Abstract
Study Objectives
To investigate sex differences in the effect of sleep deprivation on performance, accounting for menstrual phase in women.
Methods
We examined alertness data from 124 healthy women and men (40 women, 84 men; aged 18–30 years) who maintained wakefulness for at least 30 hr in a laboratory setting using a constant routine protocol. Objective alertness was assessed every 2 hr using a 10 min psychomotor vigilance task. Subjective alertness was assessed every hour via the Karolinska Sleepiness Scale.
Results
Women in the follicular phase of the menstrual cycle demonstrated the poorest level of performance. This poor performance was most pronounced at times corresponding to the typical sleep episode, demonstrating a window of vulnerability at night during this menstrual phase. At 24 hr awake, over 60 per cent of their responses were lapses of >500 ms and over one-third of their responses were longer lapses of at least 3 s in duration. Women in the luteal phase, however, were relatively protected from alertness failure, performing similar or better than both follicular-phase women and men.
Conclusions
These results have important implications for education and intervention programs for shift workers, specifically during times of vulnerability to attentional failure that increase risk of injury.
Keywords: sex differences, sleep deprivation, alertness, performance, sex hormones, shift work
Statement of Significance
Women have more attentional failures during acute sleep deprivation, an effect that is particularly strong in the night. The degree to which the sex difference in attentional failure after prolonged wakefulness is associated with menstrual phase is not well understood. Under highly controlled laboratory conditions, we demonstrate that sex differences in attentional failures were due to the poor performance of women in the follicular phase of the menstrual cycle, and that women in the luteal phase were not more impaired than men. This finding has important implications for education and training programs for shift workers, and identifying windows of increased vulnerability to attentional failure and workplace accidents.
Introduction
Shift work has become a prevalent part of modern, industrialized society. Approximately 16%–20% of the workforce engages in work outside of the normal working day [1–4]. Night shift work is particularly challenging, as it requires individuals to engage in work activity while their circadian system promotes sleep. There are marked interindividual differences in the extent to which endogenous circadian rhythms adapt to shift work, with most individuals showing maladaptation [5–7]. This physiological maladaptation to an inverted schedule results in diminished alertness and performance during night shift work, with associated increases in fatigue-related accidents during night shift hours. This in turn leads to impaired job performance and high rates of accidents and injuries [4].
Women appear to respond more poorly than men to shift work schedules. Women have been reported to have more health complaints, higher absenteeism from work, a higher prevalence of sleep disturbances, and more drowsiness at work than men [8–15]. Women have also been found to have higher rates of work-related injuries than men on night shifts, despite having nearly identical injury rates during the day, indicating that women may be more vulnerable to sleepiness-related risks [4, 16]. In agreement with these findings, alertness has been shown to be more affected by acute sleep deprivation in women than in men both in the laboratory setting [17] and in the field [18]. Although women appear to be more adversely affected by chronic and acute sleep insufficiency inherent in shift work, the degree to which this sex difference is due to menstrual phase is not known.
The present study examined the effects of sleep deprivation on alertness during 30 hr of continuous wakefulness in a sample of healthy young men and women (N = 124), studied under highly controlled in-laboratory conditions. Furthermore, we analyzed the impact of menstrual phase on alertness during sleep deprivation. Given that progesterone is released during the luteal phase and appears to mitigate the effects of sleep deprivation on cognitive performance in women [19, 20], we hypothesized that alertness would be influenced by menstrual phase, with women in the follicular phase demonstrating greater effects of acute sleep deprivation on alertness.
Methods
Participants
Participants were 40 healthy women and 84 healthy men who participated in protocols with identical screening and in-laboratory procedures carried out over an ~10 year period. Participants maintained a fixed, self-selected 16:8 hr wake:sleep schedule for at least 1 week at home prior to admission in the laboratory. This was verified with wrist actigraphy (Actiwatch-L; Minimitter, Inc., Bend, OR), time stamped call-ins, and sleep diaries. All participants were asked to refrain from the use of drugs, medications, dietary supplements, nicotine, and the consumption of caffeine and alcohol. Upon admission, toxicology screens for drugs of abuse, alcohol, caffeine, and nicotine were conducted. All women reported regular menstrual cycles (29.23 days ± 1.60; 27–35 day range). Menstrual phase was calculated based on the self-report of menses onset prior to admission in the laboratory. Women reported their last menses during prelaboratory screening and their average menstrual cycle length. Menstrual phase was estimated using a count-forward method, with last menses onset being the beginning of the follicular phase and the luteal phase starting half-way through the average cycle length. The estimation of menstrual phase was made for the beginning of the sleep deprivation portion of the study. The average time from self-reported menses onset to the in-laboratory study was 1.7 months (±1.1 months). No women in the present analysis were taking hormonal contraception. Of the 40 women, 19 were estimated to be in the follicular phase at the time of testing and 21 were estimated to be in the luteal phase. All participants provided a written informed consent. Procedures were approved by the Institutional Review Board at the Brigham and Women’s Hospital and were in compliance with the Health Insurance Portability and Accountability Act regulations and the Declaration of Helsinki.
In-laboratory protocol
Participants were admitted to the Intensive Physiological Monitoring Unit of the Center for Clinical Investigation (Brigham and Women’s Hospital, Boston, MA) where they lived individually in windowless rooms, free from time cues. On the first 3 baseline days, participants’ sleep was scheduled based on the average of the 16:8 hr wake:sleep schedule in the 7 days prior to laboratory admission. Upon waking on day 4, participants began a 30–50 hr “constant routine” (CR) protocol. For those participants with a >30 hr CR, only the first 30 hr were analyzed. During the CR, participants remained awake in bed in a semirecumbent posture in constant dim light (<3 lux, 0.01 W/m2; 4100K fluorescent lamps, Philips Lighting, The Netherlands) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA, USA). Daily nutritional intake was divided into hourly portions (150 mEq Na+/100 mEq K+ (± 20%) controlled nutrient, isocaloric [basal energy expenditure × 1.3] diet, 2500 mL fluids/24 hr).
To measure objective alertness, a 10 min visual psychomotor vigilance task (PVT) simple reaction time (RT) test (2–10 s interstimulus interval) was administered every 2 hr. A total of 1,835 PVTs were analyzed. Self-reported sleepiness was measured every hour using the Karolinska Sleepiness Scale (KSS). The KSS is a 9-point Likert scale, from 1 = “very alert” to 9 = “very sleepy, fighting sleep.” Core body temperature (CBT) was recorded at 1 min intervals via disposable rectal thermistor (Measurement Specialties TPG, Dayton, OH).
Data analysis
Raw PVT data were initially cleaned by excluding RT below 100 ms (errors of commission) and those greater than 10000 ms (errors of omission) [21, 22]. Several indices of performance were extracted from PVT data based on their sensitivity to sleep loss [21]. Firstly, we calculated mean reciprocal RT by dividing every valid RT into 1000 and taking the average of these values. Secondly, we calculated the proportion of responses within a PVT that were lapses (RT ≥ 500 ms) or long lapses (RT ≥ 3000 ms). A threshold of 3000 ms was chosen to create a long lapse variable as such responses under sleep deprivation have a >95 per cent chance of being due to eyes being closed (likely due to microsleeps), as opposed to inattention [23]. Finally, the proportion of RTs (excluding time-outs of 10000 ms) that were errors of commission (RT < 100 ms) was calculated.
A linear mixed effect model (Proc MIXED, SAS, 9.4) was used for analyzing PVT and KSS data. Time (hours since wake), group (men, follicular-phase women, and luteal-phase women), and their interaction were entered into the model as fixed effects and a random intercept was included for each participant. Time was included as a repeated factor and the covariance structure was modeled as first-order autoregressive (ar[1]). The first-order autoregressive covariance structure models autocorrelation in repeated measures data as being highest between adjacent time points and decaying with increasing distance between time points. For all models, this covariance structure produced the lowest Schwarz’s Bayesian information criterion (BIC) value [24]. Planned contrasts (LSMESTIMATE statement) were used to examine between-groups differences in the change in PVT indices from participants’ typical wake episode (“day”; average of PVTs during hours 0–16) to their typical sleep episode (“night”; average of PVTs during hours 18–24). A separate model was used to assess PVT performance and KSS scores on baseline days 2 and 3. Group (men and follicular- and luteal-phase women), time (hours since wake), and day (2 vs. 3) were entered into a full-factorial model as fixed effects and a random intercept was included for each participant. Day and time were included as repeated factors and the covariance structure was unstructured at first-order autoregressive (UN@ar[1]).
Full RT distributions were constructed by first taking the log base 10 of each RT within a PVT and calculating the 5th to 95th percentiles in 0.05-step quantiles. The quantiles were averaged across PVTs within a period (day, hours 0–16; night, hours 18–24). Independent samples t-tests were carried out to compare men and follicular- and luteal-phase women at the 5th, 25th, 75th, and 95th percentiles.
CBT amplitude was assessed by the maximum-likelihood fit of a two-harmonic regression model with first-order autoregressive noise [25]. The first 5 hr and the final 30 min of temperature data were excluded from analysis to eliminate the masking effects of waking and changing posture at the beginning and end of the CR. Body temperature amplitude was used to confirm menstrual cycle phase estimates were accurate for the groups. A linear-mixed model was used to compare CBT between luteal- and follicular-phase women during the CR. Individual data were aligned at the CBT minimum (0°) and averaged into two hourly phase bins. Circadian phase, group (luteal or follicular phase), and their interaction were entered as fixed effects and a random intercept was included for each participant. Phase was included as a repeated effect and the covariance structure was the first-order autoregressive (ar[1]).
Results
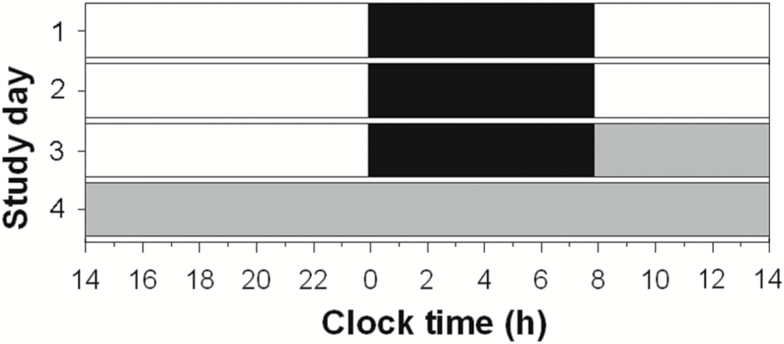
A total of 124 healthy young men and women (84 men, 19 follicular-phase women, and 21 luteal-phase women) completed the CR protocol following 3 baseline days with 8 hr sleep and 16 hr wake (Figure 1). There were no group differences in age or diurnal preference (morningness–eveningness score [26]) or self-selected bed and wake times (pall > 0.05).
Figure 1.
Representative Raster plot of the study protocol. Black bars represent 8 hr self-selected scheduled sleep episodes. White areas represent scheduled wake on baseline days. Gray bars represent the 30–50 hr constant routine in dim light (30 hr CR pictured). Baseline sleep periods were timed based on typical sleep time for the week preceding admit to the study.
PVT performance
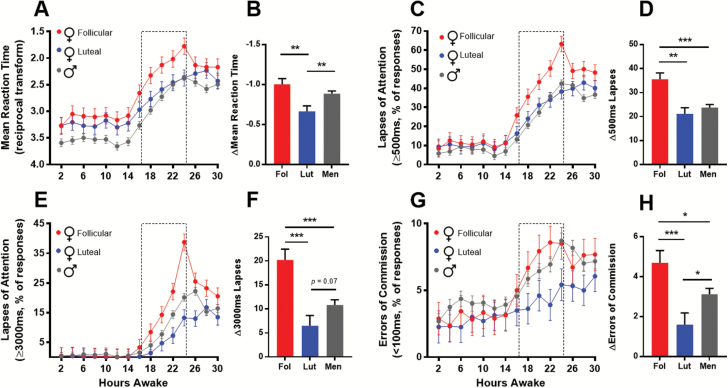
Linear-mixed models were used to analyze performance indices extracted from the PVT. Time (hours since wake), group (men, follicular-phase women, and luteal-phase women), and their interaction were included as fixed effects. The time courses of performance indices across the 30 hr sleep deprivation protocol are shown in Figure 2. There was a significant main effect of group (p = 0.003) on mean reciprocal RT and a trend for a group*time interaction (p = 0.07; Figure 2A). Planned contrasts were used to investigate between-groups differences in the deterioration of performance from day (hours 0–16 awake) to night (hours 18–24 awake). Planned contrasts identified a greater decrement in RT from day to night in men and follicular-phase women, compared with luteal-phase women (pboth < 0.01; Figure 2B). For reference, mean reciprocal RT by sex (without dividing women by menstrual phase) is presented in Supplementary Figure S1A. When the menstrual phase of women is not known, a general, but weak impairment in women is seen in the night, masking a large impairment in follicular-phase women due to similar performance in men and luteal-phase women.
Figure 2.
PVT performance by group. Line graphs (A, C, E, and G) depict least-square means and standard error of the means (SEMs) of PVT performance as a function of time awake for men (gray circles), follicular (red), and luteal (blue) phase women. Dotted-line bars represent typical sleep periods (16–24 hr). Bar graphs (B, D, F, and H) adjacent to each line graph depict the least-square means and SEMs for the change (Δ) in each performance index from PVTs during typical wake to those during typical sleep, within-groups. *p < 0.05, **p < 0.01, ***p < 0.001 for independent sample t-tests.
There were both a significant main effect of group (p = 0.01) and a group*time interaction (p = 0.002) for lapses of attention (% of RTs ≥500 ms; Figure 2C). Although all groups had a similar proportion of lapses during the typical wake episode (hours 0–16 awake), the increase in the proportion of lapses across the night (hours 18–24 awake) was much larger in follicular-phase women compared with men and luteal-phase women. At 24 hr awake, 63.2 per cent of responses made by follicular-phase women took 500 ms or longer, compared with 42.5 per cent in men and 39.9 per cent in luteal-phase women. Planned contrasts revealed an increase of 35.5 per cent in the follicular-phase women from daytime to the night, compared with a 23.7 and 21.1 per cent increase in men and luteal-phase women, respectively (Figure 2D; pboth < 0.001). For reference, lapses by sex are presented in Supplementary Figure S1B. Again, when the menstrual phase of women is not known, a weak impairment in women is seen in the night, due to the averaging of poorer performance in follicular-phase women with the better performance in luteal-phase women that is similar to the men.
For long lapses of attention (% of RTs ≥ 3000 ms; Figure 2E), there were also a significant main effect of group (p = 0.02) and a highly significant group*time interaction (p < 0.0001). A threshold of 3000 ms was chosen to create a long-lapse variable as such responses under sleep deprivation have a >95 per cent chance of being due to eyes being closed (likely due to microsleeps), as opposed to inattention [23]. This interaction is driven by similar performance of all groups during the typical wake episode and a strong dissociation with increased wakefulness, into the typical sleep episode. At 24 hr awake, 38.7 per cent of responses made by follicular-phase women took 3 s or longer, compared with 20.1 per cent in men and 13.3 per cent in luteal-phase women. Planned contrasts showed that the performance decrement from day to night among follicular-phase women was larger than that of both men and luteal-phase women (Figure 2F; pboth < 0.001). There was also a trend for a larger increase in long lapses among men, relative to luteal-phase women (Figure 2F; p = 0.07). For reference, long lapses by sex (without dividing women by menstrual phase) is presented in Supplementary Figure S1C. Given the opposing effect of sleep deprivation on long lapses between luteal- and follicular-phase women, analysis by sex alone eliminates the true sex difference between men and follicular-phase women.
Finally, errors of commission (anticipatory errors; % of RTs < 100 ms) did not show a main effect of menstrual phase (p = 0.24) or a group*time interaction (p = 0.44). Although men and follicular-phase women make more errors of commission with cumulative wakefulness, especially at times matching the typical sleep episode, the increase among luteal-phase women is negligible (Figure 2G). Planned contrasts showed that follicular-phase women showed a larger increase in errors of commission from day to night relative to both men and luteal-phase women (Figure 2H; p < 0.05 and p < 0.001, respectively). Furthermore, men showed a significantly larger increase in errors of commission relative to luteal-phase women (p < 0.05). For reference, errors of commission by sex (without dividing women by menstrual phase) are presented in Supplementary Figure S1D. Without menstrual-phase information, there appears to be a slight increase in errors of commission in men, which is driven by the low rate of errors of commission in the luteal-phase women.
Pseudoeffect sizes (reflecting the relative importance of fixed effects in the model) are reported in the Supplementary Table S4.
Reaction time distributions
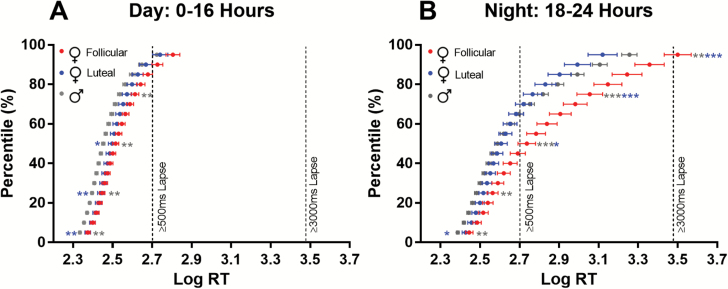
To compare the full RT distributions between-groups during the day (0–16 hr) and night (18–24 hr), log RT quantile plots were produced. During the day, luteal- and follicular-phase women have similar performance at the faster end of the distribution (5th and 25th percentiles) and are slower compared with men (Figure 3A; pboth < 0.01). At the slower end of the distribution (75th and 95th percentiles), the follicular-phase women, but not the luteal women, show poorer performance relative to men (75th percentile; p < 0.01).
Figure 3.
Log reaction time distributions for men (gray) and women in either the luteal (blue) or follicular (red) phase of the menstrual cycle during the day (A) and night (B). Group means and standard error of the means (SEMs) at each percentile are presented. Pairwise comparisons were performed at the 5th, 25th, 75th, and 95th percentiles. Asterisks to the left of the curves indicate comparisons of men with luteal-phase women. Asterisks on the right-hand side of the curves indicate comparisons of follicular-phase women with both men (gray asterisks) and luteal-phase women (blue asterisks). *p < 0.05, **p < 0.01, ***p < 0.001 for independent sample t-tests.
During the night, luteal- and follicular-phase women again show similar performance at the fastest end of the distribution while being significantly slower than men (Figure 3B). Moving towards the slower end of the distribution (75th and 95th percentiles), the luteal-phase women drift in performance towards men, being intermediate at the 25th percentile and overlapping at the 50th percentile. Towards the slower end of the distribution, luteal-phase women show, on average, faster RT than men, though these differences were not significant. Follicular-phase women were significantly slower than men at both the fastest and slowest ends of the distribution (Figure 3B). Although follicular-phase women are similar to luteal-phase women at the fastest end of the distribution, they were significantly slower at the slowest end (75th and 95th percentiles).
Subjective alertness
Subjective sleepiness was measured using the KSS and neither the main effect of group nor the group*time interaction significantly affected this measure (p = 0.12 and p = 0.16, respectively; Supplementary Figure S2A).
Core body temperature
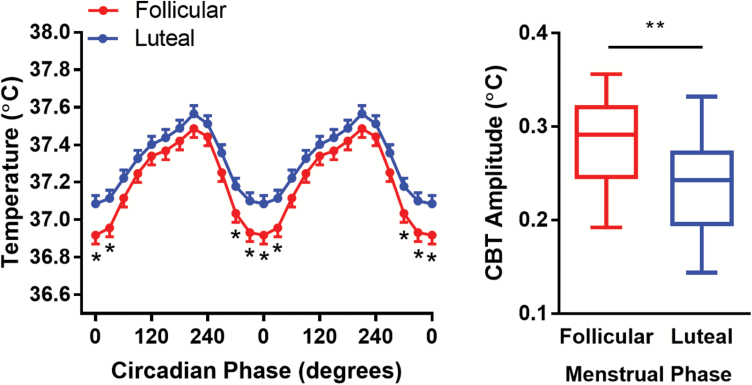
As the amplitude of CBT differs by menstrual phase [27], with a lower amplitude during the luteal phase, we compared CBT amplitude over the CR to confirm self-reported menstrual phase. Consistent with known effects on temperature, we found a significant difference in CBT amplitude, with follicular-phase women having a higher amplitude than luteal women (Mean ± SEM: 0.28 C° ± 0.05 vs. 0.24 C° ± 0.05; p = 0.009; Figure 4, A and B). Furthermore, linear-mixed model analysis identified a marginally significant group*phase interaction (p = 0.05) such that follicular-phase women had a lower absolute temperature around the CBT minimum (Figure 4A). There was a trend for a main effect of group (p = 0.08), with follicular-phase women showing a generally lower CBT.
Figure 4.
Core body temperature in women in the luteal or follicular phases of the menstrual cycle. (A) CBT profiles for follicular (red) and luteal (blue) women. Data were aligned with CBT minimum for every individual as 0°. Asterisks indicate significant comparisons (p < 0.05). (B) Box-plots of CBT amplitude in follicular and luteal women. Whiskers show the minimum and maximum values.
Baseline PVT performance and subjective alertness
PVT performance and subjective alertness of men and follicular- and luteal-phase women across baseline wake periods were assessed. Irrespective of baseline day or time awake, there was a significant effect of group on both mean reciprocal RT and lapses of attention (pboth < 0.05; Supplementary Figure S3). The between-groups differences observed at baseline are consistent with those observed under CR conditions during the normal waking day hours (Figure 2) such that men on average had faster RT and fewer lapses of attention. There was no significant effect of group (follicular, luteal, and men) on KSS scores (p > 0.05).
Discussion
Our findings demonstrate that sex differences in the effect of sleep deprivation on alertness depend on menstrual phase. Women in the follicular phase of the menstrual cycle are most vulnerable to attentional failure at night compared with men and compared with women in the luteal phase, consistent with other reports [28]. The importance of separating women by menstrual phase in understanding the impact of extended wakefulness on alertness is especially evident when menstrual-phase groups are combined, resulting in diluted or completely obscured effects (Supplementary Figure S1).
Our findings are consistent with aspects of two earlier laboratory studies. Wright and Badia [20] found that some measures of cognitive performance (including spatial memory, recognition memory, and addition) differed in women by menstrual phase across 24 hr of continuous wakefulness. In that study, women in the follicular phase (n = 8) had poorer performance than women in the luteal phase (n = 9), especially during the late night/early morning hours. Analysis of PVT data in that study did not reveal significant differences, however, likely due to insufficient power in that sample. Moreover, performance in men was not examined in that study, and thus, the reduced risk of attentional failure in the luteal phase compared with men was not recognized. Blatter et al. [17] found that alertness was more impaired in women across 40 hr of wakefulness when compared with men. Menstrual-phase differences were not studied, however, as all women were reported to be in the follicular phase. Our results, in a larger sample, both replicate and extend these findings, demonstrating that when women are in the luteal phase, their risk of attentional failures is less than that of men, and their RT is comparable. We thus conclude that sex differences in the response to sleep deprivation fundamentally depend on menstrual phase.
Sex and menstrual-phase differences in performance were especially striking for attentional failures. Women in the follicular phase demonstrated extreme impairment such that over 60 per cent of the responses were ≥500 ms at 24 hr awake. At the end of the night, women in the follicular phase had lapses of attention that were at least 3 s long for over one-third of their responses. Such long intervals in which women in the follicular phase did not respond represent a serious safety concern. For example, while driving at 100 km/h, a 3 s lapse of attention would represent traveling greater than 18 car lengths without responding to the environment. Driving home after 16–24 hr of wakefulness is common for some shift workers. A shift worker who wakes at a normal time, but then remains awake to start the first night shift in a sequence, will encounter sleep deprivation similar to that experienced in this protocol. Thus, the up-to-24 hr of sleep deprivation that leads to the poorest performance seen in this study may be commonplace in shift working women. In addition, long-duration shifts (>24 hr) are common practice in medical training and firefighting, ensuring that approximately half the time (during the follicular phase), women in these professions are likely to have a much higher vulnerability to attentional failure. Knowledge that menstrual phase confers a large risk of attentional failures in women could potentially help to counter this serious risk by either promoting increased countermeasures (e.g., caffeine and light) or potentially avoiding activities that could result in serious accidents of injury at that time.
Our study also includes the novel finding that women in the luteal phase of the menstrual cycle are at reduced risk of the most serious attentional failures (RTs >3 s). Men demonstrated a greater disintegration of performance on the first night than luteal women, relative to each group’s baseline. For mean RT, men and luteal women reach the same poor level of performance at 22–24 hr awake (Figure 2). As baseline performance in men is better, this represents an overall greater decrement in performance from baseline to poorest performance. The potentially superior performance of luteal women over men can also be seen for longer lapses. For lapses of attention 3 s or longer, luteal women demonstrate a lower proportion of long lapses than men (13% vs. 20% of responses at 24 hr). Thus, women in the luteal phase appear to be protected from the impact of sleep deprivation, both relative to women in the follicular phase and to men.
The benefits of examining performance in women by menstrual phase are most apparent for lapses of attention that reached 3 s or longer. Women in the follicular phase demonstrated a dramatic increase in these long lapses, relative to men, whereas women in the luteal phase demonstrated fewer. When menstrual-phase groups are collapsed, therefore, it appears that there is no sex difference (Supplementary Figure S1C). This result demonstrates that for measures of extreme attentional failure, grouping by sex alone could lead to the false conclusion that there are no sex differences. Knowledge of menstrual phase is thus critical to understand performance, as grouping together women at all menstrual phases can lead to the conclusion that the performance of women is always worse at night [28], whereas our results indicate that the risk of attentional failures is lower in women at the luteal phase.
The dramatic difference in performance in women in the follicular and luteal phases is most likely driven by the different hormonal milieu during these menstrual phases. The effect of sex hormones on body temperature may contribute to the difference in performance in women. The relationship between body temperature and RT has long been known [29]. With increases in body temperature, coincident RTs are faster. Forced desynchrony studies demonstrate a strong relation of circadian rhythms in CBT and performance, with poorest performance following lowest temperature and best performance close to the temperature peak [30]. This relation of temperature and performance can be seen independently of circadian time [31]. Thus, body temperature and alertness are associated with increases in temperature that are potentially protective against alertness failure. During the follicular phase of the menstrual cycle, body temperature decreases, whereas in the luteal phase, body temperature increases. As we demonstrate in Figure 4, the differences in body temperature by menstrual phase are most apparent in the biological night, when temperature is at its lowest. This overlaps with the strongest differences in performance between the menstrual-phase groups. These differences in temperature are likely due to the increase in progesterone during the luteal phase. Progesterone has a hyperthermic effect on CBT [32–36]. By raising body temperature in the biological night, this hormone may act as protection from attentional failure at this vulnerable biological time. It is possible that individual differences in both the relative levels of progesterone, or sensitivity to the hormones, may confer different susceptibility to attentional failure. The examination of hormones within individuals may help us to predict which women are most likely to respond poorly to sleep deprivation.
The finding of superior performance in women in the luteal phase is somewhat surprising, considering the impact of menstrual phase on sleep. Studies have consistently shown that women in their luteal phase report poorer subjective sleep quality, more awakenings after sleep onset, more slow-wave sleep after sleep loss (suggesting that there is a greater build-up of homeostatic sleep pressure), a decrease in REM sleep, an increase in sleep onset latency, a decrease in total sleep time, and a decrease in sleep efficiency [37–40]. Therefore, sleep quality is poorer during the luteal phase. Thus, it seems counterintuitive that the women in the luteal phase performed better than women in the follicular phase (and at times better than men), since it would be expected that there would be greater sleep insufficiency at that time. This is similar to findings in older people, however, who show a greater number of awakenings and reduced depth of sleep [41, 42], yet have a reduced risk of attentional failure when sleep deprived during the biological night [43]. Our results may reflect a wake bias in the luteal phase (perhaps due to increased body temperature at night as a consequence of increased progesterone levels) that results in poorer sleep, but also an increased ability to maintain wakefulness and alertness. Alternatively, poorer sleep and improved alertness at night in the luteal phase may reflect a decreased homeostatic sleep pressure build-up in the luteal phase. Conversely, it is possible that poor sleep quality in luteal phase women is another manifestation of increased arousal during the night time, as also reflected in increased body temperature and alertness.
In a previous study demonstrating a greater effect of time awake on RT in women, it was suggested that this was potentially due to a difference in response strategy [17]. As women in that study had fewer errors of commission (anticipations) than men, women were thought to be choosing accuracy over speed. We did not replicate this result in this larger sample. Blatter et al. [17] reported that all women were studied in the follicular phase. When we compare only women in the follicular phase with men (Figure 2G), women have slightly more anticipations, not fewer. We did, however, find that women in the luteal phase made fewer errors of commission. For our sample, therefore, there is no evidence for an accuracy/speed trade-off in follicular-phase women. For women in the luteal phase, there may be a tendency to choose accuracy over speed. This trade-off does not appear to negatively affect performance, as the luteal phase women show similar performance to men and less of a decrement in the first night, relative to their baseline.
One sex difference that did not seem highly affected by menstrual phase was the difference in RT during the regular waking day (~hours 0–16; Figure 2A and Supplementary Figure S3). Under rested conditions, women were slower than men. This was also apparent in the RT distribution analysis (Figure 3A). The RT distribution demonstrates that at the fastest RT (5th percentile), men were faster than women. Menstrual phase appeared to have no influence on these fast RTs in women. This overall sex difference is consistent with other studies comparing men and women [44].
Although we found highly significant effects of menstrual phase and sex on PVT performance, as measured with the PVT, we found no evidence of differences in self-reported sleepiness. As can be seen in Supplementary Figure S2, subjective sleepiness measures were almost entirely overlapping in both groups of women and the men across the entire duration of sustained wakefulness. The results confirm and extend multiple previous findings demonstrating that self-reported sleepiness is not an accurate reflection of objective performance (e.g. Refs. 45 and 46). As the subjective ratings of alertness poorly reflect the susceptibility to attentional failure, our results suggest that we should not rely on subjective sleepiness ratings to try and predict and avoid these failures, especially in women working night shifts while in the follicular phase of the menstrual cycle.
There are some limitations of note in this study. First, we examined a limited age range (18–30 years) which does not reflect the age of typical shift workers. For example, a recent examination of health in >189000 nurses working rotating shift work had an average age of 50 years [47]. Thus, wider age ranges are needed to understand the impact of menstrual phase on alertness failure in a typical shift working–age range. Second, this study in young healthy women not on hormonal contraception may be limited in its generalizability to women who are pregnant, on hormonal contraception, or perimenopausal. Finally, our method of determining menstrual phase by self-report is less ideal than hormone-based tracking. As our study was a retrospective analysis of existing data, this was not possible. The use of self-report, however, has been found to be nearly as accurate (67% accuracy in targeting the mid-luteal phase) vs. 3 to 5 days of blood sampling following a positive urinary ovulation test (58% to 75%) [48]. Although the self-report method used was likely to result in menstrual phase misclassification of some women, our group temperature data demonstrates that as a group, we were accurate. Finally, our study was a retrospective analysis of existing data. A prospective study, with a wider age range and hormonally determined menstrual phase, would be ideal for confirming and expanding on these findings.
Conclusions
Our study shows that previously reported differences between women and men in alertness and performance with sleep deprivation are most likely due to the powerful influence of menstrual phase. We also demonstrated that these differences are most pronounced during the night. As women in the luteal phase perform comparably or better than men, reports of sex differences that fail to account for menstrual phase are likely to be inadvertently misleading. Our results demonstrate that there is a highly predictable window of vulnerability to attentional failures in shift working women: working night shifts with insufficient prophylactic sleep while in the follicular phase of the menstrual cycle. Use of this information could allow workers to prepare for night shift more effectively using countermeasures such as light or caffeine, or potentially guide shift work scheduling. This information is especially important for professions in which attentional failure may lead to accidents and injuries in the workplace. Night shift work has become a necessary part of modern society, with women comprising approximately half of the shift working population; it is thus important for individuals to understand when they may be most vulnerable to the impact of sleep deprivation and misalignment of circadian phase so that personalized countermeasures can be deployed to improve safety and performance for themselves and those around them.
Supplementary Material
Supplementary material is available at SLEEP online.
Funding
This work was supported by grants from the National Institute of Mental Health (R01 MH45130; Czeisler/Lockley), National Center for Complementary and Alternative Medicine (R01 AT002129; Czeisler/Lockley), National Institute of Neurological Disorders and Stroke (R01NS36590; Brainard), and was performed in the Brigham and Women’s Hospital General Clinical Research supported by M01 RR02635 and the Harvard Clinical and Translational Science Center (1 UL1 RR025758), from the National Center for Research Resources. Dr. Gooley was supported by a National Heart, Lung, and Blood Institute fellowship in the program of training in Sleep, Circadian and Respiratory Neurobiology at Brigham and Women’s Hospital (T32 HL079010). Dr. Lockley was supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. Ms. Vidafar was supported by a scholarship from the National Health and Medical Research Council, via the Neurosleep Centre for Research Excellence.
Acknowledgments
We wish to thank the research volunteers, recruiters, and research staff at the Division of Sleep and Circadian Disorders and the Center for Clinical Investigation of the Brigham and Women’s Hospital, for their assistance with the studies.
Notes
Conflict of interest statement. P.V., J.J.G., E.V.R., A.C.B., M.R., and S.W.C. report no conflicts of interest. S.M.W.R. reports that he has served as a consultant through his institution to Vanda Pharmaceuticals, Philips Respironics, EdanSafe, The Australian Workers’ Union, National Transport Commission, Transport Accident Commission, New South Wales Department of Education and Communities, and has through his institution received research grants and/or unrestricted educational grants from Vanda Pharmaceuticals, Shell, Teva Pharmaceuticals, Rio Tinto, Seeing Machines, Takeda Pharmaceuticals North America, Philips Lighting, Philips Respironics, Cephalon, and ResMed Foundation, and reimbursements for conference travel expenses from Vanda Pharmaceuticals. His institution has received equipment donations or other support from Optalert, Compumedics, and Tyco Healthcare. He has served as an expert witness and/or consultant to shift work organizations. S.M.W.R. also serves as a Program Leader in the Cooperative Research Centre for Alertness, Safety and Productivity. C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for the following: Bose Corporation; Boston Celtics; Columbia River Bar Pilots; Institute of Digital Media and Child Development; Klarman Family Foundation; Quest Diagnostics, Inc.; Vanda Pharmaceuticals and V-Watch/PPRS. He has also received education/research support from Cephalon Inc., Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Optum, Philips Respironics, Inc., ResMed Foundation, San Francisco Bar Pilots, Schneider Inc., and Sysco. He has received lecture fees from American Academy of Sleep Medicine (AASM), CurtCo Media Labs LLC, Global Council on Brain Health/AARP, Hawaii Sleep Health and Wellness Foundation, National Sleep Foundation, University of Michigan, University of Washington, and Zurich Insurance Company, Ltd. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine (which CAC directs) has received Educational Grant funding from Cephalon, Inc., Jazz Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., and Wake Up Narcolepsy. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker). Since 1985, he has served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Bombardier, Inc.; Continental Airlines; FedEx; Greyhound; and United Parcel Service (UPS). C.A.C. owns or owned an equity interest in Somnus Therapeutics, Inc. and Vanda Pharmaceuticals. He received royalties from McGraw Hill, and Koninklijke Philips Electronics, N.V. for the Actiwatch-2 and Actiwatch-Spectrum devices. C.A.C.’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. S.W.L. has no conflicts of interests related to the research or results reported in this paper. In the interests of full disclosure, commercial interests from the last 3 years (2015–2017) are listed below. S.W.L. has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Pegasus Capital Advisors LP, Serrado Capital, and Slingshot Insights, and has current consulting contracts with Akili Interactive, Consumer Sleep Solutions, Delos Living LLC, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, PlanLED, OpTerra Energy Services Inc., and Wyle Integrated Science and Engineering. S.W.L. has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and F.Lux Software LLC; has equity in iSLEEP, Pty; advance author payment and/or royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Lightfair, Informa Exhibitions (USGBC), Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from DIN, FASEB, Lightfair, SLTBR, and USGBC. S.W.L. has completed an investigator-initiated research grant from Biological Illumination LLC and has an ongoing investigator initiated grant from F. Lux Software LLC. S.W.L. holds a process patent for “Systems and methods for determining and/or controlling sleep quality,” which is assigned to the Brigham and Women’s Hospital per Hospital policy. S.W.L. has also served as a paid expert for legal proceedings related to light, sleep, and health. S.W.L. is also a Program Leader for the CRC for Alertness, Safety and Productivity, Australia.
References
- 1. U.S. Congress Office of Technology Assessment. Biological Rhythms: Implications for the Worker. Wachington, DC: U.S: Government Printing Office; 1991. [Google Scholar]
- 2. Monk TH, et al. Making Shift Work Tolerable. London: Taylor & Francis; 1992. [Google Scholar]
- 3. McMenamin TM. A time to work: recent trends in shift work and flexible schedules. Mon Lab Rev. 2007;130(12):3–15. [Google Scholar]
- 4. Australia SW. Australian Work-Related Injury Experience by Sex and Age, 2009–10. Canberra: Safe Work Australia; 2012. [Google Scholar]
- 5. Gibbs M, et al. Adaptation of the circadian rhythm of 6-sulphatoxymelatonin to a shift schedule of seven nights followed by seven days in offshore oil installation workers. Neurosci Lett. 2002;325(2):91–94. [DOI] [PubMed] [Google Scholar]
- 6. Dumont M, et al. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001;16(5):502–511. [DOI] [PubMed] [Google Scholar]
- 7. Ftouni S, et al. Ocular measures of sleepiness are increased in night shift workers undergoing a simulated night shift near the peak time of the 6-sulfatoxymelatonin rhythm. J Clin Sleep Med. 2015;11(10):1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogińska H, et al. Gender, ageing, and shiftwork intolerance. Ergonomics. 1993;36(1-3):161–168. [DOI] [PubMed] [Google Scholar]
- 9. Kandolin I. Burnout of female and male nurses in shiftwork. Ergonomics. 1993;36(1-3):141–147. [DOI] [PubMed] [Google Scholar]
- 10. Dekker DK, et al. Gender differences in permanent sleep behaviour. In: Costa G, Cesana G, Kogi K, Wedderburn A, eds. Shiftwork: Sleep, Work and Performance. Frankfurt: Peter Lang; 1990:77–82. [Google Scholar]
- 11. Tepas DI, et al. Shiftwork and the older worker. Exp Aging Res. 1993;19(4):295–320. [DOI] [PubMed] [Google Scholar]
- 12. Saksvik IB, et al. Individual differences in tolerance to shift work–a systematic review. Sleep Med Rev. 2011;15(4): 221–235. [DOI] [PubMed] [Google Scholar]
- 13. Marquié JC, et al. Sleep, age, and shiftwork experience. J Sleep Res. 1999;8(4):297–304. [DOI] [PubMed] [Google Scholar]
- 14. Admi H, et al. Shift work in nursing: is it really a risk factor for nurses’ health and patients’ safety?Nurs Econ. 2008;26(4):250–257. [PubMed] [Google Scholar]
- 15. Rouch I, et al. Shiftwork experience, age and cognitive performance. Ergonomics. 2005;48(10):1282–1293. [DOI] [PubMed] [Google Scholar]
- 16. Safe Work Australia. Work-Related Injuries in Australia, 2005–06: The Impact of Shiftwork on Work-Related Injuries in Australia. Canberra: Safe Work Australia; 2009. [Google Scholar]
- 17. Blatter K, et al. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168(2):312–317. [DOI] [PubMed] [Google Scholar]
- 18. Axelsson J, et al. Tolerance to shift work-how does it relate to sleep and wakefulness?Int Arch Occup Environ Health. 2004;77(2):121–129. [DOI] [PubMed] [Google Scholar]
- 19. Urrila AS, et al. Psychomotor vigilance task performance during total sleep deprivation in young and postmenopausal women. Behav Brain Res. 2007;180(1):42–47. [DOI] [PubMed] [Google Scholar]
- 20. Wright KP Jr, et al. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103(2):185–194. [DOI] [PubMed] [Google Scholar]
- 21. Basner M, et al. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myerson J, et al. Aging and intraindividual variability in performance: analyses of response time distributions. J Exp Anal Behav. 2007;88(3):319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson C, et al. PVT lapses differ according to eyes open, closed, or looking away. Sleep. 2010;33(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarz G. Estimating dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 25. Brown EN, et al. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7(3):177–202. [DOI] [PubMed] [Google Scholar]
- 26. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 27. Boivin DB, et al. Diurnal and circadian variation of sleep and alertness in men vs. naturally cycling women. Proc Natl Acad Sci. 2016;113(39):10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santhi N, et al. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci. 2016;113(19):E2730–E2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleitman N, et al. The effect of body temperature on reaction time. Am J Physiol. 1938;121(2):495–501. [Google Scholar]
- 30. Dijk DJ, et al. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1(2):112–117. [DOI] [PubMed] [Google Scholar]
- 31. Wright KP Jr, et al. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283(6):R1370–R1377. [DOI] [PubMed] [Google Scholar]
- 32. Baker FC, et al. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol.. 2001;530(Pt 3):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MARSHALL J, et al. Transient focal cerebral Ischaemia. Br Med J. 1963;2(5365):1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shibui K, et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48(11):1062–1068. [DOI] [PubMed] [Google Scholar]
- 35. Halbrecht I. Ovarian function and body temperature. Lancet. 1945;2(6380):668. [DOI] [PubMed] [Google Scholar]
- 36. Israel SL, et al. The thermogenic property of progesterone. Fertil Steril. 1950;1(53):532–533. [Google Scholar]
- 37. Driver HS, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. [DOI] [PubMed] [Google Scholar]
- 38. Baker FC, et al. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. [DOI] [PubMed] [Google Scholar]
- 39. Baker FC, et al. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. [DOI] [PubMed] [Google Scholar]
- 40. Driver HS. Sleep in women. J Psychosom Res. 1996;40(3): 227–230. [DOI] [PubMed] [Google Scholar]
- 41. Dijk DJ, et al. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31(2):130–140. [DOI] [PubMed] [Google Scholar]
- 42. Dijk DJ, et al. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24(5):565–577. [DOI] [PubMed] [Google Scholar]
- 43. Duffy JF, et al. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57(7):1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Der G, et al. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychol Aging. 2006;21(1):62–73. [DOI] [PubMed] [Google Scholar]
- 45. Van Dongen HPA, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 46. St Hilaire MA, et al. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vetter C, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315(16):1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wideman L, et al. Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sports Health. 2013;5(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.