Abstract
Objectives:
Preclinical studies have shown that blueberry supplementation can improve cognitive performance and neuronal function in aged animals and have identified associations between anthocyanins and such benefits. Preliminary human trials also suggest cognitive improvement in older adults, although direct evidence of enhancement of brain function has not been demonstrated. In this study, we investigated the effect of blueberry supplementation on regional brain activation in older adults at risk for dementia.
Methods:
In a randomized, double-blind, placebo-controlled trial we performed pre- and post-intervention functional magnetic resonance imaging during a working memory task to assess the effect of blueberry supplementation on blood oxygen level-dependent (BOLD) signal in older adults with mild cognitive impairment, a risk condition for dementia.
Results:
Following daily supplementation for 16 weeks, blueberry-treated participants exhibited increased BOLD activation in the left pre-central gyrus, left middle frontal gyrus, and left inferior parietal lobe during working memory load conditions (corrected p < 0.01). There was no clear indication of working memory enhancement associated with blueberry supplementation. Diet records indicated no between-group difference in anthocyanin consumption external to the intervention.
Discussion:
These data demonstrate, for the first time, enhanced neuronal response during working memory challenge in blueberry-treated older adults with cognitive decline and are consistent with prior trials showing neurocognitive benefit with blueberry supplementation in this at-risk population.
Keywords: Blueberries, Brain activation, fMRI, Aging, MCI, Dementia
Introduction
The prevalence of Alzheimer’s disease (AD), the most common form of dementia, is projected to escalate substantially by mid-century. Because there is no effective treatment for AD, intervention to mitigate progressive cognitive decline in at-risk individuals is an essential approach to this growing public health problem. Mild cognitive impairment (MCI) is a clinical condition in which individuals exhibit greater than expected cognitive decline with aging in the context of relatively preserved everyday function. Those with MCI have increased risk for dementia, [1] and represent a population targeted for preventive interventions.
Modifiable, environmental factors influence risk for late-life AD, and nutritional approaches have been cited as particularly important in this regard. [2] Anthocyanins are a class of flavonoid polyphenols that are strongly represented in berry fruits such as blueberries, blackberries, and strawberries. Anthocyanin compounds have been identified in several brain regions following blueberry supplementation in animals and have been associated with cognitive performance. [3] A relatively large body of preclinical research has established both functional and mechanistic effects of blueberry supplementation such as reversal of age-related decrements in cognitive and motor function and enhancements of neuronal resilience, cerebral blood flow, and endothelial protection. [4–6] Further, cognitive and neurotrophic benefits specific to hippocampus have been shown with blueberry treatment, [7,8] a particularly pertinent effect with respect to late-life dementia. There has been much less data concerning the neurocognitive response to blueberry supplementation in humans, although the application of functional and structural neuroimaging in young and older adults in the context of other flavonoid-containing products has indicated alterations of neuronal function that are broadly consistent with animal studies. High-flavanol cocoa treatment has been associated with enhancement of cerebral blood flow, [9] Concord grape juice [10] and pomegranate juice [11] supplementation with increased regional brain activation, and resveratrol treatment with enhanced hippocampal connectivity. [12]
In the current study, we investigated the neuronal effects of blueberry supplementation in a sample of older adults with increased risk for dementia. Our intent was to investigate cerebral response to whole fruit blueberry taken daily for several weeks. We performed in-vivo functional neuroimaging before and after supplementation to assess changes in regional brain activation during performance of a working memory (WM) task.
Methods
The study protocol was approved by the University of Cincinnati Medical Institutional Review Board and registered with the Clinical Trials Identifier: NCT00599508. Each participant reviewed and signed the approved informed consent documents prior to study enrollment. In a randomized, double-blind, placebo-controlled trial, older adults with MCI were assigned to receive either freeze dried, whole fruit blueberry powder or placebo powder daily for 16 weeks. Functional magnetic resonance imaging (fMRI) was performed at pre- and post-intervention study visits while participants were engaged in a WM challenge.
Participants
Participants 68 years and older were recruited from the region in and around Cincinnati, OH USA with print notices that solicited older adults with awareness of age-related memory decline. The age range of the sample was 68 to 92 years. Each prospective participant was involved in an initial telephone contact to discern interest in the study, to inquire about awareness of cognitive decline with aging, and to identify exclusionary factors. A subsequent in-clinic visit involved further assessment to determine the extent of cognitive impairment and other aspects of study eligibility. Those with dementia or other neurological condition, diabetes, kidney disease, liver disease, serious psychiatric disorder, substance abuse, claustrophobia, and implanted ferromagnetic devices were excluded. We enrolled a sample of men and women who met clinical criteria for MCI. [1]
Clinical assessment
Demographic information, medical history, and medication and supplement use were assessed with the Academic and Medical History Questionnaire. [13] Level of cognitive decline was determined with the modified Clinical Dementia Rating (mCDR) [14] and corroborated with other cognitive assessments. The mCDR classification involved systematic evaluation of the participant’s cognitive capability by an informant, such as a spouse or adult child, who provided information concerning functioning in six domains of everyday activities with greatest emphasis on memory ability. We included individuals classified as having mild impairment consistent with MCI. Individuals classified with no impairment and those with mild, moderate, and severe dementia were excluded. We also administered the Montreal Cognitive Assessment (MoCA) [15] and the California Verbal Learning Task (CVLT) [16] to obtain objective measures of general cognitive function and memory ability, respectively. We included those who scored below the established cut score of 26 for MCI on the MoCA and at least 1 SD below the age-corrected mean on the learning trials of the CVLT. Further, the Geriatric Depression Scale (GDS) [17] and the Geriatric Anxiety Inventory (GAI) [18] were used to assess level of mood disturbance, a potentially confounding factor with respect to cognitive-cerebral function. Individuals with GDS scores > 15 or GAI scores > 13 were evaluated further and excluded from study participation if a depressive or anxiety disorder was suspected. We also measured height, body weight, and waist circumference.
Blueberry powder and placebo
Powder prepared from whole freeze-dried blueberry fruit (Vaccinium) and a placebo powder were provided by the US Highbush Blueberry Council (Folsom, CA USA). The blueberry fruit powder was a 50%:50% mixture of blueberry cultivars V. ashei Reade ‘Tifblue’ and V corymbosum L “Rubel.’ Fruit was frozen immediately after harvest, freeze-dried and milled to a 40 mesh powder. The berry powder had a phenolic concentration of 20.37 ± 0.31 gallic acid equivalents/g DW powder, [19] anthocyanin content of 14.53 ± 0.04 mg cyanidin 3-glucoside equivalents/g DW powder [20], and an ORAC value of 248 ± 20.61 µmole Trolox equivalents/g DW powder. [21] The caloric content of the blueberry powder was 399 calories/100 g, and the macronutrient composition was 95.0% carbohydrate, 0.96% fat, and 2.47% protein. The placebo powder was matched for color, taste, and sugar content as closely as possible and milled similarly. The placebo was composed of food-grade ingredients, including artificial and natural blueberry flavor, artificial purple and red coloring, maltodextrin, fructose, and citric acid, and contained 389 calories/100 g with 97.3% carbohydrate, 0.02% fat, and less than 0.78% protein. Fiber was not included in the placebo because of uncertainty as to how it may have contributed to health and neurocognitive effects in the control group participants.
Based on extrapolation from quantities used in animal and past human experiments, we chose a daily dose equivalent of 1 c (approximately 148 g) whole blueberry fruit. [22] Blueberry and placebo powders were packaged in identical, individual dose foil packets containing one-half the daily dosage, the blueberry powder at 12.5 g per packet and placebo at 12 g per packet. HPLC analysis indicated that approximately 25% of the anthocyanins was still present in the powder after three years (data not shown). Colorimetric analysis indicated no decline in total phenolics and ORAC antioxidant capacity during the three-year period. Compared to the blueberry powder the placebo powder had an apparent phenolic content of about 3.5%, while the ORAC of the placebo was about 2.4%. Anthocyanin compounds were not detected in the placebo.
To achieve the daily dose of 1 c whole fruit equivalent, participants were instructed to take one packet of powder two times a day, with the morning and evening meal. Therefore, for participants taking blueberry powder, the daily intake of phenolics was 417 gallic acid equivalents, of anthocyanins was 269 mg cyanidin 3-glucoside equivalents, and ORAC 6525 μM Trolox equivalents based on average values for the blueberry powder over the three years. The general recommendation for consumption was that the powder be mixed with water, although mixing with other foods or beverages was not prohibited. Our prior flavonoid intervention trials included an intervention period of 12 weeks. [10,11] In this trial we chose to extend the intervention to 16 weeks in order to investigate the possibility of greater benefit with a longer period. All participants were given a list of anthocyanin-containing foods including berries and other red, purple, or blue plant-based food and their processed products such as red wines, juices and jams that were to be avoided for the duration of the intervention.
Participants were instructed to record all food and beverage intake for three consecutive days, including one weekend day and two weekdays, during the week before enrollment, during week 8, and during the final week of the intervention.[23] The polyphenol module of the Nutrition Data Systems for Research (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) was used to calculate anthocyanin consumption in the diet external to the study intervention as a means of evaluating adherence to the prescription against berry fruit product consumption and to assess potential group differences in consumption outside the study.
Functional neuroimaging
Functional magnetic resonance imaging (fMRI) data were acquired while participants performed a sequential letter n-back working memory task programmed and administered using EPrime (www.pstnet.com). This task has been applied extensively in neuroimaging studies and shown to elicit regional activation characteristic of WM processing, [24] and we have used it in a prior fMRI experiment involving older adults and berry fruit supplementation. [10] The paradigm involved visual presentation of a series of upper and lower case letters of the alphabet with an inter-stimulus interval of 2500 ms. The participants responded by pressing one of two buttons to indicate either ‘yes’ or ‘no’ in accordance with task instructions. In the 0-back condition, participants were instructed to respond ‘yes’ when the letter ‘X’ was displayed and ‘no’ otherwise. Accordingly, the 0-back condition represented a sustained attention task with minimal working memory load as there was no trial-to-trial retention requirement. Working memory load was elicited by the 1- and 2-back conditions in which participants were instructed to respond to each letter presentation indicating whether or not that letter had appeared n items previously. In the 1-back condition, the participant was required to recognize when the currently displayed letter was the same as the letter displayed one item previously. In the 2-back condition, the participant was required to recognize when the current letter matched the letter presented two items earlier in the sequence. Four runs, each consisting of the three n-back blocks containing 34 trials were administered, and the order in which the condition blocks were presented within each run was pseudo-randomized such that each block type appeared once per run. All participants were acquainted with the instructions and the procedure and performed a practice version of the n-back task at a work station before placement in the scanner.
Stimulus event times were used to model activation in the fMRI data. For each participant, the event times were extracted from E-Prime for hits (correctly identifying a target), correct rejections (rejecting a non-target), misses (failing to identify a target), and false alarms (identifying a non-target as target) for each n-back condition (0-, 1- and 2-back). Instruction screens were displayed for 15 seconds separating blocks of events and served as the baseline against which event-related activation was estimated. Slice timing correction was achieved by aligning the midpoint of each trial run repetition time (TR) to the behavioral event times.
Brain imaging data were acquired on a 4.0 Tesla Varian INOVA Whole Body MRI/MRS system (Varian Inc., Palo Alto, CA, USA). Prior to entering the scanner room, participants underwent screening for MRI safety to identify hazards such as metallic implants, irremovable jewelry, and claustrophobia. Visual stimuli for the paradigm were presented through non-ferromagnetic high-resolution video goggles (Resonance Technologies, Inc., Northridge, CA, USA). We acquired four runs of T2*-weighted gradient-echo echo planar images (EPI) consisting of 35 contiguous 4 mm axial slices covering the entire brain (TR/TE 3000/25 ms, FOV 256 × 256 mm, flip angle 85º) with 126 EPI acquisitions, the first two of which were discarded to account for T1 overshoot. A multi-echo reference scan was obtained to correct for ghost and geometric distortions. [25] After the fMRI data acquisition, a T1-weighted 3-D anatomical image was acquired using a modified driven equilibrium Fourier transform (MDEFT) sequence, [26] (TMD=1.1 s, TR=13 ms, TE=5.3 ms, FOV=25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96, flip angle 20º) to provide anatomical co-registration of fMRI data.
Raw (binary) MRI data were reconstructed using in-house software developed with the Interactive Data Language program (IDL; www.ittvis.com) with Hamming filtering in the X, Y, and Z planes. Images were subsequently processed, analyzed, and visualized with the AFNI software (Analysis of Functional Neuroimages, afni.nimh.nih.gov). [27] All datasets were normalized to standard space using tools in AFNI to match each participant’s image to the International Consortium for Brain Mapping’s ICBM452 template from UCLA’s Laboratory of Neuroimaging (www.loni.ucla.edu). Co-registration of functional to anatomical images was completed using the align_epi_anat python script through AFNI. The anatomical image was normalized to the TT_icbm452 template, functional runs were co-registered to a sub-brick acquired closest in time to the anatomical image using a six-parameter rigid-body transformation with Fourier interpolation, [28] and all functional EPI datasets were normalized to the anatomical dataset. The transformations were calculated stepwise and then combined into a single transform applied to the EPI data in order to minimize distortion of the fMRI data. Co-registered images were visually inspected and individually censored from further analysis if uncorrectable head movement or image artifacts were detected. This eliminated the need to exclude whole datasets on the basis of global movement characteristics. Binary masking from the anatomical template was applied to each volume of the functional dataset to remove data points outside the brain. Voxel-wise signal scaling was performed to convert the data to percent signal change on a per-run basis prior to deconvolution. Individual activation maps were then generated for each participant from the stimulus event times using the AFNI program 3dDeconvolve. This algorithm compares the magnitude of the hemodynamic response during stimulus event times. In this case, the event times of interest included hits and correct rejections, as these responses indicated engagement with the working memory task while incorrect responses might be associated with lapses in task engagement. Hits and correct rejections data were combined to yield an Accuracy percentage score for each condition. We also recorded response time (RT) as the number of ms from the onset of the stimulus to the participant response for each trial as an index of processing time. We included motion correction parameters described above as regressors of no interest, which allowed for the definition of the magnitude of the hemodynamic response relative to the average signal intensity at each location (voxel) in the datasets. These individual maps were then used for group-wise analyses.
Statistical analyses
Analysis of demographic, clinical, and cognitive data, including behavioral performance during the n-back task was completed using SPSS version 21 (Statistical Package for the Social Sciences, Chicago, IL, USA). Assessment of pre-intervention group differences was made via independent sample t-tests and differences between percentages for continuous and nominal variables, respectively. Group by treatment effects were evaluated by analyses of covariance (ANCOVA), which adjusted final values by pre-intervention values prior to group comparisons to isolate the effect of the intervention.
We performed separate, within group analyses initially via 3dtest++ in the AFNI toolbox comparing baseline and final visit BOLD signal during working memory events. We then assessed possible treatment by visit effects with GroupAna from the AFNI/Matlab toolbox. In this model, we used a three factor mixed design with Group (blueberry or placebo powder) and Visit (baseline or final) fixed, and a third variable, participant number, nested in Group. Monte Carlo simulations were conducted to determine corrections for multiple comparisons across voxels. Voxel significance threshold was set at p < 0.01, and cluster thresholds were set at 86 contiguous (faces touching) voxels, for a corrected significance level of p < 0.01 for all clusters.
Results
Of the 21 enrolled participants, three did not complete a final study visit because of voluntary discontinuation. Two others were eliminated because performance on the WM task suggested lack of engagement. For one such participant working memory performance accuracy was more than two SD below the sample mean and the other exhibited insufficient responding overall. Because of scheduling exigencies, two participants, one from each group, completed the final study visit earlier than expected (week 9 and week 11). WM performances of these latter two participants were within two SD of the mean for each n-back condition. The final sample consisted of eight placebo- and eight blueberry-treated participants.
Table 1 contains data on the participant sample characteristics. The groups did not differ with respect to age, gender and race distribution, educational level, BMI, and distribution of diagnosed hypertension. All participants with hypertension were treated with anti-hypertensive medications, and there was no difference with regard to cognitive performance on the MoCA, CVLT, and CDR between those with and without hypertension. There was no group difference for depressive symptom scores (GDS) and anxiety symptom scores (GAI) and both groups exhibited low levels of mood symptom.
Table 1.
Participant sample characteristics by group
| Measure | Blueberry (n=8) |
Placebo (n=8) |
p |
|---|---|---|---|
| Age, y | 80.4 (7.3) | 75.5 (4.8) | 0.14 |
| Gender, % female | 62.5 | 50 | 0.30 |
| Race, % White (% Black) | 87.5 (12.5) | 100 (0) | 0.15 |
| Education, y | 14.4 (2.1) | 15.5 (3.0) | 0.40 |
| Waist circumference, cm | 88.6 (6.5) | 92.7 (13.1) | 0.45 |
| BMI, kg/mm2 | 26.2 (3.6) | 26.4 (2.4) | 0.86 |
| HTN | 5 | 3 | 0.31 |
| mCDR sum of boxes | 0.7 (0.5) | 0.8 (0.4) | 0.57 |
| MoCA | 22.3 (1.4) | 21.8 (1.7) | 0.53 |
| CVLT cumulative learning score | 39.0 (12.5) | 37.2 (6.5) | 0.73 |
| GDS | 5.0 (3.9) | 4.0 (2.1) | 0.53 |
| GAI | 1.5 (2.1) | 2.7 (3.6) | 0.20 |
Note. Probability values derived from independent sample t-tests for continuous variables and difference test for percentages.
HTN = number of participants diagnosed with hypertension at study enrollment. mCDR = modified Clinical Dementia Rating. MoCA = Montreal Cognitive Assessment. CVLT = California Verbal Learning Test-II. GDS = Geriatric Depression Scale. GAI = Geriatric Anxiety Inventory.
N-back working memory performance
Table 2 contains performance data by group for the Accuracy and RT measures of the n-back task. At the pre-intervention enrollment visit, response times during the WM task increased with increasing memory load as expected. While the placebo group exhibited significantly briefer RT in the 1-back condition, there was no baseline between-group difference with respect to Accuracy in any n-back condition. ANCOVA comparing final visit scores, with control for baseline measures, indicated marginally improved Accuracy for the blueberry group in the 1-back condition (p = 0.08). The large effect size for this finding (d = 1.02), indicated that increasing sample size to 17 per group would be sufficient to achieve significance at p = 0.05 with power = 0.80.
Table 2.
Working memory performance by group
| Baseline | Final | |||
|---|---|---|---|---|
| Condition | Blueberry | Placebo | Blueberry | Placebo |
| Acc, 0-back | 0.98 (0.01) | 0.94 (0.05) | 0.99 (0.01) | 0.96 (0.03) |
| Acc, 1-back | 0.74 (0.15) | 0.85 (0.17) | 0.88 (0.11) | 0.86 (0.09) |
| Acc, 2-back | 0.72 (0.11) | 0.78 (0.17) | 0.79 (0.08) | 0.79 (0.09) |
| RT, 0-back | 663 (135) | 592 (81) | 657 (131) | 623 (77) |
| *RT, 1-back | 921 (181) | 740 (142) | 902 (183) | 788 (130) |
| RT, 2-back | 1037 (272) | 860 (149) | 1019 (272) | 916 (247) |
Note. Marginal means at baseline and final visits. Acc = accuracy, percent. RT = response time, ms.
indicates statistical difference at baseline, p = 0.04.
Functional MRI
Dependent t-test analyses within the blueberry-treated group indicated signal change for Accuracy responses at the final visit relative to baseline during the 1- and 2- back conditions marked by increased signal in the left pre-central gyrus, left middle frontal gyrus, and left inferior parietal lobe (corrected p < 0.01; Figure 1). Within the placebo group, a small region of decreased activation was identified near the left post-central gyrus at follow-up relative to baseline.
Figure 1.
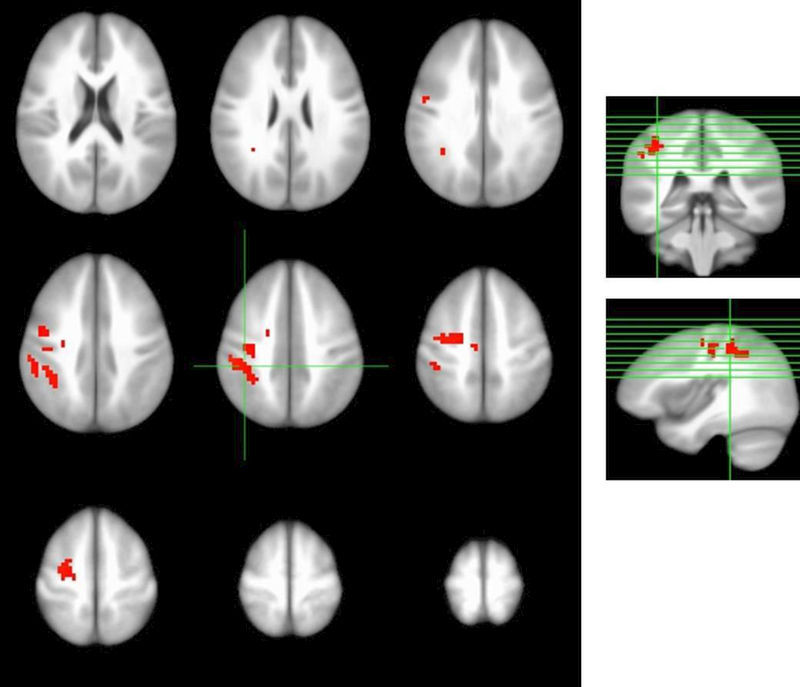
Increased blood oxygen level-dependent (BOLD) signal during memory loading conditions in blueberry-treated group in the left pre-central gyrus, left middle frontal gyrus, and left inferior parietal lobe after intervention relative to pre-intervention baseline (corrected p < 0.01).
The three factor model (Group, Visit, and Participant), which allowed for evaluation of Group by Visit interaction, also identified the left inferior parietal and left pre-central gyri as regions showing significantly greater signal at the final visit in the 2-back condition in the blueberry-treated group (corrected p < 0.01, Figure 2, Table 3). The effect size estimates for these findings were d = 1.82 (left inferior parietal gyrus) and d = 1.94 (left pre-central gyrus). [29] There was no effect of the intervention evident in BOLD contrasts for the 0- and 1-back conditions. Table 3 identifies these regions in stereotaxic (ICBM452) space.
Figure 2.
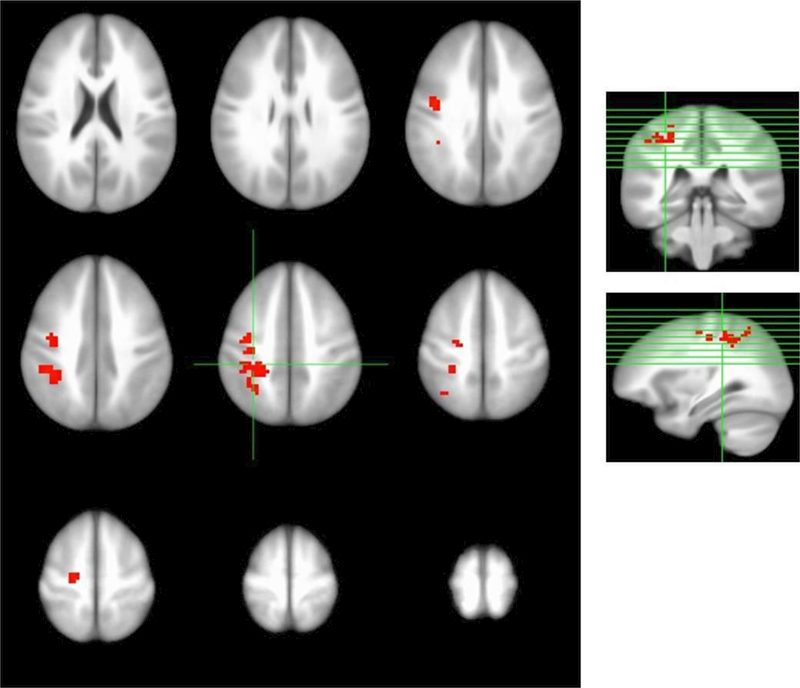
Evaluation of Group-by-Visit interaction; 2nd order, 3-factor (Group, Visit, and Participant), identified two regions of significantly higher blood oxygen level-dependent (BOLD) signal after blueberry supplementation (corrected p < 0.01), left inferior parietal lobule and left pre-central gyrus.
Table 3.
Coordinates of regions of increased signal after blueberry treatment
| Cluster | #Voxels | CM x | CM y | CM z | Peak x | Peak y | Peak z | Anatomical Region |
|---|---|---|---|---|---|---|---|---|
| 1 | 126 | 32.4 | 39.1 | 46.2 | 37.5 | 40.5 | 38.5 | Left inferior parietal lobule |
| 2 | 91 | 32.7 | 13.3 | 47.3 | 34.5 | 13.5 | 50.5 | Left pre-central gyrus |
Note: Identification of regions in stereotaxic (Talairach) space. CM = Center of Mass, Peak = location of the peak signal contrast in the region.
Dietary intake
Figure 3 shows data on daily anthocyanin consumption outside the study extracted from diet records obtained prior to the intervention, at the midpoint of the intervention, and during the final week. Separate repeated measures analyses for each anthocyanin compound indicated main effects for time for cyanidin (p = 0.01), malvidin (p = 0.05), peonidin (p = 0.05), and a trend for petunidin (p = 0.08). However, there was no group by time interaction that would indicate a between-group difference in anthocyanin consumption in the background diet.
Figure 3.
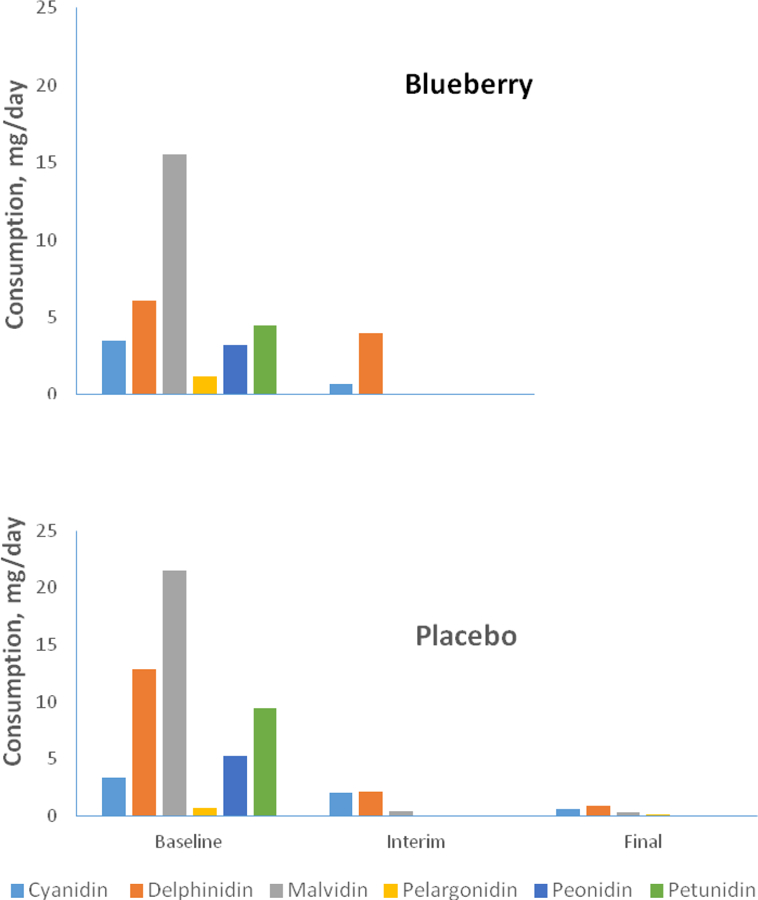
Mean daily consumption (mg/day) of anthocyanin compounds in the background diet for the blueberry- and placebo- treated groups calculated from diet diaries obtained during the week before enrollment (baseline), week 8 of the intervention (interim), and during the final week of the intervention. Separate repeated measures analyses for each anthocyanin compound indicated main effects for time for cyanidin (p = 0.01), malvidin (p = 0.05), peonidin (p = 0.05), and a trend for time for petunidin (p = 0.08). However, there was no group by time interaction that would indicate a betweem-group difference in such consumption outside the intervention.
Discussion
The three-factor (Group, Visit, and Participant) model, identified two regions of significantly different BOLD signal at follow-up relative to baseline in blueberry-treated older adults with MCI (Figure 2), which overlapped with the left inferior parietal lobule and pre-central gyrus exhibiting increased BOLD signal within the blueberry group following 16 weeks’ supplementation (Figure 1). Accordingly, the Group by Visit by Participant effect is consistent with the observed increase in regional activation within the blueberry group. This fact and the large magnitude of the BOLD effect sizes in both of these regions indicate that these findings reflect robust changes associated with the intervention. More generally, such observations support the notion that flavonoid compounds modulate central (neuronal, glial) signaling cascades contributing to alterations in neuro-vascular supply as well as endothelial mechanisms associated with improved vascular function. [5,6,30]
These fMRI effects were obtained in the absence of statistically significant group differences in working memory task performance, although the increment favoring the blueberry group suggests that significant effects might be obtained with a larger sample. We determined that increasing the total sample size to 34 participants (17 per group) would yield a significant n-back performance effect. In light of the robust activation findings in this current study, one might infer that there was relatively greater variability among the participants in behavioral performance. It is notable that the finding of enhanced regional activation in the absence of a concomitant cognitive performance effect also was observed in an older adult MCI sample following Concord grape juice supplementation, [10] and in young, healthy participants after receiving short-term high-flavanol cocoa supplementation. [9] Further, flavanol supplementation has been shown to enhance regional hippocampal function [31] and cerebral perfusion in healthy older adults. [32] Given the apparent enhancement of brain activity in this study, it is also possible that longer term supplementation might be necessary to demonstrate improvement in working memory performance. Further, it is not clear whether such an intervention introduced at earlier epochs of cognitive aging might produce greater or more rapid performance enhancement. This is a particularly salient issue in light of the notion of an extended preclinical phase of AD during which neuropathological changes accumulate [33], so that intervention, perhaps in mid-life, may be more effective with respect to forestalling late-life dementia.
Possible confounds related to metabolic disturbance and vascular disease, risks for AD, [34,35,36] should be emphasized. In this study, obese participants were excluded because of the physical constraints of the scanner bore, and six potential participants were ineligible because of implanted vascular stents. So, there is some likelihood that participants excluded for these reasons might have demonstrated even greater neurovascular and/or neurocognitive response to the intervention. This also is relevant because BOLD signal is coupled to neurovascular function, an index of neural resource recruitment, and the observed alteration of BOLD signal may have reflected enhanced circulation. Therefore, an important consideration that could be amenable to investigation is that central activation responses may be more robust in participants generally excluded from fMRI studies on the basis of factors related to vascular health such as implanted stents, pace-makers, and/or elevated waist circumference.
In this trial, we endeavored to assess neurocognitive response to whole fruit blueberry powder rather than to specific constituents of the fruit. Accordingly, we did not include fiber in the placebo powder as this conceivably might have influenced outcomes in the control group; it is possible that fiber-related improvement in metabolic function would be associated with enhanced cerebral perfusion. However, controlled study of the influence of fiber certainly is warranted in future berry trials.
A strength of this trial was the use of diet records to sample the background diet before and during the trial. The data derived on anthocyanin intake suggested that participants in both groups maintained the prohibition against berry fruit consumption at similar levels during the intervention.
To our knowledge, this is the first human functional neuroimaging trial to identify altered BOLD signal following blueberry supplementation in a sample of MCI participants. These data extend findings from basic neuroscience studies and the extant human trials implicating flavonoid-rich berry fruits as potential agents in ameliorating age-related cognitive decline. These trials indicate that nutritional approaches such as blueberry supplementation may induce beneficial effects for brain function and cognitive behavior. Further, such nutritional interventions can be instituted readily with little or no risk. In the absence of effective pharmacotherapy, such nutritional approaches may be of particular value for aging adults with risk for late-life dementia such as AD.
Acknowledgements
This research was supported with funding and research product supplied by the US Highbush Blueberry Council, Folsom, CA USA. Additional support for research personnel was provided by the NIH Office of Dietary Supplements and the NIH National Institute on Aging grant AG034617-01S2. The authors appreciate the expert assistance in data acquisition and data processing provided by Elizabeth M. Fugate and Matthew Norris and statistical consultation provided by Jeffrey Welge and Thomas Blom.
Footnotes
Declaration of Interest
The authors report no declaration of interest.
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman JJ, Fox NC, Gamst A, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279, doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu W, Tan L, Wang H, Jian T, Tan M, Tan L, Zhao Q, Li J, Wang J, Tu J. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548 [DOI] [PubMed] [Google Scholar]
- 3.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregut O, Lamuela-Raventos R, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci 2005; 8:111–120. [DOI] [PubMed] [Google Scholar]
- 4.Shukitt-Hale B, Bielinski DF, Lau FL, Willis LM, Carey AN, Joseph JA. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br J Nutr 2015; 114:1542–1549. [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 2010;140:1582–1587, doi: 10.3945/jn.110.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paixão J, Dinis TC, Almeida LM. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: an in vitro approach. Apoptosis 2011; 16: 976–989, doi: 10.1007/s10495-011-0632. [DOI] [PubMed] [Google Scholar]
- 7.Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci 2004; 7:309–316. [DOI] [PubMed] [Google Scholar]
- 8.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med 2008; 45:295–305. [DOI] [PubMed] [Google Scholar]
- 9.Francis ST, Head K, Morris PG, Macdonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J Cardiovasc Pharmacol 2006;47:S215-220. [DOI] [PubMed] [Google Scholar]
- 10.Krikorian R, Boespflug EL, Fleck DE, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem 2012;60:5736–5742. [DOI] [PubMed] [Google Scholar]
- 11.Krikorian R, Shidler MD, Nash TA, Kalt W, Vinqvist-Tymchuk MR, Shukitt-Hale B, Joseph JA. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58:3996–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookheimer SY, Renner BA, Ekstrom A, et al. Pomegranate juice augments memory and FMRI activity in middle-aged and older adults with mild memory complaints. Evid Based Complement Alternat Med. 2013;2013:946298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci 2014;34:7862–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in Obsessive-Compulsive Disorder. Brain Cogn 2004;54:257–259. [DOI] [PubMed] [Google Scholar]
- 15.Duara R, Loewenstein DA, Greig-Custo1 MT, Raj A, Barker W. Int J Geri Psychia 2010; 25:82–289. [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 17.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test II (CVLT-II). USA: The Psychological Corporation; 2000. [Google Scholar]
- 18.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988; 24:709–711. [PubMed] [Google Scholar]
- 19.Pachana NA, Byrne GJ, Siddle H, Koloski N, Harley E, Arnold E. Development and validation of the Geriatric Anxiety Inventory. Int. Psychogeriatr 2007; 19:103–114. [DOI] [PubMed] [Google Scholar]
- 20.Singleton VL RJ. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144−−158. [Google Scholar]
- 21.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269−−1278. [PubMed] [Google Scholar]
- 22.Ou B, Hampsch-Woodill M, Prior RL. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J Agric Food Chem 2001;49:4619–4626. [DOI] [PubMed] [Google Scholar]
- 23.DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS et al. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr 2009;139:1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging 2001;20:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Garwood M, Menon R, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med 1995;34:308–312. [DOI] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 28.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med 1999;42:1014–1018. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J Statistical power analysis for the behavioral sciences, second edition Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 30.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999;100:1050–1055. [DOI] [PubMed] [Google Scholar]
- 31.Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 2014;12:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamport DJ, Pal D, Moutsiana C, Field DT, Williams CM, Spencer JP, Butler LT. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: a placebo controlled, crossover acute trial. Psychopharmac 2015;232:3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 2011;7:280–292, doi: 10.1016/jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Profenno LA, Porsteinsson AP, Faraone SV. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol Psychiatry 2010;67:505–512. [DOI] [PubMed] [Google Scholar]
- 35.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis Assoc Disord 2012;26:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassing LB, Dahl Ak, Thorvaldsson V, Berg S, Gatz M, Pedersen NL et al. Overweight in midlife and risk of dementia: A 40-year follow-up study. Int J Obes 2009;33:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]


