Abstract
Objective
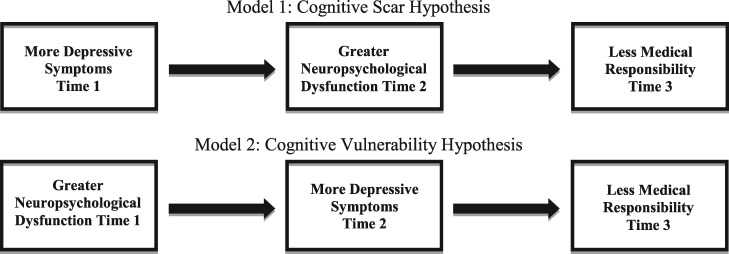
Given the increased risk for cognitive deficits and development of depressive symptoms in youth with spina bifida (SB), this study aimed to examine two pathways through which depressive symptoms and neuropsychological dysfunction may be associated with medical autonomy in this population: (1) depressive symptoms as predictors of medical autonomy as mediated by attention/executive functioning (the cognitive scarring model), and (2) attention/executive functioning as predictors of medical autonomy as mediated by depressive symptoms (the cognitive vulnerability model).
Methods
Participants were recruited as part of a larger, longitudinal study, and included 114 youth with SB (M age = 10.96 at Time 1), their parents, and teachers. Neuropsychological constructs included attention, working memory, and planning/organizing abilities, which were measured with questionnaire and performance-based data. Depressive symptoms and medical responsibility were assessed via questionnaires from multiple respondents.
Results
Bootstrapped mediation analyses revealed that teacher-reported depressive symptoms significantly mediated the relations between neuropsychological functioning (i.e., attention and working memory) and medical responsibility (all p’s < .05); neuropsychological dysfunction did not mediate the relationship between depressive symptoms and medical responsibility.
Conclusions
One way in which neurocognitive dysfunction may hinder the development of medical autonomy in youth with SB is through an increased risk for depressive symptoms.
Keywords: depression, health behavior, neuropsychology, spina bifida
Spina bifida (SB) is a relatively common congenital birth defect that results from failure of the neural tube to close during embryonic development (Mahmood, Dicianno, & Bellin, 2011). SB is a heterogeneous condition, with the spinal lesion level affecting condition severity and individual functioning across several domains, including motor and orthopedic difficulties, bladder and bowel dysfunction, and neurological complications (e.g., Chiari II malformation, hydrocephalus, and epilepsy; Copp et al., 2015). In addition, individuals with SB are at risk for secondary health complications, such as obesity, urinary tract infections, pressure sores, and shunt infections/malfunctions (Copp et al., 2015).
Youth with SB must adhere to a lifelong, daily medical regimen (Copp et al., 2015; O’Hara & Holmbeck, 2013), and the transition of responsibility for managing this medical regimen from parents to youth has become a critical component of development (Beacham & Deatrick, 2013). Although many youth with SB have an interest in becoming autonomous with respect to their medical responsibilities (e.g., bladder and bowel programs, skin checks; Holmbeck & Devine, 2010), individuals with SB often exhibit developmental delays in self-help skills, resulting in lower levels or a delay in the acquisition of independent functioning (Andren & Grimby, 2004; Holmbeck & Devine, 2010). Additionally, independence in managing medical responsibilities relies on physical (e.g., strength, dexterity), cognitive (e.g., executive functioning), and psychosocial (e.g., emotional maturity; Beacham & Deatrick, 2013; Modi et al., 2012) abilities, all of which may pose significant challenges for youth with SB. Despite these challenges, longitudinal findings support a developmental trajectory where the majority of youth with SB gradually gain responsibility for medical tasks, such as catheterization and bowel program management, over time (Psihogios, Kolbuck, & Holmbeck, 2015; Stepansky, Roache, Holmbeck, & Schultz, 2010). Given that increased responsibility for one’s medical regimen allows youth with a chronic medical condition to advance developmentally (e.g., an increase in time spent with peers), it is important to understand processes that influence the attainment of medical autonomy in youth with SB.
Few studies have been conducted to isolate modifiable risk factors that are associated with medical autonomy in youth with SB. One potentially important modifiable, individual factor is depressive symptomology. Depressive symptoms were found to be associated with decreased competency in completing self-management activities in adults with SB (Bellin et al., 2010). Although research has shown that youth with SB, especially adolescents, are at a significantly greater risk for developing depressive symptoms compared with healthy peers (Appleton et al. 1997; Holmbeck et al., 2003), the relationship between depressive symptoms and attainment of medical responsibility has yet to be studied in youth with SB. It is possible that depressive symptoms compromise medical autonomy by decreasing youth’s decision-making abilities and attention, which are required to complete health-care-related tasks on a daily basis (Modi et al., 2012). In other illness populations (e.g., type 1 diabetes), youth depressive symptoms have been associated with a decrease in motivation to complete medical tasks (Guo et al., 2013) as well as increased parent responsibility for medical tasks (Helgeson, Reynolds, Siminerio, Escobar, & Becker, 2008). Therefore, the processes through which depression may influence autonomy in medical care for youth with SB should be examined.
One possible mechanism is that depressive symptoms may disrupt neuropsychological functioning, leading to persistent cognitive deficits (the cognitive scarring model; Allott, Fisher, Amminger, Goodall, & Hetrick, 2016). Studies with otherwise healthy adolescents have found associations between the experience of acute depressive symptoms and executive functioning, memory, and attentional impairments (Wilkinson & Goodyer, 2006). Youth with SB are susceptible to neuropsychological impairments because of neurological factors (e.g., presence of hydrocephalus, Chiari II malformation, and shunt complication; Copp et al., 2015). Specifically, they experience difficulties with attention and executive functioning (e.g., problem-solving, initiation, working memory, planning, organization, and self-monitoring), and these preexisting deficits may be exacerbated by depressive symptoms. Such deficits could affect the higher-order cognitive skills needed to attain autonomy in completing medical tasks.
An alternate hypothesis is that the difficulties with executive functioning and attention experienced by youth with SB are primarily responsible for decreased medical responsibility. Executive dysfunction has been predictive of lower levels of medical responsibility for youth with SB (Psihogios et al., 2016). However, it is possible that neuropsychological difficulties reduce these youth’s ability to cope and problem solve when confronted with stressors and, as a consequence, make them more susceptible to depressive symptoms (the cognitive vulnerability model; Lee, Hermens, Porter, & Redoblado-Hodge, 2012). In other words, depressive symptoms may mediate the relationship between neuropsychological deficits and decreased attainment of medical autonomy for youth with SB. In fact, deficits in executive functioning and attention have been found to put individuals with SB at risk for the development of future depressive symptoms (Lennon, Klages, Amaro, Murray, & Holmbeck, 2015). Thus, it is also possible that neuropsychological impairment hinders the development of medical autonomy in youth with SB via increased depressive symptoms.
Despite our knowledge that both depressive symptoms and neuropsychological functioning are related to the development of medical autonomy (and each other), few studies to date have examined the interrelationships of these variables in youth with SB (Donlau et al., 2011; O’Hara & Holmbeck, 2013; Psihogios et al., 2016). Therefore, the current study examined relations between depressive symptoms, attention/executive functioning, and medical autonomy in youth with SB. Specifically, this study explored two potential pathways to delays in medical autonomy: (1) depressive symptoms as predictors of medical autonomy as mediated by attention/executive functioning (the cognitive scarring model; Figure 1, Model 1), and (2) attention/executive functioning as predictors of medical autonomy as mediated by depressive symptoms (the cognitive vulnerability model; Figure 1, Model 2). It was hypothesized that greater depressive symptoms would be associated with worse neuropsychological functioning, which, in turn, would predict lower levels of medical autonomy. With respect to the alternate pathway, it was hypothesized that poorer neuropsychological functioning would be associated with greater depressive symptoms, which, in turn, would predict lower levels of medical autonomy. Additionally, the current study sought to address gaps in the literature by testing these models with longitudinal, multimethod, and multiinformant data.
Methods
Participants
Participants were recruited for an ongoing, larger longitudinal study examining family, neuropsychological, and psychological functioning among children and adolescents with SB (Devine et al., 2012). The present study examined three waves of data that were collected every 2 years (ages 8–15 years at Time 1). Families of youth with SB were recruited from four hospitals and a statewide SB association in the Midwest. Families were sent recruitment letters and were also approached during regularly scheduled clinic visits. Interested families were screened by phone or in-person by a member of the research team, and were invited to participate if their child met the following criteria: (a) diagnosis of SB (types included myelomeningocele, lipomeningocele, and myelocystocele); (b) age 8–15 years at Time 1; (c) ability to speak and read English or Spanish; (d) involvement of at least one primary caregiver; and (e) residence within 300 miles of laboratory (to allow for home-based data collections).
In total, 246 families were approached during recruitment, of which 163 initially agreed to participate. After this initial recruitment, 21 families could not be contacted or later declined, and 2 families did not meet all of the inclusion criteria. The final sample of participants included 140 families of children with SB (53.6% female; 53.5% Caucasian; M age = 11.40 years). Children of families who declined participation did not differ from those who agreed to participate with respect to type of SB (e.g., myelomeningocele vs. other), χ2 (1) = 0.0002, p > .05, shunt status, χ2 (1) = 0.003, p > .05, or occurrence of shunt infections χ2 (1) = 1.08, p > .05.
Additionally, because self-management tasks necessitate a certain cognitive capacity, the present study did not include participants who functioned intellectually at two or more SDs below the population mean (i.e., an estimated intelligence quotient [IQ] score <70; American Psychiatric Association, 2013). At Time 1, 26 of 140 (19%) individuals had an estimated IQ < 70 or did not complete the brief neuropsychological battery because of low comprehension. Therefore, the final sample used in the analyses included 114 children and adolescents with SB (52.63% female; Mage = 10.96 years (SD = 2.43); 51.75% Caucasian, 11.40% African-American, 17.54% Hispanic, 5.26% Other; Table I).
Table I.
Youth Demographic and SB Information at Time 1
| Youth (N = 114) M (SD) or N (%) | |
|---|---|
| Gender: female | 60 (52.63%) |
| Age | 10.96 (2.43) |
| Race | |
| Caucasian | 59 (51.75%) |
| African-American/Black | 13 (11.40%) |
| Hispanic | 20 (17.54%) |
| Other | 6 (5.26%) |
| Family SES | 42.32 (14.99) |
| IQ | 92.41 (15.67) |
| SB type | |
| Myelomeningocele | 85(74.56%) |
| Lipomeningocele | 9 (7.89%) |
| Not sure/not reported | 13 (4.40%) |
| Lesion level | |
| Thoracic | 11 (9.65%) |
| Lumbar | 74 (64.9%) |
| Sacral | 23 (20.18%) |
| Unknown/not reported | 6 (5.26%) |
| Shunt: present | 73 (64.04%) |
Note. IQ = intelligence quotient; SB = spina bifida; SES = socioeconomic status.
Of the 114 participants that were included at Time 1, 92 (81%) participated at Time 2, and 84 (74%) participated at Time 3. Youth who did not participate at either Time 2 or Time 3 (n = 38, 33%) did not differ significantly from youth who participated at all three data collection waves with respect to gender, socioeconomic status (SES), type of SB, lesion level, shunt status, or IQ. However, youth who did not participate at either Times 2 or 3 were significantly older at Time 1 (M = 11.74 compared with 10.61; t(106) = −2.28, p = .03).
Procedure
This study was approved by university and hospital institutional review boards. Trained undergraduate and graduate student research assistants collected data from families during two separate 3-hr home visits at Time 1, and one 3-hr home visit at both Time 2 and Time 3. Informed consent from parents and assent from youth were obtained before the start of the first visit. Parents also filled out releases of information to permit data collection from medical charts, health professionals, and teachers. During data collection, youth and their parents completed questionnaires independently. The questionnaires were offered in both English and Spanish; questionnaires that were only available in English were adapted for Spanish speakers by a translation team using back translation procedures. Additionally, research assistants completed a brief neuropsychological battery with the child. Families received monetary compensation of $150 and small gifts (e.g., logo t-shirts, pens, water bottles) for participating.
Measures
Demographics
Parents reported on youth and family demographic information through questionnaires at Time 1, including age, gender, race, and ethnicity. The Hollingshead Index of SES was computed to assess SES based on parents’ education and occupation, with higher scores indicating higher SES (Hollingshead, 1975).
Youth IQ
At Time 1, youth were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), which were used to estimate a Full Scale IQ score. These subtests have demonstrated high levels of internal consistency for youth aged 6–16 years (α = .89 for Vocabulary, α = .92 for Matrix Reasoning; Wechsler, 1999).
Depressive Symptoms
Depressive symptoms were measured via child-, parent-, and teacher-report. Children completed the Child Depression Inventory (CDI) at Time 1 and Time 2 (Kovacs, 1992). The CDI is a 27-item self-rated measure of depressive symptoms for children and adolescents, which demonstrated acceptable levels of internal consistency at both Time 1 and Time 2 (α = .82; α = .78). Parents completed the Child Behavior Checklist (CBCL) and teachers completed the Teacher Report Form (TRF) at Time 1 and Time 2 (Achenbach, 1991; Achenbach & Rescorla, 2001). The CBCL and TRF assess behavioral and emotional problems over the past 6 and 2 months, respectively. For this study, a subscale of depressive symptoms was derived based on 15 items from in the Anxious/Depressed and Withdrawn/Depressed subscales to form a CBCL-Depression Scale (CBCL-D; Clarke, Lewinsohn, Hops, & Seeley, 1992). As this adapted scale has not been normed, raw mean total scores were calculated in lieu of T-scores, which demonstrated adequate internal consistency at Time 1 and Time 2 for mother- (α = .74; α = .64), father- (α = .69; α = .71), and teacher-report (α = .78; α=.84).
Neuropsychological Functions
Child attention and executive functions were assessed via performance-based measures, as well as parent- and teacher-report, at Time 1 and Time 2. The following areas of neuropsychological functioning were examined: (1) attention, (2) working memory, and (3) planning and organizational skills.
Attention
Parents and teachers completed the Swanson, Nolan, and Pelham Teacher and Parent Rating Scale (SNAP-IV; Swanson, 1992). The SNAP-IV comprises 18 items derived from criteria for Attention-Deficit/Hyperactivity Disorder from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). Mean subscale scores were calculated for the inattention subscale, which demonstrated high internal consistency at Time 1 and Time 2 for mother (α = .93; α = .95), father (α = .92; α=.93), and teacher-report (α = .94; α = .95). Parents and teachers also completed the Attention Problems subscale of the CBCL and TRF, respectively (Achenbach & Rescorla, 2001). This subscale demonstrated adequate levels of internal consistency in the current study (α = .73–.82). At Time 1 and Time 2, youth were administered a performance-based measure of attention, the Number Detection (ND) Subtest of the Cognitive Assessment System (CAS; Naglieri & Das, 1997). Internal consistency reliability (α = .77) and test-retest reliability (r = .77) for the ND subtest are high across age groups (Naglieri & Das, 1997).
Working Memory
Parents and teachers completed the Working Memory subscale of the Behavior Rating Inventory of Executive Functioning (BRIEF, Gioia et al., 2000a). This is a valid measure of multiple domains of executive functioning, including working memory, over the past 6 months (Gioia et al., 2000a, 2000b). For the BRIEF, higher scores indicate greater impairment. The Working Memory subscale demonstrated high internal consistency at Time 1 and Time 2 for mother- (α = .90; α = .91), father- (α = .90; α = .89), and teacher-report (α = .91; α = .92). Youth were administered the Digit Span subtest of the Wechsler Intelligence Scale for Children (WISC-IV; Wechsler, 2003) as a performance-based measure of working memory ability. The Digit Span subtest has good internal consistency (r = .87) and test-retest reliability (r = .83; Williams, Weiss, & Rolfhus, 2003).
Planning and Organizational Skills
Parents and teachers completed the Plan/Organize and the Organization of Materials subscales of the BRIEF (Gioia et al., 2000a, 2000b). The Plan/Organize subscale measures the ability to organize one’s thoughts and to plan one’s actions to achieve present and future goals. This subscale demonstrated high internal consistency at Time 1 and Time 2 for mother- (α = .92; α = .92), father- (α = .90; α = .87), and teacher-report (α = .91; α = .92). The Organization of Materials subscales measures the child’s tendency to keep his or her spaces neat and orderly. High internal consistency was found at Time 1 and Time 2 for mother- (α = .88; α = .86), father- (α = .88; α = .84), and teacher-report (α = .79; α = .78). For a performance-based measure of planning skills, youth were administered the Planned Connections (PCn) subtest of the CAS. The PCn subtest has high internal consistency (α = .77) and test-retest reliability (r = .73) (Naglieri & Das, 1997).
Medical Responsibility
Parents and youth completed the Sharing of Spina Bifida Management Responsibilities Scale (SOSBMR), which is an adaptation of the Diabetes Family Responsibility Questionnaire (Anderson, Auslander, Jung, Miller, & Santiago, 1990). The SOSBMR assesses division of SB responsibilities and health-related tasks within the family (e.g., remembering to catheterize regularly). Participants rated who was primarily responsible for each task (e.g., parent, child, equal, or not applicable). For each task item, a score of “1” indicates the parent is primarily responsible, “2” indicates responsibility is shared equally between the parent and child, and “3” indicates the child was primarily responsible. Mean scores were calculated for the total responsibility scale. Items that participants rated as “not applicable” were excluded from the total scale score. Previous studies have not included internal consistency scores for the total scale score of this measure, as reliability software uses listwise deletion when computing alpha coefficients, and several items include a “not applicable” response (Psihogios, Kolbuck, & Holmbeck, 2015).
The mean scores of the CDI, CBCL, and SNAP-IV at Time 1 and Time 2 fell within the average range relative to the normative data samples. Across participant gender and age, BRIEF Working Memory subscale mean t-scores fell between 54 and 61 for parent-report and 47 and 60 for teacher-report, Plan/Organize subscale mean t-scores fell between 53 and 61 for parent-report and 59 and 73 for teacher-report, and Organization subscale mean t-scores fell between 51 and 55 for parent-report and 47 and 59 for teacher-report. It should be noted that these mean scores may be an overestimation of the overall study sample's executive functioning abilities, as lower functioning individuals were excluded from analyses. At Times 1 and 2, respectively, 24 and 18% of participants were reported to have borderline or clinically significant attention problems via the CBCL. Mean performance on both CAS subtests fell in the low average range at Time 1 and Time 2 (mean scaled scores = 6–7) relative to the normative data. Finally, mean performance on the Digit Span subtest was average at both Time 1 (M = 8.06; SD = 2.88) and Time 2 (M = 8.64, SD = 2.96).
Statistical Treatment
All analyses included the following covariates: child lesion level, age, SES, and target variables at previous waves of data collection. Given the concerns about statistical overcorrection and the contention that IQ should not be controlled for in examinations of specific cognitive processes in neurodevelopmental disorders, IQ was not included as a covariate in this study (Dennis et al., 2009). To decrease the number of analyses and reduce the possibility of shared method variance, composite scores were created when possible that included multiple reporters and/or measures (Holmbeck et al., 2002). Composite scores were created if they met the following criteria: Pearson correlation coefficients were run to assess for adequate associations (r ≥ .40) between two reporters and/or measures, and Cronbach alphas were computed to assess for adequate internal consistency (α > .60) among three or more reporters and/or measures.
Two meditational models were tested using Preacher and Hayes’ (2008) bootstrapping methods. The cognitive scarring model examined the impact of youth depressive symptoms at Time 1 on SB medical autonomy at Time 3, as mediated by neuropsychological functioning (i.e., attention, working memory, and planning/organizing ability) at Time 2. The cognitive vulnerability model examined the impact of neuropsychological deficits at Time 1 on SB medical autonomy at Time 3, as mediated by depressive symptoms at Time 2. Bootstrapping has been validated in the literature and is preferred over other methods, as bootstrapping is less conservative and reduces the possibility of type II errors (Preacher & Hayes, 2008).
Results
Preliminary Analyses
Ms, SDs, and bivariate correlations among study variables are displayed in Table II. Mother-report, father-report, teacher-report, and performance-based assessment of youth attention, working memory, and planning/organizing abilities were aggregated to form global composite variables. Medical responsibility data were also aggregated across parent and youth reports. While mother- and father-report of youth depressive symptoms could be combined across reporters, they were not adequately correlated with self- or teacher-report of youth depressive symptoms. Thus, self-, parent-, and teacher-report of youth depressive symptoms were examined separately in the analyses.
Table II.
Correlations among Depressive Symptoms, Neuropsychological Variables, Medical Responsibility Variables, and Covariates
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. CDI | – | .12 | .14 | .17 | .31** | .27* | .15 | −.03 | −.18 | −.16 |
| 2. CBCL-P–Depressiona | .24* | – | −.03 | .30** | .12 | .23* | −.09 | .03 | .15 | −.02 |
| 3. TRF–Depressiona | .09 | .30* | – | .51** | .50** | .42** | −.40** | −.16 | −.09 | .01 |
| 4. Attentiona | −.05 | .28** | .52** | – | .80** | .82** | −.41** | −.17 | .08 | .03 |
| 5. Working memorya | .08 | .21* | .48** | .84** | – | .79** | −.38** | −.28** | −.11 | −.13 |
| 6. Plan/organizinga | .01 | .30** | .44** | .78** | .76** | – | −.24* | −.28* | .02 | −.09 |
| 7. Medical responsibility | .04 | −.15 | −.28* | −.33** | −.26* | −.19 | – | .54** | −.03 | −.15 |
| 8. Ageb | .17 | −.02 | −.02 | −.09 | −.12 | −.06 | .54** | – | .05 | .01 |
| 9. SESb | −.12 | .21 | .02 | −.07 | −.08 | .04 | −.03 | .05 | – | .03 |
| 10. Lesion levelb | −.01 | .03 | .16 | −.07 | −.09 | −.07 | −.15 | .01 | .03 | – |
| M (SD) T1 depressive, T2 neuropsychological factors | 1.1(.1) | −.03(1) | −.08(.9) | −.01(.8) | −.01(.7) | .01(.7) | 2.2(.4) | 10.9(2.4) | 42.3(15) | – |
| M (SD) T2 depressive, T1 neuropsychological factors | 1.2(.2) | .00(.9) | .00(1.0) | −.01(.8) | .02(.7) | −.02(.7) | – | – | – | – |
Notes. Values above the diagonal reflect variables from the first model (i.e., Time 1 depressive symptoms, Time 2 neuropsychological factors). Values below the diagonal reflect variables from the alternate model (i.e., Time 1 neuropsychological factors, Time 2 depressive symptoms). CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory; P = parent-report; SES = socioeconomic status measured by Hollingshead Four Factor Index; TRF = Teacher Report Form; T = teacher-report. All cognitive variables were scored such that higher scores represent greater neuropsychological deficits in attention, working memory, and planning/organizing abilities.
These variables are based on standardized Z scores.
These variables are covariates. Descriptive statistics for lesion level are presented in Table 1. *p < .05, **p < .01.
Listwise deletion was used to handle missing data. Sample sizes for models with child-, parent-, and teacher-reported depressive symptoms at Time 1 were 67, 70, and 65, and at Time 2 were 67, 68, and 56, respectively. Missing data were because of attrition across time points. Further, while composites for parent-reported variables could accommodate missing data across time points from either mothers or fathers, fewer teachers participated at each time point. Assuming a power of .80, and an alpha of .05, a sample size of 78 is required to detect medium effect sizes, and a sample size of 36 is required to detect large effect sizes (Fritz & MacKinnon, 2007). Thus, the current study had enough power to detect effects between medium and large.
Mediation Analyses
Mediation analyses were conducted to examine the indirect effects of neuropsychological deficits and depressive symptoms on medical responsibility. Time 1 mediators and Time 2 medical responsibility scores were also entered as covariates. To maximize sample size and investigate differential relationships among depressive symptoms and individual cognitive deficits, each model was tested separately with the three neuropsychological factors (i.e., attention, working memory, and planning/organizing) and self-, parent-, and teacher-report of child depressive symptoms, for a total of nine models.
Model 1 (Cognitive Scar Hypothesis)
The first objective of this study was to examine if neuropsychological functioning mediated the impact of child depressive symptoms on medical responsibility in youth with SB longitudinally. Results indicated no significant indirect effects (all p’s > .05). When attention was examined as a mediator, there was a significant direct, positive effect of parent-reported child depressive symptoms at Time 1 on child medical responsibility at Time 3 (b =.27, SE = .12, t = 2.20, p = .03). This effect was only significant in the model examining attention as a mediator. The lack of significant bivariate correlation between these variables likely indicates statistical suppression; as a result, this finding will be regarded as a statistical artifact and will not be interpreted further (Pandey & Elliott, 2010). In the model using self-reported child depressive symptoms as the independent variable, greater dysfunction in working memory (b = −0.12, SE = .05, t = −2.32, p = .02) predicted less child medical responsibility at Time 3.1
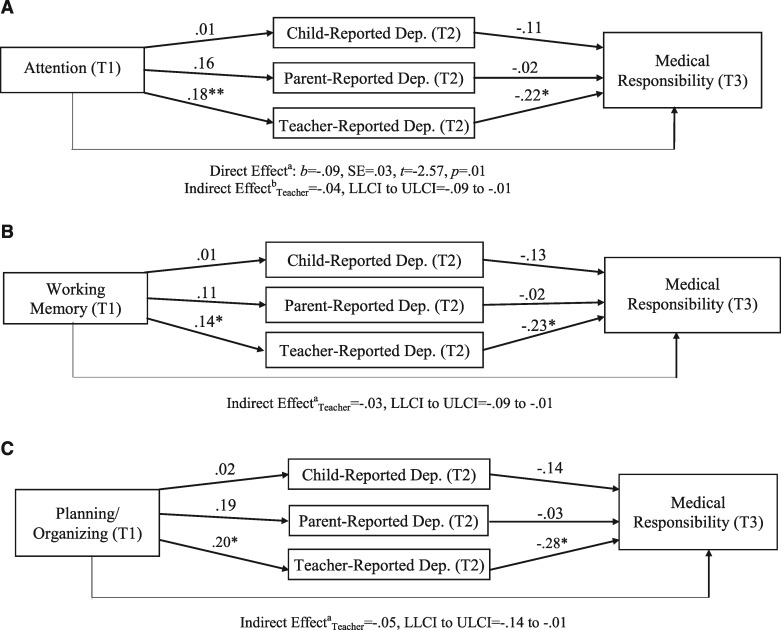
Model 2 (Cognitive Vulnerability Hypothesis)
The second model examined if child depressive symptoms mediated the longitudinal impact of neuropsychological functioning on medical responsibility in youth with SB. The results are presented in Figure 2a–c. Teacher-reported depressive symptoms at Time 2 significantly mediated the relationship between attention at Time 1 and child responsibility for medical care at Time 3 (estimated indirect effect = −.04, SE = .02, 95% LLCI to ULCI = −.09 to −.01). Teacher-reported depressive symptoms at Time 2 also significantly mediated the relationship between working memory at Time 1 and child medical responsibility at Time 3 (estimated indirect effect = −.03, SE = .02, 95% LLCI to ULCI = −.09 to −.01). The indirect effect of planning/organizing abilities on medical responsibility through teacher-reported depressive symptoms was significant (estimated indirect effect = −.05, SE = .03, 95% LLCI to ULCI = −.14 to −.01). However, because the magnitude of the direct effect of planning/organizing skills when adjusting for depressive symptoms was greater than the total effect, results likely indicated statistical suppression (MacKinnon, Krull & Lockwood, 2000). Therefore, this finding will be regarded as a statistical artifact.2
Figures 2.
Mediation models of child neuropsychological functioning at Time 1, depressive symptoms at Time 2, and medical responsibility at Time 3.1 (a) aDirect effect of attention on medical responsibility in model controlling for parent-reported depressive symptoms as a mediator; bindirect effect of attention on medical responsibility through teacher-reported depressive symptoms. Neither the total effect nor the direct effect was significant for the model controlling for teacher-reported depressive symptoms as a mediator. *p < .05; **p < .01. (b)cIndirect effect of working memory on medical responsibility through teacher-reported depressive symptoms. Neither the total effect nor the direct effect was significant for the model with a significant indirect effect. *p < .05. (c) dIndirect effect of planning/organizing on medical responsibility through teacher-reported depressive symptoms. Neither the total effect nor the direct effect was significant for the model with a significant indirect effect. *p < .05.
1For Figure 2a–c, analyses were tested separately for each of the three mediators and three independent variables. In all models, attention, working memory, and planning/organizing represent global, composite factors.
Figure 1.
Mediational models of alternate pathways among depressive symptoms, neuropsychological functioning, and medical responsibility.
Discussion
The current study examined depressive symptoms and neurocognitive deficits in relation to medical responsibility over time in youth with (SB). To clarify the ambiguous relationship between depressive symptoms and neurocognitive deficits, two mediation pathways were tested. In the first pathway (i.e., the cognitive scarring model), neurocognitive deficits were expected to mediate the relationship between depressive symptoms and medical responsibility, such that more severe depressive symptoms would predict greater deficits in attention and executive functioning, and reduced cognitive abilities would predict lower medical responsibility. In the second pathway (i.e., the cognitive vulnerability model), depressive symptoms were expected to mediate the relationship between neurocognitive deficits and medical responsibility, such that more profound cognitive deficits would predict greater depressive symptoms, which would in turn predict lower levels of medical responsibility. This study found support for the latter model, in that deficits in attention and working memory were associated with medical responsibility via increased depressive symptoms. As medical autonomy constitutes a key developmental goal for many youth with SB (Holmbeck & Devine, 2010), it is important to understand how relevant cognitive and psychological factors, together, play a role in this gradually unfolding process.
Results indicated that the hypotheses were partially supported, and clarify the directional relationships among these individual factors in youth with SB. By demonstrating that greater deficits in attention and working memory were associated with less medical autonomy, these findings align with previous research and provide further support for the bio-neuropsychosocial model of medical autonomy and adherence in youth with SB (Holmbeck & Devine, 2010; Psihogios et al., 2016; Tuminello, Holmbeck, Olson, 2012). Although poor psychological adjustment in adolescents with other chronic illnesses has been found to complicate the transition of health-care responsibilities (Reed-Knight, Blount, & Gilleland, 2014), depressive symptoms have not been examined as a predictor of medical responsibility in youth with SB. Thus, the finding from the current study that youth with more severe depressive symptoms struggled to develop independence in their medical care represents a unique contribution to the literature.
Further, mediation results suggest that one way in which certain neurocognitive deficits may hinder the development of medical autonomy in youth with SB prospectively is through an increased risk for experiencing depressive symptoms. From a clinical perspective, it is possible that youth with poor attention and working memory have difficulty following instructions, completing multistep tasks, and planning for long-term goals (Kelly et al., 2012). This difficulty may lead to increased challenges across multiple environments (e.g., home, school, community) followed by decreased self-esteem and greater depressive symptoms, which may act as a barrier to achieving higher levels of medical autonomy. Thus, when conceptualizing the development of medical autonomy in SB, it is important to consider not only the neurocognitive impairments associated with SB, but also how these deficits may lead to increased depressive symptoms.
As neuropsychological functioning did not mediate the relationship between depressive symptoms and medical responsibility, the exact mechanism through which depressive symptoms may influence future medical autonomy remains unclear. Given the developmental stage of the participants in this study, it is also plausible that family or peer factors may better explain the relationship between depressive symptoms and medical responsibility. Indeed, peer conflict in adolescents has recently been identified as a barrier to medical autonomy in youth with SB (Psihogios et al., 2016). Other individual factors, such as lowered intrinsic motivation or self-efficacy in managing one’s medical condition, may explain this relationship as well. Moreover, executive functioning and attentional skills are not fully developed until the mid-twenties, and youth with SB continue to experience delays in the growth of these abilities through adolescence and emerging adulthood (Tarazi, Mahone, & Zabel, 2007). Given these differing developmental trajectories, it is possible that relations between cognitive deficits and depressive symptoms in SB change over time.
Interestingly, main effects in the mediation model were only found for teacher-report of youth depressive symptoms, but not self- or parent-report. Lennon, Klages, Amaro, Murray, and Holmbeck (2015) similarly found that neuropsychological functioning predicted teacher-report, but not self- or parent-report of youth internalizing symptoms. It is speculated that teachers may be more objective reporters of depressive symptoms in youth with SB than parents, as they are more readily able to compare a child with SB with other typically developing, same-aged peers (Lennon et al., 2015). On the other hand, it is possible that teachers who are unfamiliar with SB may misinterpret certain cognitive and behavioral features of SB (e.g., poor initiation, amotivation) as symptoms of depression. However, teachers may also be at a unique advantage, as they are more likely to observe depressive symptoms that have emerged because of cognitive challenges because they observe the child daily in a school setting. Additionally, the cognitive deficits present in youth with SB may impair their ability to accurately report on their own depressive symptoms (Wasserman, Holmbeck, Lennon, & Amaro, 2012). Future research should explore the different perceptions of depressive symptoms in SB based on reporter and environment.
This study had several strengths, including the utilization of multiple methods and reporters, performance- and questionnaire-based assessments of executive and attentional functioning, and a longitudinal, mediational design. However, there are several limitations that should be addressed in future work. As cognitive deficits are a direct consequence of SB itself, the unique relationships among neuropsychological factors, depressive symptoms, and medical autonomy may not generalize to youth with chronic illnesses that do not congenitally impact the central nervous system. While a strength of this study was its multimethod assessment of cognitive variables, only three domains of executive functioning were assessed with both performance and questionnaire measures. Future research should examine how other executive functions (e.g., inhibition, cognitive flexibility) relate to depressive symptoms and medical autonomy in SB, as these skills have been implicated in both the broader depression and self-management literatures (Bagner, Williams, Geffken, Silverstein, & Storch, 2007). Other limitations to consider include a small sample size that limited the potential to identify small mediation effects, and a relatively wide age range. Additionally, some individual subscales had relatively low internal consistency scores (e.g., the CBCL-D scale).
Finally, while this study aimed to investigate two pathways in depth, it did not examine other potentially important factors related to the medical autonomy process, such as peer relationships or parenting influences (Modi et al., 2012; O’Hara & Holmbeck, 2013). Indeed, past research has shown that peer and family factors, such as peer conflict and family cohesion, have a unique impact on medical autonomy in youth with SB (Psihogios et al., 2016). To date, no studies have examined the influence of community or macro-level (e.g., health-care system) factors on SB self-management outcomes. Inclusion of these broader dyad- and community-level influences in future research would help build a more comprehensive picture of how cognitive and affective functioning impacts medical autonomy over time in SB.
The results of the current study have important implications for promoting medical autonomy in youth with SB. First, building off of Modi et al.’s (2012) comprehensive model of pediatric self-management and Psihogios et al.’s (2016) bio-neuropsychosocial model for self-management in youth with SB, it appears that depressive symptoms, attention, and executive functioning are intertwined and have a unique impact on medical autonomy in this population. Second, depressive symptoms appear to be one pathway through which attention and executive impairment may hinder medical autonomy. This key finding paves the way for further research on other pathways that may mediate the impact of neuropsychological functioning on medical autonomy in SB. Further, given the increased prevalence of depressive symptoms in youth with SB (Holmbeck et al., 2003), this study serves as a guide for research on other factors that may explain the relationship between depressive symptoms and medical autonomy (e.g, intrinsic motivation, self-efficacy).
Clinical interventions aimed at facilitating the transfer of health-care responsibilities to the child may maximize treatment success by taking into account an individual’s level of depressive symptoms and executive and attentional skills. Psychological screenings have been shown to predict disease management in adolescents with type 1 diabetes (Hilliard, Herzer, Dolan, & Hood, 2011). Results from this study suggest that regular psychological screenings could help clinicians identify depressive symptoms early on that may be negatively impacting health autonomy in adolescents with SB. As families begin the transfer process, providers may also choose to incorporate specialized cognitive training programs (Stubberud, Langenbahn, Levine, Stanghelle, & Schanke, 2014) or assistive technologies for executive weaknesses (e.g., visual schedules) to support an adolescent with SB who is struggling in these areas.
Acknowledgments
The authors thank the Illinois Spina Bifida Association as well as staff of the spina bifida clinics at Ann & Robert H. Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Medical Center; Jenna Shapiro, M.A., for her assistance with the statistical analyses; the numerous undergraduate and graduate research assistants who helped with data collection and data entry; and finally, the parents, children, and teachers who participated in this study. Correspondence regarding this manuscript can be sent to gholmbe@luc.edu.
Footnotes
Consistent with reported results, mediation models using maximum likelihood estimation in MPlus; (Muthén & Muthén, 1998-2017) a direct effect of parent-reported child depressive symptoms on medical responsibility in the model with attention as a mediator (B = .06, SE = .03, 95% LLCI to ULCI = .004 to .11). An additional negative direct effect of teacher-reported child depressive symptoms on medical responsibility in the model with planning and organization skills as the mediator emerged (B = −.06, SE = .03, 95% LLCI to ULCI = −.14 to −.01).
Consistent with reported results, mediation models using maximum likelihood estimation in MPlus identified a significant indirect effect of teacher-reported child depressive symptoms on the relations between attention and medical responsibility (B = −.04, SE = .02, 95% LLCI to ULCI = −.09 to −.01) and working memory and medical responsibility (B = −.03, SE = .02, 95% LLCI to ULCI = −.09 to −.004). Also consistent with reported results, there was a significant indirect effect of teacher-reported child depressive symptoms on the relation between planning and organizing skills and medical responsibility (B = −.05, SE = .03, 95% LLCI to ULCI = −.13 to −.01), which was best explained by statistical suppression, as the direct effect (B = .05, SE = .05) was greater than the total effect (B = .00, SE = .05). Significant total and direct effects emerged for the relations between parent-reported child depressive symptoms and both attention (B = −.08, SE = .03, total effect 95% LLCI to ULCI = −.14 to −.01; B = −.08, SE =.03, direct effect 95% LLCI to ULCI = −.15 to −.02) and working memory (B = −.07, SE = .03, total effect 95% LLCI to ULCI = −.13 to −.002; B = −.07, SE = .03, direct effect 95% LLCI to ULCI = −.14 to −.003).
Funding
This research was supported in part by grants from the National Institute of Nursing Research and the Office of Behavioral and Social Sciences Research (grant number R01 NR016235), National Institute of Child Health and Human Development (grant number R01 HD048629), and the March of Dimes Birth Defects Foundation (grant number 12-FY13-271). This study is part of an ongoing, longitudinal study.
Conflicts of interest: None declared.
References
- Achenbach T. M. (1991). Manual for the child behavior checklist/4-18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- Achenbach T. M., Rescorla L. A. (2001). ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA. [Google Scholar]
- Allott K., Fisher C. A., Amminger G. P., Goodall J., Hetrick S. (2016). Characterizing neurocognitive impairment in young people with major depression: State, trait, or scar? Brain and Behavior, 6, e00527.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. J., Auslander W. F., Jung K. C., Miller J. P., Santiago J. V. (1990). Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology, 15, 477–492. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Andren E., Grimby G. (2004). Dependence in daily activities and life satisfaction in adult subjects with cerebral palsy or spina bifida: A follow-up study. Disability and Rehabilitation, 26, 528–536. 10.1080/09638280410001672490 [DOI] [PubMed] [Google Scholar]
- Appleton P. L., Ellis N. C., Minchom P. E., Lawson V., Böll V., Jones P. (1997). Depressive symptoms and self-concept in young people with spina bifida. Journal of Pediatric Psychology, 22, 707–722. [DOI] [PubMed] [Google Scholar]
- Bagner D., Williams L., Geffken G., Silverstein J., Storch E. (2007). Type 1 diabetes in youth: The relationship between adherence and executive functioning. Children's Health Care, 36, 169–179. [Google Scholar]
- Bellin M., Zabel T., Dicianno B., Levey E., Garver K., Linroth R., Braun P. (2010). Correlates of depressive and anxiety symptoms in young adults with spina bifida. Journal of Pediatric Psychology, 35, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham B., Deatrick J. (2013). Health care autonomy in children with chronic conditions: Implications for self-care and family management. The Nursing Clinics of North America, 48, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G. N., Lewinsohn P. M., Hops H., Seeley J. R. (1992). A self-and parent-report measure of adolescent depression: The Child Behavior Checklist Depression scale (CBCL-D). Behavioral Assessment, 14, 443–463. [Google Scholar]
- Copp A., Adzick N., Chitty L., Fletcher J., Holmbeck G., Shaw G. (2015). Spina bifida. Nature Reviews Disease Primers, 1, 15007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine K. A., Holmbeck G. N., Gayes L., Purnell J. Q. (2012). Friendships of children and adolescents with spina bifida: Social adjustment, social performance, and social skills. Journal of Pediatric Psychology, 37, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Francis D. J., Cirino P. T., Schachar R., Barnes M. A., Fletcher J. M. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlau M., Imms C., Glad Mattsson G., Mattsson S., Sjörs A., Falkmer T. (2011). Children and youth with myelomeningocele’s independence in managing clean intermittent catheterization in familiar settings. Acta Paediatrica, 100, 429–438. [DOI] [PubMed] [Google Scholar]
- Fritz M. S., MacKinnon D. P. (2007). Required sample size to detect the mediated effect. Psychological Science, 18, 233–239. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. (2000a). BRIEF behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. (2000b). Test review: Behavior rating inventory of executive function. Child Neuropsychology, 6, 235–238. [DOI] [PubMed] [Google Scholar]
- Guo J., Whittemore R., Grey M., Wang J., Zhou Z., He G. (2013). Diabetes self management, depressive symptoms, quality of life and metabolic control in youth with type 1 diabetes in China. Journal of Clinical Nursing, 22, 69–79. [DOI] [PubMed] [Google Scholar]
- Helgeson V. S., Reynolds K. A., Siminerio L., Escobar O., Becker D. (2008). Parent and adolescent distribution of responsibility for diabetes self-care: Links to health outcomes. Journal of Pediatric Psychology, 33, 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Herzer M., Dolan L. M., Hood K. K. (2011). Psychological screening in adolescents with type 1 diabetes predicts outcomes one year later. Diabetes Research and Clinical Practice, 94, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G., Devine K. (2010). Psychosocial and family functioning in spina bifida. Developmental Disabilities Research Reviews, 16, 40–46. 10.1002/ddrr.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G., Li S., Schurman J., Friedman D., Coakley R. (2002). Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology, 27, 5–18. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Westhoven V. C., Phillips W. S., Bowers R., Gruse C., Nikolopoulos T., Totura C. M., Davison K. (2003). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology, 71, 782–796. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor index of social status. New Haven, CT: Yale University. [Google Scholar]
- Kelly N., Ammerman R., Rausch J., Ris M., Yeates K., Oppenheimer S., Enrile B. (2012). Executive functioning and psychological adjustment in children and youth with spina bifida. Child Neuropsychology, 18, 417–431. [DOI] [PubMed] [Google Scholar]
- Kovacs M. (1992). Children's depression inventory—Manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Lee R. S., Hermens D. F., Porter M. A., Redoblado-Hodge M. A. (2012). A meta-analysis of cognitive deficits in first-episode major depressive disorder. Journal of Affective Disorders, 140, 113–124. [DOI] [PubMed] [Google Scholar]
- Lennon J., Klages K., Amaro C., Murray C., Holmbeck G. (2015). Longitudinal study of neuropsychological functioning and internalizing symptoms in youth with spina bifida: Social competence as a mediator. Journal of Pediatric Psychology, 40, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood D., Dicianno B., Bellin M. (2011). Self-management, preventable conditions and assessment of care among young adults with myelomeningocele. Child: Care, Health and Development, 37, 861–865. [DOI] [PubMed] [Google Scholar]
- MacKinnon D. P., Krull J. L., Lockwood C. M. (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science, 1, 173–181. 10.1023/A:1026595011371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., Guilfoyle S. M., Gray W. N., Drotar D. (2012). Pediatric self-management: A framework for research, practice, and policy. Pediatrics, 129, E473–E485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998-2017). Mplus user’s guide, 8th edn. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Naglieri J. A., Das J. P. (1997). Cognitive assessment system administration and scoring manual. Itasca, IL: Riverside Publishing. [Google Scholar]
- O'Hara L., Holmbeck G. (2013). Executive functions and parenting behaviors in association with medical adherence and autonomy among youth with spina bifida. Journal of Pediatric Psychology, 38, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Elliott W. (2010). Suppressor variables in social work research: Ways to identify in multiple regression models. Journal of the Society for Social Work and Research, 1, 28–40. [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Psihogios A. M., Murray C., Zebracki K., Acevedo L., Holmbeck G. N. (2016). Testing the utility of a bio-neuropsychosocial model for predicting medical adherence and responsibility during early adolescence in youth with spina bifida. Journal of Pediatric Psychology, 42, 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A., Kolbuck V., Holmbeck G. (2015). Condition self-management in pediatric spina bifida: A longitudinal investigation of medical adherence, responsibility-sharing, and independence skills. Journal of Pediatric Psychology, 40, 790–803. 10.1093/jpepsy/jsv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Knight B., Blount R. L., Gilleland J. (2014). The transition of health care responsibility from parents to youth diagnosed with chronic illness: A developmental systems perspective. Families, Systems and Health: The Journal of Collaborative Family Healthcare, 32, 219–234. [DOI] [PubMed] [Google Scholar]
- Stepansky M., Roache C., Holmbeck G., Schultz K. (2010). Medical adherence in young adolescents with spina bifida: Longitudinal associations with family functioning. Journal of Pediatric Psychology, 35, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubberud J., Langenbahn D., Levine B., Stanghelle J., Schanke A.-K. (2014). Goal Management Training improves everyday executive functioning for persons with spina bifida: Self-and informant reports six months post-training. Neuropsychological Rehabilitation, 24, 26–60. [DOI] [PubMed] [Google Scholar]
- Swanson J. M. (1992). School-based assessments and interventions for ADD students. Irvina, CA: KC Publishing. [Google Scholar]
- Tarazi R. A., Mahone E. M., Zabel T. A. (2007). Self-care independence in children with neurological disorders: An interactional model of adaptive demands and executive dysfunction. Rehabilitation Psychology, 52, 196–205. 10.1037/0090-5550.52.2.196 [DOI] [Google Scholar]
- Tuminello E., Holmbeck G., Olson R. (2012). Executive functions in adolescents with spina bifida: Relations with autonomy development and parental intrusiveness. Child Neuropsychology, 18, 105–124. 10.1080/09297049.2011.590470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R. M., Holmbeck G. N., Lennon J. M., Amaro C. M. (2012). A longitudinal assessment of early pubertal timing as a predictor of psychosocial changes in adolescent girls with and without spina bifida. Journal of Pediatric Psychology, 37, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. O., Goodyer I. M. (2006). Attention difficulties and mood-related ruminative response style in adolescents with unipolar depression. Journal of Child Psychology and Psychiatry, 47, 1284–1291. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1999). WASI: Wechsler abbreviated scale of intelligence manual. San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]
- Wechsler D. (2003). WISC-IV administration and scoring manual. San Antonio, Texas: (WISC-IV Technical Report No. 1). San Antonio, TX: Psychological Corp. [Google Scholar]
- Williams P. E., Weiss L. G., Rolfhus E. L. (2003). Theoretical model and test blueprint (WISC-IV Technical Report No. 1). San Antonio, TX: Psychological Corp. TX: Psychological Corp. [Google Scholar]