Abstract
Aims:
Mental stress-induced myocardial ischemia (MSIMI) occurs in up to 70% of patients with clinically stable ischemic heart disease and is associated with increased risk of adverse prognosis. We aimed to examine the prognostic value of indices of MSIMI and exercise stress-induced myocardial ischemia (ESIMI) in a population of ischemic heart disease patients that was not confined by having a recent positive physical stress test.
Methods and results:
The Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment (REMIT) study enrolled 310 subjects who underwent mental and exercise stress testing and were followed annually for a median of four years. Study endpoints included time to first and total rate of major adverse cardiovascular events, defined as all-cause mortality and hospitalizations for cardiovascular causes. Cox and negative binomial regression adjusting for age, sex, resting left ventricular ejection fraction, and heart failure status were used to examine associations of indices of MSIMI and ESIMI with study endpoints. The continuous variable of mental stress-induced left ventricular ejection fraction change was significantly associated with both endpoints (all p values < 0.05). For every reduction of 5% in left ventricular ejection fraction induced by mental stress, patients had a 5% increase in the probability of a major adverse cardiovascular event at the median follow-up time and a 20% increase in the number of major adverse cardio vascular events endured over the follow-up period of six years. Indices of ESIMI did not predict endpoints (ps > 0.05).
Conclusion:
In patients with stable ischemic heart disease, mental, but not exercise, stress-induced left ventricular ejection fraction change significantly predicts risk of future adverse cardiovascular events.
Keywords: Mental stress, exercise stress, ejection fraction
Introduction
Mental stress-induced myocardial ischemia (MSIMI) occurs in up to 70% of patients who have clinically stable ischemic heart disease (IHD) and a recent history of physical stress-induced myocardial ischemia.1 A recent pooled analysis of five studies of IHD patients found that MSIMI is associated with a twofold increased risk of combined cardiac events and mortality.2 However, it remains unknown if MSIMI contributes independent prognostic value to indicators obtained from conventional physical stress testing.
A common feature of previous studies examining the prognostic value of MSIMI is that enrollment was limited to IHD patients with a recent positive physical stress test. This limits the generalizability of findings to a broader patient population as MSIMI occurs in a significant portion of IHD patients who show no signs of ischemia in response to physical stress.3–5 This study aims to further elucidate the clinical value of mental stress testing by examining indices of MSIMI as predictors of a major adverse cardiovascular event (MACE) in clinically stable IHD patients utilizing data from the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment (REMIT) study,6 a clinical trial that demonstrated the efficacy of escitalopram treatment as a therapy for reducing MSIMI. REMIT study participants were not selected based on having a recent positive physical stress test. All study participants completed a mental stress test, followed by a conventional treadmill exercise stress test. We hypothesized that indices of MSIMI would yield significant prognostic information in IHD patients independent of exercise stress-induced myocardial ischemia (ESIMI).
Methods
Subjects
The present study population consists of all participants of the REMIT study.7 Briefly, clinically stable adult patients with documented IHD by history of myocardial infarction, history of revascularization procedures such as coronary artery bypass graft surgery (CABG) or percutaneous coronary intervention (PCI) greater than three months, and/or had angiographic evidence of coronary artery stenosis ⩾70%, were recruited. The study protocol was reviewed and approved by the Duke Institutional Review Board, and all participants provided written informed consent.
Study design
All participants underwent mental stress testing followed by exercise stress testing at the Duke Cardiac Diagnostic Unit. Our protocol required that only beta-blockers be withheld for up to five half-lives before stress testing. Other anti-angina medications were continued per patients’ home routine. Following an initial 20-minute rest period, patients completed a battery of three mental stress tests in sequence (mental arithmetic, mirror trace, and anger recall public speech) with a six-minute rest period following each mental stress test. After completion of the mental stress testing and a 20-minute rest period, patients performed an exercise stress treadmill test using standard Bruce protocol.8 Exercise stress was terminated according to standard guidelines.
Assessment of myocardial ischemia
Transthoracic echocardiography (echo) and electrocardiography were used to assess for myocardial ischemia.9 Echo images were acquired during the last three minutes of baseline resting period, for three minutes during each mental stress task, and immediately following the cessation of exercise stress. Images (parasternal long- and short-axis views and apical four- and two-chamber views) were acquired using a 3 MHz transducer while in the harmonic imaging mode of the Philips iE33 system (Philips Ultrasound, Bothell, Washington, USA). Left ventricular wall motion was assessed using the American Society of Echocardiography’s recommended 16-segment model and determined from 30–40 frames of systole from a cardiac cycle.10 Assessments of echocardiographic images were made by two experienced cardiologists who were blinded to any patient characteristics and the nature of the tests. Left ventricular ejection fraction (LVEF) was calculated by measuring images of the two apical windows (parasternal long-axis, apical four-chamber, and apical two-chamber) from a three- to five-beat loop using the biplane Simpson’s method.11 Blood pressure, heart rate, and standard 12-lead electrocardiography were recorded simultaneously during echo image acquisition.
Definition of stress-induced myocardial ischemia
MSIMI was defined by the presence of one or more ischemic markers: compared to at rest, the development or worsening of any wall motion abnormality (WMA), reduction of LVEF ⩾ 8%, or ischemic ST-segment change on electrocardiography (horizontal or downsloping depression ⩾ 1 mm in two or more leads, lasting for three or more consecutive beats) occurring during one or more of the three mental stress tasks compared to at rest. ESIMI was defined as the development of any or all of the above during the treadmill exercise stress testing.
Follow-up
All 310 patients were followed via telephone calls at annual intervals. Information regarding patients’ medical status, hospitalizations, and current use of anti-depressant medication(s) was obtained by study physicians and verified with medical records. Information on death was obtained from patients’ family members and/or medical records. For patients who could not be reached after three telephone call attempts, follow-up information was gathered from medical records. Date and cause of death were verified via the National Death Index.
Study endpoints
Study endpoints were (a) time to first MACE, an a priori composite event term defined as all-cause mortality and all cardiovascular events that resulted in an unplanned hospitalization, (b) time to all-cause mortality, and (c) total number of MACEs experienced by each patient during the follow-up period. Hospitalization was defined as inpatient admission of a patient for ⩾ 24 h. Cause of hospitalization was identified by means of the primary discharge diagnosis. All events identified were adjudicated by study investigators (WJ and CMO) who were blinded to the subjects’ MSIMI and ESIMI status.
Statistical analysis
Sample characteristics were described using means and standard deviations for continuous variables and frequencies and percentages for categorical variables, separately for patients who experienced a MACE during follow-up and those who did not.
Multivariable Cox proportional hazards regression models were used to examine association between indices of MSIMI and ESIMI and time to first MACE and/or all-cause mortality. Initial analyses focused on modeling the dichotomous (with vs without) MSIMI and ESIMI variables as predictors of study endpoints with age, sex, resting LVEF, resting wall motion score index (WMSI) (the sum of the segmental wall motion scores divided by the total number of scored segments), and New York Heart Association (NYHA) classification of heart failure severity as adjustment covariates. Missing resting LVEF values (n = 25) were imputed using the sample median.
We then fitted models with mental stress- and exercise-induced LVEF expressed as change scores (i.e. stress value–resting value) as predictors of time to first MACE and/or all-cause mortality with the same covariates. To enhance reliability of the mental stress-induced LVEF measurements and reduce the number of statistical tests, we averaged the three mental stress measurements of the LVEF variables. Mental stress- and exercise-induced LVEF changes were modeled as continuous variables and scaled such that a one unit change reflected a 5% change in LVEF from rest in response to stress testing. Thus, hazard ratios (HRs) estimated from these variables reflected the risk of MACEs associated with clinically meaningful change in LVEF, i.e. 5%. The association between stress-induced LVEF change and time to first MACE was examined for nonlinearity using restricted cubic splines, a flexible nonparametric smoother. Schoenfeld residuals were examined to test the proportional hazards assumption, which was met for all models.
We conducted an ancillary analysis using negative binomial regression to examine associations between ischemia indices and the number of MACEs during the follow-up period. These models were controlled for age, sex, resting LVEF (and resting WMSI for dichotomous ischemia variables), NYHA severity, and an offset term, the natural logarithm of follow-up time, to account for differential follow-up duration.
In cases where an index of MSIMI was a significant predictor of a study endpoint, we re-estimated the model with additional control for the appropriate exercise variable (i.e. dichotomous ESIMI or exercise-induced LVEF). The rationale for this additional adjustment was to test whether the prognostic value of indices of MSIMI was independent of indicators of ischemia derived from exercise stress testing.
Of the 310 patients who underwent baseline stress testing, 307 had adequate data for determining the presence MSIMI (i.e. LVEF, WMSI, or both) and 290 had adequate data for determining the presence of ESIMI. Mental stress-induced LVEF data was available for 282 patients and exercise-induced LVEF data was available for 261 patients. Missing exercise data was handled through the use of median imputation in models that included an additional adjustment for an exercise ischemia variable.
Results
Patient characteristics
As shown in Table 1, demographic and clinical characteristics were similar between those with and without MACEs. The sample was predominantly white (81%) and male (83%) with a mean of age of about 63 years. As previously reported,3 the rate of MSIMI was higher than ESIMI (43.45% vs 33.79%, p = 0.003) in the REMIT study population. Relative to exercise, mental stress induced greater LVEF reduction (Figure 1). Mental stress testing did not induce electrocardiography (ECG) changes.
Table 1.
Baseline characteristics of patients with and without major adverse cardiovascular events.
| Baseline characteristics |
No event (n = 182) |
Yes event (n = 125) |
Total (n = 307) |
|
|---|---|---|---|---|
| Age, mean ± SD, years |
61.9 ±10.1 | 65.7 ±10.7 | 63.5 ± 10.5 | |
| Men, n (%) | 154 (84.6) | 100 (80.0) | 254 (82.7) | |
| White race, n (%) | 153 (84.1) | 97 (77.6) | 250 (81.4) | |
| BMI, mean ± SD kg/m2 |
28.8 ± 4.7 | 29.0 ± 4.8 | 28.9 ± 4.8 | |
| Smoking, n (%) | ||||
| Current | 16 (8.8) | 27 (21.6) | 43 (14.0) | |
| Past | 99 (54.4) | 69 (55.2) | 168 (54.7) | |
| Never | 67 (36.8) | 29 (23.2) | 96 (31.3) | |
| Prior MI, n (%) | 83 (45.6) | 53 (42.4) | 136 (44.3) | |
| Prior PTCA, n (%) | 106 (58.2) | 86 (68.8) | 192 (62.5) | |
| Prior CABG, n (%) | 73 (40.1) | 61 (48.8) | 134 (43.7) | |
| Hx of DM, n (%) | 47 (25.8) | 41 (32.8) | 88 (28.7) | |
| Hx of HTN, n (%) | 143 (78.6) | 103 (82.4) | 246 (80.1) | |
| Hx of hyperlipidemia, n (%) |
171 (94.0) | 118 (94.4) | 289 (94.1) | |
| Hx of depression, n (%) |
26 (14.3) | 17 (13.6) | 43 (14.0) | |
| NYHA functional class, n (%) | ||||
| I | 175 (96.2) | 107 (85.6) | 282 (91.9) | |
| II | 6 (3.3) | 14 (11.2) | 20 (6.5) | |
| III | 1 (0.6) | 4 (3.2) | 5 (1.6) | |
| ASA, n (%) | 175 (97.2) | 117 (93.6) | 292 (95.7) | |
| Other antiplatelet agent, n (%) |
68 (37.6) | 64 (51.2) | 132 (43.1) | |
| ACEI, n (%) | 115 (63.5) | 81 (64.8) | 196 (64.1) | |
| Angiotensin II, n (%) | 22 (12.2) | 18 (14.4) | 40 (13.1) | |
| β-Blockers, n (%) | 151 (83.4) | 105 (84) | 256 (83.7) | |
| Calcium channel blockers, n (%) |
41 (22.7) | 29 (23.4) | 70 (23.0) | |
| Statin, n (%) | 167 (92.3) | 113 (91.9) | 280 (92.1) | |
| Other lipid-lowering agent, n (%) |
51 (29.0) | 29 (24.0) | 80 (26.9) | |
| Baseline SBP, mean ± SD mm Hg |
126 ± 19 | 128 ± 18 | 127 ± 18 | |
| Baseline DBP, mean ± SD mm Hg |
73 ± 12 | 73 ± 11 | 73 ± 11 | |
| Baseline HR, mean ± SD beats/min |
67 ± 10 | 69 ± 11 | 68 ± 11 | |
| Baseline LVEF, mean ± SD |
58 ± 10 | 55 ± 11 | 57 ± 10 | |
ACEI: acetycholinesterase inhibitor; ASA: aspirin; BMI: body mass index; CABG: coronary artery bypass graft; DBP: diastolic blood pressure; DM: diabetes mellitus; HR: heart rate; HTN: hypertension; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PTCA: percutaneous transluminal coronary angioplasty; SBP: systolic blood pressure; SD: standard deviation. Hx: history.
Figure 1.
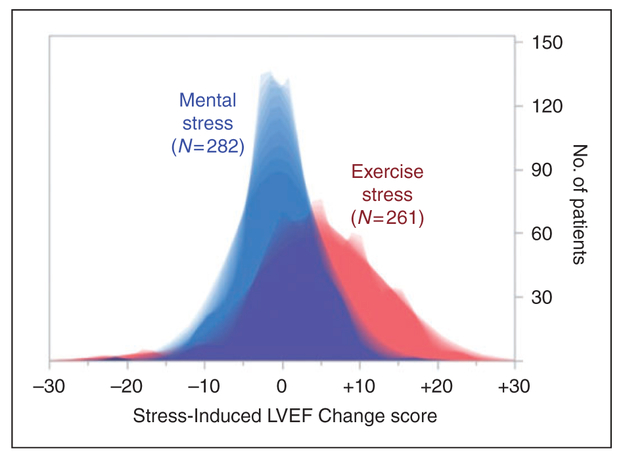
Distribution of left ventricular ejection fraction (LVEF) change scores during mental and exercise stress test. LVEF change score during mental stress shown is the average change score of three mental stress tests. On average, mental stress caused LVEF reduction (−0.505 ± 4.75 (−21.3, −0.333, + 17.0)) and exercise resulted in LVEF elevation (+4.52 ± 7.74 (−24.0, + 4.00, + 23.0)). Values are mean ± standard deviation (SD) (minimum, median, maximum).
Occurrence of MACEs
Patients were followed for up to six years with a median follow-up of four years. One hundred twenty-five patients had at least one MACE during follow-up period. There were 18 deaths attributable to various causes and 220 hospitalizations due to cardiovascular causes, including non-fatal myocardial infarction (n = 24), unstable angina resulting in intracoronary stenting procedure or change in medication management (n = 81), cardiac arrest (n = 1), acute heart failure exacerbation (n = 31), arrhythmia (n = 28), cerebrovascular accident (n = 20), stable angina (n = 7), and others (n = 51). Events included in the ‘others’ category included implantable cardioverter defibrillator (ICD) generator change (n = 2), hypertension (n = 5), peripheral vascular disease (n = 15), abdominal aortic aneurysm (n = 4), pulmonary thromboembolism or deep venous thrombosis (n = 3), cardiac valvular disease (n = 6), syncopal episode (n = 10), diabetic vasculopathy (n = 5), and pericarditis (n = 1).
MACEs occurred in 46.27% of subjects with MSIMI and in 36.42% of those without (p = 0.08). The frequency of MACEs was 41.84% in subjects with ESIMI and 38.02% in those without (p = 0.53). All-cause mortality was observed in subjects with 7.46% with MSIMI and in 4.62% of those without MSIMI (p = 0.29). The frequency of all-cause mortality was 7.14% with ESIMI and 3.65% in those without (p = 0.19).
Ischemia measures and event-free survival
Cox regression models examining ischemia measures as predictors of time to first MACE and all-cause mortality showed non-significant effects for dichotomous MSIMI (HRMACE=1.23, 95% confidence interval (CI) = 0.85–1.76, p = 0.27; HRMortality=1.70, 95% CI = 0.64–4.51, p = 0.29), dichotomous ESIMI (HRMACE=1.20, 95% CI = 0.82–1.77, p = 0.35; HRMortality=1.91, 95% CI = 0.65–5.63, p = 0.24), and continuous exercise-induced LVEF change (HRMACE=1.07, 95% CI = 0.95–1.21, p = 0.27; HRMortality=0.94, 95% CI = 0.65–1.35, p = 0.73). The mental stress-induced LVEF change variable showed a significant association with time to first MACE (Figure 2, Table 2), adjusting for covariates. In this multivariable model, older age, lower resting LVEF, and more severe heart failure were also significantly associated with time to first MACE. Further adjustment with exercise-induced LVEF change resulted in slight reduction of the HR for mental stress-induced LVEF in predicting time to first MACE (Table 2). There was a non-significant effect for mental stress-induced LVEF change in relation to time to mortality (HRMortality=1.38, 95% CI = 0.81–2.37, p = 0.24).
Figure 2.
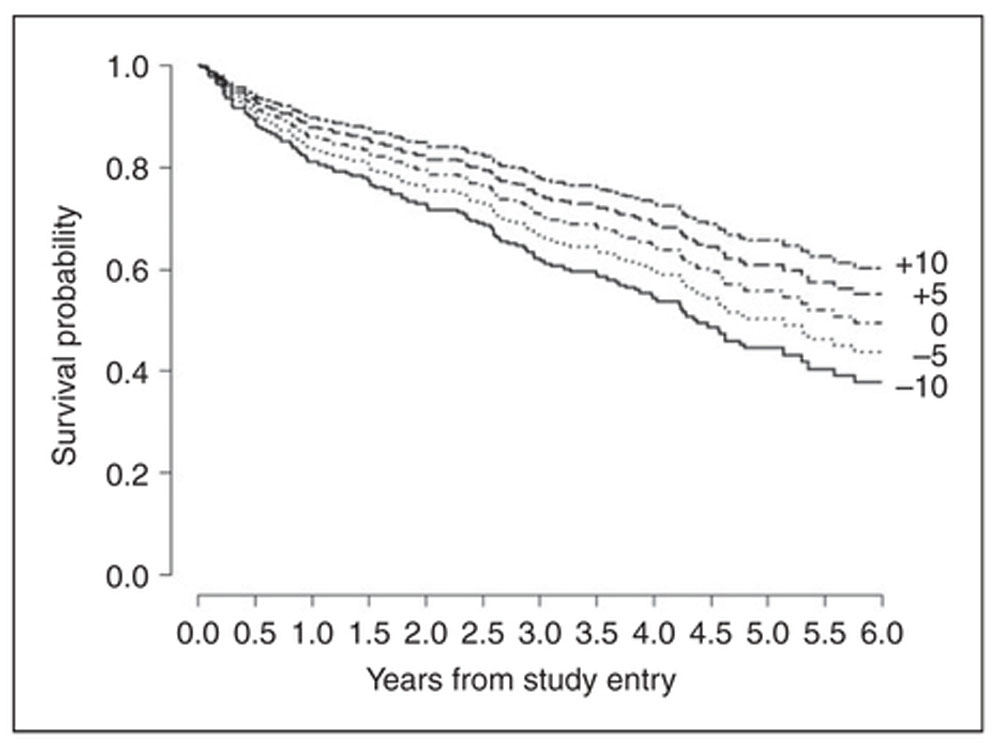
Major adverse cardiovascular event (MACE)-free probability as a function of mental stress-induced left ventricular ejection fraction (LVEF) change score. Predicted survival curves showing probability of MACE-free survival as a function of mental stress-induced LVEF change of + 10%, +5%, 0%, −5%, and −10%, compared to at rest, adjusted for age, sex, resting LVEF, and heart failure severity. The number of patients at risk was 282 at year 0, 241 at year 1, 223 at year 2, 160 at year 3, 101 at year 4, and 42 at year 5. Since the survival plot represents predicted curves from the Cox model in which ejection fraction (EF) change is modeled as a continuous variable, the number at risk we provide is necessarily for the entire sample rather than strata. Note that the Schoenfeld residual test for proportional hazards for change in EF was not significant (=0.63), thus the modeled lines are proportional.
Table 2.
Multivariate Cox proportional regression examining association between mental stress (MS)-induced ejection fraction change and major adverse cardiovascular event (MACE).
| Predictor | HR (95% CI)a | p value |
|---|---|---|
| Age | 1.03 (1.01–1.05) | 0.008 |
| Sex (male) | 0.74 (0.47–1.17) | 0.20 |
| Resting ejection fraction | 0.97 (0.96–0.99) | 0.001 |
| Heart failure severity | 1.96 (1.30–2.96) | 0.001 |
| MS-induced LVEF change (n = 282) |
1.24 (1.01–1.52) | 0.04 |
| MS-induced LVEF change, further adjusted for exercise-induced LVEF change (n = 282) |
1.23 (0.97–1.55) | 0.09 |
CI: confidence interval; HR: hazard ratio; LVEF: left ventricular ejection fraction.
HRs for ejection fraction changes represent increase in hazard associated with a 5% decrease in ejection fraction in response to stress.
The spline analysis suggested the association between the continuous mental stress-induced LVEF change variable and time to first MACE was linear (Figure 3(a) and (b)). Thus, every incremental reduction of 5% in LVEF induced by mental stress was associated with a 24% increase in MACE hazard. To express this result in more clinically meaningful terms, we estimated four-year MACE probabilities associated with three possible mental stress-induced LVEF changes compared to at rest, i.e. 5% reduction, no change, and 5% increase. The MACE probabilities were 0.36 for patients with a LVEF reduction of 5%, 0.31 for patients showing no LVEF change, and 0.25 for patients showing a 5% increase in LVEF from resting. This means that, compared to no LVEF change, each incremental reduction of LVEF by 5% induced by mental stress raised the predicted four-year MACE probability by about 5%.
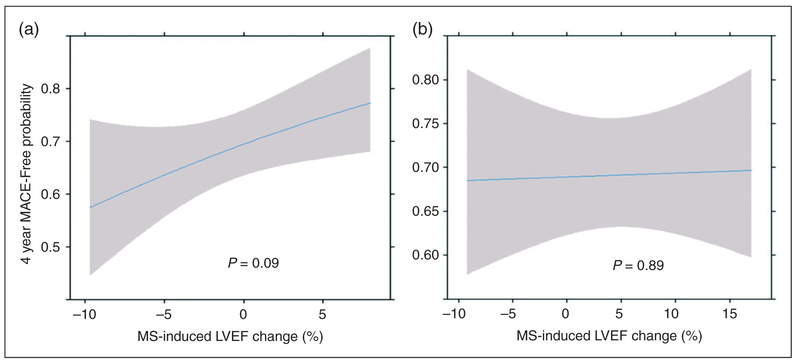
Figure 3.
Slope plots depicting mental stress (MS)-induced left ventricular ejection fraction (LVEF) change and exercise-induced LVEF change as predictors of four-year major adverse cardiovascular event (MACE)-free probability. Slope plots from multivariable cox proportional regression models examining associations of MS-induced LVEF change (a) and exercise-induced LVEF change (b) with MACE-free probability at four years (median follow-up time from study entry). Each slope is adjusted for all other variables in the model, which include age, sex, New York Heart Association congestive heart failure severity classification, and resting LVEF.
Ischemia measures and MACE category
In order to better understand the association between mental stress-induced LVEF change and MACEs, we calculated first event rates for each MACE category across tertiles of mental stress-induced LVEF change scores. Given the low number of events in each category, we decided to pursue a purely descriptive rather than a formal statistical analysis of these associations. Patients in the lowest (i.e. largest reduction in LVEF) tertile of mental stress-induced LVEF change generally showed higher event rates for most categories of MACE, whereas the lowest event rates tended to be in the groups of patients in the second and third tertiles of mental stress-induced LVEF change. This tendency was greatest for all-cause mortality, heart failure exacerbations, cerebrovascular events, and a heterogeneous category of other cardiovascular events.
Ischemia measures and total number of MACEs
Multivariable negative binomial regression examining associations between the ischemia indices and total number of MACEs suffered during follow-up time showed non-significant effects for dichotomous MSIMI, dichotomous ESIMI, and exercise-induced LVEF change. Mental stress-induced LVEF change was a significant predictor of total number of MACEs (incident rate ratio = 0.20, 95% CI = 0.004–0.35, p = 0.047), meaning that every incremental 5% reduction in LVEF change during mental stress was associated with a 20% increase in the number of MACEs experienced over the follow-up period. The association of mental stress induced-LVEF change with the number of MACEs was attenuated after further adjustment for exercise-induced LVEF change (incident rate ratio = 0.18, 95% CI=−0.05–0.35, p = 0.11).
Discussion
This is the first study to examine the prospective relationship of MSIMI and ESIMI indices with MACE occurrence in a sample of IHD patients that was not confined by having myocardial ischemia induced by physical stress testing. The major unique finding is that in these patients, mental stress-induced change in LVEF significantly predicts subsequent MACE occurrence. This association is independent of age, sex, resting LVEF, and heart failure severity.
Our finding that the continuous variable of mental stress-induced LVEF change predicts subsequent MACEs in a linear manner is in agreement with previous prognostic studies.1 Given the linear association, there does not appear to be a precise cutoff point for mental stress-induced LVEF change that clearly delineates elevated risk of MACEs. Therefore, measures of MSIMI that are, in part, defined by arbitrary cutoff points will sacrifice power. Low power due to dichotomization may in fact account for the positive, but non-significant, association between the dichotomous MSIMI variable and MACE risk observed in the present study.
A number of possible mechanisms underlying MSIMI have been examined. In IHD patients who had mental stress-induced LVEF reduction ⩾ 5% compared to those without, Jain et al. found no significant decrease in peak power or end-systolic ventricular elastance, two reliable indexes of LV contractility that are independent of afterload.12 Additional observations from that study and others suggest that mental stress-induced LVEF reduction is accompanied by increased systemic vascular resistance.13,14
Mental stress has also been observed to induce reversible segmental wall motion and perfusion abnormalities in the same regions as exercise stress.15,16 Yeung et al. observed paradoxical vasoconstriction in large, diseased epicardial coronary arteries during coronary angiography in response to mental arithmetic.17 More recently it has been proposed that mental stress over time may lead to diffuse microvascular insufficiency in response to vasodilators that is limited to the sub-endocardium and midwall region, leading to left ventricular diastolic dysfunction.18,19 The same process has been proposed to drive heart failure with preserved ejection fraction,20 suggesting that heart failure sits at the intersection of mental stress-induced LVEF reduction and a poor cardiovascular prognosis. This speculation appears to be supported by our event category specific analysis that showed that patients in the lowest tertile of mental stress-induced LVEF response had the highest rate of heart failure exacerbation. Expanded studies are needed to explore this intriguing relationship.
Our results indicate the prognostic power of mental stress-induced LVEF change is independent of exercise-induced LVEF change as the HR remained essentially the same when exercise-induced LVEF change was included as a covariate, though the p-value was attenuated (p = 0.09). The p-value attenuation might reflect a loss of power due to the inclusion of an additional covariate in the model, or to mental stress-induced LVEF changes sharing predictive variance with exercise-induced LVEF changes, or both. It is important to mention that all patients underwent exercise stress testing after completion of mental stress testing, raising concerns of a carryover effect. The possibility of a carryover effect is supported by the observation that ESIMI was most prevalent in patients showing MSIMI to all three mental tasks.21 Mental stress preceding exercise stress has been found to reduce exercise capacity and potentiate the development of myocardial ischemia during physical exercise in IHD patients.22 Therefore controlling for exercise-induced LVEF changes measured under the conditions of the current study may overestimate the impact of that variable on the prognostic power of mental stress-induced LVEF change. Additional studies using a different study protocol are needed to explore this possibility. Ultimately, it will be necessary to replicate the present findings in a larger sample of coronary patients in order to establish mental stress-induced LVEF changes as an independent prognostic indicator in this population.
No ESIMI indices were associated with occurrence of MACEs or all-cause mortality in our study. Previous studies looking at MSIMI and ESIMI also found exercise treadmill testing to have low prognostic power in IHD patients, but those conclusions were believed to be primarily due to the requirement that all study patients must have had a recent positive exercise stress test.1,23 The REMIT study population, nevertheless, was not restricted by this criterion and was a larger sample compared to previous studies. Our finding may reflect the clinical reality that patients with ESIMI had received appropriate interventions whereas mechanisms underlying daily ischemia episodes due to mental stress may not be well addressed.
Previous studies examining MSIMI and prognosis primarily focused on endpoints such as mortality and/or non-fatal acute coronary syndrome events. We included hospitalizations due to all cardiovascular causes in our a priori definition of MACEs because we are motivated by testing the hypothesis that mental stress-induced myocardial dysfunction worsens event-free survival through multiple mechanisms. It has been proposed that in addition to increased peripheral vascular resistance and coronary perfusion abnormalities, IHD patients with MSIMI also manifest broader systemic consequences of psychological stress, including elevated platelet aggregation and adhesion, changes in fibrinolysis activity or coagulation state, enhanced pro-inflammatory processes, and many other biochemical changes.14,24 In light of this, our analysis of ischemia measures and individual MACE categories lends support to the growing appreciation that mental stress-induced myocardial dysfunction worsens patients’ overall prognosis through increased disease burden across a number of cardiovascular pathologies that may not be limited to acute plaque rupture events.
Our conclusions are limited by several considerations. In examining specific MACE categories, analyses were limited by the low number of events in most categories as well as differential follow up duration, making it difficult to draw definite conclusions. The REMIT trial randomized 127 of 132 patients with MSIMI to receive escitalopram or placebo.7 We chose not to include REMIT trial participation as a covariate in our models because of the short duration of intervention (six weeks) relative to the median follow-up period of 209 weeks. The REMIT study was not intended to study the long-term effect of selective serotonin receptor inhibitor (SSRI) treatment on prognosis. Our results show that only 15% (n = 47) of subjects took an SSRI at some point, with the majority of them taking an SSRI for less than 50% of their follow-up duration. We believe this is a negligible factor for the purposes of the current study.
The clinical implications of our findings are several fold. First, the finding that mental stress-, but not exercise-, induced LVEF change predicts MACEs independent of other risk factors suggests that mental stress testing adds unique prognostic value to the current risk stratification algorithm of IHD patients. Second, although earlier studies have defined MSIMI using a criterion of either ⩾ 5% or ⩾ 8% reduction in LVEF, any reduction in LVEF under mental stress is detrimental to the prognosis of clinically stable IHD patients. Lastly, our results are especially relevant given that, in recent years, behavioral interventions and SSRI treatment have been shown to reduce the incidence of MSIMI in susceptible patients.6,25 Whether this will improve patient engagement in comprehensive cardiovascular rehabilitation remains to be seen.26
Conclusion
Our results suggest that mental stress-induced LVEF change may be a clinically useful predictor of MACEs in patients with IHD, independent of conventional exercise stress-induced ischemic indices.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Heart, Lung, and Blood Institute, Bethesda, Maryland (RO1HL085704, RO1HL118077).
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Z Samad has received research funding from Boston Scientific-Duke University Strategic Alliance for Research and the American Society of Echocardiography. RC Becker has received research grant support from AstraZeneca and Johnson and Johnson; and consulting/lecture fees from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Portola, Johnson and Johnson, and Regado Biosciences. TL Ortel is a consultant for Instrumentation Laboratory, Boehringer Ingelheim, and Bayer; and has received or has grants pending from GlaxoSmithKline, Eisai, Pfizer, Daiichi-Sankyo, Instrumentation Laboratory, and Stago. RB Williams holds a US patent on the 5HTTLPR L allele for use as a marker of increased cardiovascular risk in stressed persons and is a founder and major stockholder of Williams LifeSkills Inc. JG Rogers has received funding from Boston Scientific Corporation, HeartWare, and Thoratec Corporation. CM O’Connor has received funding from Actelion Pharmaceuticals Ltd., Amgen Inc., Biscardia LLC, Faculty Connection, GE Healthcare, Ikaria, Novella Clinical Inc., Pfizer Inc., Pozen, and Roche Diagnostics; serves as a consultant for Novartis, HeartWare, ResMed, Johnson and Johnson, Gilead, Critical Diagnostics, BG Medicine, Otsuka, Astellas, Cytokinetics, and Capricor; and holds stock or stock options in Neurotronik/Interventional Autonomics Corporation. EJ Velazquez has received research grants from Abbott Laboratories, Evalve, and Ikaria; and consulting fees from Boehringer Ingelheim, Gilead, and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Jiang W, Babyak M, Krantz DS, et al. Mental stress-induced myocardial ischemia and cardiac events. JAMA 1996; 275: 1651–1656. [DOI] [PubMed] [Google Scholar]
- 2.Wei J, Rooks C, Ramadan R, Shah AJ, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol 2014; 114: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol 2013; 61: 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramachandruni S, Fillingim RB, McGorray SP, et al. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol 2006; 47: 987–991. [DOI] [PubMed] [Google Scholar]
- 5.Hassan M, York KM, Li Q, et al. Variability of myocardial ischemia responses to mental stress versus exercise or adenosine stress in patients with coronary artery disease. J Nucl Cardiol 2008; 15: 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: Results of the REMIT trial. JAMA 2013; 309: 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang W, Velazquez EJ, Samad Z, et al. Responses of mental stress-induced myocardial ischemia to escitalopram treatment: Background, design, and method for the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment trial. Am Heart J 2012; 163: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce RA, Pearson R, Lovejoy FW, et al. Variability of respiratory and circulatory performance during standardized exercise. J Clin Invest 1949; 28: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottdiener J, Livengood SV, Meyer PS, et al. Should echocardiography be performed to assess effects of anti-hypertensive therapy? Test-retest reliability of echocardiography for measurement of left ventricular mass and function. J Am Coll Cardiol 1995; 25: 424–430. [DOI] [PubMed] [Google Scholar]
- 10.Schiller N, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367. [DOI] [PubMed] [Google Scholar]
- 11.Lang R, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 12.Jain D, Shaker SM, Burg M, et al. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol 1998; 31: 1314–1322. [DOI] [PubMed] [Google Scholar]
- 13.Becker L, Pepine CJ, Bonsall R, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study. Circulation 1996; 94: 2768–2777. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg A, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 1996; 94: 2402–2409. [DOI] [PubMed] [Google Scholar]
- 15.Deanfield J, Shea M, Kensett M, et al. Silent myocardial ischaemia due to mental stress. Lancet 1984; 2: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 16.Giubbini R, Galli M, Campini R, et al. Effects of mental stress on myocardial perfusion in patients with ischemic heart disease. Circulation 1991; 83: S100–S107. [PubMed] [Google Scholar]
- 17.Yeung A, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 1991; 325: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 18.Elhabyan A, Reyes BJ, Hallak O, et al. Subendocardial ischemia without coronary artery disease: Is elevated left ventricular end diastolic pressure the culprit. Curr Med Res Opin 2004; 20: 773–777. [DOI] [PubMed] [Google Scholar]
- 19.Pepine C, Petersen JW and Bairey Merz CN. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: A revised concept of ischemia during daily life. JACC Cardiovasc Imaging 2014; 7: 362–365. [DOI] [PubMed] [Google Scholar]
- 20.Paulus W and Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 21.Boyle S, Samad Z, Becker RC, et al. Depressive symptoms and mental stress-induced myocardial ischemia in patients with coronary heart disease. Psychosom Med 2013; 75: 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanovic J, Ostojic M, Beleslin B, et al. Mental stress-induced ischemia in patients with coronary artery disease: Echocardiographic characteristics and relation to exercise-induced ischemia. Psychosom Med 2012; 74: 766–772. [DOI] [PubMed] [Google Scholar]
- 23.Sheps D, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease:Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002; 105: 1780–1784. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Boyle SH, Ortel TL, et al. Platelet aggregation and mental stress induced myocardial ischemia: Results from the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) study. Am Heart J 2015; 169: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozanski A, Blumenthal JA and Kaplan J Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99: 2192–2217. [DOI] [PubMed] [Google Scholar]
- 26.Pogosova N, Saner H, Pedersen SS, et al. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the cardiac rehabilitation section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. EJPC 2015; 10: 1290–1306. [DOI] [PubMed] [Google Scholar]