Abstract
Early weaning results in intestinal dysfunction in piglets, while sulfur amino acids (SAA) are involved in improving intestinal functions. We tested a hypothesis that dietary supplementation with SAA can improve intestinal functions of weaning piglets and analyzed the effects of different dietary SAA levels on intestinal functions. A total of 80 piglets (Duroc × Landrace × Yorkshire) were weaned at 21 d of age and randomly assigned to one of the five diets that contained 0.53%, 0.63%, 0.74%, 0.85%, or 0.96% SAA, which corresponded to 70%, 85%, 100%, 115%, or 130% of the SAA:Lys ratio recommended by the National Research Council (2012). The 14 d feeding experiment involved 16 pens per diet and one piglet per pen. Eight randomly selected piglets from each treatment were euthanized for tissue sampling on day 7 and 14 post weaning. Supplementation with SAA led to a rise over time in G:F (linear, P = 0.001; quadratic, P = 0.001). Between day 0 and 14 of treatment, the jejunal crypt depth decreased (linear, P = 0.018; quadratic, P = 0.015), while that of the duodenal villus (linear, P = 0.049) and ileal villus width (linear, P = 0.029; quadratic, P = 0.034) increased. The activities of jejunal alkaline phosphatase (ALP) were quadratically increased (P = 0.040) from day 0 to 14 due to dietary SAA. Dietary SAA also elevated the activities of jejunal lactase (linear, P = 0.003; quadratic, P = 0.004), jejunal sucrase (linear, P = 0.032; quadratic, P = 0.027), and jejunal contents of glutathione (GSH) from day 0 to 7, as well as the activity of jejunal maltase (linear, P = 0.014; quadratic, P = 0.001) between day 0 and 14. During the first wk, dietary SAA linearly increased the amounts of intestinal-type fatty acid-binding protein (I-FABP) (P = 0.048) and SGLT-1 (P = 0.021) and linearly decreased the amount of GLUT2 (P = 0.029) proteins in the jejunum. The abundance of jejunal I-FABP (P = 0.044) and PEPT1 (P = 0.049) protein linearly increased from day 0 to 14 in response to this supplementation. These findings indicate that there is a dose-dependent response to dietary SAA on feed efficiency and intestinal parameters of weanling pigs.
Keywords: function, intestine, piglet, sulfur amino acids, weaning
INTRODUCTION
Weaning is one of the most stressful events in the pig life cycle because weaned pigs must rapidly adapt to dramatic alterations in their physical and social environments, including a shift from milk to a solid diet, separation from the sow and littermates, and the establishment of a social hierarchy with unfamiliar pigs (van Beers-Schreurs et al., 1998; Moeser et al., 2007a, 2007b). The combined effects of these stressors induce structural and functional changes in the small intestine, such as villous atrophy and crypt hyperplasia, which lead to poor performance and reduce the capacity for digestion and absorption in the gut (Pluske et al., 1997; Montagne et al., 2007).
In addition to their functions in protein synthesis, sulfur amino acids (SAA), methionine (Met), and cysteine (Cys) play key roles in biological functions (e.g., cellular redox function, cell survival and proliferation) and protection against diseases (e.g., liver cirrhosis, Alzheimer’s disease, and diabetes; Bauchart-Thevret et al., 2009b). The gastrointestinal tract is a metabolically significant site of SAA metabolizes in the body and metabolizes about 20% of the dietary Met (Bauchart-Thevret et al., 2009a). For neonatal piglets, dietary supplementation with SAA leads to improved intestinal growth and morphology, increased proliferation of epithelial cells, and increased goblet cell numbers (Bauchart-Thevret et al., 2009b). Weaning stress results in severe intestinal damage in piglets during the first 2 wk post weaning (Montagne et al., 2007). Therefore, we hypothesized that dietary supplementation with SAA would improve intestinal functions of weaning piglets, and the requirement of SAA for maintaining intestinal functions is different from that for improving growth performance of piglets during the first 2 wk post weaning. Our primary objective was to explore the effects of SAA on growth, intestinal index and morphology, the activities of intestinal enzymes, and the synthesis of proteins related to nutrient absorption in those animals.
MATERIALS AND METHODS
The experimental design and procedures in this study were reviewed and approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
Animals and Experimental Treatments
Eighty piglets (Duroc × Landrace × Yorkshire) were weaned at 21 d of age and randomly assigned each to one of the five diets that contained 0.53%, 0.63%, 0.74%, 0.85%, or 0.96% SAA. Those levels corresponded to 70%, 85%, 100%, 115%, or 130% of the SAA:Lys ratio that had been recommended by National Research Council (2012). The doses of SAA used in the present experiment were based on results of previous studies (Matthews et al., 2001; Chen et al., 2014). The 14 d feeding experiment required 16 pens per diet treatment, with each pen holding one piglet. Except for SAA levels, these experimental diets (Table 1) were formulated to meet National Research Council (2012) nutrient requirements (Table 1). Piglets had free access to feed and drinking water at all times throughout the experimental period. Following the method of Yin et al. (2001), we assessed growth performance based on ADG, ADFI, and G:F and covered two periods of measurement, i.e., day 0 to 7 (first wk) and day 0 to 14 (overall experimental period).
Table 1.
Ingredient and chemical composition of experimental piglet diets (as-fed basis)
| Item | Dietary SAA, % | ||||
|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | |
| Ingredient | |||||
| Extruded soybean | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Soybean meal | 7.00 | 6.90 | 6.90 | 6.70 | 6.50 |
| Extruded blood meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Whey powder | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Corn | 53.23 | 53.21 | 53.07 | 53.12 | 53.19 |
| Dicalcium phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Limestone | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Lys | 0.26 | 0.26 | 0.26 | 0.27 | 0.27 |
| Met | 0.00 | 0.12 | 0.25 | 0.39 | 0.52 |
| Thr | 0.08 | 0.08 | 0.09 | 0.09 | 0.09 |
| Try | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Soybean oil | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Zinc oxide | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin and mineral premixa | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calculated composition | |||||
| CP, % | 20.82 | 20.82 | 20.89 | 20.87 | 20.84 |
| DE, MJ/kg | 14.60 | 14.60 | 14.60 | 14.60 | 14.60 |
| Lys,b % | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 |
| Met,b % | 0.27 | 0.37 | 0.48 | 0.59 | 0.70 |
| SAA,b,c % | 0.53 | 0.63 | 0.74 | 0.85 | 0.96 |
| Thr,b % | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Trp,b % | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 |
| Analyzed composition | |||||
| Met, % | 0.33 | 0.49 | 0.53 | 0.71 | 0.83 |
| SAA, % | 0.65 | 0.76 | 0.89 | 0.94 | 1.15 |
aVitamin-mineral premix supplied per kilogram of feed: 10,000 IU of Vitamin A, 1,000 IU of Vitamin D3, 80 IU of Vitamin E, 2.0 mg of Vitamin K3, 0.03 mg of Vitamin B12, 12 mg of riboflavin, 40 mg of niacin, 25 mg of d-pantothenic acid, 0.25 mg of biotin, 1.6 mg of folic acid, 3.0 mg of thiamine, 2.25 mg of pyridoxine, 300 mg of choline chloride, 150 mg of Fe (FeSO4), 100 mg of Zn (ZnSO4), 30 mg of Mn (MnSO4), 25 mg of Cu (CuSO4), 0.5 mg of I (KIO3), 0.3 mg of Co (CoSO4), 0.3 mg of Se (Na2SeO3), and 4.0 mg of ethoxyquin.
bStandardized ileal-digestible.
cSAA = Met + Cys.
Sample Collection and Measurements
At 28 and 35 d of age, the BW of individual piglets was measured immediately before feeding. After BW measuring was completed, eight piglets (four males and four females; one per replicate) were randomly selected from each feeding treatment for tissue sampling as described by Yin et al. (2001). They were euthanized with an overdose of sodium pentobarbital solution (40 mg kg−1 BW), followed by exsanguination (Ren et al., 2014). Then, the small intestine was removed and its length obtained by holding it vertically against a ruler after the mesenterium was stripped away. The net weight of the small intestine was determined after gently squeezing out the contents and removing the mesenterium and fat. Intestinal tissues from middle sections of the duodenum, jejunum, and ileum (approximately 20 cm of each tissue) were isolated using sterilized instruments, flushed with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH = 7.4), and divided into two sections. One segment (approximately 2 cm long) was fixed with 4% formaldehyde-phosphate buffer and kept at 4 °C for a microscopic assessment of the mucosal morphology; the remaining was used for the collection of mucosa on a glass slide. Those mucosal tissue samples were immediately frozen in liquid nitrogen and stored at –80 °C until they were required for western blot analysis and monitoring of enzyme activity (Tan et al., 2011).
Examination of Intestinal Morphology
The fixed intestinal segments were prepared according to standard paraffin-embedding techniques. Three cross sections of each segment were sectioned at 5 μm thickness and stained with hematoxylin and eosin. The villus height and crypt depth of each intestinal segment were determined under a microscope with 40×-combined magnification, using an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, UK). At least 10 well-oriented intact villi and their associated crypts were examined in each intestinal section of each piglet. The mean villus height and crypt depth of each section was then calculated per piglet and used for further analysis (Yang et al., 2016).
Analysis of Intestinal Enzyme Activities and Glutathione
Mucosal tissue samples from the jejunum and ileum were homogenized in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH = 7.4) and centrifuged (2,500 × g, 4 °C, 10 min). The activities of intestinal alkaline phosphatase (ALP), lactase, sucrase, and maltase were analyzed using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The contents of glutathione (GSH) and glutathione disulfide (GSSG) in jejunal mucosa were analyzed using commercial kits (Beyotime Biotechnology, Haimen, China) according to the manufacturer’s instructions.
Western Blotting Analysis
Total protein of jejunal mucosa was extracted using ice-cold RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris–HCl; pH = 7.4; Beyotime Biotechnology, Shanghai, China), plus a protease inhibitor cocktail (Roche, Shanghai, China) and phosphatase inhibitors (Thermo Scientific, Bremen, Germany). After centrifugation (10,000 × g, 4 °C, 10 min), the protein concentration in the supernatant fluid was determined using a bicinchoninic acid assay (Beyotime Biotechnology). All samples were adjusted to an equal protein concentration then diluted with 5× loading buffer (Beyotime Biotechnology) to a final volume of 1 mL and heated in boiling water for 5 min. Soluble proteins were subjected to SDS-PAGE and were transferred to PVDF membranes (Millipore, Billerica, MA). They were then blocked with 5% nonfat milk in TBS-0.05% Tween-20 for 1 h and incubated overnight with primary antibodies followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The bound antibodies were detected via enhanced chemiluminescence (Applygen Technologies Inc., Beijing, China; Yang et al., 2013). For western blot analysis, we used antibodies for the Na+-dependent glucose transporter 1 (SGLT-1), intestinal-type fatty acid-binding protein (I-FABP), GLUT2, PEPT1, and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The abundance of the target proteins was normalized by β-actin, and AlphaImager 2200 software (Alpha Innotech Corporation, CA, USA) was used to quantify the bands of each protein per sample.
Statistical Analysis
All data were subjected to GLM procedures (Version 9.2; SAS Institute, Inc., Cary, NC, USA). Linear and quadratic contrasts were used to examine the effects of SAA on growth performance, intestinal index and morphology, intestinal enzyme activities, GSH and GSSG contents, and the amounts of jejunal I-FABP, PEPT1, SGLT-1, and GLUT2 proteins in weaning piglets. Data were presented as means ± SEM, and P < 0.05 indicated that differences were statistically significant.
RESULTS
Growth Performance and Intestinal Index
No significant differences in growth performance were observed among the five dietary treatments from day 0 to 7 (Table 2). However, from day 0 to 14, dietary SAA supplementation tended to increase both ADG (linear, P = 0.087; quadratic, P = 0.082) and G:F (linear, P = 0.001; quadratic, P = 0.001). The addition of SAA also tended to lead to the production of longer intestines during the first wk (linear, P = 0.080; quadratic, P = 0.075). However, differences were not significant among treatments for intestinal weights or for the ratios of intestinal length:body weight and intestinal weight:body weight (Table 3).
Table 2.
Effects of dietary supplementation with SAA on growth performance of weaning pigletsa
| Item | Dietary SAA, % | SEM | Contrast, P< | |||||
|---|---|---|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | Linear | Quadratic | ||
| ADG, g | ||||||||
| day 0 to 7 | 85.71 | 94.64 | 101.79 | 123.21 | 60.71 | 23.78 | 0.417 | 0.462 |
| day 0 to 14 | 68.75 | 78.57 | 131.25 | 119.64 | 130.36 | 19.81 | 0.087 | 0.082 |
| ADFI, g | ||||||||
| day 0 to 7 | 160.77 | 191.16 | 193.88 | 207.79 | 186.57 | 12.70 | 0.148 | 0.145 |
| day 0 to 14 | 241.80 | 267.02 | 260.38 | 264.88 | 271.01 | 11.05 | 0.351 | 0.389 |
| G:F, g/g | ||||||||
| day 0 to 7 | 0.60 | 0.55 | 0.54 | 0.60 | 0.52 | 0.07 | 0.909 | 0.921 |
| day 0 to 14 | 0.28 | 0.37 | 0.55 | 0.52 | 0.47 | 0.04 | 0.001 | 0.001 |
aValues are means of eight individually caged pigs.
Table 3.
Effects of dietary supplementation with SAA on intestinal index in weaning pigletsa
| Item | Dietary SAA, % | SEM | Contrast, P< | |||||
|---|---|---|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | Linear | Quadratic | ||
| Intestinal length, cm | ||||||||
| day 0 to 7 | 625.50 | 687.13 | 627.00 | 682.38 | 594.63 | 26.17 | 0.080 | 0.075 |
| day 0 to 14 | 740.63 | 674.75 | 703.25 | 696.63 | 785.57 | 33.24 | 0.208 | 0.201 |
| Intestinal length:body weight, cm:kg | ||||||||
| day 0 to 7 | 78.88 | 86.37 | 77.22 | 84.84 | 79.42 | 4.09 | 0.369 | 0.442 |
| day 0 to 14 | 90.13 | 79.04 | 83.43 | 81.32 | 85.64 | 4.42 | 0.406 | 0.468 |
| Intestinal weight, g | ||||||||
| day 0 to 7 | 363.38 | 391.13 | 386.00 | 384.14 | 322.50 | 26.90 | 0.388 | 0.384 |
| day 0 to 14 | 440.63 | 480.00 | 489.88 | 422.25 | 464.86 | 29.23 | 0.472 | 0.469 |
| Ratio of intestinal weight:body weight, g:kg | ||||||||
| day 0 to 7 | 44.92 | 49.41 | 47.10 | 48.14 | 42.49 | 3.07 | 0.550 | 0.548 |
| day 0 to 14 | 53.87 | 55.38 | 57.28 | 49.55 | 50.70 | 3.17 | 0.419 | 0.417 |
aValues are means of eight individually caged pigs.
Intestinal Morphology
From day 0 to 7, no significant differences were observed in crypt depths or villus widths. However, dietary SAA was associated with quadratic increases in jejunal villus height (P = 0.003) and the ratio of duodenal villus height:crypt depth(P = 0.098) during that period (Table 4). From day 0 to 14, dietary SAA decreased the jejunal crypt depth (linear, P = 0.018; quadratic, P = 0.015) as well as the duodenal villus width (linear, P = 0.049), but increased the ileal villus width (linear, P = 0.029; quadratic, P = 0.034; Table 4). No significant differences in the ratio of villus height:crypt depth were found for the duodenum, jejunum, and ileum.
Table 4.
Effects of dietary supplementation with SAA on morphology of small intestines from weaning pigletsa
| Item | Dietary SAA, % | SEM | Contrast, P< | |||||
|---|---|---|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | Linear | Quadratic | ||
| day 0 to 7 | ||||||||
| Villus height, μm | ||||||||
| Duodenum | 186.33 | 203.87 | 209.32 | 242.24 | 181.39 | 14.00 | 0.353 | 0.103 |
| Jejunum | 177.94 | 214.77 | 220.94 | 215.74 | 172.63 | 9.06 | 0.390 | 0.003 |
| Ileum | 157.24 | 136.98 | 161.25 | 146.91 | 142.19 | 9.43 | 0.390 | 0.384 |
| Crypt depth, μm | ||||||||
| Duodenum | 228.78 | 258.56 | 231.56 | 238.65 | 226.48 | 13.69 | 0.587 | 0.585 |
| Jejunum | 149.30 | 172.16 | 152.47 | 159.22 | 146.01 | 9.83 | 0.373 | 0.420 |
| Ileum | 176.33 | 177.29 | 165.50 | 167.90 | 168.02 | 8.70 | 0.765 | 0.869 |
| Villus width, μm | ||||||||
| Duodenum | 106.75 | 107.23 | 108.46 | 104.86 | 98.81 | 2.69 | 0.205 | 0.300 |
| Jejunum | 85.99 | 88.29 | 93.33 | 90.95 | 84.52 | 2.60 | 0.243 | 0.235 |
| Ileum | 79.14 | 83.39 | 88.82 | 85.28 | 83.38 | 2.54 | 0.301 | 0.294 |
| Ratio of villus height:crypt depth, μm:μm | ||||||||
| Duodenum | 0.83 | 0.80 | 0.92 | 1.02 | 0.81 | 0.06 | 0.108 | 0.098 |
| Jejunum | 1.20 | 1.28 | 1.50 | 1.36 | 1.26 | 0.10 | 0.353 | 0.440 |
| Ileum | 0.90 | 0.80 | 0.99 | 0.89 | 0.87 | 0.07 | 0.321 | 0.528 |
| day 0 to 14 | ||||||||
| Villus height, μm | ||||||||
| Duodenum | 226.77 | 207.90 | 233.83 | 198.33 | 214.02 | 15.16 | 0.325 | 0.503 |
| Jejunum | 230.47 | 212.92 | 225.64 | 194.66 | 212.69 | 13.89 | 0.325 | 0.425 |
| Ileum | 168.30 | 161.84 | 142.08 | 145.17 | 181.40 | 11.52 | 0.140 | 0.132 |
| Crypt depth, μm | ||||||||
| Duodenum | 263.07 | 270.11 | 266.24 | 262.49 | 274.47 | 11.42 | 0.947 | 0.947 |
| Jejunum | 220.50 | 170.72 | 189.44 | 184.40 | 192.43 | 8.87 | 0.018 | 0.015 |
| Ileum | 196.47 | 189.57 | 185.99 | 191.63 | 197.36 | 7.88 | 0.760 | 0.856 |
| Villus width, μm | ||||||||
| Duodenum | 113.16 | 113.12 | 110.92 | 104.23 | 115.21 | 2.71 | 0.049 | 0.075 |
| Jejunum | 107.24 | 105.17 | 107.13 | 101.66 | 108.56 | 3.87 | 0.763 | 0.763 |
| Ileum | 94.42 | 95.85 | 104.55 | 97.92 | 106.84 | 3.05 | 0.029 | 0.034 |
| Ratio of villus height:crypt depth, μm:μm | ||||||||
| Duodenum | 0.88 | 0.77 | 0.90 | 0.76 | 0.79 | 0.06 | 0.447 | 0.443 |
| Jejunum | 1.04 | 1.24 | 1.20 | 1.08 | 1.12 | 0.07 | 0.254 | 0.247 |
| Ileum | 0.87 | 0.88 | 0.77 | 0.76 | 0.94 | 0.07 | 0.321 | 0.404 |
aValues are means of eight individually caged pigs.
Intestinal Enzyme Activities
Supplementation with SAA increased the activities of jejunal lactase (linear, P = 0.003; quadratic, P = 0.004), sucrase (linear, P = 0.032; quadratic, P = 0.027) and tended to influence jejunal ALP (quadratic, P = 0.054) and ileal maltase (quadratic, P = 0.058) from day 0 to 7 but had no significant impact on the activities of jejunal maltase, as well as ileal ALP, lactase, and sucrase when the five dietary treatments were compared (Table 5). From day 0 to 14, SAA affected the activity of jejunal ALP (quadratic, P = 0.040) and jejunal maltase (linear, P = 0.014; quadratic, P = 0.001), but no significant differences among treatments were observed for jejunal lactase and sucrose or for ileal ALP, lactase, sucrase, and maltase from day 0 to 14.
Table 5.
Effects of dietary supplementation with SAA on activity of digestive enzymes in weaning pigletsa
| Item | Dietary SAA, % | SEM | Contrast, P< | |||||
|---|---|---|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | Linear | Quadratic | ||
| day 0 to day 7 | ||||||||
| ALP, U/mg of protein | ||||||||
| Jejunum | 245.40 | 172.38 | 217.87 | 238.80 | 242.87 | 24.52 | 0.814 | 0.054 |
| Ileum | 220.26 | 163.17 | 219.41 | 203.98 | 144.65 | 38.51 | 0.989 | 0.291 |
| Lactase, U/mg of protein | ||||||||
| Jejunum | 17.61 | 9.82 | 15.58 | 30.49 | 25.06 | 3.46 | 0.004 | 0.003 |
| Ileum | 1.43 | 2.10 | 2.08 | 2.05 | 2.36 | 0.26 | 0.207 | 0.278 |
| Sucrase, U/mg of protein | ||||||||
| Jejunum | 27.33 | 14.47 | 28.66 | 45.76 | 37.23 | 6.31 | 0.032 | 0.027 |
| Ileum | 12.40 | 8.52 | 7.71 | 9.21 | 8.16 | 1.83 | 0.579 | 0.576 |
| Maltase, U/mg of protein | ||||||||
| Jejunum | 57.62 | 53.97 | 65.98 | 80.29 | 62.91 | 7.22 | 0.311 | 0.273 |
| Ileum | 48.66 | 27.77 | 47.08 | 41.49 | 22.84 | 9.93 | 0.894 | 0.058 |
| day 0 to day 14 | ||||||||
| ALP, U/mg of protein | ||||||||
| Jejunum | 170.81 | 92.07 | 160.23 | 83.73 | 75.47 | 27.47 | 0.796 | 0.040 |
| Ileum | 228.17 | 231.71 | 327.07 | 208.73 | 281.09 | 49.15 | 0.177 | 0.474 |
| Lactase, U/mg of protein | ||||||||
| Jejunum | 18.62 | 15.45 | 19.92 | 21.78 | 14.64 | 4.19 | 0.791 | 0.792 |
| Ileum | 4.02 | 3.69 | 6.31 | 6.46 | 5.05 | 1.80 | 0.748 | 0.748 |
| Sucrase, U/mg of protein | ||||||||
| Jejunum | 9.70 | 7.09 | 10.62 | 10.50 | 6.70 | 2.22 | 0.718 | 0.718 |
| Ileum | 51.38 | 57.36 | 57.40 | 75.28 | 66.94 | 16.53 | 0.856 | 0.865 |
| Maltase, U/mg of protein | ||||||||
| Jejunum | 36.48 | 26.25 | 51.24 | 35.92 | 21.31 | 4.61 | 0.014 | 0.001 |
| Ileum | 38.88 | 34.97 | 44.21 | 28.31 | 42.30 | 12.13 | 0.688 | 0.559 |
aValues are means of eight individually caged pigs.
Intestinal Nutrients Transporters Synthesis
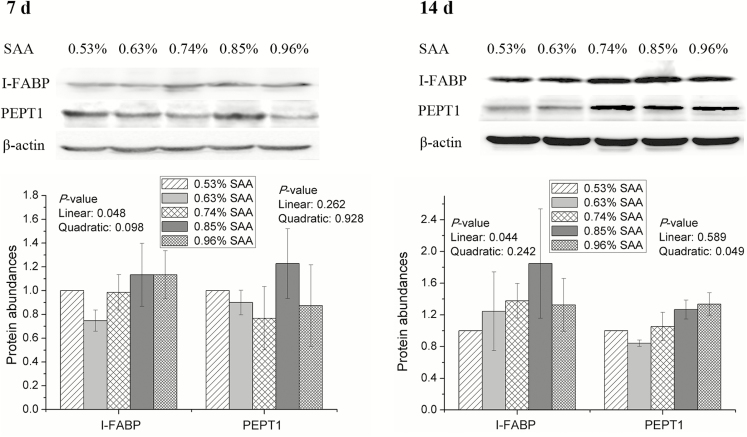
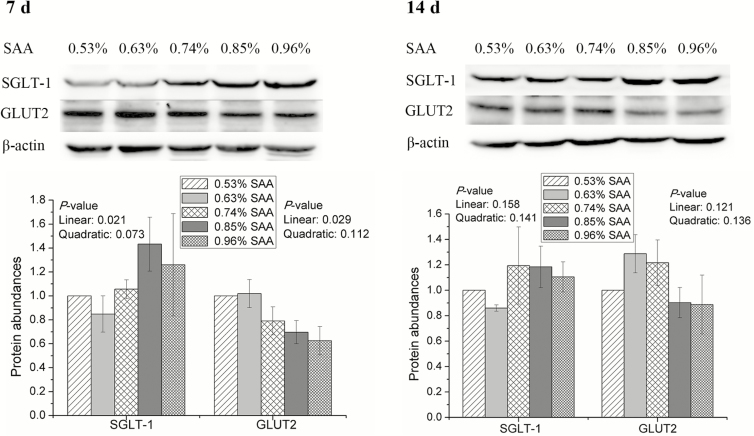
The amounts of I-FABP were linearly increased by dietary SAA from day 0 to 7 (P = 0.048) and from day 0 to 14 (P = 0.044; Figure 1). Dietary SAA was associated with quadratic increases (P = 0.049) in the abundance of PEPT1 from day 0 to 14, but the levels of PEPT1 did not differ significantly among treatments from day 0 to 7 (Figure 1). From day 0 to 7, dietary SAA linearly increased the amounts of SGLT-1 (P = 0.021) but linearly decreased the abundance of GLUT2 (P = 0.029) in jejunal mucosa of weaning piglets (Figure 2). There are no significant differences in the amounts of SGLT-1 and GLUT2 among treatments from day 0 to 14 (Figure 2).
Figure 1.
Effects of dietary supplementation with SAA on protein abundance of jejunal I-FABP and PEPT1 in weaning piglets. β-Actin was used as internal control to normalize abundance. Data are means ± SEM, n = 8.
Figure 2.
Effects of dietary supplementation with SAA on protein abundance of jejunal SGLT-1 and GLUT2 in weaning piglets. β-Actin was used as internal control to normalize abundance. Data are means ± SEM, n = 8.
Intestinal GSH and GSSG Contents
From day 0 to 7, dietary SAA was associated with quadratic increases (P = 0.009) in jejunal contents of GSH, and the contents of GSSG was tended (P = 0.082) to be linearly decreased in the jejunal mucosa of weaning piglets (Table 6). No significant differences among treatments were observed for ileal contents of GSH and GSSG during the first wk and overall experimental period (Table 6).
Table 6.
Effects of dietary supplementation with SAA on intestinal mucosal GSH and GSSG contents in weaning pigletsa
| Item | Dietary SAA, % | SEM | Contrast, P< | |||||
|---|---|---|---|---|---|---|---|---|
| 0.53 | 0.63 | 0.74 | 0.85 | 0.96 | Linear | Quadratic | ||
| Jejunal GSH, μM/mg of protein | ||||||||
| day 0 to 7 | 5.23 | 5.84 | 13.25 | 6.92 | 6.26 | 2.58 | 0.182 | 0.009 |
| day 0 to 14 | 1.36 | 2.01 | 2.10 | 1.07 | 1.02 | 0.46 | 0.362 | 0.683 |
| Jejunal GSSG, μM/mg of protein | ||||||||
| day 0 to 7 | 2.82 | 3.67 | 2.67 | 0.99 | 0.63 | 0.50 | 0.081 | 0.802 |
| day 0 to 14 | 4.08 | 4.59 | 5.36 | 4.97 | 4.33 | 1.07 | 0.903 | 0.313 |
| Ileal GSH, μM/mg of protein | ||||||||
| day 0 to 7 | 0.72 | 0.73 | 0.62 | 0.72 | 0.83 | 0.21 | 0.755 | 0.822 |
| day 0 to 14 | 0.88 | 0.88 | 1.08 | 0.90 | 0.85 | 0.23 | 0.572 | 0.713 |
| Ileal GSSG, μM/mg of protein | ||||||||
| day 0 to 7 | 2.69 | 2.72 | 2.49 | 1.66 | 1.45 | 0.76 | 0.830 | 0.868 |
| day 0 to 14 | 2.86 | 1.77 | 1.98 | 1.50 | 1.86 | 0.71 | 0.306 | 0.362 |
aValues are means of eight individually caged pigs.
DISCUSSION
Increasing evidence indicates that SAA, including Met and Cys, play important metabolic and functional roles in human health and diseases (e.g., liver cirrhosis, Alzheimer’s disease, inflammatory bowel disease, and diabetes; Shoveller et al., 2005). The Met is commonly provided when feeding piglets because it is the second limiting amino acid in typical diets. Dry DL-Met or liquid DL-Met hydroxy analog-free acids are frequently used in basal diets to optimize the growth performance of pigs (Matthews et al., 2001; Kim et al., 2006; Ettle et al., 2010; Kaewtapee et al., 2010, 2016; Ly et al., 2012; Krutthai et al., 2015). This response might be attributed to an increase in the rate of feed conversion (Kim et al., 2006; Kaewtapee et al., 2010, 2016; Ly et al., 2012). Similar to those earlier studies, we found here that dietary supplementation with SAA (via DL-Met) improved G:F in our weaning piglets. Chen et al. (2014) showed that dietary Met did not significantly affect growth performance of weaning piglets during the first week post weaning. However, dietary supplementation with Met increased G:F of piglets from day 0 to 14. The results of the present study also showed that dietary SAA levels did not affect growth performance of weaning piglets from day 0 to 7, but increased G:F from day 0 to 14. That dietary SAA did not affect growth performance of piglets during the first week post weaning might be because the intestine of piglets had severe damage in intestinal architecture and digestive enzyme activities during this period. However, only few piglets (less than 10 animals per treatment) were used in Chen et al. (2014) and the present experiments, more studies with a larger number of animals should be conducted to confirm the results of growth performance. Most experiments conducted to estimate SAA requirement used piglets with a BW greater than 10 kg and based on their growth performance (National Research Council, 2012). The intestines of these piglets were recovered from weaning stress as the intestinal architecture and digestive enzyme activities were recovered, thereby demonstrating that this supplement helps support intestinal functions. Therefore, the requirement of intestine should be considered when estimating dietary SAA requirement for weaning piglets.
The significant changes that occur in intestinal structure and functioning after weaning, such as villous atrophy, crypt hyperplasia, and a decline in enzyme activities, are generally associated with poor performance (Pluske et al., 1997). However, piglets fed a diet supplemented with L-Met show an increase in the villus height in the jejunum and a decrease in crypt depth in the duodenum (Chen et al., 2014). Likewise, a higher level of dietary dl-methionine hydroxy analog free acid causes a quadratic increase in jejunal villous height (Kaewtapee et al., 2016). We also observed that, here, a quadratic increase in the ratio of jejunal villus height when our piglets were fed with dietary SAA for 7 d post weaning, demonstrating that SAA can be used to improve the intestinal morphology of weaning piglets. Moreover, this beneficial effect on intestinal morphology was attenuated when SAA:Lys reached 130% of the NRC recommendation.
Weaning stress results in a decline in the activities of most digestive enzymes in piglets (Montagne et al., 2007). Besides the improvement of intestinal morphology, dietary SAA also improved the activities of various enzymes. Intestinal ALP is a marker of enterocyte differentiation (Hodin et al., 1995; Lackeyram et al., 2010). Early weaning can reduce the capacity for its digestion because enzyme activity is maximized primarily in the jejunal region in pigs (Lackeyram et al., 2010). Our results showed that dietary SAA increased the activity of jejunal ALP from day 0 to 14 and tended to lead to an increase from day 0 to 7; further studies are needed to test the effects of SAA on jejunal enterocyte differentiation. Moreover, the intestinal ALP was regarded as a key marker enzyme when considering changes in intestinal primary digestive and absorptive functions, which suggests dietary SAA may improve intestinal digestive and absorptive functions (Lackeyram et al., 2010). In addition, dietary supplementation with SAA has been reported to increase the activities of jejunal lactase, sucrose, and maltase. For example, Tsukahara et al. (2016) showed that weaning seriously influences disaccharidase activity in piglets weaned at 14 d old. This suggests, therefore, that dietary SAA can improve intestinal digestive capacity by increasing the activity of jejunal enzymes. However, we did not analyze nutrient digestibility in the present investigation and will have to perform further experiments to address this component. Similar to the results we found from our morphological comparisons, we learned that the beneficial effects of dietary SAA on enzyme activity also tended to be attenuated when SAA:Lys was 130% of the NRC recommendation.
The piglet jejunum is the major site in the intestine for absorbing most dietary nutrients, including fatty acids, glucose, peptides, and amino acids (Alexander and Carey, 1999). One protein, I-FABP, is specifically and abundantly expressed in the epithelial cells of the mucosal layer in the small and large intestines and plays a key role in fatty acid absorption (Pelsers et al., 2003). Glucose uptake in epithelial cells depends upon two types of glucose transporters: the apically expressed Na+-dependent SGLT-1 and the basolaterally expressed GLUT2 (Dai et al., 2016). The former mediates the majority of D-glucose transport across the brush-border membrane of enterocytes (Gorboulev et al., 2012). The intestinal peptide transporter PEPT1, with high expression in apical membranes of jejunum, plays key roles in absorbing dietary di- and tripeptides (Ma et al., 2011). We found here that dietary SAA increased the abundance of I-FABP, PEPT1, and SGLT-1 in the jejunal mucosa, which again suggested that this supplement can improve the intestinal absorptive capacity of weaning piglets. This enhancement was attenuated as the SAA:Lys ratio reached 130% of the NRC recommendation. Redox mechanisms function in the regulation of proliferation, differentiation, and apoptosis of intestinal enterocytes (Turan et al., 2009). The SAA plays a key role in cellular redox function as a precursor for GSH biosynthesis (Deplancke and Gaskins, 2002). The results of the present study showed that dietary supplementation with SAA increased jejunal GSH contents and tended to decrease jejunal GSSG contents. Therefore, dietary SAA may act through affecting mucosal antioxidant systems to improve intestinal functions of weaning piglets.
In conclusion, dietary supplementation with SAA improved growth performance and intestinal digestive and absorptive functions of weaning piglets, and this beneficial effect of SAA on intestinal functions may via affecting the antioxidant function. Moreover, the requirement of SAA for maintaining intestinal functions may be different from that for improving growth performance of piglets during the first 2 wk post weaning.
Footnotes
This work was supported by National Natural Science Foundation of China (No. 31402089, 31330075, 31472106, 31272261), Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW- SMC008).
LITERATURE CITED
- Alexander A.N., and Carey H.V.. 1999. Oral IGF-I enhances nutrient and electrolyte absorption in neonatal piglet intestine. Am. J. Physiol. 277:G619–G625. [DOI] [PubMed] [Google Scholar]
- Bauchart-Thevret C., Stoll B., and Burrin D.G.. 2009a. Intestinal metabolism of sulfur amino acids. Nutr. Res. Rev. 22:175–187. doi: 10.1017/S0954422409990138 [DOI] [PubMed] [Google Scholar]
- Bauchart-Thevret C., Stoll B., Chacko S., and Burrin D.G.. 2009b. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 296:E1239–E1250. doi: 10.1152/ajpendo.91021.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li D., Dai Z., Piao X., Wu Z., Wang B., Zhu Y., and Zeng Z.. 2014. L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids. 46:1131–1142. doi: 10.1007/s00726-014-1675-5 [DOI] [PubMed] [Google Scholar]
- Dai L., Hu W.W., Xia L., Xia M., and Yang Q.. 2016. Transmissible gastroenteritis virus infection enhances SGLT1 and GLUT2 expression to increase glucose uptake. PLoS One. 11:e0165585. doi: 10.1371/journal.pone.0165585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B., and Gaskins H.R.. 2002. Redox control of the transsulfuration and glutathione biosynthesis pathways. Curr. Opin. Clin. Nutr. Metab. Care. 5:85–92. [DOI] [PubMed] [Google Scholar]
- Ettle T., Rademacher M., Htoo J.K., and Roth F.X.. 2010. Dietary preference for methionine sources in weaned pigs. Anim. Feed. Sci. Tech. 155:201–205. doi: 10.1016/j.anifeedsci.2009.11.001 [Google Scholar]
- Gorboulev V., Schurmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R.,. et al. 2012. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 61:187–196. doi: 10.2337/db11-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin R.A., Chamberlain S.M., and Meng S.. 1995. Pattern of rat intestinal brush border enzyme gene expression changes with epithelial growth state. Am. J. Physiol. 269:C385–C391. [DOI] [PubMed] [Google Scholar]
- Kaewtapee C., Krutthai N., Poosuwan K., Poeikhampha T., Koonawootrittriron S., and Bunchasak C.. 2010. Effects of adding liquid DL-methionine hydroxy analogue-free acid to drinking water on growth performance and small intestinal morphology of nursery pigs. J. Anim. Physiol. Anim. Nutr. 94:395–404. doi: 10.1111/j.1439-0396.2009.00920.x [DOI] [PubMed] [Google Scholar]
- Kaewtapee C., Krutthai N., and Bunchasak C.. 2016. Effects of supplemental liquid DL-methionine hydroxy analog free acid in diet on growth performance and gastrointestinal functions of piglets. Asian-Australas. J. Anim. Sci. 29:1166–1172. doi: 10.5713/ajas.15.0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.G., Lindemann M.D., Rademacher M., Brennan J.J., and Cromwell G.L.. 2006. Efficacy of DL-methionine hydroxy analog free acid and DL-methionine as methionine sources for pigs. J. Anim. Sci. 84:104–111. doi: 10.2527/2006.841104x [DOI] [PubMed] [Google Scholar]
- Krutthai N., Vajrabukka C., Markvichitr K., Choothesa A., Thiengtham J., Sawanon S., Kaewtapee C., and Bunchasak C.. 2015. Effect of source of methionine in broken rice-soybean diet on production performance, blood chemistry, and fermentation characteristics in weaned pigs. Czech J. Anim. Sci. 60:123–131. doi: 10.17221/8077-CJAS [Google Scholar]
- Lackeyram D., Yang C., Archbold T., Swanson K.C., and Fan M.Z.. 2010. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J. Nutr. 140:461–468. doi: 10.3945/jn.109.117267 [DOI] [PubMed] [Google Scholar]
- Ly N.T.H., Ngoan L.D., Verstegen M.W.A., and Hendriks W.H.. 2012. Pig performance increases with the addition of DL-methionine and L-lysine to ensiled cassava leaf protein diets. Trop. Anim. Health. Prod. 44:165–172. doi: 10.1007/s11250-011-9904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Hu Y., and Smith D.E.. 2011. Peptide transporter 1 is responsible for intestinal uptake of the dipeptide glycylsarcosine: studies in everted jejunal rings from wild-type and Pept1 null mice. J. Pharm. Sci. 100:767–774. doi: 10.1002/jps.22277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.O., Southern L.L., and Bidner T.D.. 2001. Estimation of the total sulfur amino acid requirement and the effect of betaine in diets deficient in total sulfur amino acids for the weanling pig. J. Anim. Sci. 79:1557–1565. doi: 10.2527/2001.7961557x [DOI] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huërou-Luron I., Lallès J.P., and Sève B.. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi: 10.1017/S000711450720580X [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Klok C.V., Ryan K.A., Wooten J.G., Little D., Cook V.L., and Blikslager A.T.. 2007a. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G173–G181. doi: 10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Ryan K.A., Nighot P.K., and Blikslager A.T.. 2007b. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G413–G421. doi: 10.1152/ajpgi.00304.2006 [DOI] [PubMed] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine. 11th ed Washington (DC): National Academies Press. [Google Scholar]
- Pelsers M.M.A.L., Namiot Z., Kisielewski W., Namiot A., Januszkiewicz M., Hermens W.T., and Glatz J.F.C.. 2003. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 36:529–535. doi: 10.1016/S0009-9120(03)00096-1 [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Hampson D.J., and Williams I.H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. [Google Scholar]
- Ren W.K., Yin J., Wu M.M., Liu G., Yang G., Xion Y., Su D., Wu L., Li T.J., Chen S.,. et al. 2014. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One. 9:e88335–e88340. doi: 10.1371/journal.pone.0088335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoveller A.K., Stoll B., Ball R.O., and Burrin D.G.. 2005. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J. Nutr. 135: 1609–1612. [DOI] [PubMed] [Google Scholar]
- Tan B.E., Yin Y.L., Liu Z.Q., Tang W.J., Xu H.J., Kong X.F., Li X.G., Yao K., Gu W.T., Smith S.B.,. et al. 2011. Dietary L-arginine supplementation differentially regulates expression of fat-metabolic genes in porcine adipose tissue and skeletal muscle. J. Nutr. Biochem. 22:441–445. doi: 10.1016/j.jnutbio.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Tsukahara T., Inoue R., Nakatani M., Fukuta K., Kishino E., Ito T., and Kazunari U.. 2016. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim. Sci. J. 87:67–75. doi: 10.1111/asj.12399 [DOI] [PubMed] [Google Scholar]
- Turan A., Gill R., Dudeja P.K., Mohan H., and Mahmood A.. 2009. Effect of fat feeding on pro-oxidant and anti-oxidant enzyme systems in rat intestine: possible role in the turnover of enterocytes. Dig. Dis. Sci. 54:1229–1236. doi: 10.1007/s10620-008-0490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers-Schreurs H.M., Nabuurs M.J., Vellenga L., Kalsbeek-van der Valk H.J., Wensing T., and Breukink H.J.. 1998. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J. Nutr. 128:947–953. [DOI] [PubMed] [Google Scholar]
- Yang H.S., Fu D.Z., Kong X.F., Wang W.C., Yang X.J., Nyachoti C.M., and Yin Y.L.. 2013. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal AA transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012–5795 [DOI] [PubMed] [Google Scholar]
- Yang H.S., Wu F., Long L.N., Xiong X., Liao P., and Liu H.N.. 2016. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J. Zhejiang Univ. Sci. B. 17:752–762. doi:10.1631/jzus.B1500192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.L., Baidoo S.K., Schulze H., and Simmins P.H.. 2001. Effect of supplementing diets containing hulless barley varieties having different levels of non-starch polysaccharides with β-glucanase and xylanase on the physiological status of gastrointestinal tract and nutrient digestibility of weaned pigs. Livest. Prod. Sci. 71:97–107. [Google Scholar]