Abstract
Background
Insomnia is two to three times more prevalent in cancer survivors than in the general population, where it is estimated to be 10% to 20%. Cognitive-behavioral therapy for insomnia (CBT-I) is the recommended treatment for chronic insomnia, but meeting survivor needs remains a challenge. Internet-delivered CBT-I (iCBT-I) has been shown efficacious in otherwise healthy adults. We tested the efficacy of iCBT-I in breast cancer survivors with clinically significant sleep disturbance.
Methods
Women from a national sample of Danish breast cancer survivors who experienced clinically significant sleep disturbance were randomly allocated to iCBT-I or waitlist control (55:45). The fully automated iCBT-I program consisted of six cores. Online measures of insomnia severity, sleep quality, and fatigue were collected at baseline, postintervention (nine weeks), and follow-up (15 weeks). Online sleep diaries were completed over two-week periods pre- and postintervention. Intention-to-treat analyses (time × group interactions) were conducted with mixed linear models and corrected for multiple outcomes. All statistical tests were two-sided.
Results
A total of 255 women were randomly allocated to iCBT-I (n = 133) or waitlist control (n = 122). Statistically significant (P ≤ .02) time × group interactions were found for all sleep-related outcomes from pre- to postintervention. Effect sizes (Cohen’s d) ranged from 0.33 (95% confidence interval [CI] = 0.06 to 0.61) for wake after sleep onset to 1.17 (95% CI = 0.87 to 1.47) for insomnia severity. Improvements were maintained for outcomes measured at follow-up (d = 0.66–1.10).
Conclusions
iCBT-I appears to be effective in breast cancer survivors, with additional benefit in terms of reduced fatigue. This low-cost treatment could be incorporated in cancer rehabilitation programs.
Insomnia is prevalent in the general population, with an annual prevalence of 10% to 20% (1), and the rates in cancer survivors have been found to be two to three times higher (2). In breast cancer, treatments such as chemotherapy and radiotherapy—and possibly surgery and hormonal therapy—may be partly responsible (3). Although the precise causal pathways are unclear, poor sleep is associated with higher levels of cancer-related fatigue, reduced quality of life (4,5), and perhaps even increased risk of all-cause mortality (6) and cancer recurrence (7).
While pharmacotherapy remains the most commonly used option for insomnia (8,9), hypnotics such as benzodiazepine receptor agonists are associated with side effects, dependence, and tolerance over time and are usually not curative, requiring maintenance treatment over many years (10). Cognitive-behavioral therapy for insomnia (CBT-I) (11) is an efficacious nonpharmacological alternative (12,13) and is the recommended first-choice treatment for chronic insomnia (14). Short-term effects are comparable or superior to those found for pharmacotherapy, and long-term effects are maintained for up to three years (15), both in individuals with insomnia as their primary problem and in patients with co-occurring diagnoses such as cancer (16).
Due to limited availability of trained therapists and the relatively high costs of face-to-face-delivered CBT-I (17), a considerable challenge remains to make it available and accessible to meet population needs. Delivering CBT-I over the internet (iCBT-I) is a possible solution. A meta-analysis (18) of 11 RCTs of iCBT-I found statistically significant effects on key sleep outcomes such as insomnia severity, sleep efficiency (SE), wake after sleep onset (WASO), total sleep time (TST), sleep onset latency (SOL), number of nocturnal awakenings (NA), and subjective sleep quality at post-treatment, with effect sizes (Hedges's g) ranging from small (0.2) to large (1.1). Furthermore, the effects were generally maintained up to several months postintervention and comparable to those found for face-to-face-delivered individual and group-based CBT-Is (18).
So far, only two feasibility trials have examined the efficacy of iCBT-I in cancer survivors: a small controlled pilot study with 28 survivors with mixed cancer diagnoses (19) and an uncontrolled feasibility trial with 171 breast cancer patients (20). While both studies found statistically significant effects for several sleep outcomes, there is a need to evaluate the efficacy of iCBT-I in cancer survivors in large and well-designed RCTs. We therefore tested the efficacy of Sleep Healthy Using The Internet (SHUTi), previously shown efficacious in otherwise healthy adults with insomnia (21) and with promising pilot results in cancer survivors (19), in a national sample of Danish breast cancer survivors experiencing clinically significant sleep disturbances.
Methods
Study Design and Participants
A national sample of 255 Danish breast cancer survivors experiencing clinically significant sleep disturbances (scores >5 on the Pittsburgh Sleep Quality Index [PSQI]) (22) were randomly assigned to iCBT-I (SHUTi) (21) or a waitlist control condition and assessed at baseline, postintervention (nine weeks), and follow-up (15 weeks). Eligible women were age 18 to 75 years and surgically treated according to the Danish Breast Cancer Group (DBCG) guidelines for locoregional breast cancer between June 1, 2011, and December 31, 2013. Exclusion criteria included recurrence of breast cancer, second cancer, severe psychological or physical comorbidity, other sleep disorders (sleep apnea, parasomnia, narcolepsy), and inability to read Danish. The study was approved by the Regional Science Ethical Committees (Registration No. 1-10-72-553-12) and the Danish Data Protection Agency and preregistered at ClinicalTrials.gov (NCT02444026).
Random Assignment and Masking
Recruitment, screening, and randomization were done in two waves. Based on names and addresses in the DBCG registry, research assistants mailed out invitations, screened the women who responded, sent out links to the online baseline questionnaire, and allocated women who completed baseline questionnaires using computer-generated lists (PASS v.12). All outcomes were assessed by online questionnaires (Qualtrics, Provo, UT) and automated online collection of sleep diaries integrated in the SHUTi platform. Researchers had no contact with study participants and no knowledge about allocation until after study completion.
Procedure
Sample size and allocation ratio were based on the average effect (d = 0.38) across sleep outcomes reported in six trials of iCBT-I available at the time of planning the study (23). To detect a statistically significant (P < .05) effect of a d value of 0.38 with a statistical power of 80% required a sample of 2 × 109 participants. Based on the available trials (23), we anticipated an uneven dropout of 30% and 15% in the intervention and control groups, respectively, and planned to randomly assign 268 women in a ratio of 55:45 to intervention and control. Expecting a response rate of 50% and a prevalence of sleep disturbance of 50% (24), we anticipated a need to contact approximately 1600 women. In March 2015, letters including study information and a link to an online version of the Pittsburg Sleep Quality Index (PSQI) (22) were mailed out to a random sample of 1607 women in the DBCG registry fulfilling the age, surgery date, and clinical inclusion criteria. Women with PSQI scores greater than 5 who had provided their telephone number were contacted by research assistants, who conducted a structured screening interview with the aim of excluding women with comorbidities and other sleep disorders. Eligible women were mailed an informed consent form, and those who returned the signed form were e-mailed a link to the online baseline questionnaire. Those who completed the baseline questionnaire were then randomly assigned to intervention (SHUTi) or waitlist control, as described above. Because of the lower response rate and prevalence of sleep disturbance than expected, only 114 women had been included after the first recruitment wave. In September 2015, a second recruitment wave was initiated, in which 1599 letters were mailed out to another random sample from the same cohort. Following the same procedure as described above, this resulted in a final sample of 133 and 122 participants in the intervention and control groups, respectively.
SHUTi (25), a fully automated interactive iCBT-I based on well-established face-to-face CBT-I (26), was adapted into Danish. The program, which has been described in detail elsewhere (25), consists of six successively delivered cores: introduction and treatment rationale (Core 1), sleep restriction and stimulus control (Core 2 and 3), cognitive restructuring (Core 4), sleep hygiene (Core 5), and relapse prevention (Core 6). Each core takes 45 to 60 minutes to complete, with new cores becoming available one week after the completion of the previous core. Participants receive automated e-mail prompts when it is time to complete a new core and reminders to complete sleep diaries. To receive updated sleep restriction recommendations, participants must complete at least 5 days of sleep diaries in a seven-day period. Based on this information, participants receive automatically computed tailored recommendations for sleep restriction. The fully automated program presents information through text, graphics, interactive activities, vignettes, and video clips. Participants were not in contact with researchers or research assistants unless they requested technical help. The program can be completed in as little as six weeks; however, participants were given nine weeks before postassessment was administered. At pre- and postintervention, all participants were instructed to access the online program and complete 10 sleep diaries over a two-week period, but access to the SHUTi intervention was not made available to waitlist controls until week 15, that is, six weeks after the postintervention assessment.
Measures
Participant Characteristics
Data obtained from the DBCG registry included age at surgery and cancer and treatment characteristics. Additional information obtained through the baseline questionnaire included sociodemographic data, health behaviors, work status and type, insomnia duration, sleep medication use, and chronotype (the reduced Morningness-Eveningness Questionnaire [rMEQ]; score range = 5–25) (27). Motivation and expectancy was measured with five ad hoc items (see Table 1 for further details on patient characteristics).
Table 1.
Participant and nonparticipant characteristics
| Nonparticipants* | Participants† | P‡ | Intervention | Control | P‡ | |
|---|---|---|---|---|---|---|
| No. | 2952 | 255 | 133 | 122 | ||
| Stage (0–III), % | .86 | .23 | ||||
| 0 | 0.3 | 0.0 | – | 0.0 | 0.0 | – |
| I | 25.3 | 25.9 | – | 26.4 | 25.2 | – |
| II | 44.3 | 46.1 | – | 41.6 | 51.4 | – |
| III | 29.4 | 27.6 | – | 32.0 | 22.4 | – |
| Tumor size, mean (SD), mm | 19.4 (29.1) | 19.6 (13.5) | .93 | 20.5 (13.1) | 18.6 (13.8) | .29 |
| ER status, mean (SD), % pos. cells | 77.1 (38.0) | 80.7 (35.1) | .12 | 81.6 (34.7) | 79.6 (35.7) | .66 |
| Lymph node positive, % | 39.5 | 45.8 | .06 | 47.6 | 43.6 | .54 |
| Premenopausal, % | 51.5 | 62.0 | .002‖ | 59.2 | 64.7 | .37 |
| Mastectomy, % | 29.2 | 32.8 | .23 | 31.6 | 33.9 | .70 |
| Chemotherapy, % | 47.9 | 56.7 | .03¶ | 50.4 | 63.6 | .07 |
| Radiotherapy, % | 74.5 | 74.0 | .92 | 74.5 | 85.9 | .13 |
| Endocrine therapy, % | 76.0 | 77.6 | .21 | 77.4 | 81.0 | .45 |
| Age at surgery, mean (SD)§ | 52.3 (11.2) | 50.2 (8.8) | <.001‖ | 50.3 (8.8) | 50.1 (8.9) | .89 |
| Age group, y | <.001 | .95 | ||||
| 18–40 | 16.3 | 14.6 | - | 12.8 | 14.9 | – |
| 41–50 | 32.9 | 45.3 | - | 46.6 | 46.3 | – |
| 51–60 | 25.0 | 24.7 | - | 25.6 | 23.1 | – |
| 61–75 | 25.8 | 15.4 | - | 15.0 | 15.7 | – |
| Age at inclusion, mean (SD)§ | – | 53.1 (8.8) | – | 53.2 (8.8) | 52.9 (8.9) | .81 |
| Time from surgery, mean (SD), y | – | 2.9 (2.9) | – | 2.9 (1.8) | 2.9 (3.8) | .98 |
| Married/partnered, % | – | 79.6 | – | 78.2 | 81.1 | .56 |
| Education | .24 | |||||
| Lower (<2 y of higher ed.) | – | 23.9 | – | 22.3 | 25.7 | – |
| Medium (2–4 y of higher ed.) | – | 57.8 | – | 55.4 | 60.6 | – |
| Higher (≥5 y of higher ed.) | – | 18.3 | – | 22.3 | 13.8 | – |
| Body mass index, mean (SD), kg/m2 | – | 25.7 (4.8) | – | 25.3 (4.2) | 26.1 (5.3) | .17 |
| Alcohol, mean (SD), drinks/wk | – | 7.2 (5.3) | – | 6.9 (5.2) | 7.7 (5.3) | .32 |
| Smoker, % | – | 8.2 | – | 8.3 | 8.2 | .98 |
| Working, % | – | 76.1 | – | 77.4 | 74.6 | .59 |
| Work type: day, evening, shift, % | – | 91.4, 2.3, 6.3 | – | 90.0, 2.2, 7.8 | 92.9, 2.4, 4.8 | .72 |
| Insomnia duration, mean (SD), y | – | 3.4 (2.7) | – | 3.3 (2.8) | 3.4 (2.7) | .77 |
| Sleep medication, last 30 d, % | – | 15.3 | – | 15.8 | 14.8 | .82 |
| Sleep aids: herbal supplements, % | – | 10.6 | – | 13.5 | 7.4 | .11 |
| Sleep aids: relaxation, % | – | 46.3 | – | 46.6 | 45.9 | .91 |
| Sleep aids: other, % | – | 50.2 | – | 51.1 | 49.2 | .76 |
| Morningness (rMEQ), mean (SD)# | – | 16.7 (3.5) | – | 16.7 (3.6) | 16.8 (3.5) | .86 |
| Motivation and expectancy, mean (SD)** | – | 10.6 (2.5) | – | 10.6 (2.6) | 10.5 (2.5) | .85 |
Includes nonresponders, decliners, and excluded. ER = estrogen receptor; rMEQ = reduced Morningness-Eveningness Questionnaire.
Participants who consented and completed baseline measures.
t test for independent samples or chi-square tests (two-sided), as appropriate, after adjusting for multiple comparisons with the Benjamini-Hochberg procedure (35).
Age at surgery differs from age at inclusion (mean = 2.9 years later).
When entering participants vs nonparticipants as the dependent variable and age and menopausal status (pre/post) as independent variables in a logistic regression, neither remained statistically significant (P = .37 and .22).
When adjusting for multiple comparisons (35), chemotherapy did not reach the adjusted statistical significance level (P < .02).
reduced Morningness-Eveningness Questionnaire (27).
Five-item ad hoc scale.
Outcomes
Primary sleep-related outcomes included insomnia severity and sleep quality at baseline, postintervention (nine weeks), and follow-up (15 weeks), and sleep diaries completed at pre- and postintervention. The secondary outcome of fatigue was assessed at baseline, postintervention, and follow-up. All instruments had either previously been translated or were translated into Danish for the present study using recognized approaches (28).
Insomnia severity was assessed with the Insomnia Severity Index (ISI) (29). Total scores range from 0 to 28, with higher scores indicating more severe insomnia. The ISI is a valid and reliable measure shown to be sensitive to changes in treatment studies (30). A cutoff of 10 has been found optimal for detecting insomnia (30). Internal consistency was 0.80 in the present sample.
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) (22), which measures a broader concept than the ISI. The PSQI yields seven component scores summed to produce a global measure of sleep disturbance, with higher scores denoting poorer sleep quality (range = 0–21). A cutoff of 5 has been suggested for the presence of sleep disturbance (22). Internal consistency was 0.77.
An online version of the consensus sleep diary (31) was completed daily over a two-week period by both groups at pre- and postintervention (but not at follow-up). The diary measures time to fall asleep; number and length of any awakenings at night; time of awakening and arising from bed in the morning; length of any naps; subjective sense of how refreshed they felt on awakening as well as soundness of sleep during the night; and amount of medication and alcohol used as a sleep aid. Each sleep diary period yields the following outcomes: 1) SOL (minutes), 2) NA, 3) WASO (minutes), 4) early morning awakening (EMA; minutes), 5) time in bed (TIB; hours), 6) TST (hours), 7) SE (calculated as TST/TIB)×100, and 8) the proportion of nights on which participants took sleep medication.
Fatigue was assessed with the 13-item Functional Assessment of Chronic Illness Therapy for Fatigue (FACIT-F) (32). Raw scores range from 0 to 52, with higher scores indicating less fatigue. A cutoff score of 34 was used to determine the level of clinically significant fatigue (33). Internal consistency was 0.91.
Statistical Analysis
Data were analyzed with IBM SPSS statistics, v.24 (IBM, Chicago, IL) when both waves had completed follow-up. Baseline differences between nonparticipants and participants and between the intervention and control groups were explored with t tests or χ2 tests. Intervention adherence was quantified as the number of SHUTi cores (1 to 6) completed. Study dropouts were defined as participants failing to complete questionnaires at postintervention and follow-up. For scales with internal consistencies of at least 0.70, mean substitution of missing values with the respondent’s average response on the remaining scale items was used if respondents had completed at least 50% of the items (34). Mixed linear models (MLMs) based on the intent-to-treat sample were used to compare groups over time on all outcome variables, all treated as continuous variables. MLMs can be fit to all available data, including data from participants with missing values. An intervention effect was indicated by a statistically significant group × time interaction. The false discovery rate (FDR) for baseline comparisons was controlled for multiple comparisons at baseline with the Benjamini-Hochberg procedure (35), and the familywise error rate (FWER) for multiple outcomes was controlled with Holm’s procedure (36). Generalized estimating equation (GEE) models were used to examine between-group differences in changes over time (time × group interaction) in dropout and proportions of participants above suggested clinical cutoffs (ISI, PSQI, SE, FACIT-F). Effect sizes are presented as Cohen’s d, based on absolute between-group differences at postintervention and follow-up, with 0.2, 0.5, and 0.8 considered a small, medium, and large effect size, respectively (37). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Participants
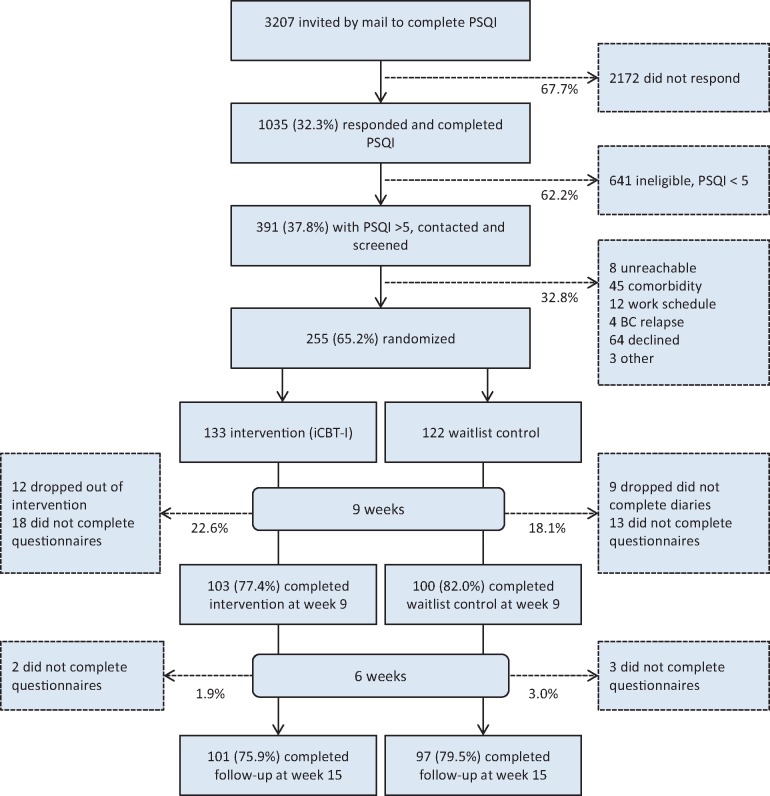
Between March 26 and Oct. 4, 2015, a total of 255 women were enrolled in the study. Clinical and demographic characteristics of participants and nonparticipants and the intervention and control groups are shown in Table 1. Participants were on average two years younger and more likely to be premenopausal than nonparticipants. The two recruitment waves did not differ in background variables or outcome measures at baseline (P ≥ .11 for all). Attrition in the intervention and control groups from baseline to postintervention (22.6% and 18.1%) and follow-up (1.9% and 3.0%) was evenly distributed (time × group interaction; Wald χ2 = 0.80, P = .67) (Figure 1). Dropouts and participants who completed postintervention assessments did not differ on any background or outcome variables at baseline (data not shown).
Figure 1.
Study flow diagram. iCBT-I = internet-delivered cognitive-behavioral therapy for insomnia; PSQI = Pittsburgh Sleep Quality Index.
Sleep Outcomes and Fatigue
Statistically significant group × time effects were found at postintervention for all but one outcome, with effect sizes (Cohen’s d) ranging from 0.33 (95% confidence interval [CI] = 0.06 to 0.61) for wake after sleep onset to 1.17 (95% CI = 0.87 to 1.47) for insomnia severity (Table 2). Changes in the proportion of nights on which participants took sleep medication did not reach statistical significance (P = .09). Large effect sizes were found for improvements in insomnia severity (ISI), sleep quality (PSQI), and sleep efficiency; medium effect sizes for increased total sleep time, less time in bed, and fewer EMAs; and small effect sizes for shorter SOL, fewer NAs, reductions in fatigue (FACIT-F), and less time spent awake after sleep onset (WASO). Improvements were maintained for outcomes measured at follow-up, with effect sizes from 0.66 (95% CI = 0.38 to 0.95) to 1.10 (95% CI = 0.80 to 1.40). Effects for ISI and PSQI were large, and the effect for fatigue (FACIT-F) was of medium magnitude (Table 2).
Table 2.
Insomnia severity, sleep quality, fatigue, and sleep variables at baseline, postintervention, and 6-month follow-up
| Baseline |
Postintervention (9 wk) |
Group × time interaction* | Follow-up (15 wk) |
Group × time interaction* | ||||
|---|---|---|---|---|---|---|---|---|
| Variable | Interv. | Control | Interv. | Control | P†; Cohen’s d (95% CI)‡ | Interv. | Control | P†; Cohen’s d (95% CI) |
| No. who completed questionnaires | 133 | 122 | 103 | 100 | 101 | 97 | ||
| Insomnia severity by ISI§ | 14.9 (4.8) | 14.7 (4.5) | 7.1 (4.4) | 12.8 (5.3) | <.001; 1.17 (0.87 to 1.47) | 6.1 (4.5) | 11.6 (5.5) | <.001; 1.10 (0.80 to 1.40) |
| Sleep quality by PSQI‖ | 10.2 (3.6) | 10.2 (3.0) | 6.5 (2.8) | 9.3 (3.4) | <.001; 0.90 (0.61 to 1.19) | 6.1 (3.2) | 8.9 (3.3) | <.001; 0.86 (0.57 to 1.15) |
| Fatigue by FACIT-F¶ | 35.8 (9.4) | 35.1 (9.6) | 40.8 (8.5) | 36.8 (10.6) | <.001; 0.42 (0.14 to 0.70) | 43.0 (7.9) | 37.6 (9.1) | <.001; 0.66 (0.38 to 0.95) |
| No. with ≥5 sleep diaries | 116 | 99 | 90 | 91 | ||||
| Sleep onset latency, min | 18.8 (21.3) | 19.0 (24.8) | 7.7 (10.5) | 14.9 (24.0) | .02; 0.39 (0.11 to 0.67) | – | – | – |
| No. of awakenings | 2.6 (1.2) | 2.4 (1.2) | 1.7 (1.0) | 2.2 (1.2) | <.001; 0.45 (0.18 to 0.73) | – | – | – |
| Wake after sleep onset, min | 37.7 (38.0) | 26.5 (27.5) | 11.9 (15.8) | 18.0 (20.5) | <.001; 0.33 (0.06 to 0.61) | – | – | – |
| Early morning awakenings, min | 83.3 (57.0) | 73.4 (51.5) | 31.9 (41.2) | 71.9 (62.9) | <.001; 0.76 (0.47 to 1.04) | – | – | – |
| Time in bed, h | 8.0 (0.8) | 7.9 (0.7) | 7.4 (0.8) | 7.9 (0.7) | <.001; 0.67 (0.38 to 0.95) | – | – | – |
| Total sleep time, h | 5.7 (1.2) | 5.9 (1.2) | 6.5 (1.1) | 6.1 (1.3) | <.001; 0.64 (0.34 to 0.94) | – | – | – |
| Sleep efficiency#, % | 71.2 (15.0) | 75.2 (14.2) | 88.3 (10.3) | 78.1 (15.0) | <.001; 0.80 (0.51 to 1.08) | – | – | – |
| Sleep medication** | 14.8 (31.3) | 8.9 (24.4) | 11.0 (28.8) | 9.7 (26.2) | .09; 0.05 (–0.23 to 0.32) | – | – | – |
Mixed linear models (two-sided significance testing).
All statistically significant effects remained significant after controlling the familywise error rate with Holm’s method (36).
Cohen’s d conventions: 0.2 (small), 0.5 (medium), 0.8 (large) (37).
Insomnia Severity Index (29), higher scores = more severe insomnia. CI = confidence interval; FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index; Interv. = intervention. Values are reported as means (with SD) unless noted otherwise.
Pittsburgh Sleep Quality Index (22), higher scores = higher levels of sleep disturbance.
Functional Assessment of Chronic Illness Therapy Fatigue Scale (32), higher scores = lower levels of fatigue.
Sleep efficiency (total sleep time/time in bed × 100).
Proportion (%) of nights on which participants took sleep medication.
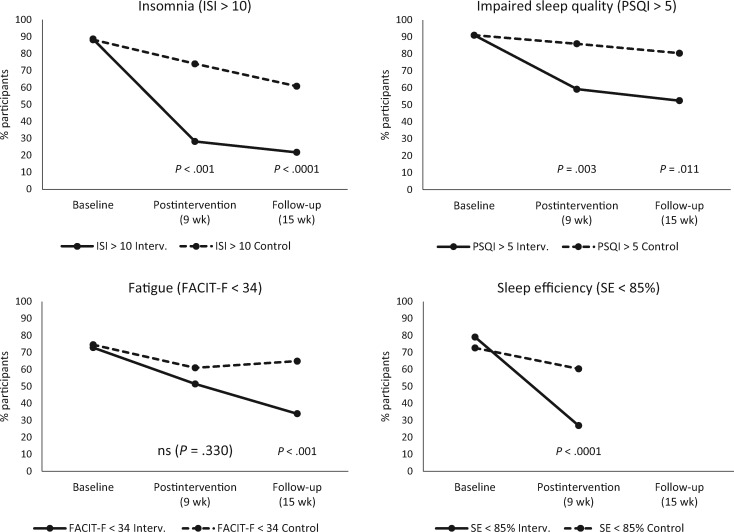
Statistically significantly fewer participants in the intervention group than in the control group showed signs of clinically impaired sleep (cutoffs: ISI > 10 and PSQI > 5) at both postintervention (P < .001 and P = .003) and follow-up (P < .001 and P = .011) (Figure 2; see Supplementary Table 1, available online, for further details). The reduction from baseline to postintervention in the proportion of participants with SE of less than 85% was statistically significantly larger in the intervention group than in controls (P < .001). Group differences in the change in the proportion of participants who were clinically fatigued (scores < 34) only reached statistical significance at follow-up (P < .001) (Figure 2).
Figure 2.
Proportion of participants reporting clinically significant insomnia severity (Insomnia Severity Index > 10), impaired sleep quality (Pittsburgh Sleep Quality Index > 5), clinically significant fatigue (FACIT Fatigue Scale < 34), and poor sleep efficiency (sleep efficiency < 85%) at baseline, post-treatment, and follow-up (generalized estimating equation; time × group interactions). All statistical tests were two-sided. FACIT-F = Functional Assessment of Chronic Illness Therapy Fatigue Scale; GEE = generalized estimating equation; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index; SE = sleep efficiency.
Adherence
On average, intervention group participants completed 4.1 (SD = 2.5) cores, with 82.1%, 75.1%, 67.2%, 64.2%, 62.7%, and 59.7% completing cores 1, 2, 3, 4, 5, and 6, respectively. Motivation and expectancy scores at baseline did not predict the number of completed cores (Beta = .08; P = .19). There were no between-group differences in the mean number of completed sleep diaries at baseline (mean [SD] = 8.7 [3.2] vs mean [SD] = 8.03 [3.9], P = .12) or postintervention (mean [SD] = 6.7 [4.7] vs mean [SD] = 7.3 [4.4], P = .35; data not shown).
Statistically significant group × time × adherence effects were found for ISI (F = 19.6, d.f. = 260, P < .001), PSQI (F = 10.1, d.f. = 262, P < .002), and SE (F = 8.1, d.f. = 119, P < .001). Cores completed were associated with larger improvements in ISI scores, PSQI scores, and SE from baseline to postintervention. No effects of adherence were seen for the remaining sleep outcomes or fatigue. No adverse effects were reported (data not shown).
Discussion
Our results extend previous findings (16,18) to cancer survivors by demonstrating that iCBT-I can reduce insomnia severity and improve overall sleep quality in breast cancer survivors. Not only were the effects highly statistically significant, but also clinically relevant when comparing changes in proportions of participants with clinically significant sleep problems as determined by the suggested cutoffs for ISI, PSQI, and SE.
The postintervention effect sizes found for insomnia severity and sleep diary outcomes, ranging from 0.33 (95% CI = 0.06 to 0.61) to 1.17 (95% CI = 0.87 to 1.47), were generally comparable to those found in a previous pilot study with cancer survivors (95% CI = 0.22 to 1.85) (19) and the aggregated effects found for face-to-face CBT-I in cancer survivors, ranging from 0.41 (95% CI = 0.24 to 0.59) to 0.77 (95% CI = 0.60 to 0.93) (16). Sleep medication use was the only outcome not reaching statistical significance. It should be noted that SHUTi does not focus on medication titration, and participants were instructed to contact their doctor if they wished to change their sleep medication use. Sleep effects were maintained over time. For insomnia severity and sleep quality, which were assessed at the follow-up, the effects remained statistically significant and tended to show continued improvement. The effect size at follow-up for insomnia severity (effect size = 1.10, 95% CI = 0.80 to 1.40) was larger than effect sizes found at follow-up for both iCBT-I in general population samples (effect size = 0.68, 95% CI = 0.42 to 1.30) (18) and face-to-face CBT-I in cancer survivors (effect size = 0.55, 95% CI = 0.37 to 0.73) (16). It should here be mentioned that the time to follow-up in our study was relatively short.
Cancer-related fatigue is a prevalent and distressing symptom among cancer survivors (5), and, at baseline, on average 2.9 years after surgery, a considerable proportion (73.7%) of the breast cancer survivors in our study reported clinically severe fatigue (33). While the relative decrease in the proportion experiencing severe fatigue (<34) did not reach statistical significance at post-treatment, fatigue continued to decrease, with only 34% reporting severe fatigue at follow-up, compared with 65% of controls, suggesting that the indirect effects of sleep improvement on fatigue may take more time to consolidate.
The study dropout rates of 22.6% and 18.1% in the intervention and control groups, respectively, are comparable to the average study dropout of 24.7% found in previous iCBT-Is with general population samples (18) and dropout rates of up to 33% found in face-to-face-delivered CBT-I (13). The average number of completed program cores was high (67%), and higher than the 52% reported in a meta-analysis of adherence to technology-mediated insomnia treatments (38). While the association found between adherence and improvement in insomnia severity, sleep quality, and SE at postintervention suggests potential benefits of attempts to further increase adherence, our results also indicate that adherence and effects can be substantial even with a fully automated program with no therapist contact.
Our trial has several strengths, including a national sample of breast cancer survivors who were generally comparable with nonparticipants, commonly used sleep measures enabling comparisons with existing studies of face-to-face and internet-delivered CBT-I, and study attrition and intervention adherence comparable to those found in similar studies with general population samples. Some limitations should be mentioned. First, participants were younger and more likely to be premenopausal at surgery than nonparticipants. However, the small mean age difference (two years) is unlikely to be a major threat to generalizability. Second, we used self-report sleep diaries rather than more objective assessments such as polysomnography. However, given the night-to-night variability seen in insomnia, sleep diaries over multiple nights may provide a more accurate picture of sleep disturbance (26), and standardized sleep diaries provide a valid and important perspective on the individual’s sleep. Third, the follow-up was relatively short (six weeks after postassessment) so as to not delay the treatment in the control group. Fourth, while the effects were similar to those reported for face-to-face CBT-I in cancer survivors (16), comparability is limited by the lack of direct comparison with a face-to-face CBT-I condition. Finally, it is unknown whether our findings for breast cancer survivors can be generalized to other types of cancer survivors.
In conclusion, iCBT-I appears to be an efficacious treatment option for breast cancer survivors, with robust and clinically relevant postintervention effects found for multiple sleep outcomes that were maintained six weeks after completion of the intervention. With increasing Internet penetration rates (eg, 96.6% in Denmark and 84.2% in the United States in 2017) (39), delivering CBT-I over the Internet appears to be a viable method to overcome the challenges of disseminating this efficacious treatment to the considerable number of cancer survivors with insomnia in need.
Funding
This work was funded by TrygFonden (grant No. 7-12.0736) and the Danish Cancer Society (grant No. R48-A2585-11-S3).
Notes
The funders had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Drs. Ritterband and Thorndike have equity ownership in BeHealth Solutions, LLC, which has licensed the SHUTi program and the software platform on which it was built from the University of Virginia. The company had no role in preparing this manuscript. The terms of this arrangement have been reviewed and approved by the University of Virginia in accordance with its conflict of interest policy. The remaining authors have no conflicts of interest to declare.
The authors wish to thank the Danish Breast Cancer Group (DBCG) for their help with identifying potential participants for the study; David Zachariae, Benjamin Zachariae, and Jonas Sørensen for their help with adapting SHUTi into Danish; BeHealth Solutions, LLC, and Carolyn Caruso for implementing the translated version of SHUTi; and Marlene Lyby for help with recruiting participants.
Supplementary Material
References
- 1. Buysse DJ. Insomnia. JAMA. 2013;3097:706–716. 10.1001/jama.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;254:791–800. 10.1093/annonc/mdt506 [DOI] [PubMed] [Google Scholar]
- 3. Costa AR, Fontes F, Pereira S, et al. Impact of breast cancer treatments on sleep disturbances - a systematic review. Breast. 2014;236:697–709. 10.1016/j.breast.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 4. Minton O, Alexander S, Stone PC.. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast Cancer Res Treat. 2012;1362:513–520. 10.1007/s10549-012-2284-1 [DOI] [PubMed] [Google Scholar]
- 5. Ancoli-Israel S, Moore PJ, Jones V.. The relationship between fatigue and sleep in cancer patients: A review. Eur J Cancer Care (Engl). 2001;104:245–255. 10.1046/j.1365-2354.2001.00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trudel-Fitzgerald C, Zhou ES, Poole EM, et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses' Health Study. Br J Cancer. 2017;1169:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marinac CR, Nelson SH, Flatt SW, et al. Sleep duration and breast cancer prognosis: Perspectives from the Women's Healthy Eating and Living Study. Breast Cancer Res Treat. 2017;1623:581–589. 10.1007/s10549-017-4140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore TA, Berger AM, Dizona P.. Sleep aid use during and following breast cancer adjuvant chemotherapy. Psychooncology. 2011;203:321–325. 10.1002/pon.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;252:91–100. 10.1097/YIC.0b013e328334e5e6 [DOI] [PubMed] [Google Scholar]
- 10. Riemann D, Perlis ML.. The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;133:205–214. 10.1016/j.smrv.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 11. Morin CM. Insomnia. Psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 12. Montgomery P, Dennis J.. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2009;1:CD003161. [DOI] [PubMed] [Google Scholar]
- 13. Okajima I, Komada Y, Inoue Y.. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;91:24–34. 10.1111/j.1479-8425.2010.00481.x [DOI] [Google Scholar]
- 14. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;1652:125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 15. Mitchell MD, Gehrman P, Perlis M, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam Pract. 2012;13:40. 10.1186/1471-2296-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. 10.1016/j.smrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 17. Thomas A, Grandner M, Nowakowski S, et al. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med. 2016;146:687–698. 10.1080/15402002.2016.1173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zachariae R, Lyby MS, Ritterband LM, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 19. Ritterband LM, Bailey ET, Thorndike FP, et al. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;217:695–705. 10.1002/pon.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dozeman E, Verdock-de-Leeuw IM, Savard J, et al. Guided web-based intervention for insomnia targeting breast cancer patients: Feasibility and effect. Internet Interventions. 2017;9:1–6. 10.1016/j.invent.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: A randomized clinical trial. JAMA Psychiatry. 2017;741:68–75. 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- 22. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psych Res. 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 23. Cheng SK, Dizon J.. Computerised cognitive behavioural therapy for insomnia: A systematic review and meta-analysis. Psychother Psychosom. 2012;814:206–216. 10.1159/000335379 [DOI] [PubMed] [Google Scholar]
- 24. Colagiuri B, Christensen S, Jensen AB, et al. Prevalence and predictors of sleep difficulty in a national cohort of women with primary breast cancer three to four months postsurgery. J Pain Symptom Manage. 2011;425:710–720. 10.1016/j.jpainsymman.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 25. Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psych. 2009;667:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morin CM, Espie CA.. Insomnia: A Clinical Guide to Assessment and Treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 27. Adan A, Almirall H.. Horne and Ostberg Morningness Eveningness Questionnaire - a reduced scale. Pers Individ Diff. 1991;123:241–253. 10.1016/0191-8869(91)90110-W [DOI] [Google Scholar]
- 28. Behling O, Law KS.. Translating Questionnaires and Other Research Instruments. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 29. Bastien CH, Vallieres A, Morin CM.. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;24:297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 30. Morin CM, Belleville G, Belanger L, et al. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;345:601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;352:287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;132:63–74. 10.1016/S0885-3924(96)00274-6 [DOI] [PubMed] [Google Scholar]
- 33. Minton O, Stone P.. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;201:17–25. [DOI] [PubMed] [Google Scholar]
- 34. Schafer JL, Graham JW.. Missing data: Our view of the state of the art. Psycho Methods. 2002;72:147–177. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- 35. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;B571:289–300. [Google Scholar]
- 36. Holm S. A Simple sequentially rejective multiple test procedure. Scand J Stat. 1979;62:65–70. [Google Scholar]
- 37. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 38. Horsch C, Lancee J, Beun RJ, et al. Adherence to technology-mediated insomnia treatment: A meta-analysis, interviews, and focus groups. J Med Internet Res. 2015;179:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miniwatts Marketing Group. Top 50 countries with highest internet penetration rates. 2017. http://www.internetworldstats.com/top25.htm. Accessed September 24, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.