling sites were the contralateral S1-Scrotum site, the S2-Penis location and the primary somatosensory cortex ear region.
Figure 4.
Ipsilateral and contralateral corticocortical connections in males and females. (A) Drawing of all ipsilateral labeling sites seen in a tangential cortical section of a flattened hemisphere with an anterograde tracer (BDA) into the cortical penis representation of an adult male rat. Labeling sites are named with their putative cortical locations. Superimposed is cytochrome oxidase reactivity based drawing of the somatosensory map, primary somatosensory cortex S1, secondary somatosensory cortex S2, posterior ventral cortical area PV, primary visual cortex V1, primary auditory cortex A1, caudal parietal area caud parietal, primary motor cortex M1, secondary motor cortex M2 and the medial entorhinal cortex MEC. Eight or 9 sites were detected in an arrangement very similar to the case shown in Figure 3, cortex. All labels are putative as indicated by question marks. (B) Drawing of all contralateral labeling sites seen in a tangential cortical section of a flattened hemisphere in the same male animal. Note that the strongest label is seen exactly at the contralateral penis representation. For clarity only sites not already labeled in A are named, conventions as in A. (C) Drawing of all ipsilateral labeling sites seen in a tangential cortical section of a flattened hemisphere with an anterograde tracer (BDA) into the cortical clitoris representation of an adult female rat. For clarity only sites not already labeled in A are named. Nine labeling sites at the same locations observed in the male animals shown in A and Figure 3 were observed, conventions as in A. (D) Drawing of all contralateral labeling sites seen in a tangential cortical section of a flattened hemisphere in the same female animal. Note that the strongest label is seen exactly at the contralateral clitoris representation. Conventions as in A. (E) Surface area quantification of different labeling sites. Data are given as means ± SD and refer to ipsilateral labeling 5 experiments (3 males/2 females). See Table 1 for details. a = anterior, m = medial. Note that area assignments are putative.
Cortical Projection Pattern of Clitoris Cortex
Corticocortical connections of the cortical clitoris representation in females were very similar to the corticocortical connectivity observed for the cortical penis representation in males. A representative experiment on clitoris cortex connectivity is shown in Figure 4C. In this experiment we also stained the layer 4 body map (see drawing) and areas V1, MEC and A1 by cytochrome oxidase reactivity. As judged from the body map the tracer injection was well centered on the expected location of the cortical clitoris representation. We again observed 3 strong (putatively: S1-Vulva, M1-Genital, S2-Genital) and 6 weak labeling spots (putatively: area PV, posterior parietal cortex, primary somatosensory cortex ear region, caudal parietal area, area M2 and ectorhinal cortex). The similarity of the labeling pattern after clitoris cortex injection to the labeling after penis cortex is astounding. Thus, 8 out of 8 corticocortical labeling sites seen in the male example in Figure 4A were also observed in the female example shown in Figure 4C. In Figure 4D we show the corresponding contralateral labeling pattern. The strongest contralateral labeling was observed at the expected location of the contralateral cortical clitoris representation. Weak labeling was observed at the putative locations of M1-Clitoris and S2-Genital. Two further cortical clitoris representation injection experiments led to very similar results.
We noted one corticortical projection target, where the strength of genital cortex projections was possibly quantitatively different between sexes. Thus, it appeared that in females the projection to the primary somatosensory cortex ear region was more pronounced than in males. When we subjectively quantified the relative strength of projections to S2 and the primary somatosensory cortex ear region in 8 males and 6 females, this difference almost reached significance (P = 0.059) in a permutation test.
Quantification of Projection Sites and Targeting Specificity of Injections
An overall quantification of the areal extent of corticocortical labeling sites is given in Figure 4E. Here we quantified ipsilateral projections in terms of labeled surface area from 5 experiments, which had good labeling and in which we had prepared tangential sections. With exception of the relatively large injection halo surrounding penis/clitoris-cortex injections all labeling sites were smaller than a square millimeter. Thus, most of these projections probably target parts of rather than complete cortical areas. Table 1 provides an overview and further information about corticocortical labeling patterns. Here we also quantify labeled areas in terms of the percentage of total cortical surface. Penis/clitoris-cortex innervates only a small fraction of the cortical sheet, the 11 consistent corticocortical labeling sites collectively ~2.8% of the cortical surface.
Table 1.
Characteristics of corticocortical labeling sites
| Putative cortical site | Strength (qualitative) | Consistency | Laminar organization | Horizontal organization | Area (mm2) | % Of cortical surface |
|---|---|---|---|---|---|---|
| S1-clitoris (females)/S1-penis (males) injection-halo | Strong | All animals | All layers, but less in L4 | One spot | 1.45 ± 0.44 | 0.83 ± 0.26 |
| S1-vulva (females)/scrotum (males) | Strong | All animals | All layers, but less in L4 | One spot | 0.25 ± 0.10 | 0.15 ± 0.07 |
| S1-ear-region | Weak | All animals | Superficial layers | One spot and diffuse | 0.68 ± 0.18 | 0.38 ± 0.10 |
| M1-genital | Strong | All animals | All layers | Multiple spots | 0.66 ± 0.30 | 0.38 ± 0.17 |
| M2 | Weak | Most animals | All layers, superficial stronger | Multiple spots | 0.24 ± 0.23 | 0.11 ± 0.12 |
| S2 | Strong | All animals | All layers, middle layers stronger | One spot | 0.40 ± 0.07 | 0.24 ± 0.06 |
| PV | Weak | All animals | ? | One spot | 0.10 ± 0.01 | 0.06 ± 0.01 |
| Posterior parital | Weak | All animals | All layers, superficial stronger | Diffuse | 0.59 ± 0.17 | 0.34 ± 010 |
| Ectorhinal | Very weak | Most animals | All layers? | Diffuse | 0.10 ± 0.03 | 0.06 ± 0.01 |
| Perirhinal | Very weak | Most animals | All layers? | Diffuse | 0.20 ± 0.04 | 0.11 ± 0.03 |
| Caudal parietal | Very weak | Most animals | All layers? | One spot | 0.15 ± 0.02 | 0.08 ± 0.01 |
| Sum of all sites | 4.88 | 2.75 |
Data refer only to ipsilateral labeling. With exception of the S1-ear-region all labeling sites had the same characteristics in female and males. S1, primary somatosensory area; S2, secondary somatosensory area; M1, primary motor cortex; M2, secondary motor cortex; PV, posterior ventral area. Area data refer to tangential sections through layer 3 of 5 flattened cortices with good labeling; the total cortical surface was approximated as the outline of the section.
The analysis of both ipsilateral and contralateral labeling patterns in flattened hemispheres suggested that our microelectrode mapping procedures led to an accurate targeting of the cortical penis and clitoris representations. Thus, in all 5 male and 3 female ipsilateral hemispheres the injection site was at the approximately correct somatotopic position of the cortical penis and clitoris representations. Moreover, in 8 out of 8 flattened and tangentially sectioned contralateral hemispheres we observed the strongest labeling in the cortical penis and clitoris representations.
We complemented our analysis of anterograde projections by a limited set of experiments of retrograde labeling in male rats. Figure S2 shows examples of such data, where we performed retrograde labeling experiments with fluorescently tagged choleratoxin-B by injections in ipsilateral genital motor cortex and in contralateral somatosensory penis cortex. After injections in ipsilateral genital motor cortex (Fig. S2A) we observed retrograde label in most layers of somatosensory penis cortex, whereby back-labeling was conspicuously weak in layer 4 (Fig. S2B). Similarly, after injections in somatosensory penis cortex (Fig. S2C), we observed retrograde label in most layers of contralateral somatosensory penis cortex (Fig. S2D), again with weaker back-labeling in layer 4.
Identification of Genital Motor Cortex
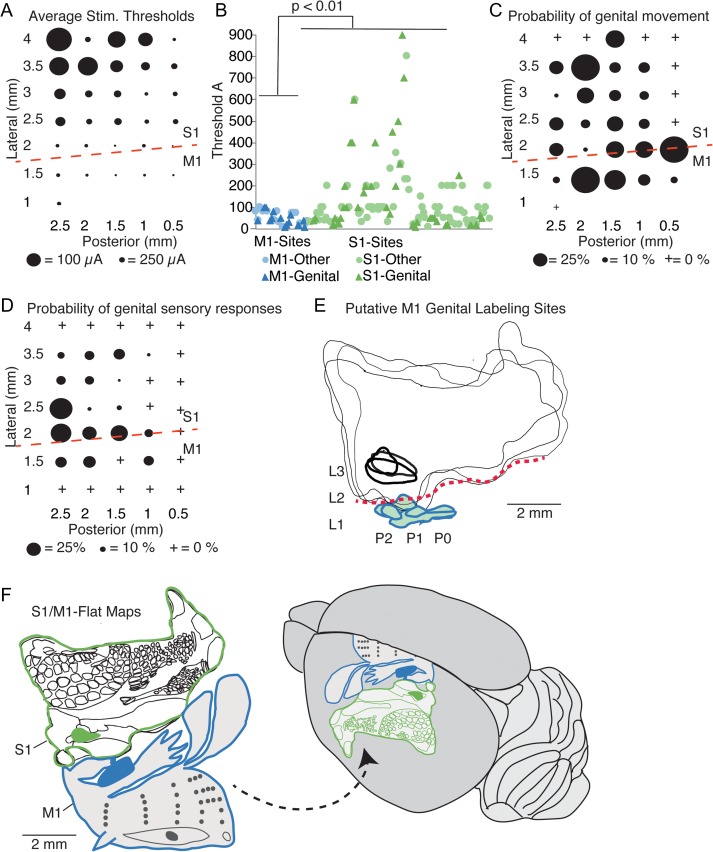
Our stimulation and labeling data along with our earlier work on sensory responses provide a strong indication for the localization of the hitherto unidentified genital primary motor cortex (Fig. 5). Average stimulation thresholds for movement varied systematically across the mapped cortical region and were lower medially (Fig. 5A). According to cytoarchitectonic alignment (Figs 1 and 2) these medial regions belong to motor cortex. At such medial coordinates in putative primary motor cortex sites (blue) we did not observe high stimulation threshold for movement, which we observed in somatosensory cortex (green) (Fig. 5B). Stimulation thresholds for genital (triangles) movements and other movements were not different. The topography of genital cortex movements sites is shown in Figure 5C, which suggests a more medial (M1) and a more lateral (S1) genital movement region. Another hallmark of primary motor cortex is the presence of sensory responses to the respective body region. Sensory responses to genitals also characterized primary genital motor cortex as quantified in Figure 5D, where we plotted tactile sensory responses to genital tapping from Lenschow et al. (2016). The medial coordinates, at which we observed low threshold genital movements and genital sensory responses received projections from S1 genital cortex as depicted in Figure 5E. In Figure 5F we show flat maps of S1 (derived from cytochrome oxidase activity staining) and M1. The flat map of M1 is pieced together from the genital cortex data (Fig. 5A–E) and the data of Neafsey et al. (1986) and Brecht et al. (2004). Genital cortex (schematically shown in blue) fits well into the overall body topography of M1 and M1 forms a mirror image of S1. A synopsis of the stimulation threshold data (Fig. 5A,B), genital motor topography (Fig. 5C), sensory responses (Fig. 5D), genital somatosensory cortex projections (Fig. 5E) and overall M1 motor topography strongly suggests that we identified primary genital motor cortex. Accordingly, primary genital motor cortex is an 0.66 ± 0.3 mm2 cortical region (Table 1), elongated in the anterior–posterior axis and centered approximately 1.5 mm lateral and 1.5 mm posterior from bregma.
Figure 5.
Identification of genital motor cortex. (A) Average stimulation threshold for movements (n = 4 motor maps from females and 4 motor maps from males). Lateral/posterior coordinates are given relative to bregma. Dashed red line, rough position of the cytoarchitectonic border between primary somatosensory cortex (S1) and primary motor cortex (M1); cytoarchitectonics refer to our data from Figures 1 and 2 and the motor mapping/cytoarchitectonics analysis of Neafsey et al. 1986. Note the lower stimulation thresholds in M1 compared with S1. (B) Univariate plot of all stimulation thresholds at M1-sites (blue) and S1-sites (green). All less than 2 mm lateral from bregma were classified as M1-sites. Significance of differences in thresholds was assessed with a Student’s t-test (P = 0.005). (C) Average probability for evoking a genital movement at different sites (same data set and conventions as in (A)). Note that there is a more medial (M1) and a more lateral (S1) region, for which genital movements are common. (D) Probability of observing a sensory response to tactile stimulation of the genitals. Data are replotted from Figure S1 in Lenschow et al. 2016; data refer to 11 sensory maps derived from 6 male and 5 female rats. Note that there is a more medial (M1) and a more lateral (S1) region, for which genital responses are seen. (E) Superimposed outlines of S1 (thin black lines) derived from cytochrome oxidase activity staining of tangential cortical sections in 3 animals, which received anterograde tracer (BDA) into genital cortex (injection area, thick black lines). Genital motor cortex labeling is shown (thick blue outlines, color fills). Dashed red line: S1/M1 border. L and P refer to approximate lateral and posterior coordinates. (F) Left, flat maps of S1 derived from cytochrome oxidase activity staining of tangential cortical sections (thin black lines, genital cortex filled black) derived from cytochrome oxidase activity staining and M1. The M1-genital cortex (schematically shown in blue) is derived from the data above, whereas the rest of the flat map is pieced together fom the data of Neafsey et al. (1986) and Brecht et al. (2004). Note that M1-genital cortex fits into the overall body topography of M1 and that M1 forms a mirror image of S1. Right, dorsolateral view of the brain with S1 and M1 map; the M1 map is partially hidden on the mesial cortical surface.
Subcortical Projections of Penis Cortex
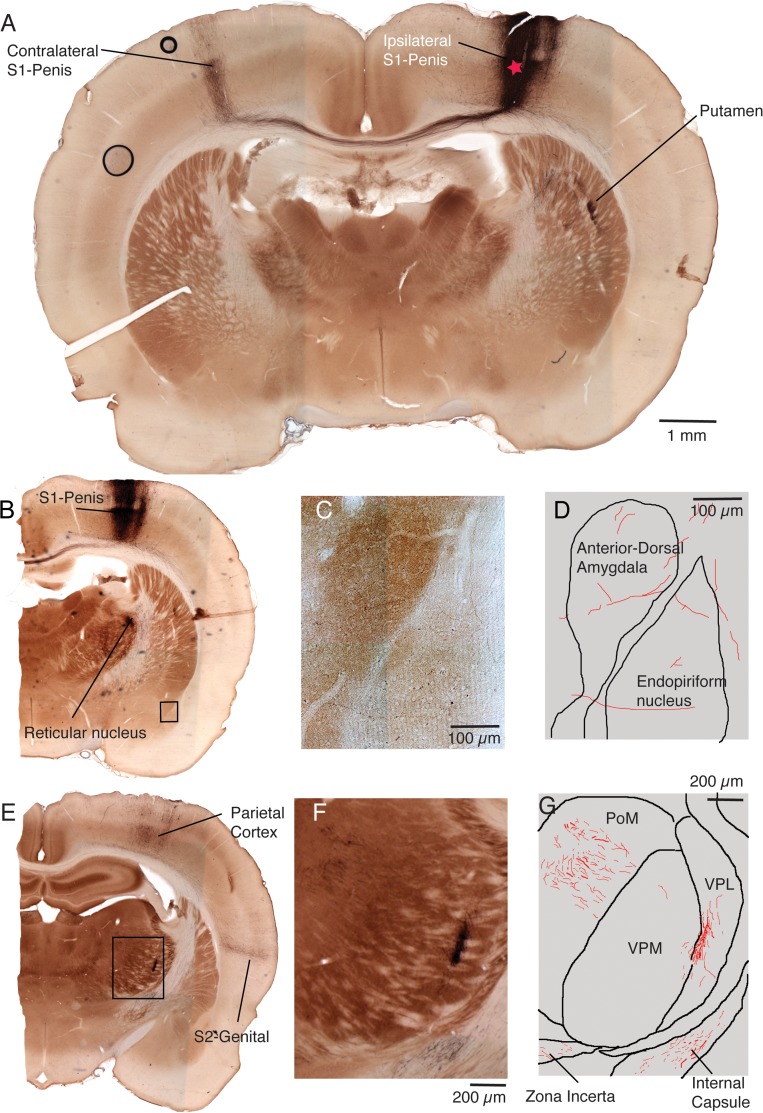
Encouraged by the results of the flattened cortical hemispheres, we analyzed cortical and subcortical projection patterns of penis cortex (as before identified by receptive field mapping during the injection experiment) in brains sectioned coronally. The section with the injection site in layer 5B of penis cortex of a male rat of one such experiment is shown in Figure 6A. Prominent labeling of the contralateral penis cortex and subcortical labeling in the putamen can also be seen in this section. Another slightly more posterior section from the same experiment is shown in Figure 6B. In this case we show only the ipsilateral hemisphere and prominent labeling in penis cortex is also present in this section. Robust subcortical label was present in the reticular nucleus of the thalamus (Fig. 6B). Weak label was also seen in the anterior-dorsal amygdala and the endopiriform nucleus (see box in Fig. 6B and micrograph Fig. 6C). The axons seen in this section are drawn in Figure 6D. An even more posterior section from the same experiment is shown in Figure 6E. In this section we observed label in the parietal cortex, the putative S2-genital cortex and at several thalamic sites. As shown in the micrograph (Fig. 6F) and the corresponding drawing (Fig. 6G) we observed prominent thalamic label in the ventral posterolateral (VPL) nucleus of the thalamus; most of this label was restricted to the VPL, but is spread along the medial part of this nucleus along the border to ventral posterior medial (VPM) nucleus of the thalamus. We also observed robust labeling in the posterior medial (PoM) nucleus of the thalamus (Fig. 6F,G) and sparse labeling in the zona incerta. A further thalamic labeling site was the dorsal part of the submedius nucleus of the thalamus.
Figure 6.
Coronal sections of a penis cortex injection site and close by cortical and subcortical projection targets. (A) Cytochrome oxidase activity stained (brownish color) coronal cortical section of both hemispheres with an anterograde tracer (BDA; blackish color) into the cortical penis representation of an adult male rat. The approximate center of the injection site is labeled with a red star. Note that the cytochrome oxidase activity stain reveals barrels and other parts of the body representation. Several labeling sites (blackish color) can be recognized and are named. This section is situated approximately 1.5 mm posterior from bregma. (B) Another slightly more posterior section from the same brain (situated approximately 1.7 mm posterior from bregma). Labeling sites (blackish color) can be recognized and are named. The black box refers to the image region shown enlarged in C. (C) Higher magnification view of labeling sites in the amygdala and the endopiriform nucleus. The label is sparse. (D) Drawing of the section shown in C. Borders of nuclei in black, axons in red. (E) Another even more posterior section from the same brain (situated approximately 2.5 mm posterior from bregma). Several labeling sites (blackish color) can be recognized and are named. The dashed box refers to the image region shown enlarged in E. (F) Higher magnification view of thalamic labeling sites. (G) Drawing of the section shown in F. Borders of nuclei in black, axons in red. In 3 structures we observed label in this section: posterior medial nucleus of the thalamus (PoM), Ventral PosteroLateral (VPL) nucleus of the thalamus and Zona incerta. Fibers passing through the internal capsule are also evident.
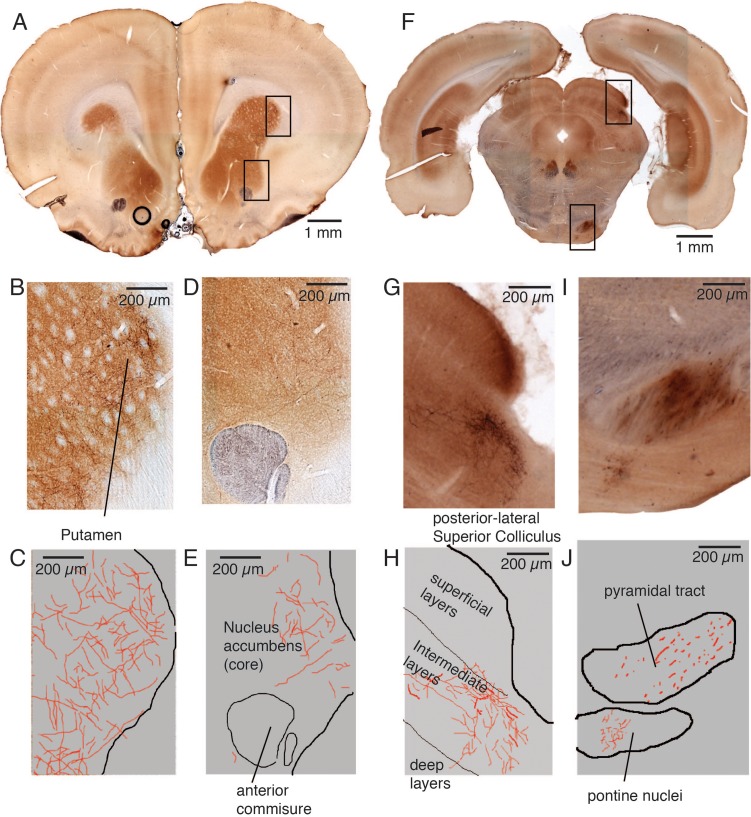
As shown in Figure 7 we also observed labeling in more anterior (Fig. 7A–E) and more posterior (Fig. 7F–J) sections. At far anterior coordinates (Fig. 7A) we again observed prominent labeling in the putamen (see micrograph; Fig. 7B and drawing; Fig. 7C). Sparse labeling was also seen in the core of the nucleus accumbens (see micrograph; Fig. 7D and drawing; Fig. 7E). At posterior coordinates (Fig. 7F) we observed prominent label in the intermediate layers of lateral–posterior parts of the superior colliculus (see micrograph; Fig. 7G and drawing; Fig. 7H). The pontine nuclei were another posterior projection target (see micrograph; Fig. 7I and drawing; Fig. 7J). Note the fibers traveling in the pyramidal tract (Fig. 7I, J). We did not analyze genital cortex projections posterior to the pyramidal tract decussation; an analysis of lower brainstem and spinal cord targets needs to be conducted in the future.
Figure 7.
Coronal sections with far anterior and far posterior projection targets after a penis cortex injection. (A) Cytochrome oxidase activity stained (brownish color) anterior coronal section situated approximately 2.6 mm anterior from bregma. The anterograde tracer (BDA; blackish color) was injected into the cortical penis representation of an adult male rat (see Fig. 5A for the injection site). The upper box refers to the image region shown enlarged in panel B/C whereas the lower box refers to panel D/E. (B) Labeling (blackish color) can be recognized in the anterior putamen. (C) Drawing of the section shown in B. Border of the putamen in black, axons in red. (D) Weak labeling (blackish color) can be recognized in the core of the nucleus accumbens. The anterior commissure can be seen in the lower part of the image. (E) Drawing of the section shown in B. Border of the accumbens in black, axons in red. (F) Cytochrome oxidase activity stained (brownish color) posterior coronal section situated approximately 6.9 mm posterior from bregma. The anterograde tracer (BDA; blackish color) was injected into the cortical penis representation of an adult male rat (see Fig. 5A for the injection site). The upper box refers to the image region shown enlarged in panel G/H, whereas the lower box refers to panel I/J. (G) Strong labeling (blackish color) can be recognized in the intermediate layers of the superior colliculus, weak label is also seen in the deep collicular layers. (H) Drawing of the section shown in F. Border of the superior colliculus and layer boundaries in black, axons in red. (I) Labeling (blackish color) axonal branching and synaptic boutons could be recognized in the pontine nuclei (lower). Fibers were seen in the pyramidal tract (upper). (J) Drawing of the section shown in I. Structural borders in black, axons in red.
Subcortical Projections of Clitoris Cortex
All the aforementioned subcortical labeling sites (Figs 6 and 7) were seen in all 3 experiments, in which we injected penis cortex in male animals and prepared coronal sections. We also performed 3 injection experiments into clitoris cortex (as before identified by receptive field mapping during the injection experiment) in females and prepared coronal sections. Injections into clitoris cortex resulted in the same subcortical labeling sites as the ones observed in males, that is, in all 3 experiments we observed labeling at the sites shown in Figures 6 and 7, and also the dorsal part of the submedius nucleus of the thalamus. Thus, at least qualitatively the subcortical projection patterns of penis and clitoris cortex are very similar.
Consistent Subcortical Projections, Inconsistent Projections and Absence of Projections
An overview of consistent subcortical labeling sites is provided in Table 2, which also provides qualitative information about labeling strength and the organization of projections.
Table 2.
Characteristics of subcortical labeling sites
| Putative subcortical site | Strength (qualitative) | Consistency | Organization of label |
|---|---|---|---|
| Putamen | Strong | All animals | Multiple spots and diffuse label |
| Nucleus accumbens | Weak | All animals | Diffuse |
| Anterior-dorsal amygdala | Very weak | All animals | Diffuse |
| Endopiriform nucleus | Weak | All animals | Diffuse |
| Reticular nucleus of the thalamus | Strong | All animals | One spot |
| Ventral PosteroLateralateral (VPL) nucleus of the thalamus | Strong | All animals | One spot |
| Posterior medial (PoM) nucleus of the thalamus | Strong | All animals | 3–4 Spots |
| Zona incerta | Weak | All animals | Diffuse |
| Dorsal part of the submedius nucleus of the thalamus | Weak | All animals | Diffuse |
| Intermediate layers of lateral–posterior parts of the superior colliculus | Strong | All animals | Multiple spots |
| Pontine nuclei | Strong | All animals | Diffuse |
Data refer to ipsilateral subcortical labeling in 6 experiments (3 males/3 females). Only sections anterior from the pyramidal tract decussation were analyzed.
In addition to the more prominent subcortical projection targets that were seen in all male and female animals there were a few less consistent subcortical labeling sites. These labeling sites were seen in a majority of animals, but not in all animals and all of these labeling sites were only weakly labeled. They included the lateral periadeductal gray, the posterior hypothalamic area, the interstitial nucleus of Cajal and the dorsal subiculum.
It is also clear from our work that genital cortex does not project to all brain structures classically implied in sexual function. Thus, there was a paucity of projections to olfactory processing regions and there were no projections to the medial preoptic area or the ventral hypothalamus.
Discussion
Summary
This study characterized physiological and anatomical outputs of rat somatosensory genital cortex. Microstimulation experiments showed that activation of genital cortex drives movements in both sexes, which usually occur in a sexual context. Our anatomical studies identified both expected as well as unexpected corticocortical projection targets of the genital cortex when compared with other parts of somatosensory cortex. A similar observation was made regarding the subcortical projection targets of the genital cortex, which also include expected as well as unexpected projection targets. We hypothesize that at least some of the unusual targets structures mediate specialized sexual functions.
Microstimulation Effects
Application of microstimulation in genital cortex evoked movements as it does in other body parts of the primary somatosensory cortex. In male rats these movements included thrusting movements and penis upward deflections. Such movements are commonly seen in male rat sexual behavior (Ågmo 1997) and can be elicited by genital reflex tests (Sachs and Garinello 1978, Hart and Melese-D’Hospital 1983). In females, genital cortex movements, different from those observed in males were evoked. Similar to the sexual character of movements in males, in female genital cortex microstimulation evoked tail upward, clitoris and trunk movements that resemble lordosis related movements in female rat sexual behavior. Still, not all sexual displays could be evoked and erections were notably absent. Given the recent evidence that the neuronal activity varies with the reproductive cycle of female rats (Bobrov et al. 2014, Nomoto and Lima 2015), we wonder whether the estrous state affects the responsivity to microstimulation in female genital cortex and to what extent different response patterns might occur. However, lordosis behavior can only be seen during the proestrous state of a natural cycling female rat and hence, we have decided consciously to first assess movement responses in female rats while having proestrous. Future experiments in awake, naturally cycling females are needed to shed light into this question.
It is important to consider the methodological limitation of the microstimulation technique. This include the stimulation of fibers of passage and more generally that it is impossible to ascertain the number and type of stimulated neurons. The methodological shortcomings are reviewed in depth in Tehovnik (1996).
All in all, our findings support the idea of a sexual role of genital cortex even though they argue against a control of erection by genital cortex. However, to what extend genital cortex function is needed in order to generate the movements, we observed in our study, needs to be evaluated. Early literature indicated that cortex is not needed for copulation or reproduction. Nevertheless, subtle changes in the pattern of reproduction (Carter et al. 1982, Wishaw and Colb 1985) are observed, however in decorticated animals. Further, somatosensory feedback from the penis was shown to be critical for the achievement of intromission and somatosensory feedback from the preputial region is needed for the execution of copulatory thrusting (Contreras and Agmo 1993). Lesioning or inactivating genital cortex might lead to those changings in the pattern of reproduction, that is, that the number of intromissions and time to ejaculation might be affected (in males); hopping, darting, rejection or place preference behavior could be affected in females.
In some motor mapping experiments, we observed genital movements not only in genital cortex, but also anterior and medial from the hindpaw representation (Fig. 1). At this location we previously also observed sensory responses to genital stimulation (Lenschow et al. 2016) and we argue—in line with the anatomical data discussed below that this area corresponds to genital motor cortex.
Corticocortical Connectivity of Rat Genital Cortex: Conventional Projection Targets
Tracer injections identified highly reproducible projection targets of genital cortex. The contralateral projections of genital cortex were less strong and more restricted than the ipsilateral ones; the contralateral target always corresponded to the strongest ipsilateral targets.
Many of the corticocortical projection targets of genital cortex are the same as those of other parts of somatosensory cortex. Thus, genital cortex much like the barrel cortex, sends strong local projections and substantial projections to topographically appropriate regions of contralateral somatosensory genital cortex, secondary somatosensory cortex and motor cortex (Welker et al. 1988). According to our retrograde labeling the multilayer connection pattern to contralateral somatosensory genital cortex and motor cortex appear to be “lateral” rather than feedforward or feedback connections (Felleman and Van Essen 1991). Also the targets of weaker projections such as perirhinal cortex, area PV and M2 appear to be similar to barrel cortex targets (Welker et al. 1988).
At the qualitative level (i.e., in terms of projection targets) we did not detect any obvious sexual differences/dimorphism in the corticocortical connectivity of genital cortex between male and female rats. Our data are insufficient to assess potential quantitative differences between the sexes.
Corticocortical Connectivity of Rat Genital Cortex: Unconventional Projection Targets
A number of projection targets of genital cortex differ from barrel cortex projection targets. These areas include the scrotum region in males and the vulva region in females, the caudal parietal area and the primary somatosensory–ear/auditory cortex region. We wonder if these projection targets reflect sexual specializations of genital cortex. Thus, scrotum and vulva are sex organs. No less interesting is the projection to the primary somatosensory cortex ear region, as in estrous females touching the clitoris can evoke ear wiggling, a sexual display in rats (Erskine 1989).
Identification of Primary Genital Motor Cortex
A key result of our study is the localization and identification of a candidate region for primary genital motor cortex (Fig. 5). The identification of this region rests on 5 coherent observations: (1) the localization of this region medial to the cytoarchitectonic border of somatosensory cortex; (2) microstimulation-data suggested low stimulation thresholds for genital movements in medial cortex; (3) the presence of sensory responses to genital tapping in medial cortex; (4) the corticocortical projections from sensory genital cortex in this region; and (5) the alignment of genital movements in this region with the overall motor topography. Collectively, these characteristics—the localization medial from primary somatosensory cortex, low movement thresholds, sensory responsiveness, input from somatosensory cortex, motor topography—makes a strong case that primary genital motor cortex does indeed map to this region.
Sucortical Projection Targets of Rat Genital Cortex: Conventional Targets
Tracer injections identified highly reproducible subcortical projection targets of genital cortex. With exception of the bilateral striatal projection, the targets were ipsilateral. As for corticocortical targets, these subcortical targets were similar to those of barrel cortex (Welker et al. 1988). Such conventional targets include the corticothalamic projections to the medial portions of the ventral posterolateral nucleus of the thalamus (VPL), the posteromedial nucleus of the thalamus (PoM), the reticular nucleus of the thalamus and the zona incerta. Other conventional targets include the pontine nuclei, the intermediate layers of the superior colliculus and as already mentioned the bilateral projection to the striatum. All of these targets have also been described for tracer injections in barrel cortex (Welker et al. 1988).
Subcortical Projection Targets of Rat Genital Cortex: Unconventional Targets
Tracer injections also identified subcortical targets not usually revealed by tracer injection in somatosensensory cortex. These include the anterior-dorsal amygdala, the endopiriform nucleus and the nucleus accumbens. Again we wonder if such projection targets reflect a specialization of genital cortex for sexual functions. The amygdala has long been implicated in sexual functions (Harris and Sachs 1975, Mascó and Carrer 1980) and the nucleus accumbens is known to be involved in the processing of hedonic stimuli such as sexual stimulation (Liu et al. 1998, Jenkins and Becker 2003, Wise et al. 2016). Another unusual subcortical target of genital cortex was the dorsal part of the submedius nucleus of the thalamus. This nucleus is poorly studied, but one of the most interesting projection targets as earlier work has observed sensory responses to penis stimulation in this nucleus (Hubscher & Johnson 2003). As genital cortex does not project to the medial preoptic area and the ventral hypothalamus—2 brain structures presumably involved in the onset of puberty—it remains unclear, how rat genital cortex mediates the hastening of puberty by sexual touch (Lenschow, Sigl-Glöckner, and Brecht 2017).
Do Genital Cortex Injections Reveal a Sex-Related Processing Network?
We noted at the introduction of our study that little is known about the processing of sexual processing in the rodent forebrain. We wonder if the data provided in our study will change this situation. Clearly we observed a reproducible network that differs in small but significant aspects from the connectivity of the rest of somatosensory cortex. The projection from genital cortex is clearly not pan-cortical, but even for large injections, it was restricted to about 10 corticortical spots encompassing not more than 3% of the cortical sheet. To ascertain, if the network revealed here is indeed a sex-related processing network, efforts should be made to characterize activity patterns in genital S1 and its projection targets during sexual behavior.
Supplementary Material
Notes
We thank Undine Schneeweiß and Juliane Steger for excellent technical assistance and Edith Chorev, Johanna Sigl-Glöckner, Rajnish Rao, Jean Simonnett, and Ann Clemens for help with the perfusions. For comments on the manuscript we thank Christian Ebbesen and Johanna Sigl-Glöckner. Conflict of Interest Statement: None declared.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Authors’ Contributions
C.L. and M.B. designed the experiments, C.L. performed the experiments, C.L. and M.B. analyzed the data and wrote the article.
Funding
Humboldt-Universität zu Berlin, the Bernstein Center for Computational Neuroscience Berlin, the German Federal Ministry of Education and Research (BMBF, Förderkennzeichen 01GQ1001A) and NeuroCure. M.B. was a recipient of the Gottfried Wilhelm Leibniz Prize.
References
- Ågmo A. 1997. Male rat sexual behavior. Brain Res Protoc. 2:203–209. [DOI] [PubMed] [Google Scholar]
- Bobrov E, Wolfe J, Rao RP, Brecht M. 2014. The representation of social facial touch in rat barrel cortex. Curr Biol. 1:109–115. [DOI] [PubMed] [Google Scholar]
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. 2004. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation an intrcellular stimulation of identified cells. J Comp Neurol. 479:360–373. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Kolb B, Whishaw IQ. 1982. Neonatal decortication and adult female sexual behavior. Physiol Behav. 4:763–766. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. 1995. Organization of the somatosensory cortex of the star-nosed mole. J Comp Neurol. 4:549–567. [DOI] [PubMed] [Google Scholar]
- Comte P. 1982. Monopolar versus bipolar stimulation. Appl Neurophysiol. 1–2:156–159. [DOI] [PubMed] [Google Scholar]
- Contreras JL, Agmo A. 1993. Sensory control of the male rat’s copulatory thrusting patterns. Behav Neural Biol. 3:234–240. [DOI] [PubMed] [Google Scholar]
- Divac I, Mojsilovic-Petrovic J, López-Figueroa MO, Petrovic-Minic B, Møller M. 1995. Improved contrast in histochemical detection of cytochrome oxidase: metallic ions protocol. J Neurosci Methods. 56:105–113. [DOI] [PubMed] [Google Scholar]
- Erskine MS. 1989. Solicitation behavior in the estrous female rat: a review. Horm Behav. 4:473–502. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. 2011. Miniaturized integration of a fluorescence microscope. Nat Methods. 10:871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris VS, Sachs BD. 1975. Copulatory behavior in male rats following amygdaloid lesions. Brain Res. 3:514–518. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D’Hospital PY. 1983. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 6:807–813. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, Lin D. 2016. The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol. 38:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. 1992. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 2:321–326. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. 2003. Responses of thalamic neurons to input from the male genitalia. J Neurophysiol. 89:2–11. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. 2003. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 7:1997–2001. [DOI] [PubMed] [Google Scholar]
- Kell CA, von Kriegstein K, Rösler A, Kleinschmidt A, Laufs H. 2005. The sensory cortical representation of the human penis: revisiting somatotopy in the male homunculus. J Neurosci. 25:5984–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisaruk B, Wise N, Frangos E, Liu WC, Allen K, Brody S. 2011. Women’s clitoris, vagina, and cervix mapped on the sensory cortex: fMRI evidence. J Sex Med. 8:2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Florea C, Schwanzel-Fukuda M, Devidze N, Kami Kia H, Lee A, Zhou J, Maclaughlin D, Donahoe P, Pfaff D. 2007. Development of a sexually differentiated behavior and its underlying CNS arousal functions. Curr Top Dev Biol. 79:37–59. [DOI] [PubMed] [Google Scholar]
- Lauer SM, Lenschow C, Brecht M. 2017. Sexually selected size differences and conserved sexual monomorphism of genital cortex. J Comp Neurol. 12:2706–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow C, Copley S, Gardiner JM, Talbot ZN, Vitenzon A, Brecht M. 2016. Sexually monomorphic maps and dimorphic responses in rat genital cortex. Curr Biol. 26:106–113. [DOI] [PubMed] [Google Scholar]
- Lenschow C, Sigl-Glöckner J, Brecht M. 2017. Development of rat female genital cortex and control of female puberty by sexual touch. PLoS Biol. 15:e2001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Sachs BD, Salamone JD. 1998. Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacol Biochem Behav. 2:585–592. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 62:609–614. [DOI] [PubMed] [Google Scholar]
- Mascó DH, Carrer HF. 1980. Sexual receptivity in female rats after lesion or stimulation in different amygdaloid nuclei. Physiol Behav. 6:1073–1080. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. 1983. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 3:277–280. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. 1986. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res Rev. 11:77–96. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Lima SQ. 2015. Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr Biol. 5:589–594. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Highland L, Karam P. 1993. Socio-sexual behavior in male rats after lesions of the medial preoptic area: evidence for reduced sexual motivation. Brain Res. 2:271–276. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. 1950. The cerebral cortex of man. New York (NY): The Macmillan Company. [Google Scholar]
- Pfaff DW, Sakuma Y. 1979. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Field PM. 1971. Sexual dimorphism in the preoptic area of the rat. Science. 173:731–733. [DOI] [PubMed] [Google Scholar]
- Ramos SD, Lee JM, Peuler JD. 2001. An inexpensive meter to measure differences in electrical resistance in the rat vagina during the ovarian cycle. J Appl Physiol. 91:667–670. [DOI] [PubMed] [Google Scholar]
- Remple MS, Henry EC, Catania KC. 2003. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol. 467:105–118. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Qi HX, Collins CE, Kaas JH. 2002. The genitals and gluteal skin are represented lateral to the foot in anterior parietal somatosensory cortex of macaques. Somatosens Mot Res. 19:302–315. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Garinello LD. 1978. Interaction between penile reflexes and copulation in male rats. J Comp Physiol Psychol. 4:759–767. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. 1996. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods. 1:1–17. [DOI] [PubMed] [Google Scholar]
- Valcourt RJ, Sachs BD. 1979. Penile reflexes and copulatory behavior in male rats following lesions in the bed nucleus of the stria terminalis. Brain Res Bull. 1:131–133. [DOI] [PubMed] [Google Scholar]
- Welker E, Hoogland PV, Van der Loos HG. 1988. Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res. 2:411–435. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Colb B. 1985. The mating movements of male decorticate rats: Evidence for subcortically generated movements by the male but regulation of approaches by the female. Behav Brain Res. 3:171–191. [DOI] [PubMed] [Google Scholar]
- Wise NJ, Frangos E, Komisaruk BR. 2016. Activation of sensory cortex by imagined genital stimulation: an fMRI analysis. Socioaffect Neurosci Psychol. 6:31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. 1979. Changes in the visual system of monocularly sutured or enucleated cats demonstratable with cytochrome oxidase. Brain Res. 171:11–28. [DOI] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 4:896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.