Abstract
Background
Existing treatments for depression are known to have only modest effects, are insufficiently targeted, and are inconsistently utilized, particularly in older adults. Indeed, older adults with impaired cognitive control networks tend to demonstrate poor response to a majority of existing depression interventions. Cognitive control interventions delivered using entertainment software have the potential to not only target the underlying cerebral dysfunction associated with depression, but to do so in a manner that is engaging and engenders adherence to treatment protocol.
Methods
In this proof-of-concept trial (Clinicaltrials.gov #: NCT02229188), individuals with late life depression (LLD) (22; 60+ years old) were randomized to either problem solving therapy (PST, n = 10) or a neurobiologically inspired digital platform designed to enhance cognitive control faculties (Project: EVO™, n = 12). Given the overlapping functional neuroanatomy of mood disturbances and executive dysfunction, we explored the impact of an intervention targeting cognitive control abilities, functional disability, and mood in older adults suffering from LLD, and how those outcomes compare to a therapeutic gold standard.
Results
EVO participants demonstrated similar improvements in mood and self-reported function after 4 weeks of treatment to PST participants. The EVO participants also showed generalization to untrained measures of working memory and attention, as well as negativity bias, a finding not evident in the PST condition. Individuals assigned to EVO demonstrated 100% adherence.
Conclusions
This study provides preliminary findings that this therapeutic video game targeting cognitive control deficits may be an efficacious LLD intervention. Future research is needed to confirm these findings.
Keywords: behavioral therapy, cognitive control, video game technology, depression, problem solving therapy
1 | INTRODUCTION
Despite decades of research into the treatment of depression, this disorder has been the second leading cause of global disability for the last 10 years (Ferrari et al., 2013). Underlying causes for this public health problem are the modest effects of existing treatments, their limited accessibility and quality when delivered in routine practice, and the high rate of early treatment discontinuation for both psychotherapies and medications (Mulder, Frampton, Luty, & Joyce, 2009; Thase et al., 1992; Warden et al., 2014). According to the National Institute of Mental Health (NIMH), this problem is due to the fact that existing treatments only manage symptoms rather than treat to the underlying causes of depression (Insel & Wang, 2009). In response to this problem, the most recent NIMH strategic plan calls for the development of novel interventions that treat the known cognitive and behavioral correlates of depression (NIMH, 2015). The plan additionally calls for the development of interventions that are portable and accessible to the general public, in order to mitigate the 20- to 30-year gap commonly found between discovery and implementation (NIMH, 2016). In summary, to reduce the burden of depression, we need to develop interventions that are both targeted to underlying causes of depression symptoms and that are easily deployed into the community.
1.1 | Cognitive control deficits and late life depression
Although there is still much to learn about the causes of depression, one area that has been well studied is late life depression (LLD), major depression that occurs after the age of 65. Research has repeatedly demonstrated that older adults with deficits in cognitive control functions have a poor response to antidepressant medications (Alexopoulos, 2001; Alexopoulos, Kiosses, Klimstra, Kalayam, & Bruce, 2002; Kiosses, Klimstra, Murphy, & Alexopoulos, 2001; Manning et al., 2015; Morimoto et al., 2011) and some forms of psychotherapy (Thompson et al., 2015). The relationship of cognitive control dysfunction in older adults to poor antidepressants is supported by structural MRI, focal cerebral activation, and functional connectivity studies (Alexopoulos et al., 2012, 2013; Bredemeier et al., 2012; Gunning-Dixon et al., 2010; Gunning-Dixon, Brickman, Cheng, & Alexopoulos, 2009; Joormann & Gotlib, 2010; Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015; Sheline, Price, Yan, & Mintun, 2010). A key deficit of cognitive control in LLD is difficulty ignoring irrelevant, especially negatively biased information. This difficulty impacts the depressed individual’s ability to attend to information central to goal-directed behavior. Cognitive control is particularly vulnerable to advancing age (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Gunning-Dixon & Raz, 2003; Gunning-Dixon et al., 2009; Nielson, Langenecker & Garavan, 2002) and at least 40% of older adults with depression suffer from cognitive control dysfunction (Lockwood, Alexopoulos, & van Gorp, 2002). Poor functioning of cognitive control networks in late life is characterized by increased susceptibility to interference from task irrelevant stimulus properties, poor response inhibition, and reduced sustained attention (Alexopoulos et al., 2005; Alexopoulos, Gunning-Dixon, Latoussakis, Kanellopoulos, & Murphy, 2008; Manning et al., 2015).
1.2 | Treating LLD characterized by cognitive control deficits
Preliminary evidence suggests that behavioral interventions that reinforce and enhance behaviors associated with cognitive control may be effective for LLD characterized by these deficits. In our own work with problem solving therapy (PST), an intervention that helps patients with goal-directed behavior, we have found that people with this presentation of LLD benefit significantly from PST, demonstrating very large effect sizes (NNT = 4 (Arean et al., 2010)). However, a primary limitation with this intervention and those like it, is that PST is hardly ubiquitous, and as has been detailed in a recent IOM report regarding the quality of psychotherapy, the chance that an intervention like PST is both available and delivered with high quality is very low (NAS, 2015).
An alternative approach to treating patients with LLD with such deficits is through computerized cognitive control interventions. An advantage to treatment using video game technologies is that they can be (1) standardized, and as result are not subject to the skill drift seen in the delivery of other behavioral interventions, (2) personalized in that they are set to the baseline functioning of the individual and adapt as the individual improves, and (3) are easily accessed and can be delivered in the individual’s home, minimizing typical access barriers older adults contend with when accessing traditionally delivered mental health care (Anguera & Gazzaley, 2015; Mishra, Anguera, & Gazzaley, 2016). The utilization of these types of tools is especially prevalent amongst older adults, with one-third of people over 65 using digitally based cognitive exercises currently (Allaire et al., 2013). Future digital health use is predicted to increase substantially as the baby-boomer generation ages (Collier, 2014), which could lead to a new avenue for providing mental health treatment.
Recent reports indicate that commercial interventions are not evidence based (Hamilton, 2009), and that the impact these interventions have on function is limited because they do not specifically target a known underlying mechanism, nor do they thoroughly challenge the brain enough to confer positive effects on behavior. Some studies have shown that older adults demonstrate marked improvement in cognitive control abilities following directed interventions that specifically target cognitive control deficits (Anguera et al., 2013; Berry et al., 2010; Mishra, de Villers-Sidani, Merzenich, & Gazzaley, 2014). Recent work by Anguera et al. (2013) demonstrated that a video game targeting cognitive control abilities enhanced deficient age-related cognitive control processes, the very processes that are implicated in LLD (Alexopoulos et al., 2015). If a video game designed to target cognitive control deficits could improve cognitive control functions in older adults with LLD, it might be able to improve depression in this population.
In this proof-of-concept study, our exploratory hypotheses were (1) to explore the impact of a cognitive control intervention embedded into an entertainment software platform on cognitive control network, mood and disability in people with LLD, and compare the findings to patients who received PST; (2) to explore the association of cognitive control network activation (as measured by behavioral assessment) on mood and functional disability, and finally (3) determine the acceptability of such a cognitive control intervention for depressed individuals.
2 | METHODS
2.1 | Design
This was a proof-of-concept randomized clinical trial (Clinicaltrials.gov #NCT02229188) with 22 community-based participants over the age of 65 suffering from major depression. Proof-of-concept studies are widely used as a first step in human trials, most commonly in cancer trials, where a novel compound is tested in a small sample of participants to determine whether there is an effect on the underlying dynamics of a disease state, as well as an impact on a clinical outcome. The intent of these studies is to identify promising candidate interventions for larger scale study (Banerji & Workman, 2016; Kendig, 2016). Using a random number generator, participants were randomized to receive 8 weeks of PST alone or 4 weeks of a cognitive intervention (Project: EVO™) + 4 weeks of clinical management. Participants were assessed for mood (Hamilton depressive rating), mood-related disability (World Health Organization disability assessment schedule II (WHODAS-II)), and performance on behavioral emotional control measures prior to and following treatment at 4 and 8 weeks. Participants also completed a battery of cognitive control tasks at baseline and the 4-week mark. Participants were recruited from the San Francisco area between June and December 2014. This study was approved by the IRB at UCSF.
2.2 | Eligibility
To determine the presence of Major Depression Disorder (MDD) and exclusion diagnosis, we administered the Structured Clinical Interview for DSM Disorders (SCID; DSM-IV version) (First, 2015). Eligible participants met the following criteria: (1) must be 60 years old or older, (2) met criteria for Major Depression as defined by the SCID and Patient Health Questionnaire (PHQ-9), in addition to having a Hamilton Depression Rating Scale (HAM-D) score >24, 2 weeks following this initial screening, (3) an MMSE of at least 24 or above, (4) spoke and read English at a 6th grade or better level, and (5) was able to physically participate in a research protocol (e.g. were not suffering from a terminal or highly disabling illness). Exclusion criteria included: (1) history of psychosis, mania, or are currently using or abusing illicit drugs or alcohol, (2) diagnosis of dementia, or (3) a clinical diagnosis of dementia by DSM-IV (see Fig. 1 for more details on inclusion/exclusion criteria). The current use of any antidepressant medication did not act as an exclusionary criterion. Although we recognize that this will be a conservative estimate, we have used this cut-score successfully in other clinical trials and have not observed differential recruitment of people with lower educations or who are racial/ethnic minorities. Participants were reimbursed $75.00 for assessments. Randomization to each treatment protocol was determined with a random number generator. Participants were randomized after they were found to be eligible for participation and had given consent to participate.
FIGURE 1.
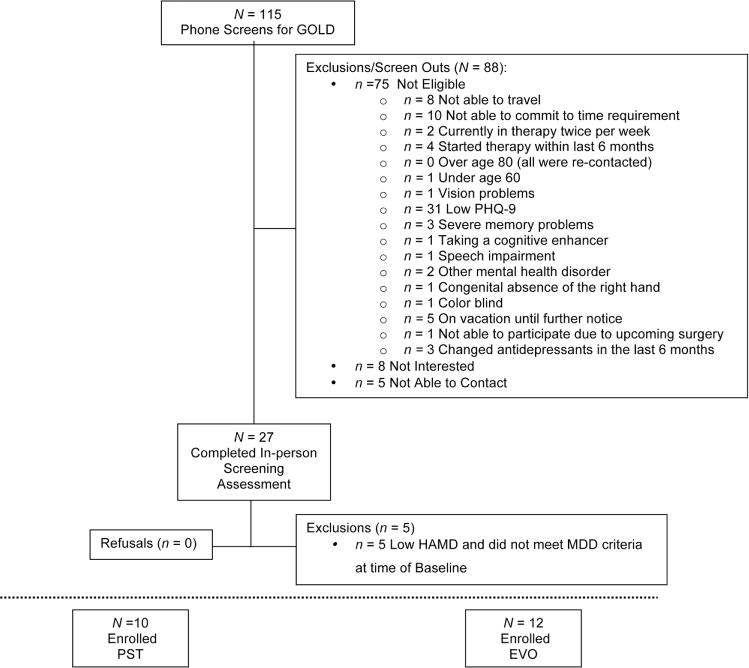
CONSORT diagram
2.3 | Intervention training and adherence
Clinicians were assigned to deliver one of the two conditions. All clinicians were trained in their respective interventions during a didactic workshop about the therapeutic rationale of the intervention and the therapeutic process. They then practiced therapy sessions through guided role-play training. All treatment sessions were recorded and rated by independent experts for treatment fidelity and adherence.
2.3.1 | Problem solving therapy
Problem solving therapy (PST) is an 8-week intervention that consists of three treatment phases: phase one lasting 3 weeks is psychoeducational, helping participants understand the problem solving steps and using the problem solving action planner for working on psychosocial problems; phase two consists of independent practice of the PST skills; phase three consists of two relapse preventions sessions, using the problem solving model to develop plans to maintain depression and functional treatment gains. Although this is a 8-week intervention, positive effects for PST are evident as early as 4 weeks (Arean, Hegel, Vannoy, Fan, & Unuzter, 2008). PST itself consists of a seven-step process to solve problems. These include problem orientation that directs patient attention to one problem at a time, problem definition that helps patients select our relevant information to determine what the root problem is in a given situation, goal setting that focuses attention to the desired outcome, brainstorming that helps patients consider multiple methods for reaching the goal, decision making, a method employed to evaluate the alternative solutions likelihood of reaching the stated goal and picking the best solution among the choices, and action planning that involves a step-by-step plan for the patient to implement his/her solution.
2.3.2 | Cognitive intervention
The cognitive intervention in this study is a mobile, iPad intervention called Project: EVO™ based on the video game used in by Anguera et al. (2013) called NeuroRacer. This game involves guiding a character through an immersive environment while responding to select targets, with the design format being ideally entertaining to children (thus it was not designed for the present population studied here; see Supporting Information for more details). The embedded adaptive algorithms titrate the experience for the user to ensure that the game is challenging, but still enjoyable and still have room for the user to improve over time. In the first session, care managers introduced participants to the intervention, explain the therapeutic rationale underlying EVO, and the rationale for the weekly check-in visits. Participants then engaged in a practice run with the iPad and EVO with the care manager present. Participants were instructed to play EVO 5 days a week for ∼20 min for 4 weeks. The 4-week duration for EVO was based upon the previous duration used by original NeuroRacer study that EVO was derived from.
Because this is a clinical population with risk, we elected to add in 8 weeks of weekly clinical management meetings for two important reasons: (1) this game had not been tested for the treatment of LLD, and is an intervention that patients use without supervision from a clinician. (2) Clinical management as it is applied here served as a means to control for the fact that participants in the PST condition were seen in person on a weekly basis, and social contact of this nature can have a positive effect on mood. During the follow up meetings, the care managers administered a PHQ-9, review adherence with participants, and assisted those who are not using the game as instructed to develop action plans that allow them to engage in regular play. No other therapeutic techniques were used beyond clinical support. Note that participants did check in with their therapist 1×/week regardless of which group they were in. However, EVO participants were simply asked about their experience and reviewed their progress with their therapist and were not given any additional treatment during these sessions.
2.4 | Clinical outcome measures
Depression symptoms were measured using HAM-D (our primary mental health outcome measure) and the PHQ-9 (our secondary mental health outcome measure) (Kroenke, Spitzer, & Williams, 2003). Note that the PHQ-9 was first administered during the screening process, with the HAM-D administered 2 weeks after screening. Physical disability was assessed through the Charleston Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987) and WHODAS (1999). Research assistants who were trained and supervised by the program manager collected these measures. Interrater reliability was calculated on a monthly basis with an effort to keep ICC to .90 or better. Corrective retraining was available to any assistant whose administration or scoring of any instrument falls below this standard, however, none fell below the standard.
2.5 | Cognitive outcome measures
The cognitive outcome measures involved tasks assessing different cognitive control domains: sustained attention (test of variables of attention, TOVA (Greenberg, 1996)) and working memory (a delayed-recognition task (Clapp, Rubens, Sabharwal, & Gazzaley, 2011)), each of which have previously shown positive transfer effects in older adults following a similar training approach (see Supporting Information Figs. 2 and 3 for images of each task). Each task is sensitive to revealing evidence of cognitive control training via task-related response times in each case. The working memory task (AID) consists of three tasks in which aspects of visual information were held constant (“Is the final face shown the same as the first?”) while task demands are manipulated. Each run begins with an instruction slide informing the participant which task conditions they will be performing for the duration of that run. In the “ignore distractor” (ID) condition, participants are instructed to ignore the distracting face stimulus while maintaining the representation of the cue face. In the “no distractor” (ND) condition, participants simply perform the WM task. TOVA is a sustained attention task that requires participants to respond to rarely occurring stimuli while ignoring all others, and has been used as a outcome measure in our previous work (Anguera et al., 2013). Finally, participants performed a basic response time task at each outcome measure testing session to confirm that any response time improvements are not solely due to heightened motoric changes, as evidenced through the absence of a change in performance over time on this test.
2.6 | Negativity bias/negative self-referential processing
Participants completed a trait adjective task to assess negativity bias (Harmer et al., 2009). During the task, participants viewed a list of adjectives one at a time that described positive and negative personality characteristics. Participants were instructed to quickly indicate whether each of these adjectives applied to them by pressing one of two response keys. Positive and negative adjectives were selected from a normed word list and matched for word length, frequency, and arousal. The degree of desirability of positive words was comparable to the degree of undesirability of negative words (Anderson, 1968). Stimuli appeared on the screen for 800 ms, preceded by a 400 ms fixation cross. Our main performance indices were the number of endorsed positive and rejected negative words as these indices of negativity bias have been observed to be sensitive to other antidepressant treatment modalities (Harmer et al., 2009; Hilimire et al., 2015).
2.7 | Data analysis
To isolate those measures that changed significantly following training we performed session × study-group ANOVAs. We also performed a repeated measures within-factor post-hoc power analysis (completed using G*Power (Faul, Erdfelder, Buchner, & Lang, 2009)) designed to reveal an effect specifically in the EVO group associated with improvements in depressive symptoms as measured by the HAM-D across the three measured time points. We also present the F-value associated with the ANCOVAs for each measure in Supporting Information Table 1 (with the dependent measure being posttraining performance, covarying by pretraining performance). The ANCOVA approach is considered to be a more suitable approach when posttest performance that is not conditional/predictable based on pretest performance is the primary outcome of interest following treatment, as opposed to characterizing gains achieved from pretraining performance (for example, group × session interaction(s)); however, both are appropriate statistical tools that have been used to assess outcomes in these types of studies. Follow-up contrasts were performed to further characterize any interactions observed, with a Greenhouse-Geisser correction utilized when assumptions of sphericity were not met. All effect size values reported for the clinical outcomes were calculated using Cohen’s d (1988) and corrected for small sample bias using the Hedges and Olkin approach (Hedges & Olkin, 1985).
3 | RESULTS
3.1 | Demographics
Twenty two participants (68 years of age ± 6.3; 16 females) were randomly assigned to either the EVO (Manning et al., 2015) or PST (Alexopoulos et al., 2002) groups (see Fig. 1). Note that the majority of people screened did not qualify because they (1) did not have major depression (2) were not willing to be randomized, and (3) were not interested in the study after it was explained to them. The mean number of years of education was 16.4. Their mean (SD) test scores for depression (Hamilton: 23.0 [3.5]) and disability (WHODAS II: 29.9 [8.1]) were in the moderate range of severity. Approximately 64% of participants had a history of antidepressant drug treatment; however, none of those enrolled reported taking such medication at the present time. Assessment of the SCID revealed that none of the participants had comorbidities involving mania, psychotic symptoms, PTSD, alcohol abuse, bipolar disorder, obsessive-compulsion, anxiety, stress, pain, or phobias. Critically, no between-group differences were observed at baseline on any neuropsychological assessments (P >.10 in each case) or pretraining data involving any of the cognitive tests (each test pretraining: P > .40).
3.2 | Participant retention and training adherence
Participants in the PST group completed all of their scheduled visits with their therapists across the 8-week period. Participants in the EVO group showed remarkable compliance with respect to game play, with participants demonstrating a 100% rate of compliance. One participant elected to drop out of the study (PST cohort) during our baseline assessment. In addition, some participants were not able to complete certain testing due to fatigue or complications during their visits to clinic, leading to differential number of participants for some of the subsequent analyses below (as seen in the reported degrees of freedom).
3.3 | Clinical outcomes
The mean MMSE score for participating individuals at baseline was 28.9, with a mean Hamilton-D score of 22.6 (±3.5) and PHQ-9 of 14.1 (±3.9). Group (Warden et al., 2014) × time point (3; baseline, week 4, week 8) ANOVAs examining changes in depression severity for both the HAM-D and PHQ-9 revealed a positive improvement in depressive symptoms across time (F ≥ 19.1, P ≤ .0001 in each case), with the absence of a group × time interaction suggesting a comparable improvement across groups over the 8-week period (F ≤ 2.14, P ≥ .15 in each case, see Fig. 2). With respect to the HAM-D, the repeated measures within-factor post-hoc power analysis resulted in a significant main effect of time (F(2,22) = 13.91, P < .0001, partial η2 = .582), with the critical F value for such an effect being 3.44, leading to an effect size of .61. Given the sample size, the post-hoc power analysis (1 − β error probability) was expectedly low, .41, to find such an effect.
FIGURE 2.
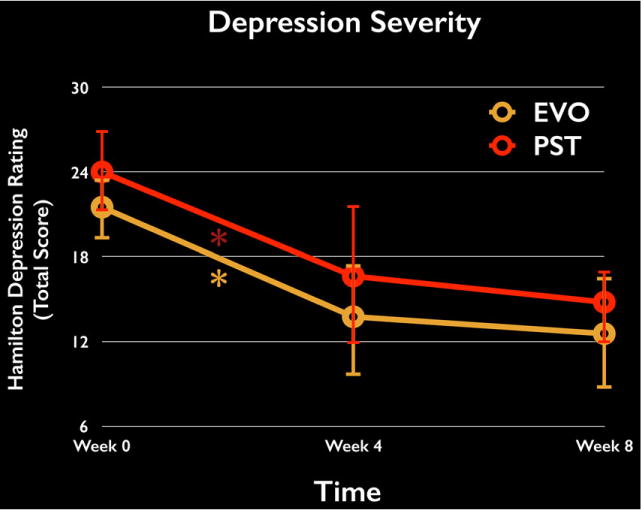
Change in depressive symptoms over time. Both the EVO and PST groups showed a comparable improvement in depressive symptoms (as measured through the Hamilton Depression Rating Scale) over the course of treatment (4 weeks), with these improvements persisting to the 8-week mark. *P < .05
Follow-up analyses revealed that each group significantly improved their HAM-D depressive symptoms from baseline (PST: P = .017, Cohen’s d = 1.03; EVO: P = .001, Cohen’s d = 1.20), with these improvements persisting to the 8-week follow-up time point (no difference from 4 to 8 week mark, P ≥ .47 for each group).
Using the same ANOVA and follow-up approach as above, an assessment of disability through the WHODAS-II test failed to reveal a group × session interaction (F(2,32) = .67, P = .52). However, a linear contrast of session was significant (F(1,16) = 13.1, P = .002), indicating a significant change in reported disability across time. Follow-up testing revealed that while there was no group difference at any time point (P > .40 in each case), the EVO group showed a trend (P = .058, Cohen’s d = .56) toward a significant improvement from baseline to the 4-week mark compared to the PST group (P = .84, Cohen’s d = .07).
3.4 | Behavioral outcomes—negativity bias/negative self-referential processing
Negativity bias was examined through the trait adjectives task at baseline, week 4, and week 8 via the number of endorsed positive self-descriptors and unendorsed/rejected negative self-descriptors (and response times for each). The trait adjective task was modeled after that used by Harmer et al. (2009). A repeated measure ANOVA for the number of unendorsed negative self-descriptors revealed a significant effect of time (F(2,28) = 3.58, P = .041) and a significant group × time interaction (F(2,28) = 3.60, P = .040), suggesting EVO participants experienced a differential decline in negativity bias over time compared to the PST participants. Follow-up contrasts revealed that while no group differences were present at either baseline or week 4 (T ≤ .17, P ≥ .86, respectively), a group difference was present at week 8 (T = 2.29, P = .035) indicative of the EVO participants showing more rejected negative self-descriptors than the PST group after the treatment period (see Table 2). Of note, the group difference in change in negativity bias may have been related to a failure of the PST group to sustain a decrease in negativity bias from week 4 to week 8. Using the same approach for the number of endorsed positive self-descriptors, similar main effects and interactions were not present (F(2,28) ≤ 1.79, P ≥ .19 in each case). However, negativity bias did decline over time in EVO-treated patients when comparing week 8 to baseline (t(8) = 4.54, P = 0.002, unlike the PST group (t(8) = .45, P = .65). Follow-up analyses examined reaction time as the dependent variables. A repeated-measures ANOVA on the RT of rejecting negative self-descriptors indicated a significant main effect of time (F(2, 26) = 0.03), with quicker responses over the course of treatment in both treatment groups. A similar main effect revealed that RTs for endorsing positive self-descriptors also became faster over time (F(2, 28) = 3.52, P = .04). However, group × time interactions did not reach significance for reaction time (P’s > .50).
TABLE 2.
Group performance on trait adjective task
| Week | EVO | PST | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Endorsed positive words | 1 | 11.4 | 4.3 | 14.7 | 4.2 |
| 4 | 13.2 | 5.0 | 16.9 | 5.3 | |
| 8 | 15.0 | 5.2 | 16 | 5.8 | |
| Rejected negative words | 1 | 13.1 | 3.9 | 12.4 | 3.9 |
| 4 | 16.3 | 4.2 | 16.9 | 5.0 | |
| 8 | 17.1 | 2.6 | 10.1 | 7.4 | |
3.5 | Behavioral outcomes—cognitive control measures
The EVO group showed improved performance on two untrained cognitive control tasks that have previously shown selective improvements in older adults participating in interference training (Anguera et al., 2013). More specifically, ANOVAs revealed group × session interactions indicative of selective improvements for the EVO group during a delayed-recognition working memory task (Clapp et al., 2011) in the setting of distraction (F(1,16) = 8.10, P = .012) and without distraction (F(1,16) = 6.15, P = .025), as well as a standard test of sustained attention (Greenberg, 1996) (F(1,16) = 10.11, P = .005; see Table 3). Planned comparisons showed that following training, the EVO group exhibited significantly faster responses to memory probes in the delayed recognition task, both with (Fig. 3A) and without distraction (Fig. 3B), and to rare target stimuli in the TOVA (Fig. 3C; P < .01 in each case), with no improvements observed in the PST group (P > .50 in each case). These cognitive improvements were specific to working memory and sustained attention processes and not the result of a generalized increase in reaction time, as a group × session interaction was not present on a basic stimulus detection task (F(1,17) = 0.19, P = .67), and neither group showed a significant improvement across time on this measure (P > .60 for each group).
TABLE 3.
Cognitive outcome measures by group
| Project: EVO™ | PST | |
|---|---|---|
| Working memory task—Distraction (baseline) | 896.19 (196) | 825.72 (142) |
| WM Distraction at the 4-week mark | 710.38 (115) | 814.60 (172) |
| Working memory task—No distraction (Baseline) | 837.16 (158) | 815.79 (160) |
| WM with no distraction at the 4-week mark | 702.66 (93) | 805.32 (158) |
| T.O.V.A (baseline) | 350.90 (67) | 375.12 (56) |
| T.O.V.A. at the 4-week mark | 313.50 (57) | 379.28 (61) |
Note: Mean and StDev presented.
FIGURE 3.
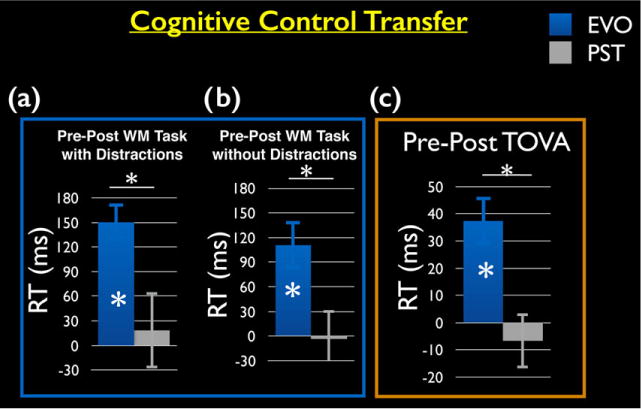
Cognitive control transfer effects. (A) Performance on the delayed-recognition working memory task: EVO participants showed a significant improvement beyond the PST group on a RT measure of working memory in the presence of distraction and (B) no distraction. (C) Performance on the sustained attention task (TOVA): EVO participants showed a significant improvement beyond the PST group on a RT measure of sustained attention. *P < .05
In follow-up analyses, we compared these results to previously published findings by Anguera et al. (2013) where healthy older adults were tested on these same measures (see Supporting Information Figs. 2 and 3). No group differences were present between the EVO-treated individuals and those in the NeuroRacer group on any of the transfer measures (P < .40 in each case), suggesting a comparable improvement was observed in cognitive control function using EVO as with NeuroRacer.
4 | DISCUSSION
To our knowledge this is the first study to compare a targeted video game intervention designed to address deficient cognitive control to a gold standard psychotherapy for LLD characterized by cognitive control deficits. Our findings provide preliminary evidence that a video game intervention targeting the cognitive control network is a potentially effective intervention for both mood and cognitive symptoms of LLD, and that the effects on mood and disability are comparable to a therapeutic gold standard and to what has been demonstrated in the literature (Arean et al., 2010). The benefits of this intervention over traditional treatments such as antidepressant medication and psychotherapy, is its ease of use, its portability, and its ability to improve cognitive symptoms. We additionally found the cognitive control intervention was highly acceptable to older adults with depression, demonstrating high retention and consistent use of the game, beyond what was expected of them.
While computerized cognitive training paradigms have been shown to improve symptoms of depression, a recent meta-analysis suggests that these approaches have only inconsistent effects on cognition (Motter et al., 2016), with the effect of such training on cognition appearing to decline slightly with age. We observed here that the EVO-trained older adult participants did indeed experience cognitive control enhancements beyond any basic motoric improvements in response time. Thus, these transfer effects are indicative that a properly designed intervention that challenges specific neural circuitry can have beneficial effects in an older population suffering from depression.
The EVO transfer effects also suggest that the improvements in depressive symptoms may have been driven by distinct underlying mechanisms of action associated with cognitive control. EVO appears to challenge similar cognitive control networks as those reported by Anguera et al. (2013) in healthy older adults who played a similar game for 1 month. In that study, the improvements in cognitive control were associated with heightened activity at the prefrontal cortex in the form of midline frontal theta, as measured through electroencephalography (EEG). This marker is of particular interest given its association with one’s response to antidepression medication (Mulert et al., 2007), and that the underlying brain region thought to be generating this signal, the anterior cingulate cortex, plays a critical role in models of depression (Pizzagalli et al., 2001; Pizzagalli, Oakes, & Davidson, 2003), particularly in response to treatment (Alexopoulos et al., 2008). Thus these findings suggest that the improved depressive symptoms in EVO may be spurred on plasticity-based changes in cognitive control networks.
The EVO group demonstrated a decline in negativity bias that continued after the end of treatment, which was not evident in the PST group. From an affective neuroscience perspective, we believe a continued decline in negativity bias/negative self-referential processing may reflect a change over time in the interaction of the cognitive control system with the functioning of other systems involved in negative self-referential processing (e.g., the default mode network). However, the basis of this interpretation would require evaluation in a larger sample and using additional measures of the cognitive control network and other networks believed to be key to the expression of depression.
We recognize that this proof-of-concept study involves a small sample size, and was clearly not powered to be a noninferiority study. Although our numbers are equal to more than half of those reported by Motter et al. (2016), the generalizability of the findings are limited and these initial results will clearly require replication in a larger cohort. Indeed, the goal of this study was not to differentiate the efficacy of the two approaches used here, but to observe whether they were comparable within a similar time period of treatment. Our sample sizes are sufficient to determine the feasibility of the trial, and to pass a proof-of-concept phase, but are too small to make firm conclusions about the impact of EVO on cognitive control network functions, negativity bias, depression, and disability outcomes. Subsequent work that interrogates the efficacy of different dosages is also warranted, as similar approaches with treatments (such as PST (Arean et al., 2008)) would provide greater insights regarding the optimal usage of EVO in future work. A related concern involves the possibility that the observed results are simply a reflection of selection bias and/or regression to the mean (Berthelot, Le Goff, & Maugars, 2011; McCarney et al., 2007). However, we do not believe this is the case here: we used a similar screening protocol in our previous work (Arean et al., 2008), ensuring comparable standards between this work. More importantly, PST has already been shown to produce effects greater than a supportive therapy control in numerous studies with depressed older adults (Alexopoulos et al., 2011, 2015, 2016; Alexopoulos, Raue, & Arean, 2003; Alexopoulos, Raue, Kanellopoulos, Mackin, & Arean, 2008; Arean et al., 1993, 2008, 2010, 2015; Chu, Huynh, & Arean, 2012; Crabb, Arean, & Hegel, 2012; Gustavson et al., 2016; Mackin et al., 2014; Mackin, Arean, & Elite-Marcandonatou, 2006; Sharpe et al., 2012). Although, we cannot claim that EVO is not significantly worse than PST, we did find the effect sizes for EVO are similar to those found in the literature and is thus worth further study as a depression intervention.
Subsequent work that interrogates the neural correlates of each type of treatment would supplement these findings by highlighting the differential behavioral effects with neural activity measures, and how these patterns of activity relate to each group. If these initial pilot findings are confirmed in larger randomized controlled trials, the use of targeted digital platforms may represent a novel therapeutic advance for the treatment of depression in a population that is notoriously underserved and susceptible to poor response to traditional depression intervention.
Supplementary Material
TABLE 1.
Group demographics
| Project: EVO™ (n = 12) | PST (n = 10) | |
|---|---|---|
| Age | 66.9 (6.8) | 69.4 (5.6) |
| Gender | 3 Males | 3 Males |
| Race# | ||
| White | 7 | 7 |
| Black | 2 | 1 |
| Asian | 1 | 2 |
| Years of education | 15.8 (2.1) | 17.1 (1.4) |
| Currently working | 6 | 1 |
| Marital status | ||
| Married | 6 | 3 |
| Divorced | 2 | 3 |
| Never married | 3 | 3 |
| Widowed | 1 | 1 |
| Baseline depression severity (HAM-D) | 21.50 (3.31) | 25.13 (2.62) |
| Week 4 | 13.75 (6.01) | 16.63 (7.06) |
| Week 8 | 12.54 (6.28) | 15.0 (2.87) |
| Baseline depression severity (PHQ-9) | 12.75 (3.52) | 15.33 (3.94) |
| Week 4 | 7.25 (3.04) | 11.22 (2.53) |
| Week 8 | 8.55 (2.83) | 9.14 (3.63) |
Notes: Mean and StDev presented.
= 2 Project: EVO individuals chose not to answer about their race.
Acknowledgments
We thank C. Han, G. Niu, J. Jordan, and M. Gross for their help with data collection and analysis; A. Piper, E. Martucci, S. Kellogg, J. Bower, and M. Omerick for assistance and insights using the Project: EVO™ platform; G. Niu, R. Crabb, J. Conant, and E. Gillung for guiding our participants through their therapy sessions. Thanks to all of our participants whose time and efforts made this work possible. Support for this research was provided by the National Institute of Mental Health (P.A.A., K24MH074717).
Grant sponsor: National Institute of Mental Health; Contract grant number: K24MH074717.
Abbreviations
- HAM-D
Hamilton Depression Rating Scale
- LLD
late life depression
- PHQ
Patient Health Questionnaire
- PST
problem solving therapy
- TOVA
test of variables of attention
- WHODAS
World Health Organization disability assessment schedule
Footnotes
Clinicaltrials.gov #: NCT02229188 “Games to overcome late life depression”
AUTHOR CONTRIBUTIONS
All authors contributed equally to the conceptualization of the study, the analysis of the data presented, and the composition of the manuscript presented.
CONFLICT OF INTEREST
All authors report no financial interests or potential conflicts of interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Alexopoulos GS. The depression-executive dysfunction syndrome of late life: A specific target for D3 agonists? American Journal of Geriatric Psychiatry. 2001;9(1):22–29. [PubMed] [Google Scholar]
- Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Murphy CF. Anterior cingulate dysfunction in geriatric depression. International Journal of Geriatric Psychiatry. 2008;23(4):347–355. doi: 10.1002/gps.1939. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: A preliminary study. Journal of Affective Disorders. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. American Journal of Geriatric Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- Alexopoulos GS, Manning K, Kanellopoulos D, McGovern A, Seirup JK, Banerjee S, et al. Cognitive control, reward-related decision making and outcomes of late-life depression treated with an antidepressant. Psychological Medicine. 2015;45(14):3111–3120. doi: 10.1017/S0033291715001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue P, Arean P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. American Journal of Geriatric Psychiatry. 2003;11(1):46–52. [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Kanellopoulos D, Mackin S, Arean PA. Problem solving therapy for the depression-executive dysfunction syndrome of late life. International Journal of Geriatric Psychiatry. 2008;23(8):782–788. doi: 10.1002/gps.1988. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: Effect on disability. Archives of General Psychiatry. 2011;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, Kiosses DN, Seirup JK, Banerjee S, Arean PA. Comparing engage with PST in late-life major depression: A preliminary report. American Journal of Geriatric Psychiatry. 2015;23(5):506–513. doi: 10.1016/j.jagp.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Raue PJ, McCulloch C, Kanellopoulos D, Seirup JK, Sirey JA, Arean PA. Clinical case management versus case management with problem-solving therapy in low-income, disabled elders with major depression: A randomized clinical trial. American Journal of Geriatric Psychiatry. 2016;24(1):50–59. doi: 10.1016/j.jagp.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, McLaughlin AC, Trujillo A, Whitlock LA, LaPorte L, Gandy M. Successful aging through digital games. Computers in Human Behavior. 2013;29(4):1302–1306. [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9(3):272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Gazzaley A. Video games, cognitive exercises, and the enhancement of cognitive abilities. Current Opinion in Behavioral Sciences. 2015;4:160–165. [Google Scholar]
- Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: Results from the IMPACT project. Gerontologist. 2008;48(3):311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- Arean PA, Mackin S, Vargas-Dwyer E, Raue P, Sirey JA, Kanellopolos D, et al. Treating depression in disabled, low-income elderly: A conceptual model and recommendations for care. International Journal of Geriatric Psychiatry. 2010;25(8):765–769. doi: 10.1002/gps.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arean PA, Perri MG, Nezu AM, Schein RL, Christopher F, Joseph TX. Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. Journal of Consulting and Clinical Psychology. 1993;61(6):1003–1010. doi: 10.1037//0022-006x.61.6.1003. [DOI] [PubMed] [Google Scholar]
- Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. American Journal of Psychiatry. 2010;167(11):1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arean PA, Raue PJ, McCulloch C, Kanellopoulos D, Seirup JK, Banerjee S, Alexopoulos GS. Effects of problem-solving therapy and clinical case management on disability in low-income older adults. American Journal of Geriatric Psychiatry. 2015;23(12):1307–1314. doi: 10.1016/j.jagp.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji U, Workman P. Critical parameters in targeted drug development: The pharmacological audit trail. Seminars in Oncology. 2016;43(4):436–445. doi: 10.1053/j.seminoncol.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, et al. The influence of perceptual training on working memory in older adults. PLoS One. 2010;5(7):e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot JM, Le Goff B, Maugars Y. The Hawthorne effect: Stronger than the placebo effect? Joint Bone Spine. 2011;78(4):335–336. doi: 10.1016/j.jbspin.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Bredemeier K, Berenbaum H, Brockmole JR, Boot WR, Simons DJ, Most SB. A load on my mind: Evidence that anhedonic depression is like multitasking. Acta Psychologica. 2012;139(1):137–145. doi: 10.1016/j.actpsy.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chu JP, Huynh L, Arean P. Cultural adaptation of evidence-based practice utilizing an iterative stakeholder process and theoretical framework: Problem solving therapy for Chinese older adults. International Journal of Geriatric Psychiatry. 2012;27(1):97–106. doi: 10.1002/gps.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Collier R. Rapid growth forecast for digital health sector. Canadian Medical Association Journal. 2014;186(4):E143. doi: 10.1503/cmaj.109-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb RM, Arean PA, Hegel MT. Sustained adoption of an evidence-based treatment: A survey of clinicians certified in problem-solving therapy. Depression Research and Treatment. 2012;2012:1–15. doi: 10.1155/2012/986547. 986547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study. PLoS Medicine. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB. The Encyclopedia of Clinical Psychology. Columbia University Department of Psychiatry and New York State Psychiatric Institute; U.S.A.: 2015. Structured clinical interview for the DSM (SCID) pp. 1–6. [Google Scholar]
- Greenberg LM. TOVA continuous performance test manual. Los Alamitos, CA: Universal Attention Disorders 1996; 1996. [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. International Journal of Geriatric Psychiatry. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, Alexopoulos GS. MRI signal hyperintensities and treatment remission of geriatric depression. Journal of Affective Disorders. 2010;126(3):395–401. doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson KA, Alexopoulos GS, Niu GC, McCulloch C, Meade T, Arean PA. Problem-solving therapy reduces suicidal ideation in depressed older adults with executive dysfunction. American Journal of Geriatric Psychiatry. 2016;24(1):11–17. doi: 10.1016/j.jagp.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. Brain games fail a big scientific test 2016. 2009 Retrieved from http://www.npr.org/sections/health-shots/2016/10/03/496120962/brain-game-claims-fail-a-big-scientific-test.
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. American Journal of Psychiatry. 2009;166(10):1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press, FL; 1985. [Google Scholar]
- Hilimire MR, Mayberg HS, Holtzheimer PE, Broadway JM, Parks NA, DeVylder JE, et al. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression. Brain Stimulation. 2015;8(2):185–191. doi: 10.1016/j.brs.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang PS. The STAR*D trial: Revealing the need for better treatments. Psychiatric Services. 2009;60(11):1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. Journal of the American Medical Association Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig CE. What is proof of concept research and how does it generate epistemic and ethical categories for future scientific practice? Science and Engineering Ethics. 2016;22(3):735–753. doi: 10.1007/s11948-015-9654-0. [DOI] [PubMed] [Google Scholar]
- Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. Executive dysfunction and disability in elderly patients with major depression. American Journal of Geriatric Psychiatry. 2001;9(3):269–274. [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The Patient health questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. American Journal of Psychiatry. 2002;159(7):1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Arean P, Elite-Marcandonatou A. Problem solving therapy for the treatment of depression for a patient with Parkinson’s disease and mild cognitive impairment: A case study. Neuropsychiatric Disease and Treatment. 2006;2(3):375–379. doi: 10.2147/nedt.2006.2.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RS, Nelson JC, Delucchi K, Raue P, Byers A, Barnes D, et al. Cognitive outcomes after psychotherapeutic interventions for major depression in older adults with executive dysfunction. American Journal of Geriatric Psychiatry. 2014;22(12):1496–1503. doi: 10.1016/j.jagp.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning KJ, Alexopoulos GS, Banerjee S, Morimoto SS, Seirup JK, Klimstra SA, Gunning-Dixon F. Executive functioning complaints and escitalopram treatment response in late-life depression. American Journal of Geriatric Psychiatry. 2015;23(5):440–445. doi: 10.1016/j.jagp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: A randomised, controlled trial. BMC Medical Research Methodology. 2007;7(30):1–8. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Anguera JA, Gazzaley A. Video games for neurocognitive optimization. Neuron. 2016;90(2):214–218. doi: 10.1016/j.neuron.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Mishra J, de Villers-Sidani E, Merzenich M, Gazzaley A. Adaptive training diminishes distractibility in aging across species. Neuron. 2014;84(5):1091–1103. doi: 10.1016/j.neuron.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto SS, Gunning FM, Murphy CF, Kanellopoulos D, Kelly RE, Alexopoulos GS. Executive function and short-term remission of geriatric depression: The role of semantic strategy. American Journal of Geriatric Psychiatry. 2011;19(2):115–122. doi: 10.1097/JGP.0b013e3181e751c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter JN, Pimontel MA, Rindskopf D, Devanand DP, Doraiswamy PM, Sneed JR. Computerized cognitive training and functional recovery in major depressive disorder: A meta-analysis. Journal of Affective Disorders. 2016;189:184–191. doi: 10.1016/j.jad.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Mulder RT, Frampton CM, Luty SE, Joyce PR. Eighteen months of drug treatment for depression: Predicting relapse and recovery. Journal of Affective Disorders. 2009;114(1–3):263–270. doi: 10.1016/j.jad.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clinical EEG and Neuroscience. 2007;38(2):78–81. doi: 10.1177/155005940703800209. [DOI] [PubMed] [Google Scholar]
- NAS. Standrards for psychosocial interventions. Washington, D.C.: NAS; 2015. [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging. 2002;17(1):56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- NIMH. NIMH strategic plan: Strategy 3. 2015 Retrieved from http://www.nimh.nih.gov/about/strategic-planning-reports/strategic-research-priorities/srp-objective-3/index.shtml.
- NIMH. NIMH strategic plan. 2016 Retrieved from https://www.nimh.nih.gov/about/strategic-planning-reports/strategic-research-priorities/index.shtml.
- Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40(6):939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. American Journal of Psychiatry. 2001;158(3):405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Gittins CB, Correia HM, Meade T, Nicholas MK, Raue PJ, Arean PA. Problem-solving versus cognitive restructuring of medically ill seniors with depression (PROMISE-D trial): Study protocol and design. BMC Psychiatry. 2012;12:207. doi: 10.1186/1471-244X-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, et al. Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. American Journal of Psychiatry. 1992;149(8):1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Thompson DG, Kesler SR, Sudheimer K, Mehta KM, Thompson LW, Marquett RM, O’Hara RM. FMRI activation during executive function predicts response to cognitive behavioral therapy in older, depressed adults. American Journal of Geriatric Psychiatry. 2015;23(1):13–22. doi: 10.1016/j.jagp.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Carmody T, Toups M, Zisook S, Lesser I, Rush AJ. Adherence to antidepressant combinations and monotherapy for major depressive disorder: A CO-MED report of measurement-based care. Journal of Psychiatric Practice. 2014;20(2):118–132. doi: 10.1097/01.pra.0000445246.46424.fe. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHODAS II, Version 3.1a Phase. Geneva: World Health Organization; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


