Abstract
The aim of this study was to investigate the effects of dietary curcumin supplementation on the performance, mitochondrial redox system, mitochondrial DNA (mtDNA) integrity, and antioxidant-related gene expression in the liver of broiler chickens after heat stress treatment. At day 21, a total of 400 Arbor Acres broiler chickens with similar body weight (BW) were divided into 5 groups with 8 replicates per group and then reared either at a normal temperature (22 ± 1 °C) or at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time) for 20 d. Broilers in the 5 groups were fed a basal diet at a normal temperature (NT group) and a basal diet with 0, 50, 100, and 200 mg/kg curcumin at a high ambient temperature (HT, CUR50, CUR100, and CUR200 groups), respectively. The serum and liver samples were analyzed for the parameters related to hepatic damage, mitochondrial function, and redox status. The results showed that the G:F was increased in the CUR50 and CUR100 groups, and the final BW was increased in CUR100 group in comparison with the HT group (P < 0.05). When compared with those in the HT group, both serum aspartate and alanine aminotransferase activities were decreased in the curcumin-supplemented groups (P < 0.05). Curcumin decreased the reactive oxygen species (ROS) production but increased the mitochondrial membrane potential in the hepatocytes of the broilers after heat stress (P < 0.05). The broilers in curcumin-supplemented groups had lower malondialdehyde and protein carbonyl concentrations as well as greater thiol concentrations (P < 0.05). The mitochondrial manganese superoxide dismutase (MnSOD) activity in the liver was increased (P < 0.05) in the CUR100 group compared with the HT group. Compared with the heat-stressed broilers, the broilers that were fed curcumin had greater (P < 0.05) mtDNA copy number and ATP concentrations than those in the HT group. Curcumin supplementation attenuated the depression of the thioredoxin 2 and peroxiredoxin-3 gene expressions (P < 0.05). The MnSOD gene expression was increased in the CUR100 and CUR200 groups, and the thioredoxin reductase 2 gene expression was increased in the CUR50 group in comparison with the HT group (P < 0.05). In conclusion, curcumin mitigated the mitochondrial dysfunction in heat-stressed broilers, as evidenced by the suppression of the ROS burst, the maintenance of the thiol pool and mtDNA content, and the enhanced mitochondrial antioxidant gene expression.
Keywords: broiler, curcumin, hepatic mitochondria, mitochondrial DNA, thioredoxin 2/peroxiredoxin-3
INTRODUCTION
With the manifestation of climate change, the occurrence of extreme heat is becoming more common, especially during the summer. Extreme heat is detrimental to the growth performance and health status of all agriculturally important species, including pigs (Yu et al., 2010), cows (Rhoads et al., 2009), and broilers (Huang et al., 2015) when ambient temperatures exceed their thermal neutral zone. Heat stress has been reported to induce a great number of adverse effects on the nervous, endocrine, metabolic, and immune systems, leading to enormous economic losses in the global animal production (Lara and Rostagno, 2013). Characterized by the reactive oxygen species (ROS), dissipation of mitochondrial membrane potential (MMP) and imbalance of mitochondrial redox status, heat stress has been found to impair the mitochondrial function of broilers (Huang et al., 2015). In addition, studies provide evidence of a link between mitochondrial dysfunction and dysregulated mitochondrial DNA (mtDNA) quality (Iida et al., 2015; Li et al., 2017). Therefore, considering the importance of mitochondria in regulating nutrient metabolism and cellular homeostasis, the defect in mitochondrial function may be a possible mechanism for the decreased feed efficiency and nutrient utilization observed in heat-stressed animals (Lucas et al., 2000; Smith, 2003).
mtDNA is a sensitive target for oxygen radical attack. Under oxidant stress, oxidized mtDNA depressed the oxidative phosphorylation function and the mitochondrial activity. Consequently, damaged mtDNA leads to consecutive ROS bursts, which further exacerbate the mitochondrial dysfunction. Therefore, alteration in mtDNA has been proposed to be a cause of mitochondrial dysfunction. Soto et al. (2009) found that heat stress induced a reduction of mtDNA copy number and suggested the dysregulation of mtDNA replication (Soto and Smith, 2009).
One possible way to overcome this situation is through the dietary modulation of the mitochondrial function. In this context, curcumin has been proposed as a possible solution. Curcumin, isolated from rhizomes, is a naturally occurring phytochemical compound (Anand et al., 2008), and it is gaining importance in the prevention of mitochondrial dysfunction (Trujillo et al., 2014). Studies reported that curcumin supplementation preceding a stressor is useful in limiting mitochondrial damage (Mythri et al., 2007; Molina-Jijon et al., 2011). Kuo et al. (2012) provided support for the protective role of curcumin in mtDNA content in obese mice. However, little is known about the effect of curcumin on the mtDNA content and mitochondrial thioredoxin 2 (Trx2) system in heat-stressed broilers. We hypothesized that curcumin could play a positive effect on the regulation of the hepatic antioxidant system at mitochondrial level when broilers are challenged by heat stress. Thus, the aim of this study was to investigate the effect of curcumin on the growth performance, mitochondrial redox system, mtDNA content, and mitochondrial antioxidant genes expression of the liver in heat-stressed broilers.
MATERIALS AND METHODS
Additives
Curcumin was provided by KeHu Biotechnology Research Center (Guangzhou, People’s Republic of China). The content of curcumin was 98% as determined by high performance liquid chromatography (HPLC) analysis (Zhang et al., 2015b).
Animals, Experimental Protocol, and Sample Collection
The experimental protocol in this study was approved by the Nanjing Agricultural University Institutional Animal Care and Use Committee, China and conducted in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, P.R. China). One-day-old Arbor Acres broiler chickens (Gallus gallus domesticus) were purchased from a commercial hatchery (Hefei, Anhui, P. R. China). The initial weight of the broilers was 42 ± 5 g and statistically insignificant. The brooding temperature of the broilers was set to 32 to 34 °C during the first week, reduced by 2 to 3 °C per week, and then fixed at 22 ± 1 °C until day 21. At the age of day 21, a total of 400 broilers with similar body weight (BW) were selected and randomly allocated into 5 groups with 8 replicates per group (n = 8). Each replicate consisted of 10 broilers. One group was raised at a normal temperature (22 ± 1 °C) and fed a basal diet (NT). Four other groups were exposed to a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time) and fed a basal diet containing 0 (HT), 50 (CUR50), 100 (CUR100), and 200 (CUR200) mg/kg curcumin, respectively. The high temperature was maintained by heated-air conditionings and electrically heated, thermostatic controllers (Zhang et al., 2015b). The ingredient composition and nutrient content of the basal diet are given in Table 1. The heat stress treatment lasted for 20 consecutive d, in which the humidity of the room was kept at 50% to 60%. All the broilers had free access to feed and water and received a light regimen of 12:12 h light:darkness throughout the experiment. Details on the heat stress treatment and the rearing condition of broilers have been previously reported (Zhang et al., 2015a; Zhang et al., 2015b).
Table 1.
Ingredient composition and calculated nutrient content of the basal diets
| 1 to 21 d | 22 to 42 d | |
|---|---|---|
| Ingredient (%) | ||
| Corn | 57.0 | 61.9 |
| Soybean meal (44.2%, crude protein) | 31.3 | 25.6 |
| Corn gluten meal (60%, crude protein) | 3.9 | 4.3 |
| Soybean oil | 3.1 | 3.8 |
| Dicalcium phosphate | 1.8 | 1.6 |
| Limestone | 1.3 | 1.2 |
| l-lysine | 0.15 | 0.2 |
| dl-methionine | 0.15 | 0.1 |
| Premixa | 1 | 1 |
| Salt | 0.3 | 0.3 |
| Total | 100 | 100 |
| Calculation of nutrients | ||
| Metabolizable energy, MJ/kg | 12.69 | 13.10 |
| Crude protein, % | 21.52 | 19.71 |
| lysine, % | 1.14 | 1.04 |
| methionine, % | 0.50 | 0.43 |
| Calcium, % | 1.00 | 0.90 |
| Available phosphorus, % | 0.46 | 0.42 |
| Arginine, % | 1.36 | 1.19 |
| Methionine + Cystine, % | 0.85 | 0.76 |
aProvided per kg of diet: vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol acetate), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 65 mg; I (from calcium iodate), 1.1 mg; and Se (from sodium selenite), 0.3 mg.
At the end of the experiment, 1 chicken was selected from each replicate (n = 8) and killed by exsanguination. Serum was obtained from the blood after centrifugation at 4,000 rpm for 15 min at 4 °C and then stored at −20 °C for further analysis of aminotransferase activities. After evisceration, liver tissue was immediately removed, weighted, and placed in ice. One part of the liver sample was snap frozen in liquid nitrogen and stored at −80 °C for redox analysis and DNA and RNA extraction. The other part of the liver sample was collected and used for the isolation of hepatic mitochondria.
Growth Performance
Bird growth performance was determined for the entire heat stress treatment period (D21–42) for the 5 replicates of 8 broilers per group. BW and feed intake were recorded on a replicate basis. The average daily body weight gain (ADG), average daily feed intake (ADFI), and feed efficiency (G:F) were calculated per bird and adjusted by mortality. The liver index, namely, the relative weight of liver, was expressed as the percentage of live BW of the selected broiler for sampling.
Determination of Serum Aminotransferases Activities
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured spectrophotometrically using the commercial kits purchased from Nanjing Jiancheng Institute Bioengineering (Nanjing, China). The results of AST and ALT activities were expressed as unit/liter (U/L).
Preparation of Hepatic Mitochondria
Isolation of hepatic mitochondria was performed according to Zhang et al. (2014), with some modifications. Briefly, the liver was washed twice to get rid of blood and finely minced. Then, the minced samples were homogenized with an ice-cold isolation buffer (pH = 7.4), containing 10-mM Trizma hydrochloride, 250-mM sucrose, and 1-mM EDTA. The above homogenate was centrifuged at 800 × g for 5 min at 4 °C, and the supernatant was centrifuged at 12,000 × g for 15 min at 4 °C to collect the mitochondrial pellets. After washing and spinning twice, the mitochondrial pellets were finally resuspended in the ice-cold isolation buffer. Aliquots of mitochondrial suspension were stored at −80 °C for the subsequent redox analysis.
Determination of Mitochondrial Membrane Potential (MMP) and ROS
The intracellular ROS concentration of liver was measured using a commercial ROS assay kit with a 2,7-dichlorofluorescein-diacetate (DCFH-DA) fluorescence probe (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s directions. Briefly, the freshly isolated hepatocytes were incubated with the DCFH-DA solution, which was oxidized to DCFH at 37 °C for 20 min. The fluorescence intensity of DCFH was measured at the excitation wavelength of 488 nm and emission wavelength of 525 nm through a FACS Calibur flowcytometer (BD, Heidelberg, Germany). The result was expressed as the percentage of the NT group taken as 100%.
Hepatic MMP was measured using a commercial assay kit with a lipophilic cation JC-1 probe (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s directions. Briefly, the freshly isolated hepatocytes were incubated with JC-1 in the dark for 20 min at 37 °C and then washed twice with buffer. The fluorescence intensity of collected hepatocytes was monitored using a FACS Calibur flowcytometer (BD, Heidelberg, Germany).The green fluorescence was measured at the emission wavelength of 530 nm, and the red fluorescence was measured at the emission wavelength of 590 nm when both were excited at 488 nm. The MMP result was determined by the ratio of red to green fluorescence intensity and expressed as the percentage of the NT group taken as 100%.
Redox Analysis in Hepatic Mitochondrial Fraction
Lipid peroxidation, expressed as the malondialdehyde (MDA) concentrations for this assay, was measured spectrophotometrically based on the reaction of thiobarbituric acid-reacting substances and performed using the commercial kit purchased from Nanjing Jiancheng Institute Bioengineering (Nanjing, China). The protein concentrations of the mitochondrial suspension were also determined using the bicinchoninic acid assay (Bainor et al., 2011). The result of the MDA concentration was corrected for the protein concentrations in each sample and expressed as nmol/100 mg protein.
The protein carbonyl (PC) concentration was measured spectrophotometrically according to the reaction of 2,4-dinitrophenylhydrazine, as described previously (Tabassum et al., 2010; Gaona-Gaona et al., 2011). Briefly, the mitochondrial suspension was prepared free from nucleic acids by overnight incubation with streptomycin sulfate. Then, 2,4-dinitrophenylhydrazine and HCl were added to produce the protein hydrazine in the presence of guanidine hydrochloride. Absorbance was recorded at 370 nm and calculated using the molar extinction coefficient of 22,000. The result of the PC concentration was corrected for the protein concentrations in each sample and expressed as millimole per milligram protein (mmol/mg protein).
The contents of total thiol (T-SH) and non-protein thiol (NP-SH) were measured using the commercial kits purchased from Nanjing Jiancheng Institute Bioengineering (Nanjing, China). The value of protein thiol (P-SH) content was calculated by subtracting the NP-SH content from the T-SH content. The results of these 3 thiol contents were corrected for the protein concentrations in each sample and expressed as micromole per milligram protein (μmol/mg protein).
The manganese superoxide dismutase (MnSOD) activity was measured using the commercial kit purchased from Nanjing Jiancheng Institute Bioengineering (Nanjing, China). The result of MnSOD activity was corrected for the protein concentrations in each sample and expressed as unit per milligram protein (U/mg protein).
Determination of ATP Concentration
The ATP concentrations in liver were determined by HPLC as described previously (Papen et al., 2013). Briefly, liver tissue in liquid nitrogen was homogenized by ice-cold saline (wt/vol, 1/1). Subsequently, 0.5 mL of the homogenate was mixed with 0.5-M perchloric acid and extracted for 20 min in an ice-water bath. After centrifugation at 10,000 rpm at 4 °C for 10 min, the supernatant was neutralized with 1-M KOH and then centrifuged to remove the potassium perchlorate. Finally, the supernatant was passed through a 0.22-μm filter and stored at −80 °C for analysis.
HPLC analysis was conducted on an Agilent 1100 with a reversed phase column (Agilent Eclipse-XDB C18, 4.6 mm × 250 mm, 5 μm). Mobile phase A was 0.1-M phosphate buffer (pH = 6.25), and mobile phase B was acetonitrile. The gradient program was 100% A and 0% B initially, 95% A and 5% B at 2 min, 80% A and 20% B at 4 min, 75% A and 25% B at 5.3 min, and 100% A and 0% B at 6 min. A 1-min additional step was included to reach the initial conditions and stabilization. The flow rate was 1.2 mL/min, and the temperature was set to 25 °C. The injection volume was 20 μL in the present study. The ATP concentration in the liver sample was identified by comparing retention times and quantified by the external standard method.
Total RNA Extraction and Real-time PCR Analysis
Total RNA from the liver tissue was collected using Trizol reagent (Takara Biotechnology Co., Dalian, China). RNA was quantified with a spectrophotometer (NanoDrop 2000c; Thermo Scientific, Camden, New Jersey) and tested by agarose gel electrophoresis. Reverse transcription was performed using a PrimeScript RT Reagent kit (Takara Biotechnology Co., Dalian, China).
The cDNA samples were amplified by quantitative real-time PCR with SYBR Premix Ex Taq II kit (Takara Bio, Inc., Dalian, China) in a 20-μL reaction system. The PCR amplification reaction of each gene was conducted in triplicate and conducted initially at 95 °C for 30 s, in 40 cycles at 95 °C for 5 s, and finally at 60 °C for 30 s in the ABI 7300 system (Applied Biosystems, Foster City, CA). The primer sequences are shown in Table 2. The relative mRNA expression level of the target genes was calculated using the 2−ΔΔCt method. β-Actin and glyceraldehyde 3-phosphate dehydrogenase were used as the reference genes for the normalization of target genes as described previously (Livak and Chmittgen, 2001).
Table 2.
Sequences for real-time PCR primers
| Genea | GeneBank ID | Primer sequence (5′→3′) | Product size (bp) |
|---|---|---|---|
| MnSOD | NM_204211.1 | AGGAGGGGAGCCTAAAGGAGA | 214 |
| CCAGCAATGGAATGAGACCTG | |||
| Trx2 | NM_001031410.1 | AGTACGAGGTGTCAGCAGTG | 141 |
| CACACGTTGTGAGCAGGAAG | |||
| Trx-R2 | NM_001122691.1 | CCGGGTCCCTGACATCAAA | 94 |
| TAGCTTCGCTGGCATCAACA | |||
| Prx3 | XM_004942320.1 | ACCTCGTGCTCTTCTTCTACC | 110 |
| ACCACCTCGCAGTTCACATC | |||
| mtD-loop | XM_015291451.1 | AGGACTACGGCTTGAAAAGC | 198 |
| CATCTTGGCATCTTCAGTGCC | |||
| β-Actin | NM_205518 | TGCTGTGTTCCCATCTATCG | 150 |
| TTGGTGACAATACCGTGTTCA | |||
| GAPDH | NM_204305 | AGAACATCATCCCAGCGTCC | 132 |
| CGGCAGGTCAGGTCAACAAC |
aMnSOD = manganese superoxide dismutase; Trx2 = thioredoxin 2; Trx-R2 = thioredoxin reductase 2; Prx3 = peroxiredoxin-3; GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Determination of mtDNA Copy Numbers
The total genomic DNA of liver was isolated using the universal Genomic DNA Extraction Kit (TakaRa Biotechnology Co., Dalian, China). The DNA concentration was quantified with a spectrophotometer (NanoDrop 2000c; Thermo Scientific, Camden, New Jersey) and diluted to the same concentration for further real-time PCR analysis. The relative mtDNA content was quantified by real-time PCR analysis under the same condition as described above. The primer specific for the mtDNA is shown in Table 2. β-Actin was chosen as the reference gene. The 2−∆∆Ct method was employed to calculate the relative mtDNA copy number (Liu et al., 2012; Jia et al., 2013).
Statistical Analyses
The data were presented as means and SE (n = 8). All data were analyzed by one-way ANOVA using SPSS 17.0 (SPSS, Inc., Chicago, IL). Data were normally distributed (Shapiro–Wilk test) and tested for homogeneity of variance (Levene’s test). Duncan’s multiple range test was used to compare the differences among groups. The level of statistical significance was considered to be P < 0.05.
RESULTS
Growth Performance
In the present study, mortality was 1.07% and not related to treatments (data not shown). The bird growth performance data during the entire heat stress treatment period (D21−42) was shown in Table 3. Compared with the NT group, the HT group had lower final BW, ADG, and G:F (P < 0.05), but its ADFI and liver index did not change (P > 0.05). A greater ADG was observed in broilers that received curcumin diets, although the difference was not of statistical significance (P > 0.05). An increased G:F was found in both the CUR50 and the CUR100 groups when compared with the HT group (P < 0.05). The broilers in the CUR100 group had a greater final BW than those in the HT group (P < 0.05), although no significant difference was found in their initial BW (P > 0.05). Curcumin administration showed no effects on the ADFI and liver index in the entire heat stress treatment period (P > 0.05).
Table 3.
Effect of curcumin on the growth performance of broilers reared under heat stress
| Group | ADGa, g bird−1 day−1 | ADFI, g bird−1 day−1 | G:F, g/g | Initial BW, g | Final BW, g | Liver indexb, % |
|---|---|---|---|---|---|---|
| The whole heat stress treatment period (21 to 42 d) | ||||||
| NTc | 70.84 ± 1.04d | 145.36 ± 2.58 | 0.4875 ± 0.0023d | 687.55 ± 6.07 | 2483.65 ± 95.85d | 2.25 ± 0.08 |
| HT | 61.72 ± 2.11e | 132.13 ± 4.32 | 0.4670 ± 0.0020f | 713.61 ± 10.29 | 2142.75 ± 67.39e | 2.23 ± 0.10 |
| CUR50 | 62.29 ± 2.25e | 130.86 ± 4.89 | 0.4761 ± 0.0020e | 707.12 ± 12.77 | 2330.96 ± 33.90d,e | 2.11 ± 0.06 |
| CUR100 | 66.92 ± 1.85d,ef | 139.07 ± 4.76 | 0.4817 ± 0.0041d,e | 683.77 ± 17.88 | 2418.38 ± 52.18d | 2.17 ± 0.06 |
| CUR200 | 67.04 ± 2.63d,e | 141.52 ± 5.75 | 0.4739 ± 0.0024e,f | 679.44 ± 11.82 | 2174.00 ± 84.13e | 2.38 ± 0.11 |
| P-value | 0.023 | 0.156 | <0.001 | 0.234 | 0.004 | 0.276 |
Results are expressed as group mean values and SE (n = 8).
aADG = average daily body weight gain; ADFI = average daily feed intake; G:F = feed efficiency.
bLiver index was expressed as the percentage of live BW of the selected broiler for sampling.
cNT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
d–fMean values followed by different letters are significantly different. Duncan’s test P < 0.05.
RNA Gene Expression in the Liver
The effects of curcumin administration and heat stress treatment on the gene expression of hepatic genes associated with mitochondrial antioxidant system are shown in Table 4. In liver tissue, heat stress led to the less expression (P < 0.05) of mitochondrial antioxidant genes, including MnSOD, Trx2, thioredoxin reductase 2 (Trx-R2), and peroxiredoxin-3 (Prx3) in the HT group compared with the NT group. Compared with those in the HT group, the broilers in the CUR100 and CUR200 groups showed a significant increase (P < 0.05) in the MnSOD gene expression. Curcumin administration significantly attenuated or reversed (P < 0.05) the depression of the Trx2 expression induced by heat stress treatment, and the Trx2 gene expression of the broilers in the CUR100 and CUR200 group was close to that of the broilers in the NT group (P > 0.05). The Trx-R2 gene expression in the curcumin-treated groups showed no significant difference compared with that in the HT group, with the exception of CUR50 group, in which the gene expression was greater than that in the HT group (P < 0.05). The dietary supplementation of curcumin significantly increased the Prx3 gene expression in comparison with that in the HT group (P < 0.05).
Table 4.
Effect of curcumin on the mitochondrial antioxidant genes expression of broilers reared under heat stress
| Group | MnSODa | Trx2 | Trx-R2 | Prx3 |
|---|---|---|---|---|
| The entire heat stress treatment period (21 to 42 d) | ||||
| NTb | 100.00 ± 2.35c | 100.00 ± 4.07c | 100.00 ± 1.79c | 100.00 ± 0.93c |
| HT | 61.24 ± 0.64d | 80.45 ± 0.88d | 77.17 ± 0.89d | 81.76 ± 1.02d |
| CUR50 | 69.15 ± 5.62d,e | 93.70 ± 1.48e | 90.06 ± 2.74e | 94.62 ± 1.23e |
| CUR100 | 75.67 ± 1.03e | 94.54 ± 0.53c,e | 85.16 ± 2.62d,e | 94.67 ± 0.58e |
| CUR200 | 72.76 ± 1.41e | 94.85 ± 0.75c,e | 82.05 ± 6.00d,e | 93.25 ± 0.50e |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Results are expressed as the group mean values and SE (n = 8).
aMnSOD = manganese superoxide dismutase; Prx3 = peroxiredoxin-3; Trx2 = thioredoxin 2; Trx-R2 = thioredoxin reductase 2.
bNT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
c–eMean values followed by different letters are significantly different. Duncan’s test P < 0.05.
Serum Aminotransferases Activities
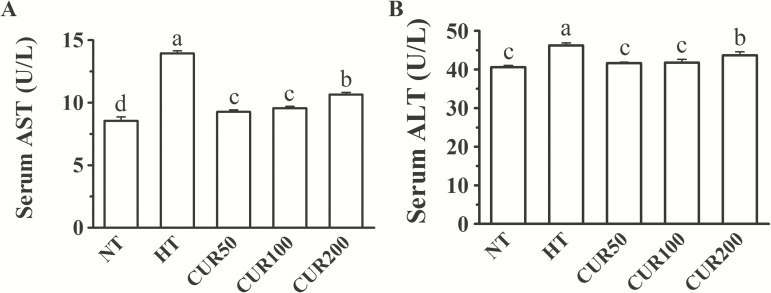
Figure 1 shows the changes in serum AST and ALT activities. Exposure to heat stress treatment caused a remarkable increase in serum AST and ALT activities (P < 0.05). Compared with those in the HT group, both serum AST and ALT activities were significantly decreased in the curcumin-supplemented groups (P < 0.05). Moreover, we found that 50- and 100-mg/kg curcumin diets returned the serum ALT activity of the heat-stressed broilers to normal status (P < 0.05).
Figure 1.
Effect of curcumin on serum (A) AST and (B) ALT activities of broilers reared under heat stress. The results are expressed as the mean ± SE (n = 8). Columns labeled with different letters are significantly different (P < 0.05). NT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
Hepatic ROS and Mitochondrial Membrane Potential
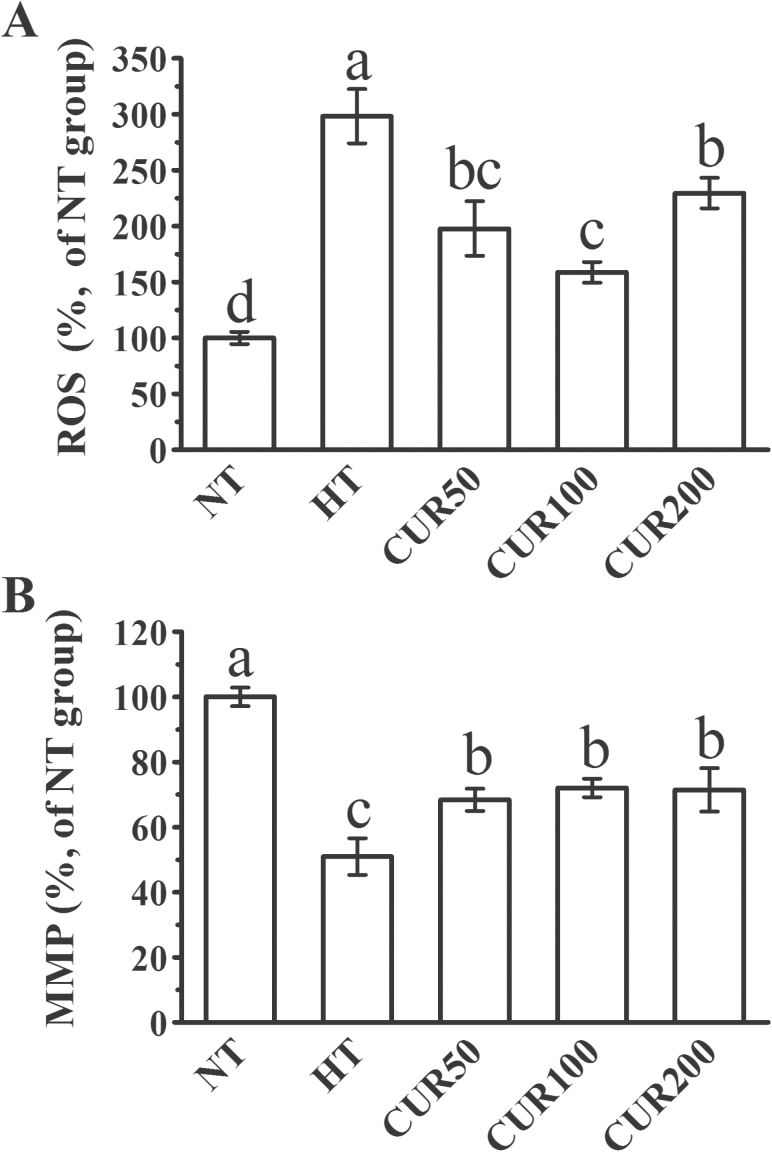
As shown in Figure 2, the long-term heat stress treatment negatively affected the ROS and MMP in liver. The ROS concentration of liver was greater, and the values of MMP were lower in the broilers from the HT group than in those from the NT group (P < 0.05). Dietary supplementation of curcumin significantly decreased the ROS production in comparison with the HT group (P < 0.05). The reduction of hepatic MMP was attenuated to a significant extent with dietary curcumin administration (P < 0.05).
Figure 2.
Effect of curcumin on the (A) ROS accumulation in hepatocytes and the (B) MMP in the hepatic mitochondrial fraction of broilers reared under heat stress. The results are expressed as the mean ± SE (n = 8). Columns labeled with different letters are significantly different (P < 0.05). NT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
Redox Parameters in Hepatic Mitochondrial Fraction
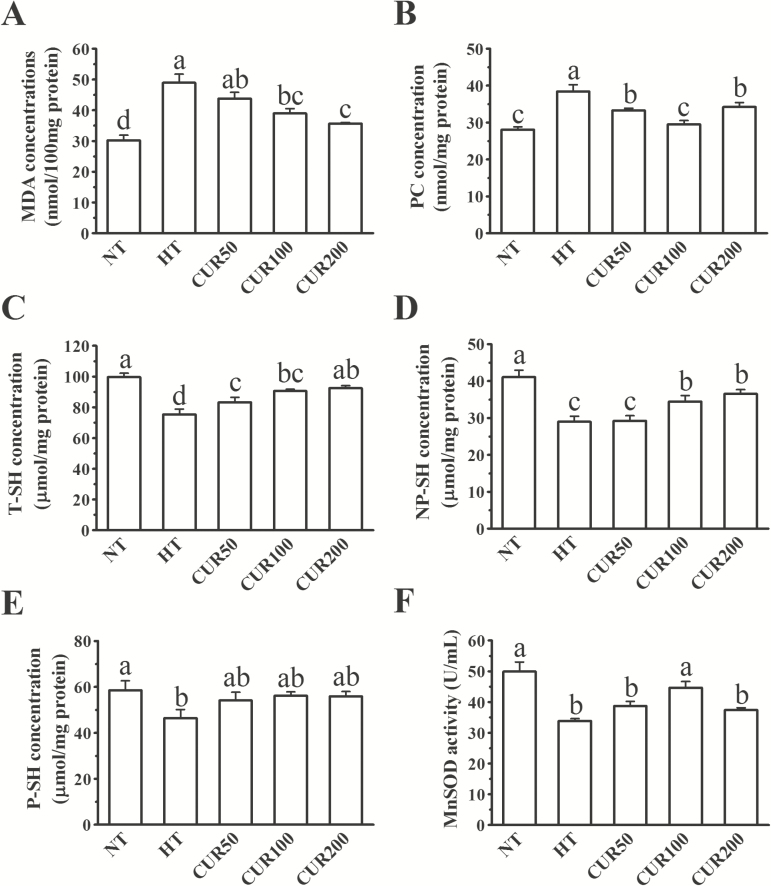
The results of the redox analysis in the hepatic mitochondrial fraction are shown in Figure 3. Compared with that in the NT group, the heat stress treatment induced significant increases (P < 0.05) in the MDA and PC concentrations and decreases (P < 0.05) in the T-SH, NP-SH, P-SH concentrations, and in the MnSOD activity of the hepatic mitochondrial fraction. Diet supplemented with 100- or 200-mg/kg curcumin significantly decreased the MDA concentrations in the hepatic mitochondria in the NT group in comparison with that in the HT group (P < 0.05). The PC concentrations were significantly decreased in the broilers subjected to the curcumin diet in comparison with those in the HT group (P < 0.05). The lowest concentration of PC was found in the CUR100 group, and it had no significance (P > 0.05) between the NT and the CUR100 groups. Broilers from the curcumin-supplemented groups exhibited a greater (P < 0.05) T-SH concentration in the hepatic mitochondrial fraction than those in the HT group. Both the 100- and 200-mg/kg curcumin diets significantly increased (P < 0.05) the NP-SH concentrations in the NT group in comparison with the HT group. However, the P-SH concentrations were not significantly different (P > 0.05) between the curcumin-treated groups and the HT group. The mitochondrial MnSOD activity in the liver was markedly increased (P < 0.05) in the CUR100 group in comparison with the HT group.
Figure 3.
Effect of curcumin on (A) MDA, (B) PC, (C) T-SH, (D) NP-SH, and (E) P-SH concentrations and on (F) MnSOD activity in the hepatic mitochondrial fraction of broilers reared under heat stress. The results are expressed as the mean ± SE (n = 8). Columns labeled with different letters are significantly different (P < 0.05). NT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
mtDNA Copy Number, DNA Fragmentation, and ATP Concentration
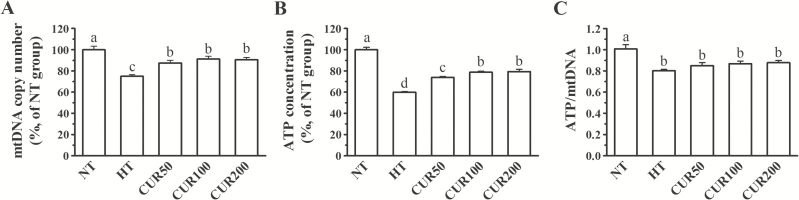
The basic indicators of mtDNA quality and ATP production are shown in Figure 4. The heat stress treatment severely affected the mtDNA quality, including decreased mtDNA content and loss of integrity. A significant difference (P < 0.05) was observed in the mtDNA copy number between the NT and HT group. The concentration of ATP in the HT group was lower (P < 0.05) than that in the NT group. Compared with the heat-stressed broilers, the broilers that fed with a curcumin diet had a greater (P < 0.05) mtDNA copy number in the hepatic mitochondrial fraction. Although dietary curcumin administration significantly increased (P < 0.05) the ATP concentrations in comparison with the HT group, it had no effect on the ratio of ATP to mtDNA copy number (P > 0.05).
Figure 4.
Effect of curcumin on the (A) mtDNA copy number, (B) ATP concentration, and (C) ATP/mtDNA ratio in the hepatic mitochondrial fraction of broilers reared under heat stress. The results are expressed as the mean ± SE (n = 8). Columns labeled with different letters are significantly different (P < 0.05). NT group = broilers fed a basal diet at a normal temperature (22 ± 1 °C); HT group = broilers fed a basal diet at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR50 group = broilers fed a basal diet with 50 mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR100 group = broilers fed a basal diet with 100-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time); CUR200 group = broilers fed a basal diet with 200-mg/kg curcumin at a high ambient temperature (34 ± 1 °C for 8 h and 22 ± 1 °C for the remaining time).
DISCUSSION
The adverse and cumulative effects of high temperature on broiler chickens have been previously reported. Growth suppression is accepted as a typical characteristic of heat stress in broiler chickens (Bottje and Carstens, 2009; Quinteiro-Filho et al., 2010; Lei et al., 2013). In the present study, the broilers exposed to heat stress treatment consumed less feed and consequently obtained a lower final BW and G:F ratio than those in the NT groups. Curcumin, a potent antioxidant with a natural origin, is capable of neutralizing the negative effects of heat stress on broilers partially or completely (Sahin et al., 2012; Zhang et al., 2015a). As expected, the increasing level of dietary curcumin increased the G:F ratio in the present study, especially at a significant extent in the CUR50 and CUR100 groups than in the HT group. However, no difference in ADG between the curcumin-treated groups and the HT groups was observed in the current study, probably because of the similar feed intake of broiler chickens. These observations agreed with the data from Sahin et al. (2012) and our previous study (Zhang et al., 2015b), which showed that oral curcumin administration exhibited no effect on the feed intake of broiler chickens. The change in the final BW in broilers under heat stress conditions as a consequence of growth suppression has also been documented in many studies (Dai et al., 2009; Quinteiro-Filho et al., 2010). Our results showed that the final BW was significantly decreased by heat stress treatment and recovered by the 100-mg/kg curcumin diet. Although no decreasing trend was found in the liver index among various groups, the abnormal increase in serum AST and ALT activities indicated the severe damage of liver tissue, especially the potential mitochondria dysfunction as AST is normally located in the cytosolic and mitochondrial fractions of the liver.
In addition to serum aminotransferase, we determined the ROS production and MMP of the liver, which further identified the mitochondrial dysfunction induced by heat stress. The depletion of MMP and the burst of ROS in heat-stressed broilers were expected on the basis of the previous findings that were consistent with our data (Del Vesco and Gasparino, 2013; Huang et al., 2015). The antioxidant mechanisms of curcumin in animals are likely to be multifaceted. The possible involvement of mitochondrial function in the observed curcumin-induced antioxidant protection has been shown (Trujillo et al., 2014). In the present study, we observed a dramatic decrease in ROS level and an elevation of MMP in broilers administrated with curcumin. A similar mitochondria-protective effect of curcumin was found in mice (Kuo et al., 2012), rats (Sivalingam et al., 2008; Waseem and Parvez, 2013), and cell models (Chen et al., 2006), suggesting that the potential antioxidant capacity of curcumin is at the mitochondrial level. Whether the above antioxidant activities of curcumin are direct or indirect is unknown because of the limited information on the bioavailability of curcumin in mitochondrial fraction. However, curcumin is likely to, at least in part, prevent mitochondrial dysfunction because of its high hydrophobicity (Zhang et al., 2014).
We also determined the oxidant damage in the mitochondria of liver tissue. The greater MDA and PC concentrations in the HT group than in the NT group indicated that lipid peroxidation and protein oxidation occurred in hepatic mitochondria. Lipids and proteins are prone to be damaged by free radical attack once the redox balance is disrupted during oxidant stress, such as heat stress. Curcumin supplementation significantly prevented the increase in MDA and PC concentrations, especially when the inclusions of curcumin were 100 and 200 mg/kg. This result is consistent with that of Molina-Jijon et al. (2011). Curcumin may beneficially influence the prooxidant–antioxidant balance system in the mitochondria through its scavenging ability against the free radicals in the microenvironment.
Mitochondria are at the heart of cellular redox signaling and are particularly rich in the thiol (-SH) function group (Nietzel et al., 2017). Thiols can be divided into 2 major groups: NP-SH, which comprises all low-molecular-weight thiol compounds with GSH as the principal one, and P-SH, which encompasses the free thiol groups present in proteins and the oxidation of which may cause an alteration of protein structure or loss of function (Grintzalis et al., 2014; Yang and Guan, 2015). In the present study, we determined both NP-SH and P-SH concentrations in hepatic mitochondria. As an important indicator of oxidant stress, the decreased T-SH concentration after the heat stress treatment supported the oxidant damage in mitochondria. Curcumin has been suggested to protect against mitochondrial dysfunction by preventing decreases in glutathione concentrations (Waseem and Parvez, 2013). Our results were consistent with the known antioxidant activities of curcumin, showing that 100- and 200-mg/kg curcumin diets significantly increased mitochondrial NP-SH, which was accompanied by an increase in T-SH concentration. The maintenance of thiol level is an effective means of protecting against oxidative damage in animals because thiols are capable of terminating free radicals by undergoing reversible oxidation to become oxidant products. Our observation strongly suggests that curcumin confers additional antioxidant protection for hepatic mitochondria aside from decreased MDA and PC concentrations in heat-stressed broilers.
As previously reported, most of the responses to heat stress have been proved to be dependent on the cumulative ROS production and mitochondrial dysfunction (Del Vesco and Gasparino, 2013; Huang et al., 2015). Only a few studies have addressed the abnormal changes in the mitochondrial content as a marker of mitochondrial injury under heat stress. Mitochondrial function is related to mitochondrial content, which can be measured quantitatively by the copy number of mtDNA and qualitatively by the degree of mtDNA fragmentation (Evdokimovsky et al., 2011; Pinto and Moraes, 2015). A lower mtDNA copy number in tissue is associated with a high level of oxidant stress and has been suggested to be a crucial contributing factor in various oxidant stress-related damages (Petersen et al., 2014). Soto et al. (2009) demonstrated evidence of heat stress driven—the depletion of mtDNA copy number in oocytes, accompanied by the loss of MMP (Soto and Smith, 2009). Similarly, in the present study, a decrease in mtDNA copy number was observed in the liver of the broilers that experienced heat stress treatment. Moreover, the ATP level was positively correlated with the mtDNA copy number as another consequence of hepatic mitochondrial dysfunction. The present study was conducted to determine whether maintaining the mtDNA copy number was one of the reasons that curcumin could protect mitochondria and reduce oxidant damage in heat stress–treated broilers. Our results show that curcumin prevented the decrease in mtDNA copy number and increased the ATP levels in all curcumin feeding groups. Although similar findings about the effects of curcumin on mitochondrial content in heat-stressed broilers are rare, we estimate that the result can be ascribed to the restoration of the mitochondrial antioxidant system by curcumin administration. We hypothesize that a link exists between the increased mtDNA copy number and the improved mitochondrial thiol pool as mitochondrial thiols can prevent mtDNA oxidation and regulate mtDNA replication, thus potentially suppressing mitochondrial dysfunction (Suliman et al., 2004). The ratio of ATP/mtDNA, which indicates the ATP production per unit mitochondria, serves as a novel indicator of the mitochondrial functionality (Hota et al., 2012). However, we found no clear trend on ATP/mtDNA among the different curcumin-treated groups, although a high temperature did lead to a sharp decline in the ratio of ATP/mtDNA, thus indicating oxidant damage of the liver mitochondria at the mtDNA level after chronic heat stress treatment.
The mitochondrial endogenous antioxidant system depends on both the glutathione/glutaredoxin and Trx/Prx families (Nakamura, 2005; Zhang et al., 2007). The mitochondrial Trx/peroxiredoxin system comprises Trx2, TrxR2, and Prx3, the specific localization of which in the mitochondria provides a primary line of defense against superoxide and hydrogen peroxide produced by the burst of mitochondrial ROS (Patenaude et al., 2004). Pérez et al. (2008) reported that the overexpression and the enhanced activity of Trx2 directly led to the increased resistance to mitochondrial dysfunction when the cells were subjected to oxidant stress (Pérez et al., 2008). In the present study, we found that curcumin administration attenuated the suppression of Trx2, TrxR2, and Prx3 gene expressions caused by heat exposure. Among them, the mRNA levels of Trx2 and Prx3 were obviously increased in all curcumin-treated groups, whereas that of TrxR2 was upregulated dramatically only when the dose of curcumin was 50 mg/kg. On the basis of our data and the results from a previous study that revealed a Prx6-related mechanism of curcumin-mediated protection against free radicals (Chhunchha et al., 2011), we consider that a possible mechanism by which curcumin improves the mitochondrial function against a stressor is due to its stimulation of the members of the mitochondrial Trx2/Prx3 family and antioxidant enzymes, such as MnSOD, localized in the mitochondria. The present study indicates that dietary supplementation with curcumin could increase the mRNA abundance of MnSOD mostly because of its stimulation of the NF-E2-related factor 2 (Nrf2) signals. The activation of Nrf2 is a key event for cellular protection against oxidative stress (Lee and Johnson, 2004). Curcumin has been found to facilitate the expression of several phase-2 detoxifying and antioxidant enzymes including MnSOD by increasing the nuclear translocation of Nrf2 (Lee and Johnson, 2004; Farombi et al., 2008; Zhao et al., 2011). Therefore, these findings may provide a potential explanation for the attenuated oxidant injury of heat-stressed broilers after curcumin administration.
In conclusion, our results demonstrate that curcumin partially mitigates mitochondrial dysfunction in heat-stressed broilers, as shown by the suppression of the ROS overproduction, maintenance of the thiol pool and mtDNA content, and improvement in the mitochondrial antioxidant function, specifically in the Trx2/Prx3 system. Therefore, this study can help us to broaden our understanding of the potential protective roles of curcumin to attenuate oxidant injury induced by heat stress in animals.
This study was funded by the Fundamental Research Funds for the Central Universities (No. KJQN201707), National Natural Science Foundation of China (No. 31601973), Natural Science Foundation of Jiangsu Province (No. BK20160739), and China Postdoctoral Science Foundation (2015M581816).
LITERATURE CITED
- Anand P., Thomas S. G., Kunnumakkara A. B., Sundaram C., Harikumar K. B., Sung B., Tharakan S. T., Misra K., Priyadarsini I. K., and Rajasekharan K. N.. 2008. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 76:1590–1611. doi:10.1016/j.bcp.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Bottje W., and Carstens G.. 2009. Association of mitochondrial function and feed efficiency in poultry and livestock species. J. Anim. Sci. 87:E48–E63. doi:10.2527/jas.2008-1379 [DOI] [PubMed] [Google Scholar]
- Chen J., Tang X. Q., Zhi J. L., Cui Y., Yu H. M., Tang E. H., Sun S. N., Feng J. Q., and Chen P. X.. 2006. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 11:943–953. doi:10.1007/s10495-006-6715-5 [DOI] [PubMed] [Google Scholar]
- Chhunchha B. K., Fatma N., Kubo E., Bhargavan B., and Singh D. P.. 2011. Sp1-regulated enhanced expression of Prdx6, an antioxidant by curcumin: a novel action of curcumin-mediated cytoprotection against oxidative stresses. Free Radic. Biol. Med. 51:S84–S84. doi:10.1016/j.freeradbiomed.2011.10.393 [Google Scholar]
- Dai S.F., Wang L. K., Wen A. Y., Wang L. X., and Jin G. M.. 2009. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 50:333–340. doi:10.1080/00071660902806947 [DOI] [PubMed] [Google Scholar]
- Del Vesco A. P., and Gasparino E.. 2013. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J. Anim. Sci. 91:582–587. doi:10.2527/jas.2012–5498 [DOI] [PubMed] [Google Scholar]
- Evdokimovsky E. V., Ushakova T. E., Kudriavtcev A. A., and Gaziev A. I.. 2011. Alteration of mtDNA copy number, mitochondrial gene expression and extracellular DNA content in mice after irradiation at lethal dose. Radiat. Environ. Biophys. 50:181–188. doi:10.1007/s00411-010-0329-6 [DOI] [PubMed] [Google Scholar]
- Farombi E. O., Shrotriya S., Na H. K., Kim S. H., and Surh Y. J.. 2008. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 46:1279–1287. doi:10.1016/j.fct.2007.09.095 [DOI] [PubMed] [Google Scholar]
- Gaona-Gaona L., Molina-Jijon E., Tapia E., Zazueta C., Hernandez-Pando R., Calderon-Oliver M., Zarco-Marquez G., Pinzon E., and Pedraza-Chaverri J.. 2011. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology. 286:20–27. doi:10.1016/j.tox.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Grintzalis K., Papapostolou I., Zisimopoulos D., Stamatiou I., and Georgiou C.D.. 2014. Multiparametric protocol for the determination of thiol redox state in living matter. Free Radic. Biol. Med. 74:85–98. doi:10.1016/j.freeradbiomed.2014.06.024 [DOI] [PubMed] [Google Scholar]
- Hota K. B., Hota S. K., Chaurasia O. P., and Singh S. B.. 2012. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus. 22:723–736. doi:10.1002/hipo.20934 [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., and Lin H.. 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 93:2144–2153. doi:10.2527/jas.2014–8739 [DOI] [PubMed] [Google Scholar]
- Iida R., Ueki M., and Yasuda T.. 2015. Identification of interacting partners of Human Mpv17-like protein with a mitigating effect of mitochondrial dysfunction through mtDNA damage. Free Radic. Biol. Med. 87:336–345. doi:10.1016/j.freeradbiomed.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Jia Y. M., Li R. S., Cong R. H., Yang X. J., Sun Q. W., Parvizi N., and Zhao R. Q.. 2013. Maternal low-protein diet affects epigenetic regulation of hepatic mitochondrial DNA transcription in a sex-specific manner in newborn piglets associated with GR binding to its promoter. Plos One 8:e63855. doi:10.1371/journal.pone.0063855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. J., Chang H. H., Tsai T. H., and Lee T. Y.. 2012. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int. J. Mol. Med. 30:673–679. doi:10.3892/ijmm.2012.1049 [DOI] [PubMed] [Google Scholar]
- Lara L. J., and Rostagno M. H.. 2013. Impact of heat stress on poultry production. Animals (Basel) 3:356–369. doi:10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M. and Johnson J. A.. 2004. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 37:139–143. doi:10.5483/BMBRep.2004.37.2.139 [DOI] [PubMed] [Google Scholar]
- Lei L., Hepeng L., Xianlei L., Hongchao J., Hai L., Sheikhahmadi A., Yufeng W., and Zhigang S.. 2013. Effects of acute heat stress on gene expression of brain-gut neuropeptides in broiler chickens. J. Anim. Sci. 91:5194–5201. doi:10.2527/jas.2013–6538 [DOI] [PubMed] [Google Scholar]
- Li H. Z., Shen L. X., Hu P. Q., Huang R., Cao Y., Deng J., Yuan W. H., Liu D. H., Yang J. f., Gu H. H., and Bai Y. D.. 2017. Aging-associated mitochondrial DNA mutations alter oxidative phosphorylation machinery and cause mitochondrial dysfunctions. Biochim. Biophys. Acta. 1863:2266–2273. doi:10.1016/j.bbadis.2017.05.022 [DOI] [PubMed] [Google Scholar]
- Liu H., Jiang Y., Luo Y., and Jiang W.. 2006. A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high performance liquid chromatography. Food Technol. Biotechnol. 44:531–534. [Google Scholar]
- Liu J. B., Yao Y., Yu B., Mao X. B., Huang Z. Q., and Chen D. W.. 2012. Effect of folic acid supplementation on hepatic antioxidant function and mitochondrial-related gene expression in weanling intrauterine growth retarded piglets. Livest. Sci. 146:123–132. doi:10.1016/j.livsci.2012.02.027 [Google Scholar]
- Livak K. J., and Chmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lucas E. M., Randall J. M., and Meneses J. F.. 2000. Potential for evaporative cooling during heat stress periods in pig production in Portugal (Alentejo). J. Agr. Eng. Res. 76:363–371. doi:10.1006/jaer.2000.0550 [Google Scholar]
- Molina-Jijon E., Tapia E., Zazueta C., El Hafidi M., Zatarain-Barron Z.L., Hernandez-Pando R., Medina-Campos O.N., Zarco-Marquez G., Torres I., and Pedraza-Chaverri J.. 2011. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 51:1543–1557. doi:10.1016/j.freeradbiomed.2011.07.018 [DOI] [PubMed] [Google Scholar]
- Mythri R. B., Jagatha B., Pradhan N., Andersen J., and Bharath M. M.. 2007. Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria?Antioxid. Redox Signal. 9:399. doi:10.1089/ars.2007.9.ft-25 [DOI] [PubMed] [Google Scholar]
- Nakamura H. 2005. Thioredoxin and its related molecules: update 2005. Antioxid. Redox Signal. 7:823–828. doi:10.1089/ars.2005.7.823 [DOI] [PubMed] [Google Scholar]
- Nietzel T., Mostertz J., Hochgrafe F., and Schwarzlander M.. 2017. Redox regulation of mitochondrial proteins and proteomes by cysteine thiol switches. Mitochondrion 33:72–83. doi:10.1016/j.mito.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Papen M. V., Gambaryan S., Schütz C., and Geiger J.. 2013. Determination of ATP and ADP secretion from human and mouse platelets by an HPLC assay. Transfus. Med. Hemo. 40:109. doi:10.1159/000350294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude A., Ven Murthy M. R., and Mirault M. E.. 2004. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J. Biol. Chem. 279:27302–27314. doi:10.1074/jbc.M402496200 [DOI] [PubMed] [Google Scholar]
- Pérez V. I., Lew C. M., Cortez L. A., Webb C. R., Rodriguez M., Liu Y., Qi W., Li Y., Chaudhuri A., and Van Remmen H.. 2008. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 44:882–892. doi:10.1016/j.freeradbiomed.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Petersen M. H., Budtz-Jorgensen E., Sorensen S. A., Nielsen J. E., Hjermind L. E., Vinther-Jensen T., Nielsen S. M., and Norremolle A.. 2014. Reduction in mitochondrial DNA copy number in peripheral leukocytes after onset of Huntington’s disease. Mitochondrion. 17:14–21. doi:10.1016/j.mito.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Pinto M., and Moraes C. T.. 2015. Mechanisms linking mtDNA damage and aging. Free Radic. Biol. Med. 85:250–258. doi:10.1016/j.freeradbiomed.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sakai M., Sa L. R., Ferreira A. J., and Palermo-Neto J.. 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89:1905–1914. doi:10.3382/ps.2010-00812 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., Van Baale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy. Sci. 92:1986–1997. doi:10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu Z., Tuzcu M., and Sahin N.. 2012. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 50:4035–4041. doi:10.1016/j.fct.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Sivalingam N., Basivireddy J., Balasubramanian K. A., and Jacob M.. 2008. Curcumin attenuates indomethacin-induced oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 82:471–481. doi:10.1007/s00204-007-0263-9 [DOI] [PubMed] [Google Scholar]
- Smith M. O. 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 82:1580–1588. doi:10.1093/ps/82.10.1580 [DOI] [PubMed] [Google Scholar]
- Soto P., and Smith L. C.. 2009. BH4 peptide derived from Bcl-xL and Bax-inhibitor peptide suppresses apoptotic mitochondrial changes in heat stressed bovine oocytes. Mol. Reprod. Dev. 76:637–646. doi:10.1002/mrd.20986 [DOI] [PubMed] [Google Scholar]
- Suliman H. B., Welty-Wolf K. E., Carraway M., Tatro L., and Piantadosi C. A.. 2004. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc. Res. 64:279–288. doi:10.1016/j.cardiores.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Tabassum H., Parvez S., Pasha S. T., Banerjee B. D., and Raisuddin S.. 2010. Protective effect of lipoic acid against methotrexate-induced oxidative stress in liver mitochondria. Food Chem. Toxicol. 48:1973–1979. doi:10.1016/j.fct.2010.04.047 [DOI] [PubMed] [Google Scholar]
- Trujillo J., Granados‐Castro L. F., Zazueta C., Andérica‐Romero A. C., Chirino Y. I., and Pedraza‐Chaverrí J.. 2014. Mitochondria as a target in the therapeutic properties of curcumin. Arch. Pharm. (Weinheim) 347:873. doi:10.1002/ardp.201400266 [DOI] [PubMed] [Google Scholar]
- Waseem M., and Parvez S.. 2013. Mitochondrial dysfunction mediated cisplatin induced toxicity: modulatory role of curcumin. Food Chem. Toxicol. 53:334–342. doi:10.1016/j.fct.2012.11.055 [DOI] [PubMed] [Google Scholar]
- Yang Y., and Guan X.. 2015. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonprotein thiols in cells. Anal. Chem. 87:649–655. doi:10.1021/ac503411p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., and Xu J.. 2010. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Phys. A. 156:119–128. doi:10.1016/j.cbpa.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang H., Go Y. -M., and Jones D. P.. 2007. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch. Biochem. Biophys. 465:119–126. doi:10.1016/j.abb.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Zhang J., Xu L., Zhang L., Ying Z., Su W., and Wang T.. 2014. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 144:1211–1218. doi:10.3945/jn.114.193573. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hu Z., Lu C., Bai K., Zhang L., and Tian W.. 2015a. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 63:3880. doi:10.1021/jf505889b [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Hu Z. P., Lu C. H., Yang M. X., Zhang L. L., and Wang T.. 2015b. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 93:1656–1665. doi:10.2527/jas.2014–8244 [DOI] [PubMed] [Google Scholar]
- Zhao S. G., Li Q., Liu Z. X., Wang J. J., Wang X. X., Qin M., and Wen Q. S.. 2011. Curcumin attenuates insulin resistance in hepatocytes by inducing Nrf2 nuclear translocation. Hepatogastroenterology. 58:2106–2111. doi:10.5754/hge11219 [DOI] [PubMed] [Google Scholar]