Abstract
Aims
Pulmonary hypertension (PH) and pulmonary vascular disease (PVD) are common and associated with adverse outcomes in heart failure with preserved ejection fraction (HFpEF). Little is known about the impact of PVD on the pathophysiology of exercise intolerance.
Methods and results
Heart failure with preserved ejection fraction patients (n = 161) with elevated pulmonary capillary wedge pressure (≥15 mmHg) at rest were classified into three groups: non-PH-HFpEF (n = 21); PH but no PVD (isolated post-capillary PH, IpcPH; n = 95); and PH with PVD (combined post- and pre-capillary PH, CpcPH; n = 45). At rest, CpcPH-HFpEF patients had more right ventricular (RV) dysfunction and lower pulmonary arterial (PA) compliance compared to all other groups. While right atrial pressure (RAP) and left ventricular transmural pressure (LVTMP) were similar in HFpEF with and without PH or PVD at rest, CpcPH-HFpEF patients demonstrated greater increase in RAP, enhanced ventricular interdependence, and paradoxical reduction in LVTMP during exercise, differing from all other groups (P < 0.05). Lower PA compliance was correlated with greater increase in RAP with exercise. During exercise, CpcPH-HFpEF patients displayed an inability to enhance cardiac output, reduction in forward stroke volume, and blunted augmentation in RV systolic performance, changes that were coupled with marked limitation in aerobic capacity.
Conclusion
Heart failure with preserved ejection fraction patients with PVD demonstrate unique haemodynamic limitations during exercise that constrain aerobic capacity, including impaired recruitment of LV preload due to excessive right heart congestion and blunted RV systolic reserve. Interventions targeted to this distinct pathophysiology require testing in patients with HFpEF and PVD.
Keywords: Heart failure with preserved ejection fraction, Pulmonary vascular disease, Right heart catheterization , Invasive exercise haemodynamics
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for approximately half of all heart failure patients, affecting millions worldwide.1 Although there are features common to all HFpEF patients, there may be substantial pathophysiologic heterogeneity as well.2 Heart failure with preserved ejection fraction is initially defined by an elevation in left-sided filling pressures, but many patients progress to develop pulmonary vascular disease (PVD) secondary to chronic left heart congestion.3–14 This cohort experiences worse outcomes when compared to HFpEF patients with isolated left heart disease, but the mechanisms explaining this observation remain poorly understood.3–14
Patients with HFpEF universally complain of exertional intolerance, but the causes may differ between patients with different phenotypes. Exercise introduces an impressive stress to the right heart and lungs, where elevations in venous return increase pulmonary blood volume by 50% while increasing lung blood flow 300%.15 The healthy pulmonary vasculature is a high compliance, low resistance circuit that can readily accommodate these marked increases in blood volume and flow.4,16 However, this reserve may be compromised in patients with HFpEF and PVD, which may lead to important differences compared to HFpEF patients with left heart disease and no PVD.
We performed invasive haemodynamic exercise testing with expired gas analysis in a well-defined cohort of HFpEF patients with and without PVD. We hypothesized that the presence of PVD in HFpEF would compromise the ability of the right heart and lungs to accommodate increased blood flow during exercise, increasing ventricular interaction, limiting right ventricular (RV) reserve, and impairing aerobic capacity.
Methods
Consecutive patients who underwent invasive haemodynamic exercise testing at the Mayo Clinic in Rochester, MN, USA between 2006 and 2016 were identified. The Mayo Clinic Institutional Review Board approved the study, and all subjects provided written informed consent. All authors had full access to the data and take full responsibility for its integrity.
Heart failure with preserved ejection fraction was defined by the presence of typical symptoms (exertional dyspnoea and fatigue), left ventricular ejection fraction (LVEF) ≥50%, and elevated left-sided filling pressures at rest [pulmonary capillary wedge pressure (PCWP) >15 mmHg].17 Heart failure with preserved ejection fraction patients with normal resting PCWP, but elevated PCWP on exercise were not included. To investigate exercise haemodynamics according to the presence of PVD, we divided HFpEF patients into pulmonary hypertension (PH) subgroups according to published recommendations: (i) non-PH [mean pulmonary artery pressure (PAP) <25 mmHg], (ii) PH with no PVD [isolated post-capillary PH, IpcPH; mean PAP ≥25 mmHg with pulmonary vascular resistance (PVR) ≤3.0 Wood units (WU) and diastolic pressure gradient (DPG) <7 mmHg], and (iii) PH with PVD (combined post- and pre-capillary PH, CpcPH; mean PAP ≥25 mmHg with PVR > 3.0 and/or DPG ≥7 mmHg).18
Patients with LVEF <50%, primary right-sided HF, valvular heart disease (>moderate left-sided regurgitation and/or >mild stenosis), unstable coronary artery disease or recent revascularization, constrictive pericarditis, high-output heart failure, and infiltrative, restrictive or hypertrophic cardiomyopathy were excluded.
Echocardiography
Echocardiography was performed at rest in a blinded fashion according to the guidelines of the European Association of Cardiovascular Imaging and the American Society of Echocardiography to assess LV diastolic function, mass, and severity of valvular heart disease.19,20 Left ventricular ejection fraction was assessed using quantitative measures based upon optimal images in each patient, including two-dimensional echocardiography using the Quinones formula from the parasternal views (n = 107), the two-dimensional biplane volumetric Simpson method (n = 23), M-mode (n = 2), or visual qualitative assessment (n = 29) if quantitative measurements could not be made. Using RV-focused views, RV basal and mid-cavity dimensions were measured at end-diastole, and RV end-diastolic and end-systolic areas were traced to calculate fractional area change (FAC = [RV end-diastolic area – end-systolic area]/end-diastolic area × 100).21 Pericardial restraint and ventricular interaction were assessed by the LV eccentricity index measured at end-diastole as recently described.22 An LV eccentricity index >1.0 indicates a leftward septal shift due to right-sided overload and enhanced ventricular interdependence.
Cardiac catheterization protocol
Patients were assessed on chronic medications, in fasted state, after minimal sedation and in supine position, without knowledge of echocardiography data, as previously described.22–26 Right heart catheterization was performed through a 9-Fr sheath via the right internal jugular vein at both rest and with exercise, with simultaneous directly measured oxygen consumption (VO2) using expired gas analysis (MedGraphic, St. Paul, MN, USA). Right atrial pressure (RAP), PAP, and PCWP were recorded at end-expiration, using the mean of ≥3 beats. Pressure tracings were digitized (240 Hz) and stored for offline analysis, performed in a blinded fashion. The left ventricular transmural pressure (LVTMP), which quantifies the net distending pressure that determines LV preload, was calculated as PCWP minus RAP.22,27–30
Arteriovenous oxygen difference (A-VO2diff) was determined from directly measured arterial and mixed venous O2 contents from blood sampling (saturation × haemoglobin × 1.34 × 10). Cardiac output (CO) was determined by the direct Fick method (CO = VO2/A-VO2diff) and indexed for body surface area to calculate cardiac index (CI). Pulmonary vascular resistance [PVR = (mean PAP − PCWP)/CO] and systemic vascular resistance [SVR = (mean arterial blood pressure − RAP)/CO], stroke volume (SV = CO/heart rate), systemic and pulmonary pulse pressure, and diastolic pressure gradient (DPG = PA diastolic − PCWP) were calculated. Pulmonary arterial compliance (PAC) and total arterial compliance (TAC) were calculated (PAC = SV/pulmonary pulse pressure; TAC = SV/systemic pulse pressure, respectively).25,31 Total pulmonary resistance (TPR) was calculated as the quotient of mean PAP and CO.32 End-systolic pressure (ESP) was taken as 0.9 × systolic blood pressure. Systemic and pulmonary arterial elastance (Ea-S, Ea-P) were calculated as ESP/SV and PA systolic pressure/SV, respectively.
Following rest measures, patients engaged in supine cycle ergometry starting at 20 W workload and increasing in 10–20 W increments (3 min per stage) until subject-reported exhaustion. Haemodynamic data were again acquired at peak exercise in all participants using the same methods.
Statistical analysis
Data are reported as mean ± standard deviation (SD), median (25th, 75th percentile) or numbers (percentages). For each parameter, between-group differences were first assessed using the one-way analysis of variance (ANOVA), Kruskal–Wallis test, or χ2 test, as appropriate. Then, the Tukey honestly significant-difference test or Steel–Dwass test were applied (as appropriate) to account for multiple comparisons between the three groups. No adjustment was made to account for multiple hypotheses testing among the different haemodynamic parameters studied. Correlations were calculated using Spearman’s or Pearson’s correlation, when appropriate. An interaction term was applied to examine whether correlations differed between two groups. To accomplish this a linear model was fit where dependent variable Y is modelled by the continuous variable X (independent variable of interest), a categorical variable (group) and the interaction between the two X variables (X × group). P-values are two-sided and predefined significance level was <0.05. Analyses were performed in JMP 10.0.0 (SAS Institute, Cary, NC, USA).
Results
Of patients with HFpEF (n = 161), the vast majority (n = 140, 87%) displayed PH (i.e. mean PA pressure ≥ 25 mmHg) at rest. Of this group, 68% (n = 95) displayed IpcPH and 32% (n = 45) had CpcPH-HFpEF. All CpcPH patients displayed elevated PVR (>240 dynes/s × cm5), but only 11 (24%) displayed elevated DPG. Of the total cohort, 50% were examined from 2006 to 2013 and 50% from 2013 to 2016. Sensitivity analysis performed separately among patients in the two eras showed similar results, suggesting that the length of the inclusion period did not significantly influence the results (Supplementary material online, Table S1).
Age, sex, body mass index, and body surface area were similar across groups (Table 1). The prevalence of AF and N-terminal pro-B-type natriuretic peptide levels were highest in CpcPH-HFpEF but other comorbidities and medication use were similar across groups. Baseline characteristics of the study cohort were similar to those from HFpEF patients enrolled in contemporary clinical trials (Supplementary material online, Table S2).
Table 1.
Baseline characteristics
| Non-PH-HFpEF (n = 21) | IpcPH-HFpEF (n = 95) | CpcPH-HFpEF (n = 45) | P-value | |
|---|---|---|---|---|
| Age (years) | 65 ± 13 | 68 ± 11 | 70 ± 11 | 0.4 |
| Female, n (%) | 13 (62%) | 60 (63%) | 29 (64%) | 1.0 |
| Body mass index (kg/m2) | 34 ± 10 | 35 ± 8 | 32 ± 6 | 0.2 |
| Body surface area (m2) | 2.02 ± 0.32 | 2.05 ± 0.29 | 1.99 ± 0.22 | 0.5 |
| Comorbidities | ||||
| Hypertension | 17 (89%) | 82 (92%) | 36 (93%) | 0.8 |
| Coronary artery disease | 6 (29%) | 32 (34%) | 13 (31%) | 0.9 |
| Atrial fibrillation | 2 (10%) | 30 (32%)a | 27 (61%)a,b | <0.0001 |
| Diabetes mellitus | 2 (10%) | 31 (33%) | 11 (25%) | 0.1 |
| Sleep apnoea syndrome | 6 (32%) | 37 (51%) | 20 (59%) | 0.2 |
| Medications | ||||
| ACEI or ARB | 10 (48%) | 42 (44%) | 20 (45%) | 1.0 |
| Beta-blocker | 11 (52%) | 59 (62%) | 25 (57%) | 0.7 |
| Diuretics | 11 (52%) | 56 (59%) | 30 (68%) | 0.4 |
| Laboratories | ||||
| Haemoglobin (gm/dL) | 12.3 ± 1.5 | 12.1 ± 1.6 | 12.1 ± 1.7 | 0.9 |
| NT-proBNP (pg/mL) | 203 (60, 713) | 809 (225, 1407) | 1056 (502, 2223)a | 0.009 |
| Pulmonary function testing | ||||
| Vital capacity (% predicted) | 90 ± 11 | 79 ± 15 | 78 ± 15 | 0.1 |
| FVC (% predicted) | 83 ± 16 | 79 ± 15 | 76 ± 15 | 0.3 |
| FEV1 (% predicted) | 77 ± 19 | 74 ± 17 | 67 ± 15 | 0.1 |
| Echocardiography | ||||
| LV ejection fraction (%) | 63 ± 4 | 62 ± 6 | 62 ± 6 | 0.8 |
| LVEDD (mm) | 48 ± 5 | 48 ± 5 | 49 ± 6 | 0.7 |
| LV mass index (g/m2) | 85 ± 16 | 96 ± 24 | 95 ± 23 | 0.2 |
| LA volume index (mL/m2) | 38 ± 23 | 40 ± 12 | 45 ± 17 | 0.2 |
| E/e′ | 10.0 (8.8, 11.5) | 13.9 (10.0, 20.0)a | 16.0 (13.0, 20.9)a | 0.001 |
| TV s′ (cm/s) | 12 ± 2 | 12 ± ± 2 | 12 ± 3 | 0.7 |
| Fractional area change (%) | 51 ± 5 | 49 ± 9 | 44 ± 11a,b | 0.02 |
| RV end-diastolic area (cm2/m2) | 6.8 ± 1.3 | 7.3 ± 2.1 | 8.3 ± 3.1 | 0.3 |
| RV basal diameter (mm) | 33 ± 5 | 34 ± 8 | 37 ± 8 | 0.1 |
| RV mid-diameter (mm) | 25 ± 3 | 26 ± 7 | 29 ± 9 | 0.1 |
| Moderate or severe TR (%) | 2 (10%) | 18 (19%) | 17 (38%)a,b | 0.02 |
| LV eccentricity index | 1.05 ± 0.13 | 1.05 ± 0.18 | 1.08 ± 0.16 | 0.7 |
Data are mean ± standard deviation, median (25th, 75th percentile), or n (%). Final column reflects overall group differences. No adjustment for multiple hypotheses testing of different variables was performed.
P < 0.05 vs. non-PH-HFpEF.
P < 0.05 vs. IpcPH-HFpEF.
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; CpcPH, combined post- and pre-capillary pulmonary hypertension; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HFpEF, heart failure with preserved ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PH, pulmonary hypertension; RV, right ventricular; TR, tricuspid regurgitation; TV, tricuspid valve.
Cardiac structure, function, and haemodynamics at rest
Left ventricular dimensions, mass and EF were similar across HFpEF groups (Table 1). Heart failure with preserved ejection fraction patients with PH displayed higher E/e′. Patients with CpcPH displayed more RV systolic dysfunction compared to the other groups, reflected by lower FAC (Figure 1A). Right ventricular dimensions tended to be increased in CpcPH, and tricuspid regurgitation (TR) was more prevalent. The LV eccentricity index tended to be increased in CpcPH-HFpEF patients with PH, indicating greater flattening of the interventricular septum towards the left ventricle at rest and thus greater ventricular interdependence (Table 1).
Figure 1.
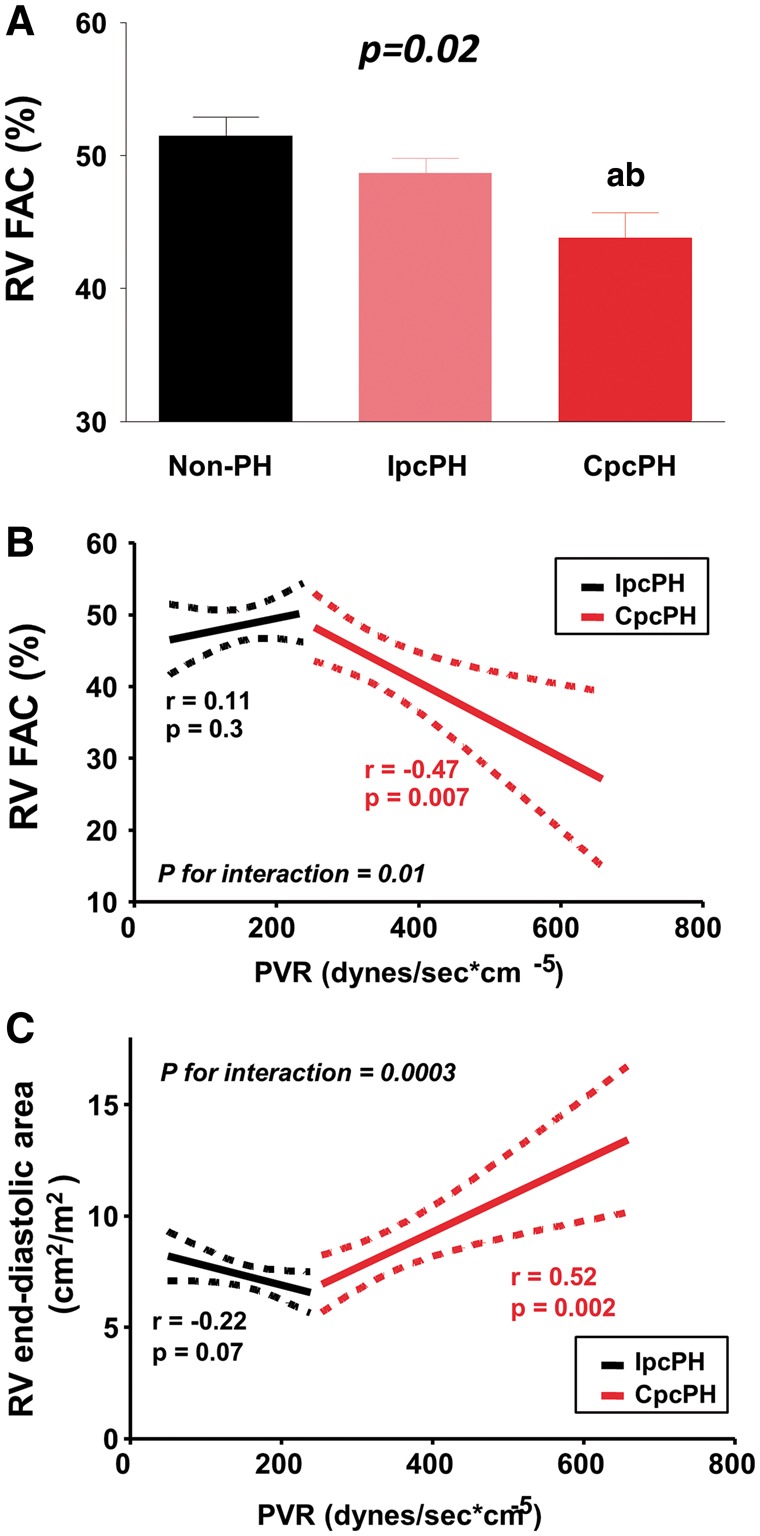
Right ventricular function and size at rest. (A) At rest, CpcPH-HFpEF patients displayed the lowest right ventricular fractional area change compared to other groups. (B–C) Higher pulmonary vascular resistance was associated with decreased fractional area change and with increased right ventricular size in CpcPH-HFpEF, while these associations were absent in IpcPH-HFpEF. Error bars reflect standard error of the mean. aP < 0.05 vs. Non-PH-HFpEF; and bP < 0.05 vs. IpcPH-HFpEF. CpcPH, combined post- and pre-capillary pulmonary hypertension; FAC, fractional area change; IpcPH, isolated post-capillary pulmonary hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular.
There were no differences in heart rate or blood pressures between the groups (Table 2). Right atrial pressure was similar among HFpEF patients with and without PH at rest. There were no statistically significant differences in RAP/PCWP ratio and LVTMP between groups at rest.
Table 2.
Resting haemodynamics
| Non-PH-HFpEF (n = 21) | IpcPH-HFpEF (n = 95) | CpcPH-HFpEF (n = 45) | P-value | |
|---|---|---|---|---|
| Vital signs | ||||
| Heart rate (bpm) | 65 ± 12 | 63 ± 11 | 62 ± 14 | 0.6 |
| Systolic BP (mmHg) | 155 ± 25 | 153 ± 33 | 159 ± 29 | 0.7 |
| Mean BP (mmHg) | 103 ± 13 | 103 ± 18 | 105 ± 18 | 0.9 |
| Central pressures | ||||
| RA pressure (mmHg) | 10 ± 4 | 12 ± 4 | 13 ± 5 | 0.1 |
| RA v wave pressure (mmHg) | 11 ± 4 | 14 ± 4a | 14 ± 5a | 0.02 |
| PA systolic pressure (mmHg) | 36 ± 11 | 46 ± 11a | 60 ± 12a,b | <0.0001 |
| PA mean pressure (mmHg) | 21 ± 4 | 31 ± 6a | 39 ± 6a,b | <0.0001 |
| PCWP (mmHg) | 18 ± 4 | 21 ± 5a | 20 ± 4 | 0.03 |
| RAP/PCWP ratio | 0.56 ± 0.19 | 0.59 ± 0.17 | 0.63 ± 0.18 | 0.3 |
| LVTMP (mmHg) | 8.2 ± 4.2 | 8.9 ± 4.5 | 7.6 ± 3.9 | 0.3 |
| Vascular and ventricular function | ||||
| SVR (dynes/s × cm5) | 1441 ± 446 | 1418 ± 519 | 1809 ± 713b | 0.01 |
| TAC (mL/mmHg) | 1.1 ± 0.3 | 1.2 ± 1.0 | 0.9 ± 0.3b | 0.01 |
| Ea-S (mmHg/mL) | 1.8 ± 0.4 | 1.7 ± 0.7 | 2.3 ± 0.9b | 0.003 |
| PVR (dynes/s × cm5) | 67 ± 50 | 154 ± 53a | 356 ± 103a,b | <0.0001 |
| TPR (mmHg × min/L) | 4.49 ± 1.46 | 6.12 ± 1.78a | 9.63 ± 2.22a,b | <0.0001 |
| PAC (mL/mmHg) | 3.9 ± 1.1 | 4.0 ± 3.0 | 2.2 ± 0.8a,b | 0.0003 |
| Ea-P (mmHg/mL) | 0.40 ± 0.09 | 0.50 ± 0.19a | 0.81 ± 0.25a,b | <0.0001 |
| RVSW index (g/m2 × beat) | 5.7 ± 3.8 | 11.3 ± 4.5a | 12.8 ± 5.6a | <0.0001 |
| Flow measures and metabolism | ||||
| Stroke volume index (mL/m2) | 40 ± 11 | 44 ± 13 | 36 ± 11b | 0.004 |
| Cardiac index (L/min/m2) | 2.6 ± 0.7 | 2.7 ± 0.6 | 2.2 ± 0.6a,b | <0.0001 |
| O2 consumption (mL/min/kg) | 2.6 ± 0.8 | 2.5 ± 0.6 | 2.4 ± 0.5 | 0.4 |
| A–V O2 difference (mL/dL) | 4.7 ± 0.9 | 4.5 ± 1.2 | 5.2 ± 1.2b | 0.005 |
Data are mean ± standard deviation. Final column reflects overall group differences. No adjustment for multiple hypotheses testing of different variables was performed.
BP, blood pressure; CpcPH, combined post- and pre-capillary pulmonary hypertension; Ea, effective arterial elastance; Ea-P, pulmonary arterial elastance; Ea-S, systemic arterial elastance; HFpEF, heart failure with preserved ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; LVTMP, left ventricular transmural pressure; PA, pulmonary artery; PAC, pulmonary arterial compliance; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RA, right atrial; RAP, right atrial pressure; RVSW, right ventricular stroke work; SVR, systemic vascular resistance; TAC, total arterial compliance; TPR, total pulmonary resistance.
P < 0.05 vs. non-PH-HFpEF.
P < 0.05 vs. IpcPH-HFpEF.
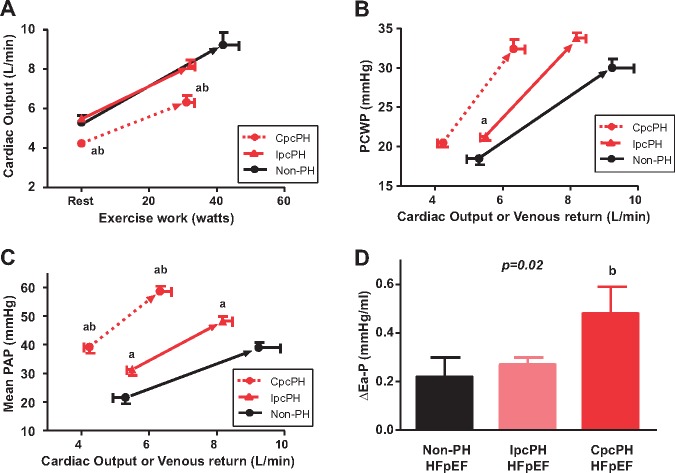
Patients with CpcPH-HFpEF displayed more deranged RV–PA coupling, with greater reduction in RV FAC and more RV dilatation as resting PVR increased (Figure 1B, C). Patients with CpcPH-HFpEF also displayed increased Ea-P, lower PA compliance, and reduced stroke volume and CI at rest, with a higher A-VO2 difference (Table 2). Patients with HFpEF and PH (regardless of PVD) displayed increased RV stroke work index, reflecting the greater pressure–volume work needed to eject blood through the pulmonary vasculature in the setting of PH. CpcPH-HFpEF patients also displayed increased systemic vascular stiffening, with higher SVR and Ea-S, and lower TAC (Table 2).
Exercise haemodynamics
Exercise capacity was reduced in HFpEF patients with PH, evidenced by lower work load achieved and decreased peak VO2 (Table 3). Cardiac output, which by definition is equal to venous return to the right heart at steady state, increased similarly with exercise in non-PH and IpcPH-HFpEF but was lower for any exercise workload in CpcPH-HFpEF (Figure 2A). All groups displayed similar absolute increases in PCWP with exercise, though PCWP elevation occurred at lesser cardiac output or venous return in CpcPH-HFpEF (Table 3, Figure 2B).
Table 3.
Exercise haemodynamics
| Non-PH-HFpEF (n = 21) | IpcPH-HFpEF (n = 95) | CpcPH-HFpEF (n = 45) | P-value | |
|---|---|---|---|---|
| Work load (W) | 42 ± 20 | 32 ± 15a | 31 ± 14a | 0.03 |
| O2 consumption (mL/min/kg) | 10.5 ± 4.6 | 8.2 ± 2.5a | 7.6 ± 2.2a | 0.003 |
| Vital signs | ||||
| Heart rate (bpm) | 102 ± 25 | 93 ± 20 | 101 ± 23 | 0.1 |
| Systolic BP (mmHg) | 195 ± 33 | 182 ± 39 | 177 ± 43 | 0.4 |
| Mean BP (mmHg) | 124 ± 19 | 118 ± 24 | 114 ± 25 | 0.5 |
| Central pressures | ||||
| RA pressure (mmHg) | 17 ± 6 | 22 ± 6a | 26 ± 8a,b | <0.0001 |
| RA v wave pressure (mmHg) | 18 ± 9 | 26 ± 7a | 29 ± 9a | 0.0005 |
| PA systolic pressure (mmHg) | 54 ± 13 | 68 ± 14a | 82 ± 19a,b | <0.0001 |
| PA mean pressure (mmHg) | 39 ± 8 | 48 ± 8a | 59 ± 11a,b | <0.0001 |
| PCWP (mmHg) | 30 ± 5 | 34 ± 6 | 32 ± 7 | 0.1 |
| RAP/PCWP ratio | 0.55 ± 0.18 | 0.64 ± 0.16 | 0.84 ± ± 0.27a,b | <0.0001 |
| LVTMP (mmHg) | 13.1 ± 5.1 | 12.6 ± 6.5 | 6.2 ± 9.0a,b | 0.0003 |
| Vascular and ventricular function | ||||
| SVR (dynes/s × cm5) | 1016 ± 261 | 1041 ± 366 | 1221 ± 556 | 0.2 |
| TAC (mL/mmHg) | 0.9 ± 0.5 | 1.0 ± 0.5 | 0.4 ± 0.9b | 0.01 |
| Ea-S (mmHg/mL) | 2.1 ± 0.9 | 2.1 ± 0.9 | 2.7 ± 1.1b | 0.02 |
| PVR (dynes/s × cm5) | 106 ± 74 | 158 ± 90 | 356 ± 158a,b | <0.0001 |
| TPR (mmHg × min/L) | 4.94 ± 2.02 | 6.57 ± 2.32 | 10.2 ± 3.67a,b | <0.0001 |
| PAC (mL/mmHg) | 2.9 ± 1.2 | 2.3 ± 1.0 | 1.4 ± 0.5a,b | <0.0001 |
| Ea-P (mmHg/mL) | 0.63 ± 0.32 | 0.77 ± 0.32 | 1.30 ± 0.55a,b | <0.0001 |
| RVSW index (g/m2 × beat) | 15.2 ± 4.8 | 16.3 ± 7.7 | 14.4 ± 6.4 | 0.5 |
| Integrated function | ||||
| Stroke volume index (mL/m2) | 49 ± 17 | 44 ± 14 | 32 ± 9a,b | <0.0001 |
| Cardiac index (L/min/m2) | 4.7 ± 1.4 | 3.9 ± 1.1a | 3.2 ± 1.0a,b | <0.0001 |
| A–V O2 difference (mL/dL) | 9.5 ± 2.1 | 9.8 ± 2.6 | 10.6 ± 2.1 | 0.3 |
Data are mean ± standard deviation. Final column reflects overall group differences. No adjustment for multiple hypotheses testing of different variables was performed.
BP, blood pressure; CpcPH, combined post- and pre-capillary pulmonary hypertension; Ea-P, pulmonary arterial elastance; Ea-S, systemic arterial elastance; HFpEF, heart failure with preserved ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; LVTMP, left ventricular transmural pressure; PA, pulmonary artery; PAC, pulmonary arterial compliance; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RA, right atrial; RAP, right atrial pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; TPR, total pulmonary resistance; RVSW, right ventricular stroke work.
P < 0.05 vs. non-PH-HFpEF.
P < 0.05 vs. IpcPH-HFpEF.
Figure 2.
Changes in central pressures with exercise. (A) Baseline and peak exercise for cardiac output. (B–C) Pulmonary capillary wedge pressure and mean pulmonary artery pressure and as a function of venous return. (D) As compared to both non-PH- and IpcPH-HFpEF, CpcPH-HFpEF patients displayed greater increase in pulmonary arterial elastance during exercise. Error bars reflect standard error of the mean. aP < 0.05 vs. non-PH-HFpEF; and bP < 0.05 vs. IpcPH-HFpEF. CpcPH, combined post- and pre-capillary pulmonary hypertension; HFpEF, heart failure with preserved ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension.
Pulmonary artery pressures increased in all groups with exercise, but the greatest increases were observed in the CpcPH group, with higher pressures relative to blood flow (Table 3, Figure 2C). Patients in the CpcPH-HFpEF group experienced greater reduction in PA compliance on exercise along with higher exercise PVR and Ea-P, in keeping with impaired pulmonary vascular reserve (Table 3, Figure 2D).
Despite similar RAP at rest, both PH-HFpEF groups developed greater increases in RAP during exercise (Table 3). The intolerance of the right heart and pulmonary circulation to elevation in venous return during exercise was most dramatic in CpcPH-HFpEF (Figure 3A).
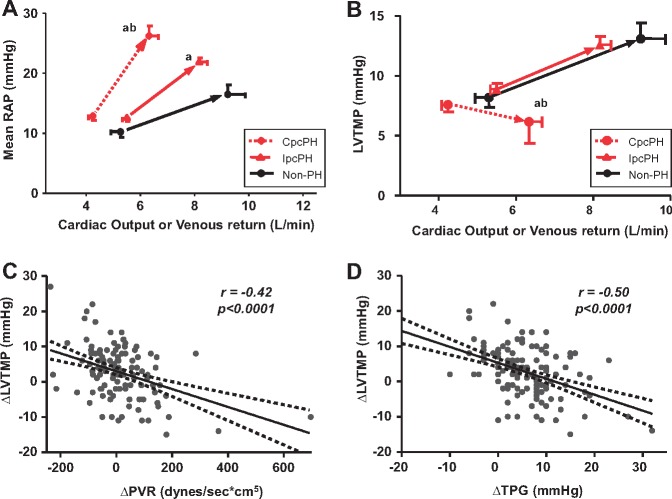
Figure 3.
Ventricular interdependence with exercise in heart failure with preserved ejection fraction and pulmonary vascular disease. (A) Increase in venous return during exercise was associated with more dramatic increase in right atrial pressure in CpcPH-HFpEF compared to the other HFpEF groups. (B) While patients with non-PH-HFpEF and IpcPH-HFpEF displayed an increase in left ventricular transmural pressure, CpcPH-HFpEF patients developed a paradoxical decrease in left ventricular transmural pressure as venous return to the right heart increased during exercise. (C–D) The reduction in left ventricular transmural pressure was increased as exercise pulmonary vascular resistance and transpulmonary gradient increased, indicating that left heart underfilling was directly related to the severity of pulmonary vascular disease. Error bars reflect standard error of the mean. aP < 0.05 vs. Non-PH-HFpEF; and bP < 0.05 vs. IpcPH-HFpEF. CpcPH, combined post- and pre-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; LVTMP, left ventricular transmural pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; TPG, transpulmonary gradient.
Increases in right heart congestion may compromise left heart filling in the setting of ventricular interdependence. Patients with non-PH-HFpEF and IpcPH-HFpEF displayed an increase in LV transmural filling pressures, with stable RAP/PCWP ratio during exercise, indicating that left heart congestion was the major pathophysiological driver (Figures 3B and 4B). In striking contrast, patients with CpcPH-HFpEF developed a paradoxical decrease in LVTMP as venous return to the right heart increased during exercise (Figure 3B), with an increase in RAP/PCWP ratio (Figure 4B).
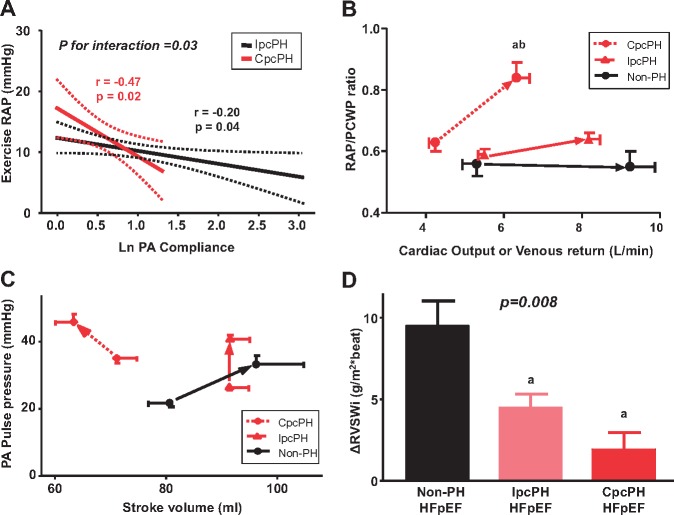
Figure 4.
Stroke volume reserve and right ventricular stroke work in heart failure with preserved ejection fraction. (A) Compared with IpcPH-HFpEF, right atrial pressure was increased to greater extent as PA compliance decreased in CpcPH-HFpEF. (B) Patients with combined post- and pre-capillary pulmonary hypertension developed a significant increase in right atrial pressure/pulmonary capillary wedge pressure ratio. (C) In CpcPH-HFpEF patients, stroke volume was decreased during exercise, coupled with an increase in PA pulse pressure. (D) Right ventricular systolic reserve was impaired in both of the PH-HFpEF groups, manifest by a blunted ability to augment right ventricular stroke work index during exercise. Error bars reflect standard error of the mean. aP < 0.05 vs. non-PH-HFpEF; and bP < 0.05 vs. IpcPH-HFpEF. CpcPH, combined post- and pre-capillary pulmonary hypertension; HFpEF, heart failure with preserved ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RAP, right atrial pressure.
The reduction in LVTMP was increased as exercise PVR and transpulmonary gradient (TPG) increased, indicating that left heart underfilling was directly related to the severity of PVD present (Figure 3C, D). This was likely related to greater increase in RAP, which was amplified to greater extent as PA compliance decreased in CpcPH-HFpEF (Figure 4A).
Thus, even as hydrostatic pressures in the pulmonary capillaries increased with exercise in CpcPH-HFpEF patients, there was effective under-distention of the LV. This reduction in LVTMP was coupled with the impairment in cardiac output in CpcPH-HFpEF (Figure 2A), explained by a reduction in stroke volume, which actually decreased with exercise in CpcPH-HFpEF, even as PA pulse pressure increased, emphasizing the marked limitation in PA compliance (Figure 4C). Right ventricular systolic reserve was impaired in both of the PH-HFpEF groups, manifest by a blunted ability to augment RV stroke work index during exercise (Figure 4D).
Discussion
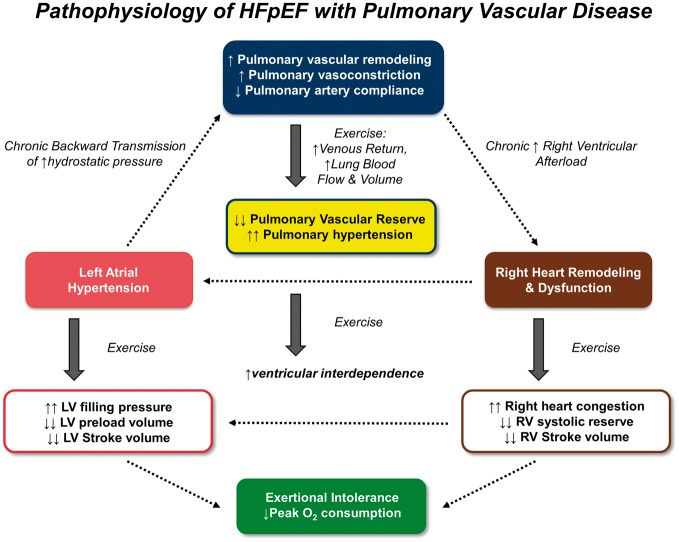
This is the first comprehensive evaluation of exercise haemodynamics in a well-defined cohort of patients with invasively verified HFpEF with and without PVD. We demonstrate that HFpEF patients with CpcPH displayed multiple features consistent with more advanced HF, including greater RV dysfunction, higher natriuretic peptide levels, and greater burden of atrial fibrillation. CpcPH-HFpEF patients displayed more abnormal RV–PA arterial interaction at rest, with greater chamber dilation and dysfunction as PVR increased. Despite similar biventricular filling pressures at rest, patients with CpcPH-HFpEF developed more dramatic increases in right heart filling pressures as venous return increased during exercise, resulting in enhanced ventricular interdependence, which compromised the transmural distending pressures that drive LV chamber filling. Together with reduced RV contractile reserve, this led to decreases in stroke volume and blunted ability to augment cardiac output with exercise in patients with CpcPH-HFpEF, which was associated with profound impairment in aerobic capacity. These data show that HFpEF patients with PVD demonstrate unique pathophysiologic features brought about by the stress of exercise that distinguish them from HFpEF patients without PVD, including impaired ability to enhance blood flow through the lungs, greater right heart congestion, failure to optimally utilize Frank–Starling reserve in the LV due to ventricular interaction, and limited capacity to augment RV systolic performance (Take home Figure). These pathophysiologic insights have important implications for clinical care and for the design of novel therapies targeted to HFpEF patients with and without PVD.
Take home figure.
Mechanistic summary of the pathophysiology contributing to exercise intolerance in patients with heart failure with preserved ejection fraction (HFpEF), see text for details. LV, left ventricle; RV, right ventricle.
Pulmonary vascular disease in heart failure with preserved ejection fraction
Accumulating evidence supports the idea that there may be pathophysiologically unique phenotypes within the broader population of patients with HFpEF.2 The presence of PH and PVD appears to identify one such phenotype of importance.5–11 Prior studies have begun to characterize PVD in HFpEF clinically and haemodynamically based upon resting data.10,12,14 Similar to the current data, these studies demonstrated that the presence of PVD in patients with HFpEF is associated with reduced exercise capacity, more severe RV dysfunction, and worse outcomes, but the mechanisms have remained unclear.
We observed that PVD in HFpEF is associated with more severe systemic arterial disease, reflected by higher mean vascular resistance and arterial elastance, and lower TAC in patients with CpcPH. This might be related in part to interdependence between the great vessels.33 Alternatively, combined systemic and PA stiffening may be related to widespread loss of NO bioavailability in both the lungs and systemic vasculature.34 Systemic vascular stiffening in HFpEF is correlated with more severe exercise-induced PH, and this is partially reversible with acute administration of NO providing therapies.31 These data support the hypothesis that endothelial dysfunction, and NO deficiency plays an important role in both the pulmonary and systemic vasculature in patients with HFpEF,35 and that therapies targeting NO metabolism may hold great promise for patients with HFpEF and PVD. Recent data also indicate that there may be substantial pulmonary vascular remodelling in patients with HFpEF, which may require additional antiproliferative therapies to restore pulmonary vascular reserve.36
Exercise unmasks a unique pathophysiology in heart failure with preserved ejection fraction with pulmonary vascular disease
We observed distinct haemodynamic responses to exercise in HFpEF patients that varied according to the presence or absence of PVD, many of which were related to the phenomenon of ventricular interdependence. We speculate that this was related to two key factors: an inability of the lung vasculature to accommodate increased blood volume and flow due to vasoconstriction and vascular remodelling, and impairments in RV function that limited the ability to eject blood through the higher impedance pulmonary circulation as metabolic demand for systemic perfusion increases.
The RV and LV are connected in series, so RV output affects LV filling in this direct way. However, the two ventricles also occupy the same space in the cardiac fossa and may also interact in parallel.27–29 Ventricular interdependence refers to the phenomenon whereby changes in pressure, filling, and volume in one chamber influences these characteristics in the other chamber. Diastolic ventricular interaction may be observed in patients with right heart failure due to acute pulmonary embolism, or severe isolated TR, where the dilated right ventricle out-competes the left ventricle for space, and the interventricular septum bows from right to left, leading to ‘underloading’ of the LV.28,30 A similar relationship is also observed in patients with the obese phenotype of HFpEF, where abnormal RV–PA interaction synergizes with volume overload and increased epicardial fat to amplify ventricular interaction.22
Exercise poses a profound stress on the heart and lungs: blood is rapidly redistributed from the abdomen and extremities to the thorax, leading in a 50% increase in lung blood volume and 300% increase in pulmonary blood flow in the healthy adult.15 Because patients with CpcPH-HFpEF display PVD that may limit this reserve, we hypothesized that the increase in systemic venous return accompanying exercise might overwhelm the right heart and lungs, leading to more severe PH, greater RV–PA uncoupling, and heightened right-sided congestion, setting the stage for conditions that promote enhanced interdependence.
Consistent with this hypothesis, we found that lower PA compliance was associated with more exuberant increases in RA pressures in CpcPH-HFpEF patients during exercise (Figure 4), while greater elevations in PVR and TPG were correlated with greater reduction in LVTMP (Figure 3), which more accurately reflects the true LV distending pressure or preload.28,29 The combination of a reduction in LV transmural distending pressure and blunted RV contractile reserve observed in the CpcPH-HFpEF group led to a striking reduction in stroke volume during exercise and impairment in cardiac output heightened venous return (Figure 4).
Clinical implications
The treatment of HFpEF is an enormous unmet public health need, and there have been valid concerns that many of the previous neutral trials might have been positive if the right patients had been enrolled. The common existence of PVD in HFpEF and its association with adverse prognosis has stimulated new interest in novel therapies targeting the pulmonary vasculature in this disorder.6,7 The present data identifying unique features to the pathophysiology of PVD provide further support for conducting trials targeting pulmonary vascular structure and function in HFpEF. For the design of such therapies, it may be best to first conduct smaller mechanistic, Phases 1 and 2 trials to specifically investigate safety and signals of efficacy for specific drugs, using invasive haemodynamic endpoints. Multiple such trials targeting pulmonary vasoconstriction and remodelling are currently underway (NCT 03153111, 02742129, 03043651, 02885636, 03015402, and 02744339).
If candidate drugs demonstrate safety and signal of efficacy in smaller invasive trials, larger clinical trials may then be conducted without the need for invasive haemodynamic phenotyping, using non-invasive surrogate criteria, for example relying upon imaging and biomarkers, and using more easily measurable endpoints such as 6 minute walk distance and quality of life assessment. This sort of staged approach may hold the greatest potential to deliver the right therapy to the patient most likely to derive benefit from this therapy, rather than the ‘one size fits all’ approach that has been used unsuccessfully thus far in HFpEF.
The current data suggest that there may be other therapeutic targets in HFpEF-PVD that merit study. The enhanced ventricular interdependence that occurs during exercise in HFpEF-PVD provides a theoretical basis for reducing pericardial restraint in order to preserve stroke volume reserve and improve cardiac output, similar to what is observed with pulmonary embolism.28,37 In this regard, we have recently shown in animals without PVD that limited anterior pericardial resection abrogates the increase in cardiac filling pressures with volume loading, improving Frank–Starling reserve.38 However, because pericardial resection can promote eccentric remodelling,39 and because we observed greater RV dilation with increasing PVR, it might be important to treat PVD in tandem with interventions targeted to the pericardial restraint in patients with HFpEF and PVD. Right ventricular contractile reserve was also impaired with exercise in this study, in agreement with previous studies performed in HFpEF patients without substantial PVD,25,40 and this also supports testing new therapies that can improve RV function and functional reserve to improve clinical status in CpcPH-HFpEF.
There is controversy on the best method to define the entity of CpcPH. Current guidelines recommend the use of either PVR or DPG criteria.18 We observed that all of the CpcPH patients displayed elevated PVR, yet only a minority demonstrated an elevated DPG. Prior studies have shown that DPG does not predict survival in HF,6 and the current data show that DPG is not superior to PVR to identify patients with this characteristic pathophysiology on exercise. Further research is needed to investigate whether other haemodynamic parameters such as PAC may provide added value in this regard.
Limitations
This study was single centre and all patients were referred for right heart catheterization, introducing selection bias. However, the baseline characteristics are similar to what is seen in general HFpEF populations enrolled in recent clinical trials (Supplementary material online, Table S1). The inclusion period for the study was extensive, but sensitivity analysis restricted to older and more recently evaluated patients revealed similar results (Supplementary material online, Table S2). Although the majority of patients had a quantitative assessment of LV ejection fraction, in a minority of patients LV ejection fraction was assessed qualitatively, and this could compromise the accuracy of LVEF assessment. Echocardiography was not performed during exercise. A relatively small number of patients with significant PVD were included in the analysis, yet multiple significant differences were identified. Adjustment of multiple hypothesis testing was not performed because the haemodynamic indices examined are highly interrelated and not independent of one another. We did not include patients with early stage HFpEF (elevated PCWP during exercise but not at rest),41 because the PH subtypes are currently only classified based on resting haemodynamics.18 Further research is needed to characterize pulmonary vascular responses to exercise in patients with early stage HFpEF.7
Conclusions
Pulmonary vascular disease in HFpEF leads to unique pathophysiologic consequences during the stress of exercise, including inadequate PA vasodilation, greater right heart congestion, left heart underfilling, heightened ventricular interdependence, and impaired RV reserve. These limitations markedly sabotage the ability of the heart to increase stroke volume and cardiac output during exercise, leading to profound limitations in aerobic capacity. Interventions targeted to this distinct pathophysiology require testing in patients with HFpEF with PVD.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
National Institutes of Health (R01 HL128526, R01 HL 126638, U01 HL125205, and U10 HL110262 to B.A.B.); research fellowship from the Uehara Memorial Foundation, Japan and the overseas research fellowship from the Japanese Society of Echocardiography, Japan to M.O.
Conflict of interest: none declared.
Supplementary Material
Footnotes
See page 2836 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy368)
References
- 1. Reddy YN, Borlaug BA.. Heart failure with preserved ejection fraction. Curr Probl Cardiol 2016;41:145–188. [DOI] [PubMed] [Google Scholar]
- 2. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ.. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM.. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guazzi M, Borlaug BA.. Pulmonary hypertension due to left heart disease. Circulation 2012;126:975–990. [DOI] [PubMed] [Google Scholar]
- 5. Opitz CF, Hoeper MM, Gibbs JSR, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grünig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S.. Pre-Capillary, Combined, and Post-Capillary Pulmonary Hypertension: a Pathophysiological Continuum. J Am Coll Cardiol 2016;68:368–378. [DOI] [PubMed] [Google Scholar]
- 6. Hoeper MM, Lam CSP, Vachiery JL, Bauersachs J, Gerges C, Lang IM, Bonderman D, Olsson KM, Gibbs JSR, Dorfmuller P, Guazzi M, Galie N, Manes A, Handoko ML, Vonk Noordegraaf A, Lankeit M, Konstantinides S, Wachter R, Opitz C, Rosenkranz S.. Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further research. Eur Heart J 2017;38:2869–2873. [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA, Obokata M.. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J 2017;38:2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA.. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM.. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, Farber-Eger EH, Sheng Q, Shyr Y, Harrell FE, Newman JH, Brittain EL.. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP.. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 2016;18:1472–1487. [DOI] [PubMed] [Google Scholar]
- 12. Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE.. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015;3:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malhotra R, Dhakal BP, Eisman AS, Pappagianopoulos PP, Dress A, Weiner RB, Baggish AL, Semigran MJ, Lewis GD.. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail 2016;9:e003011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES.. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018;19:425–432. [DOI] [PubMed] [Google Scholar]
- 15. Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S, Alpert N.. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 1990;81:1550–1559. [DOI] [PubMed] [Google Scholar]
- 16. Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M.. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470–1479. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 18. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119.26320113 [Google Scholar]
- 19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 20. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD.. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 21. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB.. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 22. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA.. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA.. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borlaug BA, Melenovsky V, Koepp KE.. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res 2016;119:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borlaug BA, Kane GC, Melenovsky V, Olson TP.. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borlaug BA, Koepp KE, Melenovsky V.. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 27. Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ.. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–432. [DOI] [PubMed] [Google Scholar]
- 28. Belenkie I, Dani R, Smith ER, Tyberg JV.. Effects of volume loading during experimental acute pulmonary embolism. Circulation 1989;80:178–188. [DOI] [PubMed] [Google Scholar]
- 29. Moore TD, Frenneaux MP, Sas R, Atherton JJ, Morris-Thurgood JA, Smith ER, Tyberg JV, Belenkie I.. Ventricular interaction and external constraint account for decreased stroke work during volume loading in CHF. Am J Physiol Heart Circ Physiol 2001;281:H2385–H2391. [DOI] [PubMed] [Google Scholar]
- 30. Andersen MJ, Nishimura RA, Borlaug BA.. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail 2014;7:911–917. [DOI] [PubMed] [Google Scholar]
- 31. Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA.. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herve P, Lau EM, Sitbon O, Savale L, Montani D, Godinas L, Lador F, Jais X, Parent F, Gunther S, Humbert M, Simonneau G, Chemla D.. Criteria for diagnosis of exercise pulmonary hypertension. Eur Resp J 2015;46:728–737. [DOI] [PubMed] [Google Scholar]
- 33. Schafer M, Ivy DD, Abman SH, Barker AJ, Browne LP, Fonseca B, Kheyfets V, Hunter KS, Truong U.. Apparent aortic stiffness in children with pulmonary arterial hypertension: existence of vascular interdependency? Circ Cardiovasc Imaging 2017;10:e005817.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farrero M, Blanco I, Batlle M, Santiago E, Cardona M, Vidal B, Castel MA, Sitges M, Barbera JA, Perez-Villa F.. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:791–798. [DOI] [PubMed] [Google Scholar]
- 35. Paulus WJ, Tschope C.. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 36. Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM.. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belenkie I, Sas R, Mitchell J, Smith ER, Tyberg JV.. Opening the pericardium during pulmonary artery constriction improves cardiac function. J Appl Physiol 2004;96:917–922. [DOI] [PubMed] [Google Scholar]
- 38. Borlaug BA, Carter RE, Melenovsky V, De Simone CV, Gaba P, Killu A, Naksuk N, Lerman L, Asirvatham SJ.. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e003612.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tischler MD, Rowan M, LeWinter MM.. Increased left ventricular mass after thoracotomy and pericardiotomy. A role for relief of pericardial constraint? Circulation 1993;87:1921–1927. [DOI] [PubMed] [Google Scholar]
- 40. Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA.. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:542–550. [DOI] [PubMed] [Google Scholar]
- 41. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM.. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.