Key Points
High-circulating AREG (≥33 pg/mL) reclassifies additional patients into HR categories and further refines the Minnesota aGVHD risk score.
Patients with aGVHD and AREG ≥33 pg/mL have lower rates of steroid response, higher NRM, and poorer OS.
Abstract
Amphiregulin (AREG) is an epidermal growth factor receptor ligand that can restore integrity to damaged intestinal mucosa in murine models of acute graft-versus-host disease (aGVHD). We previously reported that circulating AREG is elevated in late-onset aGVHD (occurring after 100 days posttransplant), but its clinical relevance in the context of aGVHD risk is unknown. We measured AREG in 251 aGVHD onset blood samples from Blood and Marrow Clinical Trials Network (BMT CTN) primary treatment trials and determined their association with GVHD severity, day 28 complete or partial response (CR/PR) to first-line therapy, overall survival (OS), and nonrelapse mortality (NRM). Every doubling of plasma AREG was associated with a 33% decrease in the odds of day 28 CR/PR (odds ratio [OR], 0.67; P < .01). An AREG threshold of 33 pg/mL or greater divided patients with Minnesota standard-risk (SR) aGVHD into a distinct group with a significantly lower likelihood of: day 28 CR/PR (72% vs 85%; P = .02); greater 2-year NRM (42% vs 15%; P < .01); and inferior OS (40% vs 66%; P < .01). High AREG ≥ 33 pg/mL also stratified patients with Minnesota high-risk (HR) aGVHD: day 28 CR/PR (54% vs 83%; P = .03) and 2-year NRM (53% vs 11%; P < .01), with a trend toward inferior 2-year OS (37% vs 60%; P = .09). High-circulating AREG (≥33 pg/mL) reclassifies patients into HR subgroups and thereby further refines the Minnesota aGVHD clinical risk score.
Visual Abstract
Introduction
Amphiregulin (AREG) is an epidermal growth factor receptor (EGFR) ligand produced by interleukin 33 (IL-33)-dependent type 2 innate lymphoid cells that can restore integrity to damaged intestinal mucosa in murine models of chemical colitis and acute graft-versus-host disease (aGVHD).1,2 AREG is released by a variety of cell types, including regulatory T cells, fibroblasts, keratinocytes, dendritic cells, CD4 T cells, neutrophils, mast cells, eosinophils, and basophils, and is a key component of type 2 immune responses involved in epithelial repair and regeneration after damage.3 We have previously described circulating AREG as a biomarker of aGVHD in humans, where high levels may reflect the host’s response to tissue damage.4 To date, AREG has not yet been evaluated as a risk-stratification biomarker in a large cohort of patients with new-onset aGVHD. To determine the association of circulating AREG with severity of aGVHD, organ involvement, response to therapy, and survival, we tested serum and plasma samples obtained at aGVHD onset from 2 aGVHD first-line treatment trials, Blood and Marrow Clinical Trials Network (BMT CTN) 03025 and 0802.6 We also determined whether incorporating AREG into the revised Minnesota GVHD risk score could further risk-stratify patients.7 The revised Minnesota GVHD risk score was derived from GVHD organ staging in 1723 new-onset aGVHD patients; 84% were classified as standard risk (SR). Complete or partial response (CR/PR) at 28 days after starting first-line systemic therapy was superior in SR (69% response) vs Minnesota high risk (HR; 43% response). In the present study, we tested whether circulating AREG could add prognostic information to the clinical risk assessment using the Minnesota risk score.
Methods
Patient cohorts
Blood samples were obtained at the onset of systemic aGVHD treatment in BMT CTN 0302 (serum) and BMT CTN 0802 (plasma), as previously reported.8 All patients from both studies who had response data and available blood samples were included in this analysis (N = 251). Of the 251 patients, 133 also had biomarker measurements for suppressor of tumorgenicity-2 (ST2) and regenerating islet-derived protein 3-α (REG3α), previously described biomarkers of aGVHD.9 Methodologic changes following the original report on these biomarkers9 yield nonlinear conversion in enzyme-linked immunosorbent assay results for ST2 and REG3α and thus preclude analysis of these biomarkers in all patients due to limited sample availability. Eleven patients in the cohorts from the 2 clinical trials had onset of aGVHD after day 100 (range, days 110-165), and removing their data had no impact on the results (not shown); thus, we studied 96% classic-onset aGVHD and 4% late-onset aGVHD patients enrolled on the 2 prospective BMT CTN trials. Samples from an independent cohort of patients with late-onset aGVHD (N = 92) were obtained from 2 other multicenter studies from the Chronic GVHD Consortium, and the Mount Sinai Acute GVHD International Consortium (MAGIC), as an independent group to test the cut point defined in the samples from BMT CTN 0302/0802. Approval for this secondary analysis was obtained from the BMT CTN and the University of Minnesota Institutional Review Board.
Statistical analysis
The χ2 test was used to perform statistical comparisons across categorical variables. Differences in continuous variables across categories were assessed using the Wilcoxon rank-sum tests for nonparametric data.10 Kaplan-Meier curves were used to estimate the probability of survival censoring patients at last follow-up or 24 months posttransplant, whichever was first. Comparison of curves was assessed by use of the log-rank test.11 The cumulative incidence function12 was used to estimate the probability of nonrelapse mortality (NRM), considering relapse as a competing risk and censoring patients without relapse or mortality at last follow-up or 24 months posttransplant. Statistical comparisons of NRM curves were assessed by use of the Gray test. Simple proportions were used to estimate day 28 response, with the χ2 test used to evaluate the comparisons. Organ staging and grading was performed and adjudicated in BMT CTN 0302 and 0802 according to consensus criteria.13 Clinical risk stratification by Minnesota criteria have been previously reported.7 AREG protein concentrations in serum (0302) and plasma (0802) samples were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions, with an assay sensitivity of 1.4 pg/mL. No sample had an AREG value below the detection limit. All samples were run simultaneously with identical standard curves and sensitivities. Methods for quantification of ST2 and REG3α were previously reported9; protein concentrations of AREG were compared with ST2 and REG3α using Spearman Rho.14
AREG biomarker analysis
The optimal cut point for dichotomized analyses of AREG was based on overall survival (OS) through 24 months posttransplant. This was determined by evaluating all possible cutpoints of AREG and choosing the value that maximized the absolute value of the log-rank statistic. The log-rank statistic was determined using Cox regression for the entire cohort.15 An unbiased estimate of the hazard ratio and its appropriate significance from this cutpoint were determined using twofold cross-validation.16,17 Twofold cross-validation randomly splits the population into 2 equal subsets in which an optimal cut point is obtained in each subset and then applied under the setting of multiple regression analysis. These cut points were then used to define HR and low risk in the opposing subset, leading to the unbiased estimate of the hazard ratio after stratifying the regression analysis by each subset. Subsequent hazard ratios based on the optimal cut point from this method were calculated, controlling for Minnesota risk, donor type (matched sibling vs other related vs unrelated donor [URD] vs umbilical cord blood [UCB]), and age. Age was modeled as a continuous factor but divided by 10 so as to show the respective increase in odds ratios (ORs) or hazards ratios per increase by decade of age. All models were stratified by trial (0302 vs 0802) using the procedure phreg from SAS version 9.4 (SAS Institute Inc, Cary, NC). Other factors considered in the regression analysis included conditioning (reduced-intensity conditioning vs myeloablative), sex (male vs female) and diagnosis (nonmalignant vs myelodysplastic syndrome/leukemia vs lymphoma vs other). Cox regression was used to assess the independent effect of AREG on OS18; Fine and Gray regression was used to assess the independent effect of AREG on NRM.19 In regression analyses, classification by the 4 levels of AREG and Minnesota risk was used to discriminate the impact and significance of the predictive significance of AREG. To further support the association between AREG and our end points, regardless of an optimal cut point, we investigated the continuous effect of AREG on clinical end points after a log transformation base 2, allowing an interpretation of the odds or risk of response per a doubling in the value of AREG. SAS version 9.4 and R version 3.4.3 were used for all statistical analyses.
Results
Characteristics of the patients from BMT CTN 0302/0802 were previously reported.8 Fifty-seven patients (23%) were HR by Minnesota criteria, with no significant difference in the proportion of HR patients between trials (P = .27). The median age of HR aGVHD patients was 6 years younger than the SR patients (47 vs 53 years; P = .01). There was no difference in underlying hematologic disease, donor source, conditioning intensity, or median days to aGVHD onset between HR and SR patients (not shown). The majority of HR aGVHD patients had overall grade 3 aGVHD (9% grade 2, 79% grade 3, and 12% grade 4), whereas the majority of SR aGVHD patients had overall grade 2 aGVHD (19% grade 1, 76% grade 2, and 5% grade 3; P < .01). There was no major skewing of severity within either risk group toward the extremes of staging. Only 4 patients (2%) with Minnesota SR aGVHD had isolated stage II lower gastrointestinal (GI) involvement, whereas 15 Minnesota HR patients (26%) had stage II lower GI involvement plus other organ involvement. Patients with Minnesota HR aGVHD had worse 2-year NRM, in agreement with previous results (supplemental Figure 1).
AREG and aGVHD clinical outcomes
We first examined AREG levels and aGVHD organ involvement. AREG levels were significantly associated with aGVHD involvement of the lower GI tract. Patients with stage II-IV lower GI tract involvement had twofold higher AREG levels at aGVHD onset than those with no lower GI involvement (median 63 vs 30 pg/mL; P < .01). We next looked at AREG and overall clinical severity of aGVHD and found that median AREG levels were 1.7-fold higher in patients with HR aGVHD compared with SR (53.4 vs 31 pg/mL; P < .01; supplemental Figure 2). Only 5 patients in this cohort had isolated upper GI aGVHD, limiting any conclusions about AREG and upper GI aGVHD.
Both the Minnesota risk score and AREG levels at aGVHD diagnosis were significantly associated with day 28 CR/PR, whereas age, donor type, and conditioning intensity were not (Table 1). As a continuous variable, every twofold increase in AREG was associated with a 33% decrease in the odds of day 28 CR/PR (OR, 0.67; 95% confidence interval [CI], 0.56-0.79; P < .01). Only one-third of patients with the highest quartile of AREG (>70.2 pg/mL) had a CR at day 28. Cox regression showed that as a continuous factor, AREG was associated with a 27% increase in risk of mortality through 2 years per each doubling in AREG (HR, 1.27; 95% CI, 1.14-1.40). Using twofold cross-validation, we determined the cutoff at GVHD onset of AREG for survival was 33 pg/mL. In subsequent analyses, an AREG of 33 pg/mL or higher is referred to as high AREG, and lower values are referred to as low AREG.
Table 1.
Factors associated with day 28 response: univariate analysis
| Factor | N | Day 28 CR/PR (%) | OR (95% CI) | P | Day 28 CR (%) | OR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| All patients | 251 | 189 (75) | 129 (51) | ||||
| Trial | .11 | .78 | |||||
| 0302 | 103 | 83 (81) | 54 (52) | ||||
| 0802 | 148 | 106 (72) | 75 (51) | ||||
| Age, y | .51 | .24 | |||||
| <18 | 10 | 8 (80) | 6 (60) | ||||
| 18-34 | 43 | 29 (67) | 19 (44) | ||||
| 35-49 | 56 | 41 (73) | 24 (43) | ||||
| ≥50 | 142 | 111 (78) | 80 (56) | ||||
| Donor type | .07 | .29 | |||||
| HLA-matched sibling | 91 | 72 (79) | 53 (58) | ||||
| Other related | 18 | 17 (94) | 11 (61) | ||||
| URD | 120 | 83 (69) | 55 (46) | ||||
| Mismatched URD | 4 | 2 (50) | 1 (25) | ||||
| UCB | 18 | 15 (83) | 9 (50) | ||||
| Conditioning | .32 | .32 | |||||
| Myeloablative | 161 | 118 (73) | 79 (49) | ||||
| Reduced-intensity | 90 | 71 (79) | 50 (56) | ||||
| Minnesota aGVHD risk | .02 | .01 | |||||
| SR | 194 | 153 (79) | 108 (56) | ||||
| HR | 57 | 36 (63) | 21 (37) | ||||
| AREG | <.01 | .01 | |||||
| First quartile, <19.8 | 62 | 58 (94) | 39 (63) | ||||
| Second quartile, 19.8-33.7 | 62 | 46 (74) | 34 (55) | ||||
| Third quartile, 33.8-70.2 | 64 | 49 (77) | 35 (55) | ||||
| Fourth quartile, >70.2 | 63 | 36 (57) | 21 (33) | ||||
| Continuous variable, OR per doubling in AREG at onset | 0.67 (0.56-0.79) | <.01 | 0.76 (0.65-0.89) | <.01 | |||
| AREG, optimal cut point, pg/mL | <.01 | .01 | |||||
| <33 | 120 | 102 (85) | 72 (60) | ||||
| ≥33 | 131 | 87 (66) | 57 (44) |
Bold values are statistically significant.
Minnesota SR aGVHD and AREG
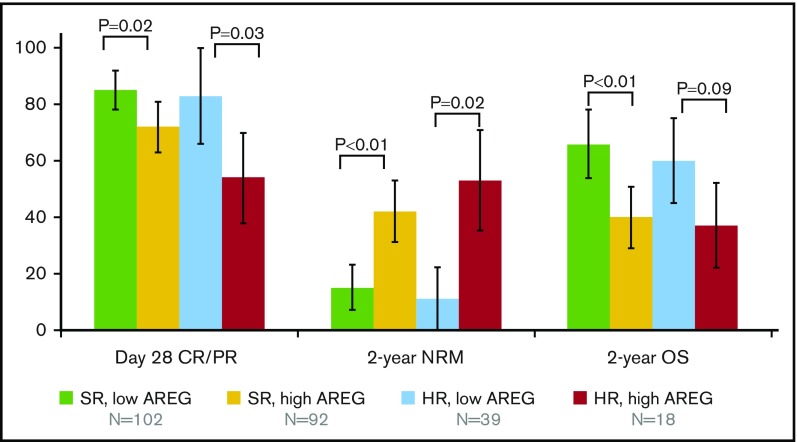
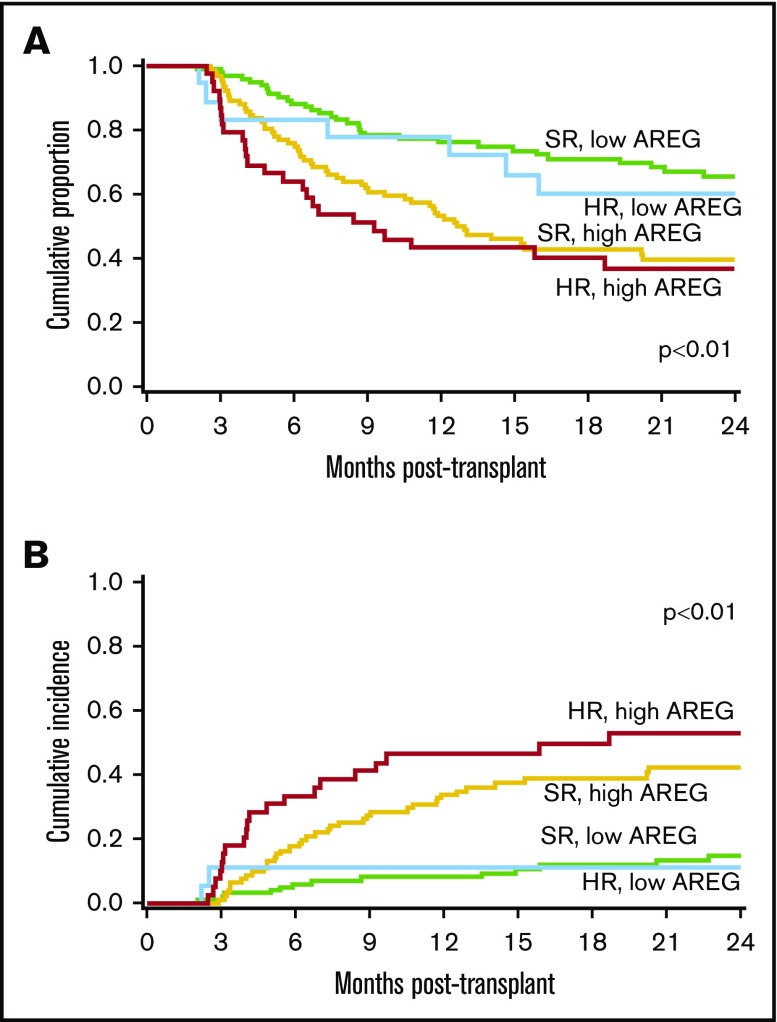
Patients with Minnesota SR aGVHD had an overall 79% day 28 CR/PR (Table 1), and almost half (92 of 194; 47%) of these patients had high AREG. Minnesota SR patients with high AREG had a significantly lower proportion of day 28 CR/PR (72% [95% CI, 63%-81%] vs 85% day 28 CR/PR [78%-92%]; P = .02; Figure 1) than Minnesota SR patients with low AREG. In addition, Minnesota SR patients with high AREG had poorer long-term outcomes with markedly higher 2-year NRM (42% [31%-54%] vs 15% [7%-22%]; P < .01), and lower 2-year OS (40% [29%-50%] vs 66% [55%-74%]; P < .01; Figure 2). In logistic regression for the end point of response, Cox regression on OS and Fine and Gray regression for NRM including the factors of interest, AREG, and Minnesota risk, controlling for donor type and age and stratified by study, we found that AREG was independently associated with a risk of poor clinical outcomes (Table 2; supplemental Figure 3). Among the Minnesota SR patients, patients with high AREG showed a 59% decrease in the odds of day 28 CR/PR (OR, 0.41; 95% CI, 0.20-0.84) when compared with patients with low AREG (reference group). Minnesota SR, high AREG patients had a twofold increased risk of death (hazard ratio, 2.30; 95% CI, 1.48-3.58; P < .01; Table 2) and a 3.6-fold higher risk of 2-year NRM (hazard ratio, 3.6; 95% CI, 1.93-6.78; P < .01) compared with Minnesota SR patients with low AREG (Table 2).
Figure 1.
Proportion of patients (±95% CI) experiencing clinical end points of day 28 CR/PR, 2-year NRM, and OS by Minnesota GVHD SR vs HR and plasma AREG at the onset of aGVHD.
Figure 2.
Patients enrolled in BMT CTN 0302/0802 by AREG plus Minnesota risk score group. (A) OS and (B) NRM.
Table 2.
AREG, Minnesota risk score, and clinical outcomes: multivariate regression
| Factor | N | OR of CR/PR | 95% CI | P | Hazard ratio of 2-y OS | 95% CI | P | Hazard ratio of 2-y NRM | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Minnesota risk/AREG | ||||||||||
| SR, low AREG | 102 | 1.00* | 1.00* | 1.00* | ||||||
| SR, high AREG | 92 | 0.41 | 0.20-0.84 | .02 | 2.30 | 1.48-3.58 | <.01 | 3.62 | 1.93-6.78 | <.01 |
| HR, low AREG | 18 | 0.89 | 0.22-3.61 | .87 | 1.44 | 0.63-3.30 | .39 | 1.14 | 0.23-5.62 | .87 |
| HR, high AREG | 39 | 0.18 | 0.08-0.44 | <.01 | 3.35 | 1.94-5.79 | <.01 | 7.17 | 3.40-15.14 | <.01 |
| Age − (/decade) | 251 | 1.18 | 0.96-1.45 | .11 | 1.20 | 1.05-1.37 | .01 | 1.44 | 1.19-1.74 | <.01 |
| Donor type | ||||||||||
| HLA-matched sibling | 91 | 1.00* | 1.00* | 1.00* | ||||||
| Other related | 18 | 4.77 | 0.58-39.7 | .15 | 1.31 | 0.63-2.72 | .47 | 1.41 | 0.58-3.41 | .45 |
| URD | 124 | 0.58 | 0.30-1.15 | .12 | 1.27 | 0.84-1.91 | .26 | 1.22 | 0.72-2.07 | .46 |
| UCB | 18 | 1.56 | 0.36-6.67 | .55 | 0.72 | 0.32-1.64 | .44 | 1.00 | 0.37-2.71 | .99 |
Bold values are statistically significant.
Reference group.
Minnesota HR aGVHD and AREG
Patients with Minnesota HR aGVHD showed a 63% day 28 CR/PR (Table 1), and over two-thirds (39 of 57; 68%) of Minnesota HR patients had high AREG. Minnesota HR patients with high AREG had significantly lower day 28 CR/PR (54% [95% CI, 38%-70%] vs 83% [95% CI, 66%-100%]; P = .03; Figure 1) and worse 2-year NRM (53% [95% CI, 35-71] vs 11% [0%-25%]; P = .02) than Minnesota HR patients with low AREG. OS at 2 years was also somewhat lower in HR patients with high vs low AREG (37% ([22%-52%] vs 60% [34%-79%]; P = .09 in univariate analysis). In multivariate regression, patients with high AREG and Minnesota HR aGVHD had the worst outcomes (Table 2; supplemental Figure 3). These patients had the lowest likelihood of response (OR, 0.18; 95% CI, 0.08-0.44; P < .01), a more than threefold higher risk of death (hazard ratio, 3.35; 95% CI, 1.94-5.79; P < .01; Figure 2A) and a more than sevenfold higher 2-year NRM (hazard ratio, 7.17; 95% CI, 3.40-15.14; P < .01). By 2 years, over half (53% [95% CI, 35%-71%]) of Minnesota HR high AREG patients experienced NRM, vs only 11% (95% CI, 0%-25%) of Minnesota HR low AREG patients (P < .01; Figure 2B). Patients with high AREG also was associated with worse outcomes among Minnesota HR aGVHD. In a separate analysis using Minnesota HR aGVHD and low AREG as the reference group, patients with Minnesota HR aGVHD and high AREG showed a lower likelihood of response (OR, 0.21; 95% CI, 0.05-0.88; P = .03), trended with a more than twofold higher risk of death (hazard ratio, 2.33; 95% CI, 0.99-5.49; P = .05) and a more than sixfold higher 2-year NRM (hazard ratio, 6.27; 95% CI, 1.26-31.30; P = .03).
AREG cutoff in late-onset aGVHD
Testing this AREG ≥ 33 pg/mL threshold in a separate cohort of 92 late-onset acute GVHD patients,4 we found that high AREG patients had poorer day 28 responses (55% vs 79% day 28 CR/PR; P = .02) and significantly lower 6-month OS (65% with high AREG vs 95% with low AREG; P = .01), although there was no difference in 2-year outcomes in this late-onset cohort (P = .3; supplemental Figure 4).
Association of AREG with other aGVHD biomarkers
We found no significant correlation between AREG and our previously reported circulating angiogenic factors at the onset of aGVHD including: epidermal growth factor, vascular endothelial growth factor-A, follistatin, endoglin, angiopoietin-2, and placental growth factor (Spearman Rho < 0.2 for all comparisons among patients with samples available for testing).8 In 133 patients with samples available for testing, AREG was moderately correlated with previously reported aGVHD biomarkers ST2 (Spearman Rho = 0.47; 95% CI, 0.33-0.59) or REG3α (Spearman Rho = 0.42; 95% CI, 0.26-0.55; supplemental Figure 5).
Discussion
Using samples from 4 different multicenter cohorts (BMT CTN 0302 and 0802, the Chronic GVHD Consortium, and MAGIC), we have identified AREG as a biomarker of aGVHD that is highly associated with clinical outcomes. High AREG concentrations are clinically predictive for worse day 28 response, OS, and NRM. Furthermore, a threshold of AREG ≥ 33 pg/mL further refined the Minnesota clinical risk score. Almost half (92 of 194; 47%) of Minnesota SR patients had high AREG levels, and these patients had a low likelihood of steroid-response and poor survival. Nearly a third (18 of 57; 32%) of Minnesota HR patients had low AREG levels, and these patients had a higher likelihood of steroid response. Patients with either Minnesota HR aGVHD or with high AREG have a low likelihood of response to steroids, poor NRM, and are in great need of novel therapies. If validated in additional large series, measurement of AREG at aGVHD onset may add value in risk-stratifying patients with aGVHD. Patients from the cohorts analyzed in this study are heterogeneous, including different ages, diseases, conditioning regimens, and treatments for aGVHD, which likely adds to the external validity of our results.
The cellular source of circulating AREG in severe aGVHD is not yet known, although we hypothesize the degree of AREG elevation reflects the host’s immune response to the severity of tissue damage caused by aGVHD. Circulating AREG was most strikingly elevated in Minnesota HR aGVHD, particularly in those with stage II-IV lower GI involvement. However, AREG was not strongly correlated with REG3α, an intestinal-specific biomarker, suggesting that circulating AREG may not be solely of gut origin.
Clinical aGVHD severity as determined by the Minnesota GVHD risk score and risk as determined by GVHD biomarkers ST2 and REG3α are currently being used to classify patients as SR or HR cohorts for GVHD treatment trials, including BMT CTN 1501 for SR patients (clinicaltrials.gov #NCT02806947). The Minnesota GVHD risk score is immediately available to the clinician (https://redcap.ahc.umn.edu/surveys/?s=bNmFhseJIf) and is readily applied using only bedside measures. It is an informative first step in personalizing aGVHD therapy, where SR patients may be candidates for less toxic investigational approaches and HR patients are candidates for investigational approaches (such as www.clinicaltrials.gov #NCT02525029).20 However, given that some patients may have extensive tissue damage or inflammatory responses that are underestimated by clinical symptoms, GVHD biomarkers may show their greatest clinical utility in identifying SR patients who are likely to have poor outcomes. In the present study, we observed that AREG reclassified nearly half of SR patients into a low steroid response, high NRM category. The GVHD prognostic algorithm in current use in clinical trials uses the levels of 2 validated GVHD biomarkers, ST2 and REG3a, for risk stratification.9,21-23 The limited correlation of AREG with ST2 and REG3α suggests that AREG levels are at least partially independent prognostic markers. This concept is supported by the observation that ST2 and REG3a are primarily derived from stromal and epithelial cell populations of the intestine, although the endothelium also contributes to ST2 levels.24 Therefore, it is possible that AREG may improve upon the current GVHD prognostic algorithm, either as a stand-alone predictor or in combination with the other biomarkers. This is a topic for future studies.
Beyond its potential clinical utility as a risk-stratification biomarker, our observation of elevated AREG in HR aGVHD lends further evidence to support the notion that aGVHD outcomes may not only be determined biologically by the donor T-cell–mediated attack, but also by the host response to that attack. The biology of wound healing may well be relevant to outcomes of aGVHD. With accumulating evidence of altered EGFR ligands in aGVHD,4,8,25,26 further investigation into epithelial repair pathways to overcome poor steroid response is needed. Novel, targeted regenerative therapies, such as systemic delivery of epithelial growth factors, or delivery of modified probiotics as luminal “factories” for such growth factors,27,28 should be tested as adjunctive therapies to standard immunosuppressive approaches in patients with Minnesota HR aGVHD, and in Minnesota SR aGVHD with elevated ST2 and REG3α (ie, AA3 risk) or those with AREG ≥ 33 pg/mL.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Michael Ehrhardt of the University of Minnesota Cytokine Reference Laboratory for technical assistance.
The ancillary study was supported by the University of Minnesota, Masonic Cancer Center. Support for Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0302 was provided by grant U10HL069294 to the BMT CTN from the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) and National Cancer Institute (NCI), along with contributions by Eisai Inc, Hospira Inc, Roche Laboratories Inc, and Immunex Corporation, a wholly owned subsidiary of Amgen Inc. Support for BMT CTN 0802 was provided by grant U10HL069294 to the BMT CTN from the NHLBI and NCI. Support for samples from the Chronic GVHD Consortium was provided by NCI grants CA163438 and CA118953. Support for samples from MAGIC was NCI grant CA039542.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the above-mentioned parties.
Footnotes
Presented as an oral abstract at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
Authorship
Contribution: S.G.H., J. Pidala, and M.L.M. conceived and designed the study, collected, assembled, analyzed, and interpreted data, and wrote and approved the final manuscript; T.E.D., N.K., S.M., M.A., C.S.C., A.L., L.F.N., A.S.R., G.Y., W.J.H., J.B.-M., V.H., A.A., and D.J.W. analyzed and interpreted data, and wrote and approved the final manuscript; A.P.-M., S.J.L., Y.I., and J.L.M.F. collected, assembled, analyzed, and interpreted data, and wrote and approved the final manuscript; J.E.L., M.E.D.F., G.L.C., J. Palmer, S.A., M.H.J., I.P., E. Hexner, F.A., E. Holler, U.B., Y.A.E., A.H., and J.W. collected and assembled data, and wrote and approved the final manuscript; W.A.W. conceived and designed the study, and wrote and approved the final manuscript; and B.R.B. conceived and designed the study, analyzed and interpreted data, and wrote and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shernan G. Holtan, Blood and Marrow Transplant Program, University of Minnesota, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: sgholtan@umn.edu.
References
- 1.Bruce DW, Stefanski HE, Vincent BG, et al. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J Clin Invest. 2017;127(5):1813-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112(34):10762-10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42(2):216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtan SG, Khera N, Levine JE, et al. Late acute graft-versus-host disease: a prospective analysis of clinical outcomes and circulating angiogenic factors. Blood. 2016;128(19):2350-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alousi AM, Weisdorf DJ, Logan BR, et al. ; Blood and Marrow Transplant Clinical Trials Network. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolanos-Meade J, Logan BR, Alousi AM, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtan SG, Verneris MR, Schultz KR, et al. Circulating angiogenic factors associated with response and survival in patients with acute graft-versus-host disease: results from Blood and Marrow Transplant Clinical Trials Network 0302 and 0802. Biol Blood Marrow Transplant. 2015;21(6):1029-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine JE, Braun TM, Harris AC, et al. ; Blood and Marrow Transplant Clinical Trials Network. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21-e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583-621. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 12.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 14.Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15(1):72-101. [PubMed] [Google Scholar]
- 15.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253-270. [Google Scholar]
- 16.Mazumdar M, Smith A, Bacik J. Methods for categorizing a prognostic variable in a multivariable setting. Stat Med. 2003;22(4):559-571. [DOI] [PubMed] [Google Scholar]
- 17.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med. 1996;15(20):2203-2213. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187-220. [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 20.Holtan SG, MacMillan ML. A risk-adapted approach to acute GVHD treatment: are we there yet? Bone Marrow Transplant. 2016;51(2):172-175. [DOI] [PubMed] [Google Scholar]
- 21.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369(6):529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118(25):6702-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartwell MJ, Özbek U, Holler E, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2(3):e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Ramadan AM, Griesenauer B, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;7(308):308ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He F, Verneris MR, Cooley S, et al. Low day +100 serum epidermal growth factor levels are associated with acute GvHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(2):301-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtan SG, Newell LF, Cutler C, et al. Low EGF in myeloablative allotransplantation: association with severe acute GvHD in BMT CTN 0402. Bone Marrow Transplant. 2017;52(9):1300-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi HJ, Ahn JH, Park SH, Do KH, Kim J, Moon Y. Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics. Infect Immun. 2012;80(3):1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Xu S, Lin Y, et al. Recombinant porcine epidermal growth factor-secreting Lactococcus lactis promotes the growth performance of early-weaned piglets. BMC Vet Res. 2014;10(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.