Abstract
The objective of the present study was to evaluate the changes in blood metabolites, AA profile, and oxidative stress markers in American Foxhound dogs fed a nutrient-fortified endurance diet while undergoing unstructured endurance exercise over several months. Thirty-six adult American Foxhound dogs (mean age: 4.5, range 2 to 10 yr and mean BW: 34.7, range: 23.1 to 46.9 kg) were selected to participate in the study. Prior to the study, all dogs consumed a commercial diet for 16 wk. After collecting baseline blood samples, dogs were assigned to a standard commercial performance diet (control) or a nutrient-fortified dog food (test). Dogs were balanced by gender, age, body weight, and athletic performance between diets. During the study, dogs underwent 78 bouts of exercise, with approximately 22 km/bout. Blood samples were collected after 40, 75, 138, and 201 d on study (October 2012 to March 2013). All blood metabolites were similar at baseline and serum chemistry profile remained within normal ranges throughout the study. Over time, plasma taurine and vitamin E concentrations decreased (P < 0.05) in dogs fed the control diet but were maintained or increased (P < 0.05) in dogs fed the treatment diet. Also, plasma creatinine and triglycerides were lower (P < 0.05) and blood phosphorus and alkaline phosphatase were higher (P < 0.05) in dogs fed the treatment diet. Vitamin E and taurine status of dogs appear to be affected by extended endurance exercise. These data suggest dogs undergoing endurance exercise may benefit from supplementation of vitamin E and taurine to minimize oxidation and maintain taurine status.
Keywords: canine, exercise, longitudinal, nutrition, oxidative stress
INTRODUCTION
Athletic dogs have unique dietary requirements to sustain bouts of repeated exercise (Hill, 1998; Wakshlag and Shmalberg, 2014). Repeated bouts of exercise in the form of racing, hunting, or herding may contribute to oxidative stress, nutrient depletion, and reduced muscle recovery. Because exercise produces free radicals, contributing to oxidative stress of the body, there should be special consideration for antioxidant inclusion in the diet of working dogs (Davies et al., 1982; Alessio, 1993; Baskin et al., 2000; Dunlap et al., 2006). This oxidative stress may not only affect the performance of the dog, increasing the potential for injuries during exercise, but can lead to long-term damage or disease (Davies et al., 1982; Slater, 1987; Bickford et al., 2000; Block et al., 2002; Hou, 2003). Active dogs, such as working dogs, may benefit from supplemental vitamin E and taurine. Feeding a balanced, highly digestible diet fortified with antioxidants may improve the health and longevity of exercising dogs (Piercy et al., 2000).
In a recent short-term study, we evaluated the acute response to unstructured mixed exercise when American Foxhound dogs were fed a test diet containing added zinc, taurine, lutein, and vitamins C and E (de Godoy et al., 2014). Dogs were fed either the test diet or a standard commercial diet for approximately 80 d before they underwent 2 to 3 h of unstructured mixed exercise. Blood samples were collected at 0, 3, and 25 h postexercise. Major findings included that dogs fed the test diet had greater plasma taurine concentrations pre- and post-acute-exercise and greater plasma branched-chain AAs (BCAA):tryptophan ratio postexercise. The purpose of the present study was to compare changes in blood metabolites and oxidative stress markers in American Foxhound dogs undergoing unstructured endurance exercise over several months when fed the same test diet compared to a control diet. Prior to investigation, it was hypothesized that dogs fed the test diet would have a lower concentration of oxidative stress markers, improved performance and distance run, and shorter recovery time following strenuous exercise as compared to the dogs fed the control diet.
MATERIAL AND METHODS
Animals and Diets
Forty adult American Foxhound dogs (32 intact males, 1 neutered male, and 7 spayed females) were used in the present experiment. Dogs were studied from October 2012 to March 2013. Power analysis was performed to determine the necessary number of dogs per treatment, using a power of 0.8 and alpha of 0.05. According to values reported by Dunlap et al. (2006) for plasma creatine kinase (CK), an SD two times greater than the observed by these authors was assumed for the determination of minimum sample size in the present study. The power analysis indicated a sample size of twenty dogs per treatment was desired. Four dogs were removed from the project for reasons unrelated to the study. Therefore, 36 adult American Foxhound dogs (29 intact males, 7 spayed females) with average age ± SD of 4.5 ± 2.7 yr of age (range: 2 to 10 yr) and average BW ± SD of 34.7 ± 5.3 kg (range: 23.1—46.9 kg) completed the study. Dogs were group housed throughout the trial, separated by dietary treatment and sex. During the day, all dogs were allowed 8 h in a play yard. Minimum dimensions for the yard were 18 m × 21 m. The outdoor runs contained trees for shade, or covered, raised platforms. All dogs had 60 min of human interaction as social enrichment daily as a group. At night, and in inclement weather, all dogs were housed in a lodge which allowed access to the outdoors should the dog prefer it. Suspended platforms and beds were available for animals in the lodge area. The indoor facility had natural and artificial lighting and was equipped with a fan that was utilized at temperatures over 32 °C. An artificial light:dark cycle of 12:12 h was used to allow early morning feeding (0500 h) and handling of the animals until later in the evening (1700 h). The animal facility and play yard were cleaned daily. The facility and all experimental methods were approved by the Waltham Centre for Pet Nutrition Animal Ethics and Welfare Committee, and an informed consent was received by the owner of the kennel and dogs prior to commencement of the study.
Prior to the study, all dogs consumed a commercial diet (NUTRO NATURAL CHOICE Chicken, Whole Brown Rice, and Oatmeal Adult Dog Food; The Nutro Company, Franklin, TN) for 16 wk. After collecting baseline blood samples, dogs were assigned to one of two groups: a standard commercial performance diet (control diet; n = 18; Sportmix High Energy; Midwestern Pet Foods, Inc., Evansville, IN) or a test diet (n = 18; NUTRO NATURAL CHOICE High Endurance Dog Food; The Nutro Company) formulated with increased concentrations of zinc, taurine, lutein, and vitamins C and E. The test diet provided 73.6 g of protein and 52.9 g of fat/4184 kJ metabolizable energy (1 kcal = 4.184 kJ) and had a metabolizable energy of 18.7 kJ/g, calculated based on National Research Council (NRC, 2006) recommendations. The control diet provided 65.7 g protein and 51.0 g of fat/4184 kJ, and a metabolizable energy of 17.9 kJ/g, calculated based on NRC (2006). Ingredient and chemical composition and guaranteed analysis information for the control and test diets are summarized in Table 1.
Table 1.
Ingredient and chemical composition and guaranteed analysis of control performance diet and high endurance dog food (test)
| Item | Control1 | Test2 |
|---|---|---|
| DM, % | 94.10 | 92.23 |
| % DM basis | ||
| Crude protein, % | 28.06 | 32.85 |
| Acid hydrolyzed fat, % | 21.79 | 23.64 |
| Crude fiber, % | 2.42 | 2.15 |
| Metabolizable energy3, kcal/kg | 4,268.86 | 4,466.01 |
| Lysine, % | 1.43 | 2.16 |
| Methionine, % | 0.51 | 0.87 |
| Cystine, % | 0.35 | 0.36 |
| Leucine, % | 2.04 | 2.32 |
| Isoleucine, % | 1.07 | 1.37 |
| Valine, % | 1.39 | 1.55 |
| Arginine, % | 1.72 | 2.20 |
| Histidine, % | 0.64 | 0.79 |
| Phenylalanine, % | 1.09 | 1.32 |
| Threonine, % | 1.13 | 1.38 |
| Tryptophan, % | 0.26 | 0.35 |
| Linoleic acid (18:1, %) | 3.90 | 4.41 |
| Zinc, mg/kg | 146.65 | 338.28 |
| Vitamin E (tocopherols), IU/kg | 86.40 | 1,507.10 |
| Vitamin C (L-ascorbyl-2-phosphate), mg/100g | 2.25 | 35.13 |
| Lutein, µg/g | 1.38 | 5.85 |
| Taurine, % | 0.07 | 0.34 |
1Chicken by-product meal, ground yellow corn, meat meal, ground wheat, chicken fat (preserved with mixed tocopherols), dried beet pulp, fish meal, flaxseed, salt, vitamin A supplement, vitamin D3 supplement, choline chloride, vitamin B12 supplement, folic acid, thiamine mononitrate, pyridoxine hydrochloride, biotin, calcium iodate, copper sulfate, ferrous sulfate, manganous oxide, zinc oxide, magnesium oxide.
2Chicken meal, whole brown rice, rice bran, chicken fat (preserved with mixed tocopherols), whole grain oatmeal, pea protein, brewers rice, chicken, dried plain beet pulp, natural flavor, fish oil (preserved with mixed tocopherols), potassium chloride, salt, choline chloride, vitamin E supplement, sunflower oil (preserved with mixed tocopherols), soybean oil (preserved with mixed tocopherols), taurine, DL-methionine, zinc sulfate, L-ascorbyl-2-polyphosphate (source of vitamin C), biotin, niacin supplement, calcium pantothenate, riboflavin supplement (vitamin B2), pyridoxine hydrochloride (vitamin B6), copper proteinate, iron proteinate, selenium yeast, vitamin B12 supplement, L-carnitine, manganese proteinate, potassium iodide, vitamin A supplement, thiamine mononitrate (vitamin B1), vitamin D3 supplement, folic acid, rosemary extract, decaffeinated green tea extract, spearmint extract.
3 NRC (2006).
Animals were systematically allotted between the two groups to match for age, sex, BW, and past performance (good = quickly catches and keeps on a scent; moderate = remains in the middle of the group, catches a scent, but does not maintain; poor = strays from the group often, does not catch scent; and unknown = usually young, inexperienced dogs) to ensure an even distribution across both groups. Unfortunately, physiological data pertaining to physical conditioning were not available in this population. Dogs were group fed once a day from a stainless steel trough, which was large enough to accommodate all dogs. Ration was adjusted as needed throughout the study to maintain baseline BW. Target BW was based on the previous feeding records, breed standards, and BCS using a nine-point scale (Laflamme, 1997). Water was accessible ad libitum. Treats (NUTRO Crunchy Treats with Real Mixed Berries, The Nutro Company) were provided at a maximum of 10% of daily caloric intake.
Blood Collection, Handling, and Analyses
Venous blood samples (20 mL) were collected via jugular venipuncture into sodium heparin- or serum-separating tube-vacutainer blood tubes (Becton Dickson, Franklin Lakes, NJ). If necessary, an additional 0.5 mL of blood was collected via jugular venipuncture using 1 mL syringes for the analysis of blood glucose. One drop of blood from syringes was immediately used to measure glucose concentration using a portable kit validated for use in dogs (AlphaTRAK 2 Blood Glucose Monitoring System, Abbott Laboratories, Abbott Park, IL). Heparin-vacutainer tubes were placed on ice for approximately 30 min. Heparin and serum tubes were centrifuged at 1,240 × g for 10 min at 4 °C. Serum, plasma, and blood samples were stored at −80 °C for plasma AA and malondialdehyde (MDA) analyses and −20 °C for serum chemistry and CK determination. Blood and plasma taurine concentrations and a complete AA profile were analyzed within 15 d of blood collection, using a norleucine standard and an automated AA analyzer (HPLC, Biochrom 30, Biochrom Ltd, Cambridge, UK) at the Amino Acid Laboratory, School of Veterinary Medicine at the University of California–Davis (Davis, CA) according to Delaney et al. (2003). Half a milliliter of 6% sulphosalicylic acid was added to 0.5 mL of plasma to precipitate the plasma proteins. All AA results were reported as nmol/mL of plasma or whole blood. Plasma MDA concentrations were determined within 1 mo of blood collection using a commercial kit (SafTest ALDESAFE, product no. 07KTAC1010, MP Biomedicals, Santa Ana, CA) developed based on the method by Hamilton and Rossell (1986). Serum tubes were kept at room temperature. Serum chemistry (creatinine, blood urea nitrogen, total protein, albumin, calcium, potassium, chloride, corticosteroid-induced alkaline phosphatase, alkaline phosphatase, alanine aminotransferase, gamma-glutamyl transpeptidase, total bilirubin, cholesterol, triglycerides, CO2, and glucose) and CK were determined using a Hitachi 911 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Illinois Veterinary Medicine Diagnostics Laboratory (Urbana, IL). EDTA-vacutainer tubes were kept on ice for a minimum of 30 min after collection, then processed in a light-protected room and stored for vitamin C (ascorbic acid) and E analyses. Values of vitamin C were determined at the Diagnostic Center for Population and Animal Health (Michigan State University, East Lansing, MI) by oxidation of ascorbic acid to di-hydro ascorbic acid, followed by a colorimetric reaction to quantify via plate reader as described by Marshall et al. (2002). Vitamin E analysis involved a lipid–lipid extraction to remove the fat-soluble vitamins from the plasma samples, and then samples were analyzed via HPLC (Diagnostic Center for Population and Animal Health, Michigan State University, East Lansing, MI).
Exercise Regimen and GPS Measurements
Dogs underwent a total of 78 unstructured bouts of exercise throughout duration of the study (average number of outings/dog ± SD: 24.5 ± 6.5). Dogs were acclimated to wearing GPS collars (DC 40, Garmin Ltd, Olathe, KS) prior to data collection days. Activity data were collected using Astro 320 Global Positioning System (Garmin Ltd). Because GPS coordinates were provided every 15 s, distance traveled and the time required were obtained and used to calculate total miles run and speeds per dog. The maximum speed was based on the intermittent measurements of distance obtained in a set length of time (e.g., every 15 s). Maximum average speed was considered to be the point of 80% of maximum speed.
Statistical Analysis
Data were analyzed as repeated measures using the MIXED procedure of SAS (version 9.3, SAS Institute Inc., Cary, NC). The statistical model included the fixed effect of diet, month, and their interaction and random effects of animal. Means were separated using a protected least squares difference, compared to each other, and a Tukey adjustment was used to control for experiment-wise error. Data normality was checked using the UNIVARIATE procedure of SAS to produce a normal probability plot based on residual data and visual inspection of the raw data. Outlier data were defined as data points 3 or more standard deviations from the mean and were removed from analysis. A probability of P ≤ 0.05 was accepted as being statistically significant. Data are presented as means with their standard errors.
RESULTS
Blood Metabolites and AA Profile
Plasma vitamin E, vitamin C, MDA, and glucose concentrations are summarized in Table 2. There was a significant diet by month interaction, in which dogs fed the control diet had lower (P < 0.0001) plasma vitamin E concentrations postbaseline and throughout the study. There was no significant diet by month interaction for plasma vitamin C, MDA, or glucose values obtained from the glucometer. However, there was a significant main effect of diet and month for plasma vitamin C, in which dogs fed the test diet had lower (P = 0.003) concentrations when compared to the control fed dogs and plasma vitamin C concentrations were lower (P < 0.0001) when collected in March. There was a significant main effect of month for plasma MDA, in which concentrations were higher (P < 0.0001) when collected in October. Dogs fed the test diet had lower (P = 0.02) blood glucose concentrations when compared to the control fed dogs throughout the study.
Table 2.
Average plasma vitamin E, vitamin C, malondialdehyde, and glucose concentrations of dogs fed either a control performance diet or high endurance dog food (test) over several months
| Month | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | August1 | October | November | January | March | SEM2 | Diet | Month | Diet × month |
| Vitamin E, µg/mL | 1.3 | <0.01 | <0.01 | <0.01 | |||||
| Control | 26.2Bx | 14.6Ax | 14.7Ax | 15.4Ax | 13.1Ax | ||||
| Test | 25.6Ax | 30.9By | 26.1Ay | 27.3Ay | 25.6Ay | ||||
| Vitamin C, mg/dL | 0.1 | 0.003 | <0.01 | 0.07 | |||||
| Control | 3.3 | 3.3 | 3.7 | 3.5 | 2.93 | ||||
| Test | 3.1 | 3.1 | 3.1 | 2.9 | 2.73 | ||||
| Malondialdehyde, nmol/mL | 0.6 | 0.21 | <0.01 | 0.33 | |||||
| Control | 7.4 | 10.03 | 7.3 | 6.2 | 7.2 | ||||
| Test | 7.8 | 11.53 | 6.9 | 7.2 | 7.0 | ||||
| Glucose (glucometer), mg/dL | 2.3 | 0.02 | 0.47 | 0.88 | |||||
| Control | n/a4 | 114.6 | 116.3 | 115.4 | 116.1 | ||||
| Test | n/a | 108.6 | 112.7 | 108.9 | 110.4 | ||||
1All dogs were fed the same diet prior to baseline (August) sample collection. Then, all dogs were randomly assigned to the control performance diet or high endurance test diet groups.
2Pooled SEM.
3Means in the same row not sharing a common superscript differ (P < 0.05) due to month.
4n/a = not available.
ABCMeans in the same row not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
xyMeans in the same column not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
Plasma taurine, whole blood taurine, and blood essential AA concentrations for both groups of dogs were within normal ranges and are summarized in Table 3 (nonessential and other blood AA concentrations can be found in Supplementary Table 1). A significant diet by month interaction was observed for most of the AA analyzed in a similar pattern, except for whole blood taurine (P = 0.17), leucine (P = 0.11), methionine (P = 0.26), phenylalanine (P = 0.42), tryptophan (P = 0.14), glutamic acid (P = 0.10), glutamine (P = 0.56), tyrosine (P = 0.08), cystathionine (P = 0.93), cysteine (P = 0.08), and 3-methyl-L-histidine (P = 0.37). There was a significant diet by month interaction, in which dogs fed the test diet had higher (P < 0.0001) plasma taurine concentrations during October, January, and March compared to baseline. Additionally, dogs fed the control diet had lower (P < 0.0001) plasma taurine concentrations when compared to the dogs fed the test diet postbaseline and throughout the study. Some essential AA concentrations were increased in dogs fed the test diet post baseline (during months October, November, January, and March), including arginine (P = 0.01), histidine (P = 0.01), isoleucine (P < 0.0001) when compared to baseline samples (August). A similar pattern was observed in the control fed dogs. Lysine concentrations were higher (P < 0.0001) in dogs fed the test diet when compared to dogs fed the control diet. There was a significant main effect of month for leucine (P < 0.0001), methionine (P = 0.0001), phenylalanine (P < 0.0001), and tryptophan (P = 0.0003), during which dogs had higher concentrations of these essential AA postbaseline. There was a significant main effect of diet for whole blood taurine and methionine concentrations. Whole blood taurine concentrations were higher (P < 0.0001) in dogs fed the test diet (478.0 nmol/mL) compared to dogs fed the control diet (363.5 nmol/mL) throughout the study. Methionine concentrations were higher (P = 0.003) in dogs fed the control diet compared to the dogs fed the test diet throughout the study.
Table 3.
Average plasma taurine, whole blood taurine, and essential blood AA concentrations of dogs fed either a control performance diet or high endurance dog food (test) over several months
| Item | Month | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| August1 | October | November | January | March | SEM2 | Diet | Month | Diet × month | |
| nmol/mL | |||||||||
| Plasma taurine | 6.6 | <0.01 | <0.01 | <0.01 | |||||
| Control | 126.2Cx | 88.1Bx | 61.8Ax | 89.9Bx | 87.6Bx | ||||
| Test | 126.1Ax | 175.4Cy | 149.4ABy | 155.8BCy | 174.1BCy | ||||
| Whole blood taurine | 17.3 | <0.01 | 0.10 | 0.17 | |||||
| Control | n/a3 | 344.8 | 355.4 | 379.3 | 374.5 | ||||
| Test | n/a | 476.5 | 473.3 | 462.8 | 499.6 | ||||
| Essential AAs | |||||||||
| Arginine | 6.1 | 0.48 | <0.01 | 0.01 | |||||
| Control | 103.4Ax | 139.3Bx | 137.6Bx | 145.2Bx | 152.7Bx | ||||
| Test | 94.1Ax | 150.4Bx | 156.7Bx | 152.9Bx | 147.5Bx | ||||
| Histidine | 2.2 | 0.30 | <0.01 | 0.01 | |||||
| Control | 78.4Ax | 83.0ABx | 82.0ABx | 88.7Bx | 82.7ABx | ||||
| Test | 73.7Ax | 77.4ABx | 85.2Cx | 81.8BCx | 84.4BCx | ||||
| Isoleucine | 2.1 | 0.002 | 0.01 | <0.01 | |||||
| Control | 65.6Bx | 59.7ABx | 56.6Ax | 64.7Bx | 65.2Bx | ||||
| Test | 62.7Ax | 67.9ABx | 71.2By | 72.6Bx | 71.2Bx | ||||
| Leucine | 3.6 | 0.22 | <0.01 | 0.11 | |||||
| Control | 117.7a | 141.6bc | 131.6b | 152.9d | 144.8cd | ||||
| Test | 112.3a | 136.9bc | 135.8b | 142.4d | 139.5cd | ||||
| Lysine | 5.6 | <0.01 | <0.01 | <0.01 | |||||
| Control | 89.7Ax | 108.9BCx | 105.1ABx | 123.4Cx | 119.8BCx | ||||
| Test | 86.3Ax | 151.3By | 146.0By | 158.9BCy | 170.4Cy | ||||
| Methionine | 3.5 | 0.003 | 0.0001 | 0.26 | |||||
| Control | 74.2a | 77.4a | 81.4b | 82.3b | 83.9b | ||||
| Test | 59.9a | 60.4a | 74.1b | 73.6b | 80.4b | ||||
| Phenylalanine | 1.6 | 0.86 | <0.01 | 0.42 | |||||
| Control | 54.1a | 58.7b | 53.4a | 56.5b | 58.8b | ||||
| Test | 52.2a | 56.3b | 53.6a | 59.3b | 58.7b | ||||
| Threonine | 11.7 | 0.95 | <0.01 | 0.02 | |||||
| Control | 219.1Ax | 238.0Ax | 246.5Ax | 238.6Ax | 283.7By | ||||
| Test | 207.5Ax | 255.0Bx | 254.2Bx | 242.5Bx | 262.3Bx | ||||
| Tryptophan | 3.4 | 0.07 | 0.0003 | 0.14 | |||||
| Control | 75.2a | 81.4ab | 69.7a | 75.9a | 82.3b | ||||
| Test | 70.0a | 67.8ab | 67.6a | 67.1a | 76.1b | ||||
| Valine | 5.5 | 0.13 | <0.01 | <0.01 | |||||
| Control | 189.6Ax | 189.0Ax | 178.1Ax | 216.8By | 237.3Cy | ||||
| Test | 179.5Ax | 197.7ABx | 199.0ABx | 187.0ABx | 204.1Bx | ||||
1All dogs were fed the same diet prior to baseline (August) sample collection. Then, all dogs were randomly assigned to the control performance diet or high endurance test diet groups.
2Pooled SEM.
3n/a = not available.
ABCMeans in the same row not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
xyMeans in the same column not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
abcdMeans in the same row not sharing a common superscript differ (P < 0.05) due to month.
Serum metabolite concentrations are summarized in Table 4. Mean serum metabolite concentrations were within reference limits for healthy dogs (Kahn, 2005). There was a significant diet by month interaction for creatinine (P < 0.0001), blood urea nitrogen (P < 0.0001), calcium (P < 0.0001), GGT (P = 0.01), triglycerides (P < 0.0001), and bicarbonate (P = 0.02). Creatinine, blood urea nitrogen, and triglyceride concentrations were higher (P < 0.0001) in dogs fed the control diet during January when compared to the test fed dogs during that collection period. Bicarbonate concentrations were higher (P = 0.02) in dogs fed the control diet when collected in November, January, and March when compared to baseline. There was a significant main effect of month for total protein (P < 0.0001), albumin (P < 0.0001), sodium (P = 0.0001), potassium (P < 0.0001), chloride (P < 0.0001), alkaline phosphatase (P < 0.0001), corticosteroid-alkaline phosphatase (P < 0.0001), alanine aminotransferase (P = 0.003), and cholesterol (P < 0.0001). Serum CK was lower (P < 0.0001) during October, November, January, and March when compared to baseline. There was a significant main effect of diet for phosphorus, alkaline phosphatase, and cholesterol concentrations. Dogs fed the test diet had higher (P = 0.004 and P = 0.01, respectively) phosphorus and alkaline phosphatase concentrations, but lower (P = 0.03) cholesterol than dogs fed the control diet throughout the study.
Table 4.
Average serum chemistry profile of dogs fed either a control performance diet or high endurance dog food (test) over several months
| Item | Month | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| August1 | October | November | January | March | SEM2 | Diet | Month | Diet × month | |
| Creatinine, mg/dL | 0.03 | 0.01 | 0.004 | <0.01 | |||||
| Control | 1.0BCx | 1.0BCx | 0.9Ax | 1.1Cy | 1.0ABx | ||||
| Test | 1.0Bx | 0.9ABx | 0.9ABx | 0.8Ax | 0.9Bx | ||||
| Urea nitrogen, mg/dL | 0.9 | 0.11 | <0.01 | <0.01 | |||||
| Control | 18.1Ax | 20.0Ax | 19.0Ax | 23.7By | 24.7Bx | ||||
| Test | 16.2Ax | 19.7Bx | 19.9Bx | 18.1ABx | 23.0Cx | ||||
| Total protein, g/dL | 0.1 | 0.35 | <0.01 | 0.92 | |||||
| Control | 6.6a | 6.9b | 6.8b | 6.6a | 6.9b | ||||
| Test | 6.8a | 7.1b | 7.0b | 6.7a | 7.1b | ||||
| Albumin, g/dL | 0.05 | 0.80 | <0.01 | 0.18 | |||||
| Control | 2.7a | 2.8b | 2.8b | 2.7a | 2.7a | ||||
| Test | 2.6a | 2.9b | 2.9b | 2.7a | 2.7a | ||||
| Calcium, mg/dL | 0.2 | 0.88 | 0.01 | <0.01 | |||||
| Control | 10.0ABx | 10.3Bx | 10.3Bx | 10.0Ax | 10.3Bx | ||||
| Test | 10.4Ax | 10.2Ax | 10.2Ax | 10.0Ax | 10.0Ax | ||||
| Phosphorus, mg/dL | 0.2 | 0.004 | 0.08 | 0.30 | |||||
| Control | 4.8 | 4.7 | 4.6 | 4.3 | 4.2 | ||||
| Test | 5.1 | 5.0 | 5.1 | 4.9 | 5.2 | ||||
| Sodium, mmol/L | 0.4 | 0.22 | 0.0001 | 0.08 | |||||
| Control | 144.4abc | 143.0a | 144.2bc | 143.7ab | 144.6c | ||||
| Test | 143.9abc | 144.0a | 145.0bc | 144.3ab | 145.1c | ||||
| Potassium, mmol/L | 0.1 | 0.97 | <0.01 | 0.99 | |||||
| Control | 4.6b | 4.3a | 4.3a | 4.4b | 4.4b | ||||
| Test | 4.4b | 4.3a | 4.3a | 4.5b | 4.5b | ||||
| Chloride, mmol/L | 0.5 | 0.11 | <0.01 | 0.13 | |||||
| Control | 110.4b | 108.7a | 109.8b | 108.3a | 110.2b | ||||
| Test | 110.2b | 109.7a | 110.5b | 109.7a | 111.4b | ||||
| Alk Phos3, U/L | 4.2 | 0.01 | <0.01 | 0.15 | |||||
| Control | 57.7b | 31.3a | 33.9a | 34.1a | 32.3a | ||||
| Test | 71.8b | 51.6a | 42.7a | 49.7a | 47.4a | ||||
| C-Alk Phos, U/L | 3.7 | 0.36 | <0.01 | 0.35 | |||||
| Control | 3.1b | 2.9b | 1.6a | 3.9b | 3.8b | ||||
| Test | 5.7b | 5.2b | 3.4a | 5.6b | 5.4b | ||||
| ALT, U/L | 3.7 | 0.40 | 0.003 | 0.45 | |||||
| Control | 36.3b | 30.0a | 34.6ab | 33.7b | 33.1ab | ||||
| Test | 37.6b | 30.6a | 35.7ab | 41.0b | 37.8ab | ||||
| GGT, U/L | 0.2 | 0.34 | <0.01 | 0.01 | |||||
| Control | 1.3Ax | 2.1BCx | 1.6ABx | 1.5ABCx | 2.2Cx | ||||
| Test | 1.6ABx | 2.1Bx | 1.1Ax | 1.4Ax | 1.6ABx | ||||
| Total bilirubin, mg/dL | 0.01 | 0.12 | 0.35 | 0.07 | |||||
| Control | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | ||||
| Test | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Cholesterol, mg/dL | 6.1 | 0.03 | <0.01 | 0.29 | |||||
| Control | 190.92d | 175.06c | 169.61b | 165.94ab | 155.48a | ||||
| Test | 175.39d | 162.61c | 149.72b | 141.06ab | 139.67a | ||||
| Triglycerides, mg/dL | 4.9 | 0.0006 | <0.01 | <0.01 | |||||
| Control | 68.1Bx | 49.8Ax | 56.7ABx | 92.0Cy | 65.2Bx | ||||
| Test | 58.5Ax | 43.4Ax | 45.8Ax | 44.7Ax | 50.4Ax | ||||
| Bicarbonate, mmol/L | 0.7 | 0.03 | <0.01 | 0.02 | |||||
| Control | 22.2Ax | 23.3ABx | 27.1Cx | 26.1BCx | 28.4Cx | ||||
| Test | 22.8Ax | 23.2Ax | 24.4Ax | 24.6Ax | 25.4Ax | ||||
| Creatine kinase, U/L | 18.6 | 0.81 | <0.01 | 0.46 | |||||
| Control | 279.8c | 187.1b | 127.6a | 147.1ab | 114.2a | ||||
| Test | 266.1c | 180.9b | 134.8a | 138.0ab | 155.0a | ||||
1All dogs were fed the same diet prior to baseline (August) sample collection. Then, all dogs were randomly assigned to the control performance diet or high endurance test diet groups.
2Pooled SEM.
3Alk Phos, alkaline phosphatase; C-Alk Phos, corticosteroid-alkaline phosphatase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase.
ABCMeans in the same row not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
xyMeans in the same column not sharing a common superscript differ (P < 0.05) due to a diet × month interaction.
abcdMeans in the same row not sharing a common superscript differ (P < 0.05) due to month.
Table 4. Continued
Athletic Performance
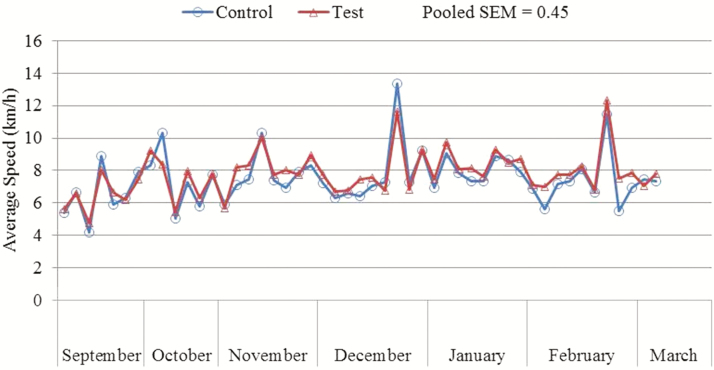
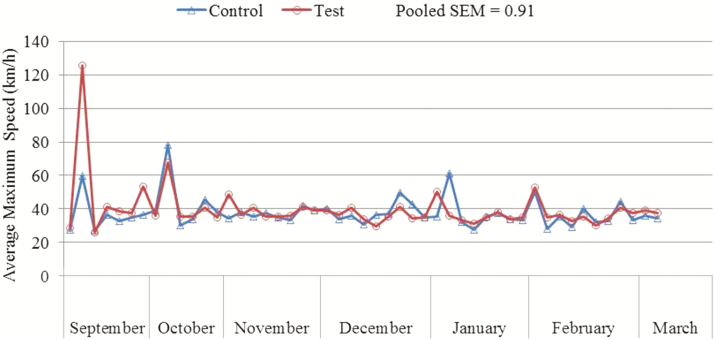
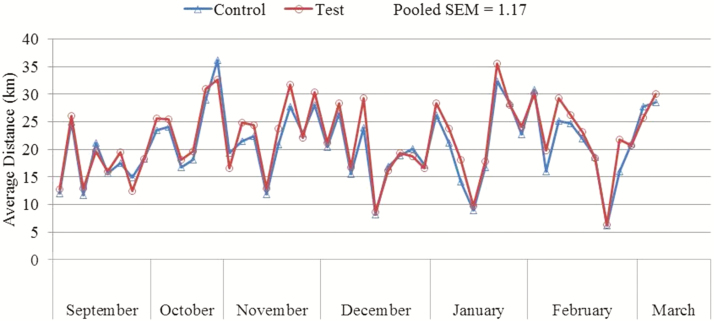
Dogs underwent variable speed exercise over extended periods of time throughout the study (Figures 1–3). There was no significant diet × month interaction for any of the tested variables, including average speed, maximum speed, and distance traveled per exercise event. There was no significant effect of diet for average speed (P = 0.47; control: 8.2 km/h, test: 8.4 km/h), maximum speed (P = 0.24; control: 34.9 km/h, test: 36.4 km/h), and distance traveled per exercise event (P = 0.23; control: 21.9 km, test: 23.1 km). Average speed was lower (P < 0.0001) during September than during October, November, December, January, February, or March. Maximum speed was lower (P = 0.0031) during January than during October or March.
Figure 1.
Mean average speed (km/h) traveled per exercise event of dogs fed either a control performance diet (blue) or a test diet (red) over several months.
Figure 2.
Mean maximum speed (km/h) traveled per exercise event of dogs fed either a control performance diet (blue) or a test diet (red) over several months.
Figure 3.
Mean distance (km) traveled per exercise event of dogs fed either a control performance diet (blue) or a test diet (red) over several months.
DISCUSSION
The exercise regimen of American Foxhounds differs from that of other athletic dogs, such as Greyhounds or sled dogs. Although other athletic dogs may consistently practice structured exercise regimens, American Foxhounds undergo bouts of unstructured exercise at variable speeds throughout a given activity. They are most active during 5 mo of the year (October to March) and may exercise for 2 to 5 h/exercise bout. Given the changes in regimen, the physiological changes may be observed over the course of several months.
The present study evaluated the longitudinal response to unstructured bouts of exercise in American Foxhounds fed a nutrient-fortified diet over a period of several months on blood metabolites, plasma indirect oxidative stress markers, and athletic performance. Although some metabolites changed over the course of the study, serum chemistry results indicated that all dogs were healthy throughout the study. Plasma AA concentrations were within the normal range for all dogs. However, once a requirement is met, plasma AA concentrations are not reflective of the degree deficiency or excess and may not be the best indicator for the status of the animal (Zimmerman and Scott, 1965; Larsen et al., 2010). Although it is unlikely to observe plasma AA concentrations outside of the normal range when a dog is fed a commercial diet, it can be beneficial to look for shifts in these values to evaluate changes in the health of the animal due to diet. Furthermore, it is likely that the greater plasma taurine concentration of dogs fed the test diet was a result of increased dietary taurine content. Taurine status is important for maintenance of cardiac function (Delaney et al., 2003), and deficiency of dietary taurine may cause dilated cardiomyopathy (DCM) in large dogs (Buchanan, 1995; Freeman et al., 1996; Sisson et al., 1999; Sanderson et al., 2001). Ko et al. (2007) compared the taurine synthetic ability of large and small dogs. These researchers controlled the intake of the diet to maintain ideal body condition and to provide similar dietary sulfur AA intake based on the dogs’ metabolic body weight. Ko et al. (2007) concluded that large-breed dogs are at a greater risk of developing DCM than small-breed dogs because they have a lower ability to synthesize taurine when precursor sulfur AAs are not in excess or when fed a low, but nutritionally adequate protein diet. Therefore, it is important to supply adequate taurine in the diet for large, exercising dogs, such as the American Foxhound. In the present study, all dogs had plasma and whole blood taurine concentrations above low critical values (concentrations considered near to cause a nutrient deficiency) reported in the literature for healthy, adult dogs (40 and 150 nmol/mL, respectively; Scott et al., 2001). However, one dog in the control group did develop plasma taurine concentrations close to the critical range (40 nmol/mL). Similarly to increased plasma taurine, the greater plasma vitamin E concentration of dogs fed the test diet was likely due to increased vitamin E in the diet. Scott et al. (2001), who evaluated the effects of feeding a vitamin E supplement to Greyhounds before and after undergoing a sprint race, also observed higher serum vitamin E concentrations following administration of supplemental vitamin E (680 units of alpha-tocopherol/d). Although the exercise regimen in the current study differs from that in Scott et al. (2001) both studies exhibit that vitamin E is reduced in the body of dogs undergoing an exercise regimen and increasing dietary vitamin E in the diet of these dogs may be beneficial.
Although several changes were observed in the current study, it is possible that the duration, frequency, and/or intensity of the exercise regimen were not strenuous enough to impact oxidative stress measurements. For example, plasma MDA, an indirect marker of oxidative stress and lipid peroxidation, was not greatly affected over time. A previous study by the current authors, evaluating acute response to bouts of unstructured exercise in these same dogs, also observed no changes in plasma MDA concentrations (de Godoy et al., 2014). Given that the current project was a field study, many factors contribute to the final results. Although food intake would have been less variable if the dogs were fed individually, the aim of the current study was to evaluate this group of dogs in their natural environment. Therefore, to keep the regimen similar to what is common for these dogs, the dogs in the current study were group housed and group fed. Therefore, it is possible that some dogs consumed more food than others, but no deficiency or adverse results were observed. Additionally, the dogs had free access to outdoors at all times, allowing for the potential consumption of unknown insects, prey, or other natural inhabitants to the environment. Finally, the exercise regimens were unstructured and were not identical each time. Although there are some factors that may negatively impact a study of this nature, the benefit is that this study is representative of the exercise regimen and habitat of exercising American Foxhounds. To account for some of the variation, age, sex, BW, and past performance were considered when allotting to test groups.
In conclusion, vitamin E and taurine status of dogs appear to be affected by variable speed exercise over extended periods of time. However, MDA concentration, a marker of oxidative stress, was not statistically different between treatment groups. Therefore, it can be concluded that both diets provided sufficient antioxidants to protect the dogs from oxidative stress that was expected from this type of exercise. Dogs undergoing variable speed exercise over extended periods of time may benefit from supplementation of vitamin E and taurine. Both diets supplied adequate concentrations of AAs, as supported by blood analysis. No decline was observed over time, which is indicative that protein concentrations were sufficient to avoid potential AA depletion due to intense exercise.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
ACKNOWLEDGMENTS
The authors express gratitude to Hard Away Whitworth Hounds for their dogs, to A. Connor, S. Evely, and R. Garrett for their animal care and assistance, and to A. Daniel for assisting with the GPS data. The Nutro Company provided funding for this research. A.N.B., M.R.C.G., Z.Y., and B.J.M. have no conflict of interest. R.A.C. is and P.R.B. was employed by The Nutro Company at the time of this study.
Presented as a poster presentation at the WALTHAM International Nutritional Sciences Symposium, Portland, OR, October 2013.
Funding provided by The Nutro Company, Franklin, TN.
LITERATURE CITED
- Alessio H. M. 1993. Exercise-induced oxidative stress. Med. Sci. Sports Exerc. 25:218–224. doi:10.1249/00005768-199302000-00010 [PubMed] [Google Scholar]
- Baskin C. R., Hinchcliff K. W., DiSilvestro R. A., Reinhart G. A., Hayek M. G., Chew B. P., Burr J. R., and Swenson R. A.. 2000. Effects of dietary antioxidant supplementation on oxidative damage and resistance to oxidative damage during prolonged exercise in sled dogs. Am. J. Vet. Res. 61:886–891. doi:10.2460/ajvr.2000.61.886 [DOI] [PubMed] [Google Scholar]
- Bickford P. C., Gould T., Briederick L., Chadman K., Pollock A., Young D., Shukitt-Hale B., and Joseph J.. 2000. Antioxidant-rich diets improve cerebellar physiology and moto learning in aged rats. Brain Res. 866:211–217. doi:10.1016/S0006-8993(00)02280-0 [DOI] [PubMed] [Google Scholar]
- Block G., Dietrich M., Norkus E. P., Morrow J. D., Hudes M., Caan B., and Packer L.. 2002. Factors associated with oxidative stress in human populations. Am. J. Epidemiol. 156:274–285. doi:10.1093/aje/kwf029 [DOI] [PubMed] [Google Scholar]
- Buchanan J. W. 1995. Causes and prevalence of cardiovascular disease. In: Kirk, R. W., and J. D. Bonagura, editors Current veterinary therapy XI: small animal practice. Philadelphia (PA): W.B. Saunders Co; p. 647–655. [Google Scholar]
- Davies J. J. A., Quintanilha T. A., Brooks G. A., and Packer L.. 1982. Free radical and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 107:1198–1205. doi:10.1016/S0006-291X(82)80124-1 [DOI] [PubMed] [Google Scholar]
- de Godoy M. R. C., Beloshapka A. N., Carter R. A., Fascetti A. J., Yu Z., McIntosh B. J., Swanson K. S., and Buff P. R.. 2014. Acute changes in blood metabolites and amino acid profile post-exercise in Foxhound dogs fed a high endurance formula. J. Nutr. Sci. 3:e33. doi:10.1017/jns.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney S. J., Kass P. H., Rogers Q. R., and Fascetti A. J.. 2003. Plasma and whole blood taurine in normal dogs of varying size fed commercially prepared food. J. Anim. Physiol. Anim. Nutr. 87:236–244. doi:10.1046/j.1439-0396.2003.00433.x [DOI] [PubMed] [Google Scholar]
- Dunlap K. L., Reynolds A. J., and Duffy L. K.. 2006. Total antioxidant power in sled dogs supplemented with blueberries and the comparison of blood parameters associated with exercise. Comp. Biochem. Physiol. 143:429–434. doi:10.1016/j.cbpa.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Freeman L. M., Michel K. E., Brown D. J., Kaplan P. M., Stamoulis M. E., Rosenthal S. L., Keene B. W., and Rush J. E.. 1996. Idiopathic dilated cardiomyopathy in Dalmatians: nine cases (1990–1995). J. Am. Vet. Assoc. 209:1592–1596. [PubMed] [Google Scholar]
- Hamilton R. S., and Rossell J. B.. 1986. Analysis of oils and fats. London (UK): Elsevier Applied Science; p. 23–32. [Google Scholar]
- Hill R. C. 1998. The nutritional requirements of exercising dogs. J. Nutr. 128:2686S–2690S. [DOI] [PubMed] [Google Scholar]
- Hou D. X. 2003. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 3:149–159. doi:10.2174/1566524033361555 [DOI] [PubMed] [Google Scholar]
- Kahn C. M, editor. 2005. Merck veterinary manual. 9th ed Whitehouse Station (NJ): Merck & Co. [Google Scholar]
- Ko K. S., Backus R. C., Berg J. R., Lame M. W., and Rogers Q. R.. 2007. Differences in taurine synthesis rate among dogs relate to differences in their maintenance energy requirement. J. Nutr. 137:1171–1175. doi:10.1093/jn/137.5.1171 [DOI] [PubMed] [Google Scholar]
- Laflamme D. P. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22:10–15. [Google Scholar]
- Larsen J. A., Fascetti A. J., Calvert C. C., and Rogers Q. R.. 2010. Bioavailability of lysine for kittens in overheated casein is underestimated by the rat growth assay method. J. Anim. Physiol. Anim. Nutr. 94:e102–e108. doi:10.1111/j.1439-0396.2010.00988.x [DOI] [PubMed] [Google Scholar]
- Marshall R. J., Scott K. C., Hill R. C., Lewis D. D., Sundstrom D., Jones G. L., and Harper J.. 2002. Supplemental vitamin C appears to slow racing greyhounds. J. Nutr. 132:1616S–1621S. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 2006. Nutrient requirements of dogs and cats. Washington (DC): The National Academies Press. [Google Scholar]
- Piercy R. J., Hinchcliff K. W., DiSilvestro R. A., Reinhart G. A., Baskin C. R., Hayek M. G., Burr J. R., and Swenson R. A.. 2000. Effect of dietary supplements containing antioxidants on attenuation of muscle damage in exercising sled dogs. Am. J. Vet. Res. 61:1438–1445. doi:10.2460/ajvr.2000.61.1438 [DOI] [PubMed] [Google Scholar]
- Sanderson S. L., Gross K. L., Ogburn P. N., Calvert C., Jacobs G., Lowry S. R., Bird K. A., Koehler L. A., and Swanson L. L.. 2001. Effects of dietary fat and L-carnitine on plasma and whole blood taurine concentrations and cardiac function in healthy dogs fed protein-restricted diets. Am. J. Vet. Res. 62:1616–1623. doi:10.2460/ajvr.2001.62.1616 [DOI] [PubMed] [Google Scholar]
- Scott K. C., Hill R. C., Lewis D. D., Boning A. J. Jr., and Sundstrom D. A.. 2001. Effect of α-tocopheryl acetate supplementation on vitamin E concentrations in Greyhounds before and after a race. Am. J. Vet. Res. 62:1118–1120. doi:10.2460/ajvr.2001.62.1118 [DOI] [PubMed] [Google Scholar]
- Sisson D., O’Grady M. R., and Calvert C. A.. 1999. Myocardial diseases of dogs. In: Fox, P. R., D. Sisson, and N. Sydney Moïse, editors Textbook of canine and feline cardiology: principles and clinical practice. 2nd ed Philadelphia (PA): W.B. Saunders Co; p. 581–619. [Google Scholar]
- Slater T. F. 1987. Free radicals and tissue injury: fact and fiction. Br. J. Cancer 55:5–10. [PMC free article] [PubMed] [Google Scholar]
- Wakshlag J., and Shmalberg J.. 2014. Nutrition for working and service dogs. Vet. Clin. Small Anim. 44:719–740. doi:10.1016/j.cvsm.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Zimmerman R. A., and Scott H. M.. 1965. Inter-relationship of plasma amino acid levels and weight gain in the chick as influenced by suboptimal and superoptimal dietary concentrations of single amino acids. J. Nutr. 87:13–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.