Abstract
To determine the effects of trace mineral (TM) supplementation and hormone implant strategy on growth and carcass characteristics of cattle, 72 Angus-cross steers (388 ± 17 kg) were blocked by body weight (six steers per pen) to a 2 × 3 factorial. Factors included growth-stimulating implant (GS): day 0 with Component TE-IS, reimplanted day 56 with Component TE-200 (IMP) or no implant (NoIMP), and TM supplementation (TM): no supplemental TM (CON), TM supplemented at national (NASEM, 2016). Nutrient requirements of beef cattle. 8th ed. Washington, DC: The National Academics Press) recommendations of 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I (mg/kg; REC), or TM supplemented at feedlot consultant recommendations of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I (mg/kg; IND). Steers received a finishing diet for 124 d in GrowSafe bunks and were harvested on day 125. Data were analyzed in SAS as a 2 × 3 factorial with steer as the experimental unit (n = 12 per combination). Day −7 liver trace mineral concentrations were used as a covariate in analysis. There were no GS × TM effects for liver Zn, Mn, Se, or Co (P ≥ 0.11) on day 70 or 125. Implanted steers had lesser liver Cu and Mn on day 70 (P ≤ 0.05) and day 125 compared with NoIMP. There was a GS × TM interaction for liver Cu on day 125 (P = 0.05) where IMP/REC, IMP/IND, and NoIMP/REC had greater liver Cu than NoIMP/CON, which had greater liver Cu than IMP/CON. There was a TM effect for liver Cu on day 70 (P < 0.01) with IND having greater liver Cu than REC and CON. There was a TM effect (P ≤ 0.01) for liver Mn and Se on day 70 where IND had greater Mn and Se than CON, with REC being intermediate. There was a TM effect (P < 0.01) on liver Mn on day 125 where IND had greater liver Mn than CON and greater (P < 0.01) liver Se than CON and REC on day 125, whereas day 125 liver Se was greater in REC vs. CON. Implanted steers had greater (P < 0.01) dry matter intake (DMI), final body weight (BW), overall average daily gain (ADG), G:F, and hot carcass weight (HCW) than NoIMP. Overall DMI was affected by TM (P < 0.01) with REC and IND being greater than CON. There was a TM effect for carcass-adjusted final BW, ADG, and DMI (P ≥ 0.03) and a tendency for TM to affect adjusted G:F (P = 0.07). There was a TM effect (P = 0.03) for HCW where IND was greater than CON and REC. There was a GS × TM (P = 0.02) for ribeye area (REA); within IMP, CON were greater than IND, whereas REC were intermediate while NoIMP had smaller REA, regardless of TM supplementation. Additional research is needed to better determine trace mineral requirements of finishing beef steers for optimal performance.
Keywords: beef, implant, trace mineral
INTRODUCTION
Combination implants improve growth rate and feed efficiency in finishing cattle (Bartle et al., 1992; Johnson et al., 1996), which ultimately can increase the profit returns of feedyards (Duckett and Pratt, 2014). With increases in cattle growth rates, supplies of critical nutrients such as trace minerals to support growth and development of feedlot animals may need to be increased. Copper and Mn are vital for the development of bone and cartilage (Liu et al., 1994; Suttle, 2010), whereas Zn plays a role in cell signaling and proliferation (Beyersmann and Haase, 2001). Some selenoproteins function in muscle (Beckett and Arthur, 2005), and both Se and I influence thyroid hormones and subsequently cellular metabolism (Meyer et al., 2008). Through vitamin B12, Co supports propionate metabolism and methyl transfer (Suttle, 2010).
Although national recommendations should prevent trace mineral deficiency and support adequate growth (NASEM, 2016), it is common among consulting feedlot nutritionists to supplement 125% to 300% of recommended trace mineral concentrations (Samuelson et al., 2016). Unfortunately, research comparing industry recommendations vs. the well-accepted national recommendations is limited (Berrett et al., 2015). Additionally, superior growth rates resulting from implant utilization may increase trace mineral requirements to support muscle and frame growth in feedlot steers. The objective of this study was to determine the effects of national or industry concentrations of supplemental trace mineral to feedlot steers not receiving hormone implants or receiving a high-potency implant strategy on steer growth, carcass performance, and mineral status. It was hypothesized that greater growth of cattle-administered hormone implants requires increased trace mineral supplementation.
MATERIALS AND METHODS
All live animal procedures and protocols for this experiment were approved by the Iowa State University Institutional Animal Care and Use Committee (#6-16-8302-B).
Animals and Experimental Design
Seventy-two Angus-cross, black hided beef steers (389 ± 17.2 kg) were utilized in a 2 × 3 factorial design experiment conducted at the Iowa State University Beef Nutrition Research Center in Ames, IA. Following arrival to the research facility, cattle were dewormed with Eprinex (Merial Limited, Duluth, GA) and given unique visual and electronic identification tags. Upon arrival, cattle were fed a 40% cracked corn, 30% hay, 25% modified distillers grains, and 5% supplement diet for 7 d. Steers were transitioned over 21 d to a corn-based finishing diet, described in Table 1. Nine days before the start of the experiment, steers were moved into pens with GrowSafe feed bunks and allowed to adapt to eating out of GrowSafe feed bunks (GrowSafe Systems Ltd., Airdire, AB, Canada) with one bunk per pen of six. On days −1 and 0, steers were weighed and then blocked by initial BW into one of 12 pens containing 6 steers per pen.
Table 1.
Ingredient and nutrient composition of control diet
| Item | % of diet DM* |
|---|---|
| Ingredient | |
| Cracked corn | 62.0 |
| MDGS† | 25.0 |
| Bromegrass hay | 8.0 |
| DDGS‡ | 3.0765 |
| Limestone | 1.5 |
| Salt | 0.31 |
| Vitamin A and E premix∥ | 0.1 |
| Rumensin 90 | 0.0135 |
| Analyzed composition | |
| Crude protein, % | 15.97 |
| Neutral detergent fiber, % | 18.02 |
| Ether extract, % | 5.62 |
| Sulfur, % | 0.33 |
| Analyzed composition,$ mg/kg DM | |
| Cu | 2.0 |
| Fe | 53.8 |
| Mn | 7.8 |
| Zn | 16.8 |
| Se | 0.22 |
| Co | 0.05 |
*Trace mineral treatments included: CON (no supplemental trace mineral), REC (2016 recommendations of 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†Modified distillers grains with solubles.
‡Dried distillers grains with solubles.
∥Premix provided 2,200 IU vitamin A and 25 IU vitamin E/kg diet.
$Analyzed mineral values reflect CON diet total.
Pens of steers were assigned to a 2 × 3 factorial arrangement, factors included growth-stimulating implant (GS): either implanted (IMP, n = 36 steers) or not (NoIMP, n = 36 steers), and dietary trace mineral (TM) supplementation. The TM treatments included 1) CON that received no additional trace mineral supplementation, 2) REC that received supplementation at the national recommendations (NASEM, 2016) for Cu (10 mg/kg), Zn (30 mg/kg), Mn (20 mg/kg), Se (0.10 mg/kg), Co (0.15 mg/kg), and I (0.50 mg/kg) from inorganic sources, and 3) IND that received supplementation at the mode value from the Samuelson et al. (2016) feedlot consulting nutritionist survey for Cu (20 mg/kg), Zn (100 mg/kg), Mn (50 mg/kg), Se (0.30 mg/kg), Co (0.20 mg/kg), and I (0.50 mg/kg) from inorganic sources. This resulted in six total treatments with 12 steers per treatment combination.
In addition to initial BW, steers were weighed on days 28, 56, 70, 84, along with days 123 and 124 to determine final BW. All BW were collected before feed delivery, and a 4% pencil shrink was applied to all live BW measurements, including those used to calculate average daily gain (ADG) and G:F. On day 0, IMP steers received a Component TE-IS implant (16 mg estradiol and 80 mg trenbolone acetate (TBA), Elanco Animal Health, Indianapolis, IN) and were reimplanted with Component TE-200 (20 mg estradiol and 200 mg trenbolone acetate, Elanco Animal Health) on day 56. On day 124, steers were shipped to a commercial abattoir in Tama, IA (Iowa Premium Beef), steers were harvested on day 125, and hot carcass weight (HCW) was collected. After a 48 h chill, marbling score (MS), quality grade (QG), ribeye area (REA), backfat, and kidney, pelvic, heart fat (KPH) data were collected. Yield grade and dressing percent were calculated based on carcass parameters collected (USDA, 2016). The carcass-adjusted performance data calculation of final BW was determined by dividing HCW by the average dressing percentage of 64.66%, and overall ADG and G:F were calculated. Specific health parameters were not measured in this experiment, but all cattle appeared healthy throughout the duration of this experiment.
Sample Collection and Analytical Procedures
Cattle were delivered feed daily at approximately 0800 h with ad libitum access to both feed and water. Ingredient and total mixed ration (TMR) samples were taken weekly to determine dry matter (DM) content. Samples were dried in a forced air oven at 70 °C for 48 h. Dried feed samples were then grounded through a 2 mm screen (Retsch Zm100 grinder; Glen Mills Inc., Clifton, NJ). TMR samples were composited by diet within implant period for determination of trace mineral concentrations. Composited feed samples were acid digested using trace metal grade nitric acid (Fisher Scientific, Fair Lawn, NJ) before trace mineral analysis.
Three steers per pen were randomly selected for liver and blood sample collection (n = 6 per treatment) to evaluate trace mineral status with the same steers used throughout the study. Before the start of treatments, cattle were preliminarily placed in GrowSafe pens, based on BW. Liver biopsies were collected 2 h after feeding before the start of the trial (day −7), 14 d after reimplantation (day 70), and liver samples were collected immediately after harvest at the abattoir (day 125). Liver biopsies were collected using the method described by Engle and Spears (2000), and samples were dried in a forced air oven at 70 °C for 7 d. Dried liver and feed samples were acid digested (CEMS MARSXpress, Matthews, NC) with trace mineral grade nitric acid before mineral analysis as described by Richter et al. (2012). Feed and liver samples were analyzed for Ca, Cu, Fe, Mg, Mn, Mo, P, K, Se, and Zn using inductively coupled plasma mass spectrometry (ICP-MS, Analytik Jena Inc., Woburn, MA) in collision reaction interface mode with hydrogen as the skimmer gas. Briefly, samples were diluted in 1% nitric acid, mixed and analyzed by ICP-MS. For quality control, Bi, Sc, In, Li, Y, and Tb were used as internal standards for the ICP-MS. A bovine National Institute of Standards and Technology (NIST) liver sample (US Department of Commerce, Gaithersburg, MD) was used to verify instrument accuracy.
Blood samples were collected on days −1, 70, and 124 into vacutainer tubes containing potassium EDTA or heparin (Becton Dickenson, Rutherford, NJ) before feeding. Samples were kept on ice and transported to the laboratory where they were centrifuged at 1,000 × g for 12 min at 4 °C. Plasma was removed and stored at −80 °C until further analysis. Plasma samples were analyzed for glucose according to commercial kit protocols, Wako Autokit Glucose (Wako Pure Chemical Industries, Ltd., Chuo-Ku Osaka, Japan) with an intra-assay coefficient of variation (CV) less than 10% and interassay CV of 9.1%. Plasma samples were also analyzed for plasma urea nitrogen (PUN) according to commercial kit protocols, urea nitrogen reagent (Colorimetric Method, Teco Diagnostics, Anaheim, CA) with an intra-assay CV less than 10% and interassay CV of 8.3%.
Statistical Analysis
Data were analyzed as a 2 × 3 factorial using the mixed procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC). Animals were assigned to treatments using a completely randomized block design. Fixed effects included block, GS, TM, and GS × TM. The experimental unit for all data was steer (n = 12 per treatment for performance and carcass data except NoIMP/REC where n = 11; for blood and liver data, n = 6 for all treatments except IMP/IND where n = 5). ADG, DMI, and G:F were summarized by implant period. Liver mineral from day −7 and plasma glucose and PUN from day −1 served as covariates in analysis for respective parameters to all subsequent sampling dates. Data were found to be normally distributed, and outliers were evaluated using Cook’s D. Data from one steer were not included in the statistical analysis because of poor overall performance (from NoIMP/REC treatment). Data reported are least squares means with standard error of the mean. Significance was determined at P ≤ 0.05, and tendencies determined when 0.05 < P ≤ 0.10.
RESULTS
Live and Carcass-adjusted Animal Performance
Live animal performance data for the first 56 d are shown in Table 2. Day 56 BW tended to be P = 0.10 and days 0 to 56 ADG was P = 0.05 affected by the GS × TM interaction, where within IMP, values for steers receiving REC or IND were greater than CON, all IMP treatments were greater than NoIMP, and no TM effect was noted within NoIMP steers. There were no GS × TM interactions on days 0 to 56 DMI or G:F data (P ≥ 0.16). Days 0 to 56 DMI and G:F were greater in implanted steers than nonimplanted steers (P ≤ 0.01). Trace mineral had no effect on days 0 to 56 G:F (P = 0.12).
Table 2.
Day 56 BW and days 0 to 56 ADG of nonimplanted or implanted beef steers fed varying concentrations* of supplemental trace minerals
| Performance | No IMP | IMP† | SEM‡ | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON, n = 12 | REC, n = 11 | IND, n = 12 | CON, n = 12 | REC, n = 12 | IND, n = 12 | GS | TM | GS × TM | ||
| BW, kg | ||||||||||
| Day 56 | 473z | 468z | 478z | 494y | 513x | 509x | 5.48 | <0.01 | 0.17 | 0.10 |
| ADG, kg/d | ||||||||||
| Days 0–56 | 1.50c | 1.45c | 1.61c | 1.88b | 2.18a | 2.18a | 0.070 | <0.01 | 0.01 | 0.05 |
a,b,cWithin rows, means without a common superscript differ (P ≤ 0.05).
x,y,zWithin rows, means without a common superscript tend to differ (P ≤ 0.10).
*Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016): 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
‡Reported SEM is greatest of all treatments because of uneven number per treatment.
SEM, standard error of the mean.
There were no GS × TM interactions for days 56 to 124 performance or DMI (P ≥ 0.13; Table 3) or overall BW, ADG, DMI, or G:F (P ≥ 0.66). Nor were there any GS × TM interactions for carcass-adjusted live animal performance (P ≥ 0.60). Days 56 to 124 ADG, DMI, final BW, as well as overall ADG, DMI, and G:F were greater in IMP steers than NoIMP steers (P ≤ 0.03). There was a GS effect on carcass-adjusted final BW and ADG (P < 0.01), where GS increased these measures over NoIMP.
Table 3.
Main effect of implant status on live animal performance and carcass-adjusted performance of nonimplanted or implanted* beef steers fed varying concentrations† of supplemental trace minerals
| Item | NoIMP, n = 36 | IMP, n = 35 | SEM | P value |
|---|---|---|---|---|
| Live animal performance‡ | ||||
| BW, kg | ||||
| Day 0 | 388 | 389 | 1.8 | 0.82 |
| Day 124 | 559 | 617 | 5.9 | <0.01 |
| DMI, kg/d | ||||
| Days 0–56 | 9.70 | 10.27 | 0.158 | 0.01 |
| Days 56–124 | 9.22 | 10.91 | 0.162 | <0.01 |
| Overall days 0–124 | 9.46 | 10.59 | 0.143 | <0.01 |
| ADG, kg | ||||
| Days 56–124 | 1.19 | 1.59 | 0.055 | <0.01 |
| Overall days 0–124 | 1.38 | 1.84 | 0.038 | <0.01 |
| G:F, kg/kg | ||||
| Days 0–56 | 0.157 | 0.203 | 0.0036 | <0.01 |
| Days 56–124 | 0.129 | 0.146 | 0.0053 | 0.03 |
| Overall G:F | 0.146 | 0.174 | 0.0032 | <0.01 |
| Carcass-adjusted performance | ||||
| Final BW∥ | 555 | 619 | 5.2 | <0.01 |
| Overall ADG∥ | 1.33 | 1.84 | 0.036 | <0.01 |
| Overall G:F∥ | 0.141 | 0.174 | 0.0030 | 0.07 |
*Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
†Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
‡No GS × TM; P ≥ 0.13.
∥Adjusted overall live performance parameters were carcass adjusted with a common 64.66% dress.
SEM, standard error of the mean.
Trace mineral supplementation effects on live animal performance are shown in Table 4. Supplementation of TM increased days 56 to 124 DMI and overall DMI (P ≤ 0.01). ADG from days 56 to 124 was not affected by TM supplementation (P = 0.59), but overall live ADG tended to be affected by TM supplementation (P = 0.07). Trace mineral supplementation had no effect on days 56 to 124 G:F or overall G:F (P ≥ 0.12). Trace mineral supplementation affected carcass-adjusted final BW and ADG (P ≤ 0.03) and tended to effect carcass-adjusted G:F (P = 0.07). Steers in the IND treatment had greater final BW than CON steers, with REC being intermediate, whereas IND had greater carcass-adjusted overall ADG than both REC and CON steers (P < 0.01).
Table 4.
Main effect of trace minerals on live animal performance and carcass-adjusted performance of nonimplanted or implanted* beef steers fed varying concentrations† of supplemental trace minerals
| Item | CON, n = 24 | REC, n = 23 | IND, n = 24 | SEM | P value |
|---|---|---|---|---|---|
| Live animal performance‡ | |||||
| BW, kg | |||||
| Day 0 | 389 | 389 | 387 | 2.34 | 0.79 |
| Day 124 | 584 | 585 | 594 | 7.24 | 0.56 |
| DMI, kg/d | |||||
| Days 0–56 | 9.66 | 10.14 | 10.15 | 0.195 | 0.13 |
| Days 56–124 | 9.42b | 10.25a | 10.53a | 0.120 | <0.01 |
| Overall days 0–124 | 9.54b | 10.20a | 10.34a | 0.177 | <0.01 |
| ADG, kg | |||||
| Days 56–124 | 1.35 | 1.38 | 1.44 | 0.069 | 0.59 |
| Overall days 0–124 | 1.57 | 1.58 | 1.67 | 0.047 | 0.07 |
| G:F, kg/kg | |||||
| Days 0–56 | 0.175 | 0.178 | 0.187 | 0.0045 | 0.12 |
| Days 56–124 | 0.143 | 0.135 | 0.137 | 0.0065 | 0.74 |
| Overall G:F | 0.165 | 0.155 | 0.161 | 0.0040 | 0.67 |
| Carcass-adjusted performance | |||||
| Final BW∥ | 577b | 584a,b | 600a | 6.4 | 0.03 |
| Overall ADG∥ | 1.50b | 1.56b | 1.70a | 0.044 | <0.01 |
| Overall G:F∥ | 0.157a,b | 0.152b | 0.164a | 0.0037 | 0.07 |
a,b,cWithin rows, means without a common superscript differ (P ≤ 0.05).
*Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
†Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
‡No GS × TM; P ≥ 0.13.
∥Adjusted overall live performance parameters were carcass adjusted with a common 64.66% dress.
SEM, standard error of the mean.
Plasma Glucose and Urea Nitrogen
Plasma glucose and urea nitrogen data are reported in Table 5. There were no GS × TM interactions for plasma glucose on day 70 (P = 0.79). There was a tendency for a GS × TM effect on day 124 for plasma glucose concentrations (P = 0.06) with NoIMP/REC tending to have greater plasma glucose concentrations than NoIMP/IND with all other treatments intermediate. There was no GS × TM effect for PUN on day 70 or 124 (P ≥ 0.85). On day 70, steers that received implants had lesser PUN than cattle that did not receive hormone implants, 8.31 and 9.68 mg/dL for implanted and nonimplanted steers, respectively (P = 0.03). There was no effect of implant on day 124 (P = 0.74), nor was there any effect of TM supplementation on PUN on day 70 or 124 (P ≥ 0.22).
Table 5.
Effects of nonimplanted or implanted beef steers fed varying concentrations* of supplemental trace minerals on plasma glucose and urea nitrogen concentrations
| Plasma | No IMP | IMP† | SEM | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON, n = 6 | REC, n = 6 | IND, n = 6 | CON, n = 6 | REC, n = 6 | IND, n = 5 | GS | TM | GS × TM | ||
| Glucose,‡ mg/dL | ||||||||||
| Day 0 | 105 | 99 | 98 | 101 | 94 | 114 | — | — | — | — |
| Day 70∥ | 96 | 97 | 95 | 98 | 102 | 103 | 4.4 | 0.14 | 0.88 | 0.79 |
| Day 124∥ | 94x,y | 108x | 93y | 104x,y | 98x,y | 107x,y | 5.1 | 0.29 | 0.72 | 0.06 |
| PUN,‡ mg/dL | ||||||||||
| Day 0 | 9.0 | 8.0 | 9.4 | 10.6 | 10.3 | 8.2 | — | — | — | — |
| Day 70∥ | 9.0 | 9.9 | 10.2 | 7.6 | 9.0 | 8.4 | 0.74 | 0.03 | 0.22 | 0.85 |
| Day 124∥ | 10.0 | 11.4 | 10.1 | 9.7 | 10.8 | 10.3 | 0.85 | 0.74 | 0.33 | 0.90 |
x,yWithin rows, means without a common superscript tend to differ (P ≤ 0.10).
*Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
‡Blood samples were collected before feeding.
∥Day 0 concentrations used as a covariate in analysis.
SEM, standard error of the mean.
Liver Mineral Status
There were no GS × TM effects for liver Cu, Zn, Mn, Se, or Co concentrations (P ≥ 0.11) on day 70 or 124; main effects of GS and TM are shown in Tables 6 and 7, respectively. There was no effect of GS or TM on liver Zn concentrations on day 70 (P ≥ 0.53). Steers that did not receive implants had greater liver Cu and Mn concentrations on day 70 (P ≤ 0.05) and lesser liver Zn concentrations (P = 0.01) on day 125, compared with IMP steers. On day 70, steers in the IND treatment had greater liver Cu concentrations than REC and CON (P < 0.01). There was a TM effect (P ≤ 0.01) on day 70 liver Mn and Se, where IND steers had greater liver Mn and Se concentrations than CON (P ≤ 0.01), with REC intermediate. There was also a TM effect (P = 0.02) on day 70 for liver Co concentrations where REC steers had greater liver Co concentrations than CON, with IND intermediate. On day 124, IND steers had greater liver Cu concentrations than CON, with REC intermediate (P < 0.01). Steers receiving REC had greater liver Zn concentrations than CON with IND being intermediate on day 124. IND and REC had greater liver Mn and Co concentrations than CON on day 124 (P ≤ 0.01). Steers receiving IND had greater liver Se concentrations than REC, and REC steers had greater liver Se than CON steers on day 124 (P < 0.01).
Table 6.
Main effect of implant on liver mineral status of nonimplanted or implanted beef steers fed varying concentrations* of supplemental trace minerals
| Mineral, mg/kg DM† | No IMP, n = 18 | IMP,‡ n = 17 | SEM | P value; GS |
|---|---|---|---|---|
| Initial (day −7) | ||||
| Cu | 219 | 234 | — | — |
| Zn | 108 | 107 | — | — |
| Mn | 9.66 | 9.14 | — | — |
| Se | 1.7 | 1.7 | — | — |
| Co | 0.241 | 0.241 | — | — |
| Day 70† | ||||
| Cu | 228 | 198 | 8.2 | 0.02 |
| Zn | 95 | 98 | 3.2 | 0.53 |
| Mn | 8.59 | 7.93 | 0.228 | 0.05 |
| Se | 2.4 | 2.4 | 0.09 | 0.91 |
| Co | 0.182 | 0.170 | 0.010 | 0.36 |
| Harvest (day 125)∥ | ||||
| Cu | 246 | 237 | 15.7 | 0.67 |
| Zn | 125 | 143 | 4.0 | 0.01 |
| Mn | 8.70 | 8.94 | 0.296 | 0.59 |
| Se | 2.4 | 2.6 | 0.09 | 0.24 |
| Co | 0.174 | 0.158 | 0.007 | 0.10 |
*Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†No GS × TM; P ≥ 0.11.
‡Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
∥Day −7 mineral concentrations were used as a covariate in analysis.
SEM, standard error of the mean.
Table 7.
Main effect of trace mineral supplementation* on liver mineral status of nonimplanted or implanted† beef steers
| Mineral, mg/kg DM‡ | CON, n = 12 | REC, n = 12 | IND, n = 11 | SEM | P value; TM |
|---|---|---|---|---|---|
| Initial (day −7) | |||||
| Cu | 224 | 221 | 233 | — | — |
| Zn | 106 | 104 | 112 | — | — |
| Mn | 9.42 | 9.25 | 9.52 | — | — |
| Se | 1.8 | 1.7 | 1.6 | — | — |
| Co | 0.232 | 0.241 | 0.249 | — | — |
| Day 70∥ | |||||
| Cu | 118c | 233b | 290a | 10.0 | <0.01 |
| Zn | 97 | 98 | 95 | 3.9 | 0.89 |
| Mn | 7.63b | 8.25a,b | 8.90a | 0.276 | 0.01 |
| Se | 2.1b | 2.3a,b | 2.7a | 0.11 | <0.01 |
| Co | 0.147b | 0.192a | 0.189a | 0.0117 | 0.02 |
| Harvest (day 125)∥ | |||||
| Cu | 137b | 277a,b | 310a | 19.0 | <0.01 |
| Zn | 126b | 144a | 133a,b | 4.9 | 0.04 |
| Mn | 7.61b | 9.10a | 9.75a | 0.359 | <0.01 |
| Se | 2.0c | 2.5b | 2.9a | 0.112 | <0.01 |
| Co | 0.135b | 0.184a | 0.179a | 0.0081 | <0.01 |
a,b,cWithin rows, means without a common superscript differ (P ≤ 0.05).
*Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
‡No GS × TM; P ≥ 0.11.
∥Day −7 mineral concentrations were used as a covariate in analysis.
SEM, standard error of the mean.
Carcass Characteristics
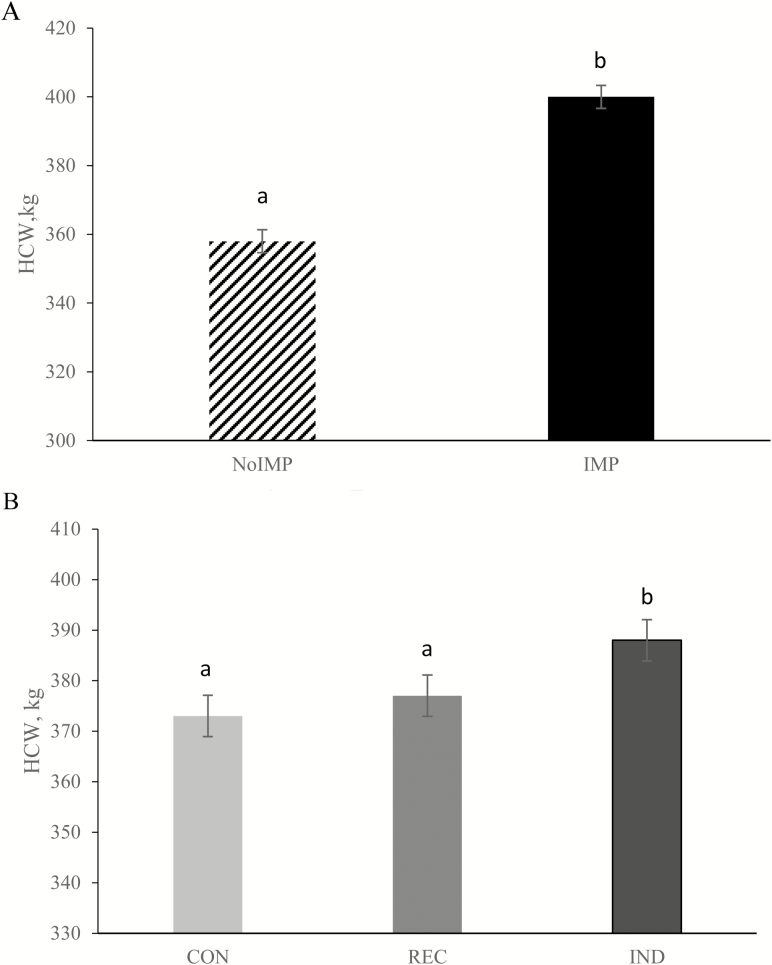
There was no GS × TM interaction for HCW (P = 0.93). Implants increased HCW by 10.5% compared with nonimplanted steers (P < 0.01; Figure 1A). There was also a TM effect (P = 0.03; Figure 1B) where IND steers had greater HCW than REC and CON steers. Carcass characteristics are described in Table 8. There were no GS × TM interactions or effects of GS or TM for dressing percent, KPH, backfat, or MS (P ≥ 0.13). There was a GS × TM effect (P = 0.02) for REA with IMP/CON being greater than IMP/IND, with IMP/REC intermediate; NoIMP had smaller REA, regardless of TM supplementation. There was also a GS × TM effect for yield grade with NoIMP/IND steers having a greater yield grade than IMP/CON with all other treatments intermediate (P = 0.02). There was no GS × TM effect on QG distribution (P ≥ 0.48; Table 8). There was also no effect of GS or TM on QG distribution of steers (P ≥ 0.18).
Figure 1.
Main effects of implant (A; P < 0.01) and trace mineral supplementation (B; P = 0.03) on HCW of beef steers. a, b indicate means differ (P ≤ 0.05). Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP steer were not implanted. Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
Table 8.
Effects of nonimplanted or implanted beef steers fed varying concentrations* of supplemental trace minerals on carcass characteristics and QG distribution
| No IMP | IMP† | SEM | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | CON, n = 12 | REC, n = 11 | IND, n = 12 | CON, n = 12 | REC, n = 12 | IND, n = 12 | GS | TM | GS × TM | |
| Dress,‡ % | 63.4 | 64.7 | 64.8 | 64.5 | 64.4 | 65.9 | 5.8 | 0.26 | 0.13 | 0.50 |
| KPH, % | 2.21 | 2.00 | 2.13 | 2.00 | 1.96 | 2.13 | 0.69 | 0.24 | 0.20 | 0.44 |
| Backfat, cm | 1.52 | 1.34 | 1.69 | 1.16 | 1.46 | 1.46 | 0.126 | 0.13 | 0.16 | 0.17 |
| REA, cm2 | 77.8c | 79.8c | 81.1c | 91.9a | 86.6a,b | 85.1b | 1.87 | <0.01 | 0.58 | 0.02 |
| YG∥ | 3.60a,b | 3.31a,b | 3.70a | 2.84c | 3.44a,b | 3.63a,b | 0.163 | 0.09 | 0.03 | 0.02 |
| MS$ | 494 | 541 | 550 | 486 | 539 | 483 | 39.5 | 0.43 | 0.46 | 0.66 |
| % QG distribution¶ | ||||||||||
| Average choice or higher | 41.7 | 58.3 | 66.7 | 33.3 | 58.3 | 33.3 | — | 0.23 | 0.34 | 0.48 |
| Low choice | 41.7 | 16.7 | 25.0 | 50.0 | 25.0 | 41.7 | — | 0.31 | 0.18 | 0.94 |
| Select | 16.6 | 25.0 | 8.3 | 16.7 | 16.7 | 25.0 | — | 0.68 | 0.88 | 0.49 |
a,b,cWithin rows, means without a common superscript differ (P ≤ 0.05).
*Supplemental trace mineral treatments: CON (no additional supplemental trace minerals), REC (2016 NASEM recommendations: 10 Cu, 30 Zn, 20 Mn, 0.10 Se, 0.15 Co, and 0.50 I; mg/kg), and IND (feedlot consultant recommendations from Samuelson et al. (2016) of 20 Cu, 100 Zn, 50 Mn, 0.30 Se, 0.20 Co, and 0.50 I; mg/kg). Sources of trace mineral included copper sulfate, zinc sulfate, manganese sulfate, calcium iodate, sodium selenite, and cobalt carbonate.
†Growth-stimulated implanted steers (IMP) received Component TE-IS (16 mg estradiol + 80 mg TBA) on day 0 and were reimplanted with Component TE-200 (20 mg estradiol + 200 mg TBA) on day 56, whereas NoIMP received no implants.
‡Dressing percent.
∥Yield grade.
$MS: small = 400, modest = 500, moderate = 600.
¶Percentage of steers in each treatment by QG within treatment total is 100%.
SEM, standard error of the mean.
DISCUSSION
Implantation is a common practice in the beef industry (Samuelson et al., 2016). Combination hormone implants containing both estradiol and trenbolone acetate can increase growth rates by 20% and improve feed efficiency by 15%, compared with cattle not receiving hormone implants (Schanbacher, 1984; Bartle et al., 1992; Johnson et al., 1996). The effects of implants on live and carcass-adjusted animal performance in this study are consistent with previous literature (Johnson et al., 1996; Sawyer et al., 2003). Because of the increase in protein accretion and frame size caused by implants, it is critical to supply cattle with adequate nutrients to support optimal growth. Along with macronutrients, micronutrients like trace minerals are essential for growth of beef animals.
In the first 56 d, steers receiving a moderate potency combination implant (16 mg estradiol + 80 mg TBA) had greater ADG than nonimplanted steers, and ADG was further improved by trace mineral supplementation within implanted steers. As expected, growth rates were greatly increased because of implant (27% increase during days 0 to 56 and a 25% increase during days 56 to 124). Within the first 56 d, trace mineral supplementation more dramatically affected growth rates in implanted cattle compared with those that were not implanted, as shown by the 20% improvement in ADG between IMP and NoIMP CON vs. the 30% improvement in ADG between IMP and NoIMP trace mineral supplemented steers (REC and IND). The added growth response could be due to a greater need for trace minerals to support growth processes in implanted steers, as the CON diet was marginally deficient in Cu, Zn, Mn, and Co.
A large response to implants on HCW was observed in this study, with implanted cattle having a 42 kg advantage in HCW compared with nonimplanted steers. This larger than expected HCW response, which is typically approximately 15 kg for combination implants (McPhee et al., 2006), may be explained by the tremendous improvements in the genetic potential for growth of beef cattle being fed today. For example, since the 1970s, ADG has improved by approximately 44% (Capper, 2011), illustrating the possibility that foundational trace mineral recommendations (NASEM, 2016) may not necessarily be optimal for modern feedlot cattle.
Blood and liver samples were collected on day 70, 14 d after reimplantation, because others have suggested that circulating estradiol and TBA concentrations increases rapidly in the days immediately following implantation (Parr et al., 2014), and thus this may be a time of greater need for nutrients to support the rapid growth that accompanies peak circulating hormone concentrations. Similar to the work by others who have shown PUN to decrease after implantation (Heitzman et al., 1997; Loy et al., 1988; Parr et al., 2014), day 70 PUN was lesser in IMP vs. NoIMP steers, suggesting a greater incorporation of N into muscle.
In this study, implanted steers had decreased Cu and Mn concentrations in the liver 14 d after reimplantation (day 70), possibly exhibiting the increased need for those minerals to support growth. Copper and Mn exert their roles in growth through the formation of cartilage and the support of the extracellular matrix (Leach and Harris, 1997; Rucker et al., 1998) and as components of the potent antioxidants, superoxide dismutase 1 and 3 (Suttle, 2010).
One of the earliest examinations of the impact of GS on trace mineral metabolism was conducted by Hufstedler and Greene (1995) in lambs. Lambs implanted with 12 mg of zeranol tended to retain greater amounts of Zn and had lesser fecal excretion of Mn and Cu compared with nonimplanted lambs (Hufstedler and Greene, 1995), suggesting that trace mineral requirements may be altered when implants are administered to support the demand for increased tissue growth. Interestingly, when Turner et al. (1995) implanted steers with incremental doses of zeranol ranging from 0 to 96 mg 140 d before slaughter and fed diets formulated to meet NRC (1984) requirements, no differences were noted in liver Cu or Zn concentrations in steers measured at slaughter. This lack of differences could be due to differences in implant type or the timing of liver sampling, which was 140 d after implantation compared with the differences noted 14 d after implantation in this study.
In this study, a trace mineral response was observed in HCW, increasing with IND trace mineral supplementation regardless of implant status. Within nonimplanted steers, IND steers had a 13 kg advantage in HCW over CON, and within implanted steers, IND steers had a 17 kg advantage in HCW over CON. When solely evaluating the advantage of trace mineral supplementation regardless of implantation, IND had an 11 kg advantage in HCW compared with REC steers, suggesting current recommendations (NASEM, 2016) may not be adequate for optimal beef production. The economic impact of the observed HCW response is of particular interest, considering the average cost of mineral supplementation was $0.67/steer for REC and $1.79/steer for IND for the entirety of the 124 d experiment.
As the mechanisms by which hormone implants influence cattle growth become better understood, it is possible to see how many trace minerals may support these processes. For example, Zn is essential for nucleic acid and protein synthesis (Hambidge et al., 1986) and increases production of insulin-like growth factor-1 and epidermal growth factor receptors in cell-signaling pathways (Beyersmann and Haase, 2001). Zinc is also a cofactor for metalloproteinases 2 and 9 (McCall et al., 2000), which are thought to increase the rate of proliferation in bovine satellite cells (Thornton et al., 2015).
The critical importance of Zn in growth processes may explain why Huerta et al. (2002) noted an approximately 0.15 kg/d ADG advantage in steers implanted with a Synovex-S implant twice during the feeding period and fed a diet containing 269 mg/kg Zn DM compared with steers that received the same implant but a basal diet of 84 mg/kg Zn DM. Additionally, Huerta et al. (2002) noted an increase in serum Zn concentration (0.835 for control vs. 0.958 mg Zn/L for supplemental Zn treatments), though these values are well within physiological norms and suggest the performance increase is not a pharmacological effect of Zn. In this study, inconsistent responses to Zn supplementation were noted in liver Zn concentrations assessed at day 70 vs. 125. This may be because Zn is involved in over 300 enzymes (Suttle, 2010), an accurate biomarker to reflect Zn status has yet to be identified. It is apparent that continued research on accumulation of Zn in the ruminant animal and the possible role Zn may be playing in hormone implant-induced growth response is warranted.
The CON diet in this study was deficient in Co but appeared to be adequate in Se (NASEM, 2016). Cobalt exerts its role in biology as a component of vitamin B12, supporting propionate metabolism and the transfer of methyl groups (Suttle, 2010), which may be necessary to support muscle cell growth. The observed increase in DMI in this study due to trace mineral supplementation could be due to the role of vitamin B12 in the metabolism of propionate, which can affect DMI in cattle (Allen et al., 2009). In the case of the CON steers with a diet deficient in Co, this could be decreasing the efficiency of propionate metabolism, which results in a greater circulating concentration of propionate, ultimately decreasing feed intake.
Selenium also plays a role in the growth and development of animals through selenoproteins, which assist in combating oxidative stress and increasing proliferation of muscle tissue, and through the conversion of the less-active thyroxine (T4) to the more active triiodothyronine (T3) (Wichtel, 1998; Suttle, 2010). No work examining the effect of dietary I on implant-induced growth has been reported but, because I is needed for the formation of thyroid hormones (Meyer et al., 2008), which play a role in the transcription of the growth hormone gene (Koenig et al., 1987), a case could be made for the importance of I in support of growth. Kahl et al. (1978) noted that a Synovex-S implant (20 mg estradiol benzoate and 200 mg progesterone) decreased the T4:T3 ratio when measured 60 and 120 d after implantation. This would suggest that estrogen is increasing thyroid hormone concentration (Kahl et al., 1978) and subsequently may also be increasing the steer’s requirement for trace minerals, particularly Se and I. Though there was no difference in dietary I supplementation between REC and IND treatment groups, it cannot be fully dismissed that I is playing a role in hormone-induced growth responses.
In comparison to CON steers, supplementation of trace minerals (REC or IND) in this study increased liver concentrations of Cu, Mn, Se, and Co on day 70 and Cu, Zn, Mn, Se, and Co on day 125. However, CON steers were considered adequate for all minerals examined in this study (Kincaid, 2000; Hansen et al., 2006; NASEM, 2016). The degree of increasing concentrations of trace minerals in the liver in response to supplementation varies, even though for most trace minerals, liver is the best indicator of status (Kincaid, 2000).
This study is not the first to examine industry vs. nationally recommended trace mineral concentrations in the diets of cattle. Berrett et al. (2015) implanted steers with Revalor-XS (40 mg estradiol and 200 mg TBA) and supplemented trace minerals at concentrations similar to those used in this study (no supplemental trace mineral, national recommendations (NRC, 1996), or consultant recommendations) to steam-flaked corn-fed feedlot steers. Trace mineral supplementation only improved G:F compared with nonsupplemented steers and a greater proportion of steers that did not receive supplemental trace minerals graded average choice or higher, with no effect on HCW (Berrett et al., 2015). The authors did not observe an increase in the concentrations of liver Cu, Mn, or Zn at slaughter (Berrett et al, 2015) in steers supplemented with trace minerals compared with steers not supplemented with trace minerals. Concentrations of Cu and Zn in the basal diet of Berrett et al. (2015) were approximately three times greater than the CON diet analyzed in this study. This may explain why no difference in liver mineral concentrations were noted at slaughter in the work of Berrett et al. (2015), whereas in this study, differences between CON and trace mineral supplemented steers were apparent. In contrast, in this study, IND-supplemented steers displayed greater liver Cu than CON, and both REC and IND had greater liver Mn than CON at slaughter. Overall, because of a lack of consistent biomarkers, there is a limited understanding of the concentrations of trace minerals in cattle tissues necessary to support optimal growth, and further refinement beyond a simple definition of deficient, adequate, or toxic, is needed.
The lack of implant effect on QG distribution in this experiment is likely contributed to the cattle only being on the terminal implant for a short duration. This could also be due to the timing of reimplantation in this experiment. Overlapping the payout times of combination implants can have detrimental effects on marbling deposition (Parr et al., 2011) and high-potency combination implants early in the feeding period decrease marbling deposition (Bruns et al., 2005). In this experiment, the hormone release of the first implant could have been decreasing at the point of reimplantation, potentially explaining why QGs were not affected by implant.
In this study, regardless of implant status, trace mineral supplementation resulted in a growth response and increased HCW. It is important to note that national recommendations are concentrations shown to prevent symptoms of mineral deficiency and should support growth of cattle (NASEM, 2016), but these may not necessarily be the concentrations needed to optimize performance or profit given the responses observed in this experiment. Further research is needed to investigate which mineral(s) have synergistic relationships with cattle growth and what concentrations are needed to optimize growth of feedlot cattle.
LITERATURE CITED
- Allen M.S., Bradford B.J., and Oba M.. 2009. Board-invited review: the hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 87:3317–3334. doi:10.2527/2009-1779 [DOI] [PubMed] [Google Scholar]
- Bartle S.J., Preston R.L., Brown R.E., and Grant R.J.. 1992. Trenbolone acetate/estradiol combinations in feedlot steers: dose-response and implant carrier effects. J. Anim. Sci. 70:1326–1332. doi:10.2527/1992.7051326x [DOI] [PubMed] [Google Scholar]
- Beckett G.J. and Arthur J.R.. 2005 Selenium and endocrine systems. J. Endocrinol. 184:455–465. doi:10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- Berrett C.J., Wagner J.J., Neuhold K.L., Caldera E., Sellins K.S., and Engle T.E.. 2015. Comparison of national research council standards and industry trace mineral supplementation strategies for yearling feedlot steers. Prof. Anim. Sci. 31:237–247. doi:10.15.232/pas.2014-01345. [Google Scholar]
- Beyersmann D. and Haase H.. 2001. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals. 14:331–341. doi:10.1023/A:1012905406548 [DOI] [PubMed] [Google Scholar]
- Bruns K.W., Pritchard R.H., and Boggs D.L.. 2005. The effect of stage of growth and implant exposure on performance and carcass composition in steers. J. Anim. Sci. 83:108–116. doi:10.2527/2005.831108x [DOI] [PubMed] [Google Scholar]
- Capper J.L. 2011. The environmental impact of beef production in the United States: 1997 compared with 2007. J. Anim. Sci. 89:4249–4261. doi:10.2527/jas.2010–3784 [DOI] [PubMed] [Google Scholar]
- Duckett S.K. and Pratt S.L.. 2014. Meat science and muscle biology symposium—anabolic implants and meat quality. J. Anim. Sci. 92:3–9. doi:10.2527/jas2013-7088. [DOI] [PubMed] [Google Scholar]
- Engle T.E. and Spears J. W.. 2000. Effects of dietary copper concentration and source on performance and copper status of growing and finishing steers. J. Anim. Sci. 78:2446–2451. doi:10.2527/jas.2013-7066. [DOI] [PubMed] [Google Scholar]
- Galbraith H. 1980. The effect of trenbolone acetate on growth, blood hormones and metabolites, and nitrogen balance of beef heifers. Cambridge Uni. Press. Animal Science. 30:389–394. [Google Scholar]
- Genther-Schroeder O.N., Branine M.E., and Hansen S.L.. 2016. The effects of increasing supplementation of zinc-amino acid complex on growth performance, carcass characteristics, and imflammatory response of beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 94:3389–3398. doi:10.2527/jas2015-0209. [DOI] [PubMed] [Google Scholar]
- Hambidge K.M., Casey C.C., and Krebs N.F.. 1986. Zinc. In: Mertz W., editor. Trace elements in human and animal nutrition. vol. 2 New York: Academic Press; pp. 1–137. [Google Scholar]
- Hansen S.L., Spears J.W., Lloyd K.E., and Whisnant C.S.. 2006. Growth, reproductive performance, and manganese status of heifers fed varying concentrations of manganese. J. Anim. Sci. 84:3375–3380. doi:10.2527/jas.2005–667. [DOI] [PubMed] [Google Scholar]
- Heitzman R.J., Chan K.H., and Hart I.C.. 1977. Live weight gain, blood levels or metabolites, proteins, and hormones following implantation of anabolic agents in steers. Br. Vet. J. 133:62–70. doi:10.1016/S007-1935. [DOI] [PubMed] [Google Scholar]
- Huerta M., Kincaid R.L., Cronrath J.D., Busboom J., Johnson A.B., and Swenson C.K.. 2002. Interaction of dietary zinc and growth implants on weight gain, carcass traits and zinc in tissues of growing beef steers and heifers. Anim. Feed Sci. Technol. 95:15–32. doi: 10.1016/S0377-8401. [Google Scholar]
- Hufstedler G.D. and Greene L.W.. 1995. Mineral and nitrogen balance in lambs implanted with zeranol. J. Anim. Sci. 73:3785–3788. [DOI] [PubMed] [Google Scholar]
- Johnson B.J., Anderson P.T., Meiske J.C., and Dayton W.R.. 1996. Effect of combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J. Anim. Sci. 74:363–371. doi:10.2527/1996.742363 [DOI] [PubMed] [Google Scholar]
- Kahl S., Bitman J., and Rumsey T. S.. 1978. Effect of Synovex-S on growth rate and plasma thyroid hormone concentrations in beef cattle. J. Anim. Sci. 46:232–237. [DOI] [PubMed] [Google Scholar]
- Kincaid R.L. 2000. Assessment of trace mineral status of ruminants: a review. J. Anim. Sci. 77:1–10. [Google Scholar]
- Kincaid R.L., Miller W.J., Fowler P.R., Gentry R.P., Hampton D.L., and Neathery M.W.. 1976. Effect of high dietary zinc metabolism and intracellular distribution in cows and calves. J. Dairy. Sci. 59:1580–1584. [DOI] [PubMed] [Google Scholar]
- Koenig R.J., Brent G.A., Warne R.L., Larsen P.R., and Moore D.D.. 1987. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc. Natl. Acad. Sci. 84:5670–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R.M. Jr. and Harris E.D.. 1997. Manganese. In: O’Dell B.L. and Sunde R.A., editors. Handbook of nutritionally essential mineral elements. New York, NY: Marcel Dekker Inc; pp. 335–356. [Google Scholar]
- Liu A.C.-H., Heinrichs B.S., and Leach R.M.. 1994. Influence of manganese deficiency on the characteristics of proteoglycans of avian epiphyseal growth plate cartilage. Poult. Sci. 73:663–669. [DOI] [PubMed] [Google Scholar]
- Loy D.D., Harpster H.W., and Cash E.H.. 1988. Rate, composition and efficiency of growth in feedlot steers reimplanted with growth stimulants. J. Anim. Sci. 66:2668–2677. [DOI] [PubMed] [Google Scholar]
- McCall K.A., Huang C., and Fierke C.A.. 2000. Function and mechanism of zinc metalloenzymes. J. Nutr. 130:1437S–1446S. [DOI] [PubMed] [Google Scholar]
- McPhee M.J., Oltjen J.W., Famula T.R., and Sainz R.D.. 2006. Meta-analysis of factors affecting carcass characteristics of feedlot steers. J. Anim. Sci. 84:3143–3154. doi:10.2527/jas.2006–175. [DOI] [PubMed] [Google Scholar]
- Meyer U., Weigel K., Schöne F., Leiterer M., Flachowsky G.. 2008. Effect of dietary iodine on growth and iodine status of growing fattening bulls. Livest. Sci. 115:219–225. doi:10.1016/j.livesci.2007.07.013. [Google Scholar]
- NASEM 2016. Nutrient requirements of beef cattle. 8th ed Washington, DC: The National Academics Press. [Google Scholar]
- NRC (National Research Council) 1996. Nutrient Requirements of Beef Cattle. 7th revised ed Washington, DC: National Academy Press. [Google Scholar]
- Parr S.L., Brown T.R., Ribeiro F.R.B., Chung K.Y., Hutcheson J.P., Blackwell B.R., Smith P.N., and Johnson B.J.. 2014. Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride. J. Anim. Sci. 92:3348–3363. doi:10.2527/jas.2013–7221. [DOI] [PubMed] [Google Scholar]
- Parr S.L., Chung K.Y., Hutcheson J.P., Nichols W.T., Yates D.A., Streeter M.N., Swingle R.S., Galyean M.L., and Johnson B.J.. 2011. Dose and release pattern of anabolic implants affects growth of finishing beef steers across days on feed. J. Anim. Sci. 89:863–873. doi:10.2527/jas.2010–3447. [DOI] [PubMed] [Google Scholar]
- Richter E.L., Drewnoski M.E., and Hansen S.L.. 2012. Effects of increased dietary sulfur on beef steer mineral status, performance, and meat fatty acid composition. J. Anim. Sci. 90:3945–3953. doi:10.2527/jas.2011-4512 [DOI] [PubMed] [Google Scholar]
- Rucker R.B., Kosonen T., Clegg M.S., Mitchell A.E., Rucker B.R., Uriu-Hare J.Y., and Keen C.L.. 1998. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 67: 996–1002. [DOI] [PubMed] [Google Scholar]
- Samuelson K.L., Hubbert M.E., Galyean M.L., and Löest C.A.. 2016. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 94:2648–2663. doi:10.2527/jas.2016-0282. [DOI] [PubMed] [Google Scholar]
- Sawyer J.E., Mathis C.P., Löest C.A., Walker D.A., Malcolm-Callis K.J., Blan L.A., and Taylor R.. 2003. Case study: niche-targeted vs conventional finishing programs for beef steers. Prof. Anim. Sci. 19:18–194. doi: 10.15232/S1080-7446(15)31398-X. [Google Scholar]
- Schanbacher B.D. 1984. Manipulation of endogenous and exogenous hormones for red meat production. J. Anim. Sci. 59:1621–1630. [Google Scholar]
- Suttle N. 2010. Mineral nutrition of livestock. 4th ed Wallingford, UK: CABI Publishing. [Google Scholar]
- Thornton K.J., Kamange-Sollo K., White M.E., and Dayton W.R.. 2015. Role of G-protein-coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor-like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate-stimulated bovine satellite cell proliferation. J. Anim. Sci. 93:4291–4301. doi:10.2527/jas2015-9191. [DOI] [PubMed] [Google Scholar]
- Turner N.D., Greene L.W., Byers F.M., and Kenison D.C.. 1995. Influence of incremental zeranol implant doses on the chemical and physical characterisitics of third metacarpal bone and chemical composition of liver and soft tissue from feedlot steers. J. Anim. Sci. 73:1–8. [DOI] [PubMed] [Google Scholar]
- Wichtel J.J. 1998. A review of selenium deficiency in grazing ruminants part 1: new roles for selenium in ruminant metabolism. N. Z. Vet. J. 46:47–52. doi:1080/00480169.1998.36055. [DOI] [PubMed] [Google Scholar]