Abstract
In the present study, we investigated the influence of weaning on antioxidant status, intestinal integrity, mitochondrial function, and the mitophagy level in piglets (weaned at 21 d) during the 1 wk after weaning. The redox status was measured by antioxidant enzymes activities, related genes expression, and malondialdehyde (MDA) content in jejunum. The intestinal barrier function was assessed by the Ussing chamber and expression of tight junction proteins in the jejunum. The function of intestine mitochondria was measured by mitochondrial DNA (mtDNA) content and activities of mitochondria oxidative phosphorylation complexes. The levels of light chain 3-1 (LC3-I), light chain 3-II (LC3-II), PTEN-induced putative kinase 1 (PINK1), and Parkin were determined to investigate whether mitophagy is involved in the weaning process. The results showed that, as compared with the preweaning phase (d 0), weaning suppressed (P < 0.05) the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) on d 3 and d 7 postweaning, decreased (P < 0.05) the expression of copper and zinc superoxide dismutase (Cu/Zn-SOD), manganese-containing superoxide dismutase (Mn-SOD) on d 3 postweaning, declined (P < 0.05) the level of glutathione peroxidase 1 (GPX-1) and glutathione peroxidase 4 (GPX-4) on d 3 and d 7 postweaning, and increased (P < 0.05) MDA content in jejunum on d 3 and d 7 postweaning. The jejunal transepithelial electrical resistance and levels of occludin, claudin-1, and zonula occludens-1 on d 3 and d 7 postweaning were reduced (P < 0.05), and paracellular flux of fluorescein isothiocyanatedextran (4 kDa) on d 3 and d 7 postweaning was increased (P < 0.05). Weaning induced mitochondrial dysfunction, as demonstrated by decreased (P < 0.05) content of mtDNA on d 3 and d 7 postweaning and declined (P < 0.05) activities of mitochondria complexes (I, II, III, IV) in jejunum on d 1, d 3, and d 7 postweaning. Weaning led to an increased (P < 0.05) expression level of mitophagy-related proteins, PINK1 and Parkin, in the intestinal mitochondria, as well as an enhancement (P < 0.05) of the ratio of LC3-II to LC3-I content in the jejunal mucosa on d 1, d 3, and d 7 postweaning. These results suggest that weaning disrupted intestinal oxidative balance, and this imbalance may impair intestinal barrier and mitochondrial function and trigger mitophagy in piglets.
Keywords: intestinal barrier function, mitochondrial function, mitophagy, oxidative stress, weaning pigs
INTRODUCTION
Weaning stress causes diarrhea, growth restriction, and intestine dysfunction in piglets, as they are abruptly compelled to adapt the nutritional, immunological, and psychological stress (Wijtten et al., 2011). Weaning may lead to intestinal inflammation (Hu et al., 2013b; Bomba et al., 2014), mast cell activation (Moeser et al., 2007b; Smith et al., 2010), and corticotrophin-releasing factor stress signaling pathways activation (Moeser et al., 2007a; Wang et al., 2015). Moreover, weaning stress disrupted free-radical metabolism and antioxidative system and caused serious oxidative stress (Yin et al., 2014). Oxidative stress is defined as an imbalance between the production of reactive oxygen species (ROS) and their elimination through antioxidative mechanisms (Bhat et al., 2015). Weaning pigs with an imbalance and immature antioxidant system in the intestine are easily attacked by oxidative stress (Xu et al., 2014). Recently, studies showed that oxidative stress and disruption of cellular redox status impair intestinal function, intestinal turnover, and cell survival (Rahal et al., 2014; Rosero et al., 2015; Li et al., 2016). However, the connection between weaning oxidative stress and intestinal barrier function is still unclear.
Intestinal tract has a high metabolic rate and a great demand for energy to support its integrity and function (Pi et al., 2014). Mitochondria are responsible for producing cellular energy (Marcu et al., 2017). However, mitochondria energy metabolism is not only the major source of ROS generation in aerobic cells but also a vital target for the damaging effects of ROS, which may result in a more severe oxidative stress (Venditti et al., 2013). Oxidative stress contributes to mitochondrial dysfunctions, including disruption of mitochondrial respiratory chain and mutation of mitochondrial DNA (mtDNA), and activates cell death pathways (Kubli and Gustafsson, 2012). In an effort to protect cells from oxidative damage, cells have developed an elaborate self-protective mechanism to selective degradation of the dysfunctional mitochondrion before it hurts the cell, calls mitochondrial autophagy, or mitophagy (Saita et al., 2013). It would be imperative to investigate the effect of weaning on intestinal mitochondria function and the level of mitophagy. To our knowledge, no information is available regarding the effect of weaning on intestinal mitochondrion function and the level of mitophagy. We hypothesized that the weaning oxidative stress leads to mitochondria dysfunction, subsequently induces an impaired intestinal barrier function, and triggers mitophagy. Therefore, the aim of the present study was to investigate the development of an antioxidant system, intestinal barrier function, mitochondria function, and level of mitophagy during weaning process of piglets.
MATERIALS AND METHODS
All procedures were approved by the Zhejiang University Animal Care and Use Committee.
Animals, Housing, and Diet
All procedures were according to previous study (Hu et al., 2013b). Six litters (Duroc×Landrace×Yorkshire, 9 to 11 piglets per litter) were selected. At 20 d of age (preweaning stage), one piglet from each of six different litters was slaughtered. At weaning day (21 d of age), three piglets from each of six different litters were allocated to one of the three experimental groups slaughtered at d 1, d 3, and d 7 postweaning (n = 6). For each group, six piglets from six different litters were removed from the sow, mixed, and housed in nursery pens. These groups had an equal numbers of barrows and females, with an average body weight of the piglets of 6.09 ± 0.2 kg. The weaned piglets were given ad libitum access to feed and water. Diets were formulated to meet or exceed requirements suggested by the National Research Council (2012). The d 7 final body weight is 7.38 ± 0.23 kg.
Sample Collection
Piglets were euthanized with an ear intravenous injection of sodium pentobarbital (200 mg/kg body weight) (Sigma-Aldrich, St. Louis, MO) as described by Luo et al. (2015) and Zhang et al. (2016) and the gastrointestinal tract quickly removed. Segments of proximal jejunum were harvested immediately and prepared for Ussing chamber studies and isolation of mitochondria. Mucosal scrapings from the adjacent jejunum were collected, rapidly frozen in liquid nitrogen, and stored at −80 °C.
Ex Vivo Ussing Chamber to Measure Intestinal Barrier Function
Tissues were mounted in EasyMount Ussing chamber system (model VCC MC6; Physiologic Instruments, San Diego, CA) as described previously (Hu et al., 2013b; McLamb et al., 2013). Jejunum mucosa was stripped from the seromuscular layer in oxygenated (95% O2–5% CO2) Ringer solution. Tissues were then mounted in Ussing chambers. Tissues were bathed on the serosal and mucosal sides with 5 mL of Ringer solution. The serosal bathing solution contained 10 mM glucose, which was osmotically balanced on the mucosal side with 10 mM mannitol. Bathing solutions were oxygenated (95% O2–5% CO2) and circulated in water-jacketed reservoirs maintained at 37 °C. The clamps were connected to acquire and analyze software (Physiologic Instruments) for automatic data collection. Briefly, data was collected automatically using Acquire and Analyze software (Physiologic Instruments). Transepithelial electrical resistance (TER) was recorded at 15-min intervals over a 1-h period after a 15-min equilibration period. The flux of fluorescein isothiocyanate dextran 4 kDa (FD4) was used to evaluate the epithelial barrier function as described previously (Jiao et al., 2015; Li et al., 2017). After a 15-min equilibration period on Ussing chambers, the fluorescein isothiocyanate dextran 4 kDa (FD4; FD4-100MG; Sigma-Aldrich, St. Louis, MO; 0.375 mg/mL) was added to the mucosal side of Ussing chamber-mounted tissues. The FD4 was allowed to equilibrate for 15 min after which 50 µL samples were taken from the serosal side of tissues at 15-min intervals over 60 min and transferred into a 96-well assay plate. The presence of FD4 fluorescence intensity of each sample was measured by Fluorescence Microplate Reader (FLx800, Bio-Tek Instruments Inc., Winooski, VT), and concentrations were determined from standard curves generated by serial dilution of FD4. FD4 flux was presented as the rate of FD4 flux (μg/cm2/h).
Redox Status
Intestinal mucosal was used for the measurement of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) as described previously (Xu et al., 2014), using an ELISA kit specific for porcine following the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Isolation of Mitochondria from Intestine
Intact mitochondria were isolated from fresh jejunum by using tissue mitochondria isolation kit (Beyotime Institute of Biotechnology, Shanghai, China). In brief, after 0.5-g intestine tissue was homogenized in ice-cold MSH buffer (10 mmol/L HEPES, pH 7.5, containing 200 mmol/L mannitol, 70 mmol/L sucrose, 1.0 mmol/L EGTA and 2.0 mg/mL serum albumin), the homogenate was centrifuged at 1,000 × g for 10 min at 4 °C. The collected supernatant was then centrifuged at 3,500 × g for 10 min at 4 °C to obtain mitochondrial pellet (Huang et al., 2013).
Determination of the Activities of Respiratory Chain Complexes I to IV in Intestine Mitochondrial
According to the previous report (Liu et al., 2012b), mitochondrial respiratory chain complexes activities quantitative determination kits were used to evaluate the enzyme activities of complexes I (NADH-CoQ reductase), II (succinate-CoQ reductase), III (CoQ-cytochrome C reductase), and IV (cytochrome C oxidase) according to the manufacturer’s instructions (GenMed Scientifics, Shanghai, China).
Analysis of mtDNA Content in the Jejunum
The content of mtDNA relative to nuclear genomic DNA was measured by amplifying the mt D-loop and the nuclear-encoded β-actin genes using real-time PCR assay as described previously (Huang et al., 2017). Total DNA was extracted from proximally jejunal mucosa using a TIANamp Stool DNA Kit (Tiangen Biotech, Beijing, China), following the manufacturer’s instructions. The primers used are presented in Tables 1 and 2. The ratio of mtDNA to genomic DNA content was calculated as ΔCt (mt CtDloop − nuclear Ctβ-actin). Relative abundance was calculated as 2−ΔΔCt method, where ΔΔCt = ΔCtmtDNA content during weaning process − ΔCtmtDNA content in the preweaning stage.
Table 1.
Ingredient and chemical composition of the basal diet as fed basis
| Items | |
|---|---|
| Ingredients, g/kg | |
| Maize | 357 |
| Extruded corn | 210 |
| Soybean meal | 113 |
| Extruded full-fat soybean | 109 |
| Fish meal | 31 |
| Spray-dried plasma protein | 40.5 |
| Dried whey | 87 |
| Soybean oil | 19 |
| Dicalcium phosphate | 11 |
| Limestone | 5 |
| Sodium chloride | 1 |
| L-Lysine HCl | 4.9 |
| DL-Methionine | 1.6 |
| Vitamin-mineral premix1 | 10 |
| Analysed composition, g/kg | |
| Digestible energy2, MJ/kg (calculated) | 14.40 |
| Crude protein (measured) | 223.57 |
| Lysine (measured) | 14.3 |
| Methionine (measured) | 3.6 |
| Calcium (measured) | 8.3 |
| Total phosphorus (measured) | 6.6 |
1Provided per kilogram of diet: vitamin A, 8000 IU; vitamin D, 2000 IU; vitamin E, 40 IU; vitamin K3, 1.5 mg; vitamin B1, 1.5 mg; vitamin B6, 1.6 mg; biotin, 0.10 mg; niacin, 30 mg; pantothenic acid, 25 mg; Zn, 100 mg; Fe, 110 mg; Cu, 15 mg; Mn, 16 mg; I, 0.3 mg; Se, 0.3 mg.
2Digestible energy was calculated from data provided by Feed Database in China (2012).
Table 2.
Primer sequences used for real-time PCR
| Gene | 5′-Primer (F) | 3′-Primer (R) | Accession number | Length |
|---|---|---|---|---|
| β-Actin | CTGCGGCATCCACGAAACT | AGGGCCGTGATCTCCTTCTG | DQ845171.1 | 147 |
| mt D-loop 1 | GATCGTACATAGCACATATCATGTC | GGTCCTGAAGTAAGAACCAGATG | AF276923 | 198 |
| Cu/Zn-SOD 2 | CAGGTCCTCACTTCAATCC | CCAAACGACTTCCASCAT | NM_001190422 | 255 |
| Mn-SOD 3 | GGACAAATCTGAGCCCTAACG | CCTTGTTGAAACCGAGCC | NM_214127 | 159 |
| GPx-1 4 | TGGGGAGATCCTGAATTG | GATAAACTTGGGGTCGGT | NM_214201 | 183 |
| GPx-4 5 | GATTCTGGCCTTCCCTTGC | TCCCCTTGGGCTGGACTTT | NM_214407.1 | 172 |
1 mt D-loop = mitochondria DNA loop.
2 Cu/Zn-SOD = copper and zinc superoxide dismutase.
3 Mn-SOD = manganese-containing superoxide dismutase.
4 GPx-1 = glutathione peroxidase 1.
5 GPx-4 = glutathione peroxidase 4.
mRNA Expression Analysis by qPCR
The mRNA levels of copper and zinc superoxide dismutase (Cu/Zn-SOD), manganese- containing superoxide dismutase (Mn-SOD), glutathione peroxidase 1 (GPX-1), glutathione peroxidase 4 (GPX-4) were analyzed as described by previous study (Yin et al., 2014). Sequence of primers used for the qPCR was shown in Table 1. Total RNA was extracted from jejunal mucosa using TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s guides. The concentration and purity of all RNA samples were measured using a Nano Drop spectrophotometer (ND-2000; NanoDrop Technologies, Wilmington, DE). Reverse transcription using the PrimeScripte RT reagent kit (TaKaRa Biotechnology, Dalian, China) was carried out following the manufacturer’s direction. Quantitative analysis of PCR was carried out on a StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA) using SYBR Green Master mix (Promega, Madison, WI), according to the manufacturer’s specification. Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. The 2−ΔΔCt method was used to analyze the relative expression (fold changes), calculated relative to the values from the preweaning phase. ΔΔCT was computed for each target gene from the treatment groups by subtracting the average ΔCT for the preweaning phase. The final fold differences were computed as 2−ΔΔCt for each target gene. All samples were run in triplicate. Our results showed that β-actin exhibited no difference among different time points.
Protein Expression Analysis by Western Blot
The Western blot analysis was performed according to the procedures outlined by previous studies (Hu et al., 2013a; Larson-Casey et al., 2016). Briefly, after electrophoresis the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated with primary Ab at 4 °C for 10 h and then with the secondary Ab for 2 h at room temperature. The primary Abs (occludin, claudin-1, zonula occludens-1 [ZO-1], light chain 3-I [LC3-I], light chain 3-II [LC3-II], PTEN-induced putative kinase 1 [PINK1], Parkin, voltage-dependent anion channel [VDAC], β-actin) were purchased from Santa Cruz Technology Inc. (Santa Cruz, CA). The secondary Ab was HRP conjugated anti- rabbit Ab (Cell Signaling Technology, Danvers, MA). Western blot was detected with an enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL), photographed by a ChemiScope 3400 (Clinx Science Instruments, Shanghai, China), and analyzed using Quantity One software. β-Actin and VDAC were used as internal reference, which exhibited no difference among each group. The relative abundance of intestinal target proteins and mitochondria target proteins were expressed as target protein/β-actin and target protein/VDAC protein ratio, respectively. The protein expression of all samples was expressed as fold changes, calculated relative to the preweaning stage (d 0 postweaning).
Statistical Analysis
One-way ANOVA was conducted using SPSS 20.0 statistical package (SPSS Inc., Chicago, IL). Differences among means were tested using Duncan’s multiple range tests. Differences were considered significant at P < 0.05.
RESULTS
Activities of SOD, GSH-Px, and MDA in Jejunum After Weaning
The activities of SOD, GSH-Px, and content of MDA in intestinal mucosa during the 1 wk after weaning are shown in Table 3. Compared with the preweaning phase (d 0 postweaning), the activities of SOD and GSH-Px in jejunal mucosa of piglets on d 3 and d 7 postweaning were decreased (P < 0.05). And then, a greater (P < 0.05) content of MDA in the jejunal mucosa was observed on d 3 and d 7 postweaning compared with d 0 values.
Table 3.
Effects of weaning on antioxidant enzymes activities and MDA content in jejunum of piglets
| Day postweaning | |||||
|---|---|---|---|---|---|
| Items | 0 | 1 | 3 | 7 | SEM1 |
| SOD2 (U/mg protein) | 222.57a | 181.21ab | 123.24b | 115.83b | 23.83 |
| GSH-Px3 (U/mg protein) | 166.36a | 158.05a | 75.52b | 97.54b | 8.61 |
| MDA4 (nmol/g protein) | 0.45b | 0.72ab | 1.07a | 0.94a | 0.15 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Standard error of means, n = 6.
2SOD = superoxide dismutase.
3GSH-Px = glutathione peroxidases.
4MDA = malondialdehyde.
Expression of Antioxidant Enzyme-Related Genes in the Jejunum After Weaning
Table 4 shows expression of antioxidant- related genes in the jejunal mucosa of piglets during the 1 wk after weaning. Compared with d 0 values, the levels of Cu/Zn-SOD, Mn-SOD, GPX-1, GPX-4 were remarkably decreased (P < 0.05) on d 3 postweaning. On d 7 postweaning, the expression levels of Cu/Zn-SOD and Mn-SOD were slightly recovered (P > 0.05). However, the level of GPX-1 and GPX-4 on d 7 postweaning was still lower (P < 0.05) than d 0 values.
Table 4.
Effects of weaning on antioxidant enzymes-related genes in the jejunum of piglets
| Day postweaning | |||||
|---|---|---|---|---|---|
| Items | 0 | 1 | 3 | 7 | SEM1 |
| Cu/Zn-SOD 2 | 1.00a | 0.72a | 0.30b | 0.65ab | 0.13 |
| Mn-SOD 3 | 1.00a | 0.90a | 0.33b | 0.61ab | 0.14 |
| GPX-1 4 | 1.00a | 0.74a | 0.22b | 0.29b | 0.10 |
| GPX-4 5 | 1.00a | 0.76a | 0.31b | 0.39b | 0.12 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Standard error of means, n = 6.
2 CuZn-SOD = copper and zinc superoxide dismutase.
3 Mn-SOD = manganese-containing superoxide dismutase.
4 GPx-1 = glutathione peroxidase 1.
5 GPx-4 = glutathione peroxidase 4.
Intestinal Barrier Function and Tight Junction Proteins Expression After Weaning
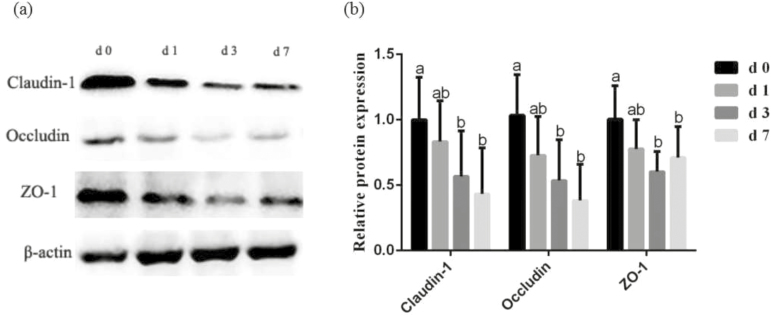
Table 5 shows intestinal barrier function and tight junction proteins expression in jejunal mucosa of piglets during the 1 wk after weaning. Compared with the d 0, the TER of jejunum was significantly reduced (P < 0.05), and the mucosal-to-serosal flux of FD4 was increased (P < 0.05) on d 3 and d 7 postweaning. Figure 1 shows the expression of occludin, claudin-1, and ZO-1 in the jejunal mucosa. Compared with the preweaning phase (d 0 postweaning), the level of claudin-1, occluding, and ZO-1 was decreased (P < 0.05) on d 3 and d 7 postweaning.
Table 5.
Effects of weaning on intestinal barrier function in jejunum of piglets
| Day postweaning | |||||
|---|---|---|---|---|---|
| Items | 0 | 1 | 3 | 7 | SEM1 |
| TER2, Ω·cm2 | 61.11a | 57.18ab | 49.53b | 46.35b | 3.72 |
| FD43 flux, µg/cm2/h | 1.06b | 1.62ab | 1.96a | 2.23a | 0.22 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Standard error of means, n = 6.
2TER = transepithelial electrical resistance.
3FD4 = fluorescein isothiocyanate dextran (4 kDa).
Figure 1.
(a) Effects of weaning on expression of tight junction proteins in jejunal mucosa. (b) Representative blots of claudin-1, occludin, zonula occludens-1 (ZO-1) and β-actin in the jejunal mucosa of piglets. Values are means and SD represented by vertical bars. a,bMeans with different letters differ significantly (P < 0.05). The protein expression of all samples was expressed as fold changes, calculated relative to the preweaning (d 0 postweaning) pigs.
Mitochondria DNA Content in Jejunum and Activities of Intestinal Mitochondrial Respiratory Chain Complexes I to IV After Weaning
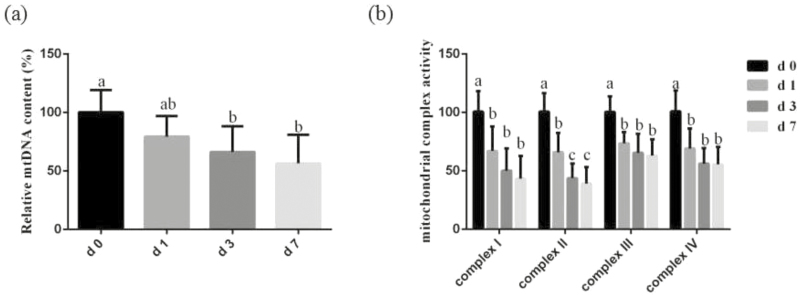
The mtDNA content in jejunum and activities of intestinal mitochondrial respiratory chain complexes during the 1 wk after weaning are shown in Fig. 2. In comparison with d 0, the content of mtDNA in the jejunum on d 3 and d 7 postweaning was decreased (P < 0.05). The activities of mitochondrial respiratory chain complexes I, II, III, IV in jejunum were significantly decreased (P < 0.05) in jejunal mitochondria on d 1, d 3, and d 7 postweaning. Moreover, the activities of mitochondrial respiratory chain complexes II in jejunal mitochondrial on d 3 and d 7 postweaning was significantly lower (P < 0.05) than d 0 and d 1 postweaning (Figure 2b).
Figure 2.
(a) Effects of weaning on mitochondrial DNA (mtDNA) content in the jejunum of piglets. (b) Effects of weaning on the activities of mitochondrial complexes in jejunal mitochondria of piglets. Values are means and SD represented by vertical bars. a,b,cMeans with different letters differ significantly (P < 0.05). The mtDNA content and mitochondrial complex activities of all samples were expressed as fold changes, calculated relative to the preweaning (d 0 postweaning) pigs.
Expression of Mitophagy-Related Proteins After Weaning
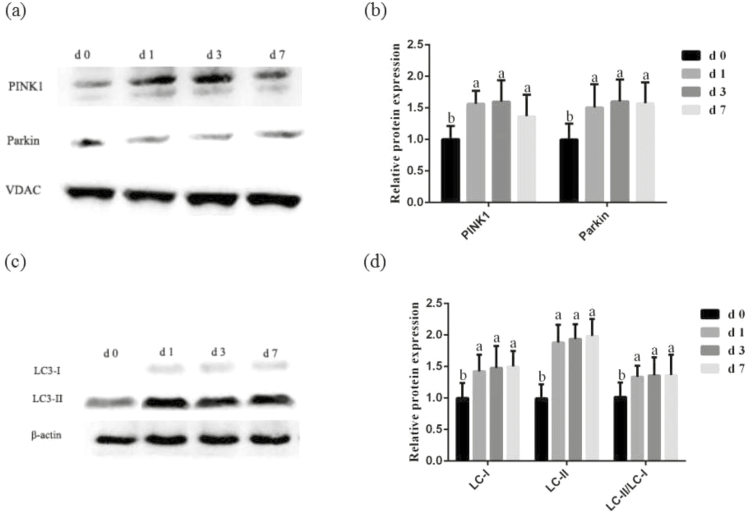
Figure 3 presents the expression of mitophagy-related protein in the mitochondrial and jejunal mucosa during the 1 wk after weaning. In comparison with the d 0, the expression of PINK1 and Parkin in the intestinal mitochondrial were striking increased (P < 0.05) on d 1, d 3 and d 7 postweaning. Correspondingly, we found that the abundance of LC3-I and LC3-II and the ratio of LC3-II to LC3-I content in the jejunal mucosa was significantly greater (P < 0.05) at d 1, d 3, and d 7 postweaning than preweaning stage (d 0 postweaning).
Figure 3.
Effects of weaning on expression of mitophagy-related protein in the intestinal mitochondrial of piglets. (a) Representative blots of PTEN-induced putative kinase 1 (PINK1) and Parkin in the intestinal mitochondria of piglets. (b) Relative PINK1 and Parkin expression of piglets. (c) Representative blots of light chain 3-I (LC3-I) and LC3-II in the jejunum mucosa weaning of piglets. (d) Relative LC3-I and LC3-II expression of piglets. Summary of Western blot for n = 6 pigs. Values are means and SD represented by vertical bars. a,bMeans with different letters differ significantly (P < 0.05). The protein expression of all samples was expressed as fold changes, calculated relative to the preweaning (d 0 postweaning) pigs.
DISCUSSION
A large amount of studies reported that early weaning stress disrupted the antioxidant system and induced a severe oxidative stress (Franco et al., 2013; Zheng et al., 2013; Xu et al., 2014). Moreover, oxidative stress and disruption of cellular redox status impair intestinal function, intestinal turnover, and cell survival (Rahal et al., 2014; Rosero et al., 2015; Li et al., 2016), due to oxidative imbalance leading to irreparable oxidative injury to the cellular macromolecules and cell apoptosis (Rani et al., 2016; Poprac et al., 2017). Generally, oxidative stress is considered to be an imbalance between the production and the elimination of ROS. ROS, such as superoxide (O2−) and hydrogen peroxide (H2O2) caused proteins, lipids, and nucleic acids oxidation (Scherz-Shouval and Elazar, 2011). Xu et al. (2014) reported that early weaning led to a striking decrease in intestinal antioxidant capacities and a remarkable increase level of MDA and free radical (−OH, H2O2) in piglets. Zhu et al. (2012) demonstrated that early weaning suppressed the activities of SOD, GSH-Px, and increased the content of MDA, NO, H2O2, and O2 in serum of piglets. Yin et al. (2014) indicated that early weaning stress led to lipid, protein, and DNA oxidative injury and increased concentrations of MDA, 8-hydroxydeoxyguanosine, and protein carbonyl in serum of piglets. Meanwhile, diet is also a major source of oxidative stress. Li et al. (2016) had reported that high dietary iron contributes to oxidative stress through the production of free radicals, which leads to lipid peroxidation, inflammation, and intestinal injury. Rosero et al. (2015) had showed that lipid peroxidation disrupted the redox environment of intestine and then impaired the small intestine structure and function. In the current study, the intestinal redox status was determined by activities of antioxidative enzymes, expression of related genes, and the product of oxidative injury. Consistently, the present study confirmed that, during 1 wk after weaning, the activities of SOD and GSH-Px were decreased, as well as the mRNA levels of related genes (Cu/Zn-SOD, Mn-SOD, GPX-1, and GPX-4) were declined. Additionally, the MDA (a product of lipid peroxidation) content in intestinal mucosa during 1 wk after weaning was significantly increased. In our present experiment, weaning induced lower activities of antioxidant enzymes and a greater content of MDA reflected an imbalance of redox status during weaning.
It has been widely reported that an intact intestinal barrier plays an important role in preventing intestinal bacteria, toxic, or allergenic substances entering the body through the intestinal tract (Liu et al., 2012a). The intestinal epithelial barrier is the first line of defense against a hostile environment within the intestinal lumen. The intestinal barrier is mainly formed by a layer of epithelial cells joined together by tight junction proteins (Pi et al., 2014). In the present study, intestinal permeability was evaluated using the Ussing chamber and expression of tight junction proteins. A decreased TER and increased flux of FD4 reflects an impaired intestinal barrier (Wijtten et al., 2011). In agreement with previous reports (Hu et al., 2013b; Xiao et al., 2014; Jiao et al., 2015), the current study indicated that weaning reduced the intestinal TER and levels of occludin, claudin-1, and ZO-1, and increased the mucosal-to-serosal flux of paracellular FD4 during 1 wk after weaning of piglets. Additionally, oxidative stress may lead to the loss of intestinal function of early-weaned pigs, promotion of free-radical generation, limitation of the antioxidant effects, and reduction in digestive enzyme activities (Zhu et al., 2012). Intestinal barrier is associated with disturbances of intestinal oxidative status including increased lipid peroxidation and protein oxidation (Yin et al., 2014). Some reports suggest that oxidative stress results in the expression of the tight junction proteins ZO-1 and occludin (Assimakopoulos et al., 2004; Liu et al., 2017). Accumulating evidence suggests that the intestine has a high requirement for ATP to support its integrity, function, and health (Blachier et al., 2009; Burrin and Stoll, 2009), and thus, energy deficits in the intestinal mucosa may play a critical role in intestinal barrier dysfunction. Mitochondria are responsible for producing cellular energy (Marcu et al., 2017). Mitochondrial function is affected by oxidative stress, including disruption of mitochondrial respiratory chain and mutation of mtDNA, and activates mitophagy (Kubli and Gustafsson, 2012).
Intestine has a high demand for energy to support its integrity and function. Mitochondrion has a pivotal role in energy metabolism through mitochondrial respiratory chain, which is the primary source of ROS (Venditti et al., 2013). Due to the mtDNA close to the mitochondrial respiratory chain and lack of protective histones, so the major attack target of ROS is mtDNA (Pinto and Moraes, 2015). Mitochondria DNA damage may result in cell death through disrupted electron transport, depolarized mitochondrial membrane, and disturbed energy production (Pinto and Moraes, 2015). Moreover, mtDNA content is a potential biomarker of mitochondrial dysfunction (Malik and Czajka, 2013). However, no data are available regarding the mtDNA content in intestine of weaning piglets during weaning process. Therefore, we determined, for the first time, the content of intestinal mtDNA of weaning piglets was remarkable decreased during 1wk after weaning. Ochoa et al. (2011) had reported that the copy number and integrity of mtDNA were damaged in human brain during oxidative stress process. Afolayan et al. (2016) had showed a significantly decreased copy number of mtDNA in pulmonary artery endothelial cells treated with nitric oxide (an oxidative stressor). The possible reason for the low content of mtDNA in jejunum in the present experiment may be that the antioxidative enzyme system had been disrupted during weaning process, so the ROS degradation is not sufficiently maintained, resulting in a disruption of mtDNA. Furthermore, mtDNA encodes multiple polypeptide subunits composed the oxidative phosphorylation complexes (I, III, IV). However, the complex II was encoded by nuclear genome (Moran et al., 2012). Hence, the mutations of mtDNA would subsequently lead to an impairment of oxidative phosphorylation complexes, overproduction of ROS and production of pro-apoptotic protein, and ultimately lead to cell death and tissue damage.
Nevertheless, no information is available related to the influence of weaning on activities of mitochondria oxidative phosphorylation complexes of weaned pigs. Therefore, in the current study, we demonstrated, for the first time, the activities of mitochondria oxidative phosphorylation complex I, II, III, IV during 1-wk postweaning was dramatically decreased. In particularly, we found that the activity of complex II was significantly lower on d 3 and d 7 postweaning than d 0 and d 1. Pramanik et al. (2011) have reported that the capsaicin-induced oxidative stress declined the activities of mitochondrial complex I and III in pancreatic cancer cells, resulting in severe mitochondrial damage leading to cell apoptosis. Zigdon et al. (2013) found a significant decrease of activity of mitochondrial complex IV under chronic oxidative stress. Tatarkova et al. (2011) have demonstrated that aging oxidative stress suppressed the activities of electron transport chain complexes in cardiac mitochondria of rats. We speculated that an imbalance between oxidant and antioxidant in intestine of piglets caused by weaning may inhibit activities of mitochondria oxidative phosphorylation complexes, resulting in declining of energy generation and disrupting of intestinal integrity.
In response to oxidative stress and disrupted mitochondria, cells degraded the dysfunctional mitochondrion before it causes activation of cell death (Springer and Macleod, 2016). This process is known as mitochondrial autophagy or mitophagy. Nevertheless, no information is available regarding the mitophagy during the weaning process of piglets. To explore whether mitophagy involved in weaning process, we determined, for the first time, whether weaning influenced expression level of mitophagy-related proteins of piglets. Previous cumulative data suggested that the PINK1 and Parkin play the crucial role in mediating mitophagy (Eiyama and Okamoto, 2015; Springer and Macleod, 2016). The PINK1 expressed in healthy, polarized mitochondria, and then it would be rapidly degraded by proteolysis. Therefore, the expression of PINK1 was maintained at very low level (Eiyama and Okamoto, 2015). Collapsing of the mitochondria function induced by oxidative stress may prevent the PINK1 degradation process and then PINK1 accumulation on the damaged mitochondria to recruit Parkin to induce mitophagy (Nguyen et al., 2016). In the present research, we revealed, for the first time, that the abundance of mitophagy-related protein (PINK1 and Parkin) in the intestinal mitochondria during 1-wk postweaning was remarkably increased. During initial phagophore formation in mitophagy process, LC3-I is modified and converted to LC3-II, which is translocated to the membrane of autophagosomes and autolysosomes (Yin et al., 2015). In the present study, we found that the ratio of LC3-II to LC3-I content was notably enhanced during 1-wk postweaning. These results demonstrated that weaning led to the formation of autophagosome, which contained dysfunctional mitochondria in the jejunum during 1-wk postweaning. Similarly, Zhang et al. (2011) have reported that a significantly increased level of LC3-I and LC3-II was observed at 12- and 24-h weaning treatments in the liver, spleen, and skeletal muscle tissues. Mitophagy in this study may protect cells against apoptosis or necrosis under weaning oxidative stress in intestine of piglets due to the damaged mitochondria to be sequestered by autophagosomes, subsequently degraded by lysosomes, which contribute to the intestinal homeostasis and reduce further oxidative damage.
In the current study, we demonstrated that weaning disrupted intestinal antioxidant balance and intestinal barrier function, and caused mitochondrial dysfunction. Furthermore, the level of mitophagy-related proteins is upregulated in the intestinal in response to weaning stress.
Footnotes
This research was supported by the National Key R & D Program (2016YFD0501210), National Natural Science Foundation of China (31472103), and Special Fund for Agroscientific Research in the Public Interest (201403047).
LITERATURE CITED
- Afolayan A. J., Eis A., Alexander M., Michalkiewicz T., Teng R. J., Lakshminrusimha S., and Konduri G. G.. 2016. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 310:L40–L49. doi:10.1152/ajplung.00392.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimakopoulos S. F., Vagianos C. E., Patsoukis N., Georgiou C., Nikolopoulou V., and Scopa C. D.. 2004. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol. Scand. 180:177–185. doi:10.1046/j.0001-6772.2003.01229.x [DOI] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., and Ganie S. A.. 2015. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74:101–110. doi:10.1016/j.biopha.2015.07.025 [DOI] [PubMed] [Google Scholar]
- Blachier F., Boutry C., Bos C., and Tome D.. 2009. Metabolism and functions ofl-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 90:814S–821S. doi:10.3945/ajcn.2009.27462S [DOI] [PubMed] [Google Scholar]
- Bomba L., Minuti A., Moisa S. J., Trevisi E., Eufemi E., Lizier M., Chegdani F., Lucchini F., Rzepus M., Prandini A.,. et al. 2014. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genomics 14:657–671. doi:10.1007/s10142-014-0396-x [DOI] [PubMed] [Google Scholar]
- Burrin D. G., and Stoll B.. 2009. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutr. 90:850S–856S. doi:10.3945/ajcn.2009.27462Y [DOI] [PubMed] [Google Scholar]
- Eiyama A., and Okamoto K.. 2015. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33:95–101. doi:10.1016/j.ceb.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Franco J. G., Lisboa P. C., Lima N. S., Amaral T. A. S., Peixoto-Silva N., Resende A. C., Oliveira E., Passos M. C. F., and Moura E. G.. 2013. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 24:960–966. doi:10.1016/j.jnutbio.2012.06.019 [DOI] [PubMed] [Google Scholar]
- Hu C., Song J., Li Y., Luan Z., and Zhu K.. 2013a. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 110:681–688. doi:10.1017/S0007114512005508 [DOI] [PubMed] [Google Scholar]
- Hu C. H., Xiao K., Luan Z. S., and Song J.. 2013b. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi:10.2527/jas.2012–5796 [DOI] [PubMed] [Google Scholar]
- Huang L., Wan J., Chen Y., Wang Z., Hui L., Li Y., Xu D., and Zhou W.. 2013. Inhibitory effects of p38 inhibitor against mitochondrial dysfunction in the early brain injury after subarachnoid hemorrhage in mice. Brain Res. 1517:133–140. doi:10.1016/j.brainres.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Huang Q., Xu W., Bai K. W., He J. T., Ahmad H., Zhou L., Zhang L. L., and Wang T.. 2017. Protective effects of leucine on redox status and mitochondrial-related gene abundance in the jejunum of intrauterine growth-retarded piglets during early weaning period. Arch. Anim. Nutr. 71:93–107. doi:10.1080/1745039x.2017.1279712 [DOI] [PubMed] [Google Scholar]
- Jiao L. F., Ke Y. L., Xiao K., Song Z. H., Hu C. H., and Shi B.. 2015. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs. J. Anim. Sci. 93:1157–1164. doi:10.2527/jas.2014–8248 [DOI] [PubMed] [Google Scholar]
- Kubli D. A., and Gustafsson A. B.. 2012. Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res. 111:1208–1221. doi:10.1161/Circresaha.112.265819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Casey J. L., Deshane J. S., Ryan A. J., Thannickal V. J., and Carter A. B.. 2016. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44:582–596. doi:10.1016/j.immuni.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hansen S. L., Borst L. B., Spears J. W., and Moeser A. J.. 2016. Dietary iron deficiency and oversupplementation increase intestinal permeability, ion transport, and inflammation in pigs. J. Nutr. 146:1499–1505. doi:10.3945/jn.116.231621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Song Z., Kerr K. A., and Moeser A. J.. 2017. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS One 12:e0171617. doi:10.1371/journal.pone.0171617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bao Z., Xu X., Chao H., Lin C., Li Z., Liu Y., Wang X., You Y., Liu N., and Ji J.. 2017. Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/Heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J. Neurotrauma 34:2119–2131. doi:10.1089/neu.2016.4764 [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Chen F., Odle J., Lin X., Jacobi S. K., Zhu H. L., Wu Z. F., and Hou Y. Q.. 2012a. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 142:2017–2024. doi:10.3945/jn.112.164947 [DOI] [PubMed] [Google Scholar]
- Liu G., Tian H., Huang Y. Q., Hu J., Ji Y. X., Li S. Q., Feng Y. Q., Guo L., and Zhu Y. G.. 2012b. Alterations of mitochondrial protein assembly and jasmonic acid biosynthesis pathway in Honglian (HL)-type cytoplasmic male sterility rice. J. Biol. Chem. 287:40051–40060. doi:10.1074/jbc.M112.382549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. H., Yang C., Wright A. D., He J., and Chen D. W.. 2015. Responses in ileal and cecal bacteria to low and high amylose/amylopectin ratio diets in growing pigs. Appl. Microbiol. Biotechnol. 99:10627–10638. doi:10.1007/s00253-015-6917-2 [DOI] [PubMed] [Google Scholar]
- Malik A. N., and Czajka A.. 2013. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction?Mitochondrion 13:481–492. doi:10.1016/j.mito.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Marcu R., Zheng Y., and Hawkins B. J.. 2017. Mitochondria and angiogenesis. Adv. Exp. Med. Biol. 982:371–406. doi:10.1007/978-3-319-55330-6_21 [DOI] [PubMed] [Google Scholar]
- McLamb B. L., Gibson A. J., Overman E. L., Stahl C., and Moeser A. J.. 2013. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One 8:e59838. doi:10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A. J., Klok C. V., Ryan K. A., Wooten J. G., Little D., Cook V. L., and Blikslager A. T.. 2007a. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G173–G181. doi:10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- Moeser A. J., Ryan K. A., Nighot P. K., and Blikslager A. T.. 2007b. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G413–G421. doi:10.1152/ajpgi.00304.2006 [DOI] [PubMed] [Google Scholar]
- Moran M., Moreno-Lastres D., Marin-Buera L., Arenas J., Martin M. A., and Ugalde C.. 2012. Mitochondrial respiratory chain dysfunction:Implications in neurodegeneration. Free Radic. Bio. Med. 53:595–609. doi:10.1016/j.freeradbiomed.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nguyen T. N., Padman B. S., and Lazarou M.. 2016. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 26:733–744. doi:10.1016/j.tcb.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Ochoa J. J., Pamplona R., Ramirez-Tortosa M. C., Granados-Principal S., Perez-Lopez P., Naudi A., Portero-Otin M., Lopez-Frias M., Battino M., and Quiles J. L.. 2011. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme q(10). Free Radic. Biol. Med. 50:1053–1064. doi:10.1016/j.freeradbiomed.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Pi D. A., Liu Y. L., Shi H. F., Li S., Odle J., Lin X., Zhu H. L., Chen F., Hou Y. Q., and Leng W. B.. 2014. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 25:456–462. doi:10.1016/j.jnutbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Pinto M., and Moraes C. T.. 2015. Mechanisms linking mtDNA damage and aging. Free Radic. Biol. Med. 85:250–258. doi:10.1016/j.freeradbiomed.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C. J., and Valko M.. 2017. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 38:592–607. doi:10.1016/j.tips.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Pramanik K. C., Boreddy S. R., and Srivastava S. K.. 2011. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One 6:e20151. doi:10.1371/journal.pone.0020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., and Dhama K.. 2014. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed. Res. Int. 2014:761264. doi:10.1155/2014/761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani V., Deep G., Singh R. K., Palle K., and Yadav U. C. S.. 2016. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 148:183–193. doi:10.1016/j.lfs.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Rosero D. S., Odle J., Moeser A. J., Boyd R. D., and van Heugten E.. 2015. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 114:1985–1992. doi:10.1017/S000711451500392X [DOI] [PubMed] [Google Scholar]
- Saita S., Shirane M., and Nakayama K. I.. 2013. Selective escape of proteins from the mitochondria during mitophagy. Nat. Commun. 4:1410. doi:10.1038/ncomms2400 [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R., and Elazar Z.. 2011. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36:30–38. doi:10.1016/j.tibs.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Smith F., Clark J. E., Overman B. L., Tozel C. C., Huang J. H., Rivier J. E., Blikslager A. T., and Moeser A. J.. 2010. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G352–G363. doi:10.1152/ajpgi.00081.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. Z., and Macleod K. F.. 2016. In brief: mitophagy: mechanisms and role in human disease. J. Pathol. 240:253–255. doi:10.1002/path.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarkova Z., Kuka S., Racay P., Lehotsky J., Dobrota D., Mistuna D., and Kaplan P.. 2011. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 60:281–289. ISSN: 0862-8408. [DOI] [PubMed] [Google Scholar]
- Venditti P., Di Stefano L., and Di Meo S.. 2013. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13:71–82. doi:10.1016/j.mito.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang C., Wu G., Sun Y., Wang B., He B., Dai Z., and Wu Z.. 2015. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 145:25–31. doi:10.3945/jn.114.202515 [DOI] [PubMed] [Google Scholar]
- Wijtten P. J. A., van der Meulen J., and Verstegen M. W. A.. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Brit. J. Nutr. 105:967–981. doi:10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Xiao K., Song Z. H., Jiao L. F., Ke Y. L., and Hu C. H.. 2014. Developmental changes of TGF-β1 and Smads signaling pathway in intestinal adaption of weaned pigs. PLoS One 9:e104589. doi:10.1371/journal.pone.0104589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., and Wang T.. 2014. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 30:584–589. doi:10.1016/j.nut.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Yin J., Duan J. L., Cui Z. J., Ren W. K., Li T. J., and Yin Y. L.. 2015. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 5:15479–15486. doi:10.1039/c4ra13557a [Google Scholar]
- Yin J., Wu M. M., Xiao H., Ren W. K., Duan J. L., Yang G., Li T. J., and Yin Y. L.. 2014. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92:612–619. doi:10.2527/jas.2013–6986 [DOI] [PubMed] [Google Scholar]
- Zhang S. J., Li X., Li L., and Yan X. H.. 2011. Autophagy up-regulation by early weaning in the liver, spleen and skeletal muscle of piglets. Br. J. Nutr. 106:213–217. doi:10.1017/S0007114511001000 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng P., Yu B., He J., Yu J., Mao X. B., Wang J. X., Luo J. Q., Huang Z. Q., Cheng G. X., and Chen D. W.. 2016. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets. J. Anim. Sci. 94:173–184. doi:10.2527/jas.2015–9530 [DOI] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Tian G., Luo Y. H., Mao X. B., Zhang K. Y., Che L. Q., and Chen D. W.. 2013. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br. J. Nutr. 109:2253–2260. doi:10.1017/S0007114512004321 [DOI] [PubMed] [Google Scholar]
- Zhu L. H., Zhao K. L., Chen X. L., and Xu J. X.. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90:2581–2589. doi:10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]
- Zigdon H., Kogot-Levin A., Park J. W., Goldschmidt R., Kelly S., Merrill A. H., Scherz A., Pewzner-Jung Y., Saada A., and Futerman A. H.. 2013. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 288:4947–4956. doi:10.1074/jbc.M112.402719 [DOI] [PMC free article] [PubMed] [Google Scholar]