ABSTRACT
Beta-keratin in poultry feathers is a structural protein that is resistant to degradation due to disulfide and hydrogen bonds. Feather meal can be a valuable feed compound if the digestibility can be increased. The objective of the present study was to analyze the effects of chemical, enzymatic, and pressure-thermic treatments for chicken feathers on solubility, in vitro protein digestibility (IVPD), and amino acid composition of solubilized and residual fractions. Two experiments were conducted. In experiment 1, models for solubility and IVPD were developed including the above factors applying a central composite face-centered design. Addition of sodium hydroxide (NaOH) and sodium sulfite (Na2SO3), and autoclaving time affected solubility and IVPD of the feather hydrolysates, but not addition of keratinolytic enzyme. In experiment 2, 7 combinations of the hydrolysis factors NaOH, Na2SO3, and autoclaving time with a predicted IVPD of 900 g/kg of DM, calculated for the sum of solubilized and residual feather fractions, were included to measure effects on IVPD and amino acid composition in each fraction. The IVPD values were higher for solubilized than residual fractions when treated with NaOH and autoclaving, but no differences were found when treated with Na2SO3 and autoclaving. Losses of cystine were substantial for all treatments, but lower for Na2SO3 than for NaOH. Furthermore, use of lower Na2SO3 concentration and longer autoclaving time reduced losses of cystine. Compared with NaOH treatments, Na2SO3 gave lower losses of threonine, arginine, serine, and tyrosine. With reference to the ideal protein profile for Atlantic salmon (Salmo salar L.), the treatments with 60 or 90 min autoclaving and 0.36 or 0.21% Na2SO3 had the highest chemical scores. The scores were generally higher for amino acids in residual than solubilized fractions, but with 90 min autoclaving and 0.21% Na2SO3 differences were small. In conclusion, hydrolysis of chicken feathers with low concentrations of Na2SO3 combined with autoclaving results in feather meal with high nutritional value for Atlantic salmon; separation of solubilized and residual fractions is not necessary.
Keywords: beta-keratin, keratinolytic protease, sodium hydroxide, sodium sulfite, Atlantic salmon
INTRODUCTION
Poultry feathers are animal by-products with a high protein content. Approximately 900 g/kg of the feather dry matter (DM) consists of beta-keratin, a structure protein rich in the essential amino acids (AA) leucine, valine, arginine, isoleucine, phenylalanine, and threonine, but with smaller proportions of lysine, methionine, histidine, and tryptophan (Yokote et al., 2007; Bandegan et al., 2010). The sulfur-containing cysteine and methionine (Glem-Hansen, 1980), together with threonine, tyrosine, and phenylalanine, are important for the synthesis of hair and feather keratin; arginine plays an important role in the urea cycle of cats (Morris and Rogers, 1978). However, a surplus of AA such as valine and isoleucine in feed can be toxic for some animals. In some countries, feather meal is used as a feed component for animals such as pigs, pets, fish, poultry, and ruminants, but due to the unbalanced AA composition, feather meal can only be a complementary feedstuff in diets for monogastrics (Papadopoulos et al., 1986) and supplementation with lysine, histidine, and other AA may be required. Because of disulfide bonds, hydrogen bonds, and hydrophobic interactions between AA, feather keratin is insoluble in water and has a low digestibility with enzymes such as pepsin (Papadopoulos et al., 1986). The digestibility of feathers can be improved and AA made biologically available through cleaving the bonds by pressure-thermic treatments, chemical hydrolysis, and steam explosion (Zhang et al., 2014), and by action of keratinolytic microorganisms and keratinolytic enzymes (Gupta et al., 2013; Lasekan et al., 2013). Degradation of keratin, as a result of pressure-thermic treatment, which is typically applied in commercial production of feather meal, is often accompanied by a decrease in cystine content and processing may also decrease the digestibility or availability of AA in general (Moritz and Latshaw, 2001). A growing volume of studies suggests improved digestibility, lower AA losses, and decreased energy requirements for cooking if enzymatic hydrolysis is included in the process (Gupta and Ramnani, 2006; Pedersen et al., 2012). Applying more gentle treatments to feathers has a potential to decrease losses of valuable AA and increase the digestibility of feather meal.
According to EU regulations, hydrolyzed proteins derived from parts of non-ruminants such as feather meal may be used as feed for non-ruminant farmed animals and aquaculture animals (EC, 2013). In 2013, 109 million tonnes of poultry meat were produced worldwide (FAOSTAT, 2017). Assuming a meat yield of 460 g/kg body weight and a feather proportion 75 g/kg of body weight (Owens et al., 2001) in the live birds, the estimated potential yield of poultry feathers accounted for 17.8 million tonnes worldwide in 2013. However, only a small proportion of poultry feather are processed to feather meal in Europe and the USA, about 175,000 and 600,000 tonnes of feather meal are produced annually, respectively (Swisher, 2012). Feather meal produced by pressure-thermic treatment has a typical protein content of over 800 g/kg of DM with a large variation in true AA digestibility depending on the processing method (Wang and Parsons, 1997). In the study of Moritz and Latshaw (2001), increased pressure from 2.1 to 5.2 bar for 36 min at 149°C increased in vitro pepsin (0.2%) digestible protein from 704 to 938 g/kg, but true available AA content, determined in White Leghorn cockerels, was reduced for most AA. In the same study, the content of cystine decreased with increased pressure during cooking and was converted to lanthionine, whereas other AA were less affected.
Efforts have been made in testing alternative treatments to increase digestibility without diminishing effects on content and availability of essential AA such as cystine (Moritz and Latshaw, 2001). Chemical hydrolysis has been studied with sodium hydroxide (NaOH), potassium hydroxide (KOH) (Mokrejs et al., 2011), or calcium hydroxide (Ca(OH)2) (Coward-Kelly et al., 2006), sodium sulfite (Na2SO3), and phosphoric acid (H3PO4) (Steiner et al., 1983). Furthermore, enzymatic hydrolysis with commercial proteases or supernatants of keratinolytic microorganisms (Tiwary and Gupta, 2012), microbial fermentation (Elmayergi and Smith, 1971), and physical extractions, by steam-flash explosion (Zhang et al., 2014) or other processes where the pressure decreases suddenly (Ferrer et al., 1999) have been tested. In most research aiming to increase the digestibility of chicken feathers, the focus has been on utilizing hydrolyzed feathers without separating solubilized and residual fractions (Steiner et al., 1983; Papadopoulos et al., 1986; Moritz and Latshaw, 2001; Grazziotin et al., 2006; Mukesh Kumar et al., 2012; Zhang et al., 2014). Others have analyzed either solubilized (Coward-Kelly et al., 2006) or residual feathers (Kim et al., 2002; Łaba and Szczekała, 2013). To our knowledge, little attention has been paid to the different characteristics of solubilized and residual fractions of feather hydrolysates.
The objective of the present study was to measure and analyze the effects of combinations of chemical, enzymatic, and pressure-thermic treatments for chicken feathers on solubility, in vitro pepsin digestibility (IVPD), and AA composition of solubilized and residual fractions, and to evaluate the usability as feed for Atlantic salmon (Salmo salar L.).
MATERIALS AND METHODS
Two experiments were conducted to study the effects of hydrolysis treatments on IVPD and AA composition of solubilized and residual feather fractions. Experiment 1 was designed to model the effects of chemical, enzymatic, and pressure-thermic hydrolysis on solubility and IVPD. Sodium hydroxide and Na2SO3 were selected as chemical agents, and 2 commercial enzymes were tested. Experiment 2 was designed to verify the model, which was established from data achieved in experiment 1, and to study effects on IVPD and AA composition in a series of treatments predicted to produce constant IVPD values for the sum of solubilized and residual fractions of hydrolyzed feathers (hereafter referred to as total IVPD).
Data Availability: All relevant raw data are within the paper and its supporting information files (http://dx.doi.org/10.17632/p62xptkt4j.1).
Materials
Feathers from white broiler chicken (Gallus gallus domesticus, breed Ross 308) were collected at a slaughterhouse in Eidsberg municipality in Norway (Nortura SA, Oslo, Norway). Chicken slaughtered at Nortura are typically 50 d old at slaughtering and feathers are removed mechanically, transported in a water bath, and collected after the water is removed mechanically. Feathers were stored frozen (–20°C) until experimental use. In experiment 1, the feathers were washed (by hand in tap water), sterilized in an autoclave (2 bar, 121°C, 15 min), dried (at 45°C for 48 h), and kept frozen (–20°C). In experiment 2, the feathers were washed, dried, and kept frozen. Prior to experiment 1, the feathers were milled in an ultrafine friction grinder (MKCA6–2, Masuko Sangyo Co. Ltd, Japan). The grinder was equipped with MKC type stainless steel fillings. During grinding the feathers were fed into the hopper and forced through a gap between rotary and stator grinding plates. Feathers were ground successively with gap widths of 7, 2, and 1 mm. Dry matter content of the milled feathers was 947 g/kg. In experiment 2, whole feathers with a DM content of 957 g/kg and a fat content of 22.6 g/kg of DM were used. These feathers were not autoclaved or ground in order to have a more realistic approach. Commercial feather meal, GoldMehl FM (GePro, Diepholz, Germany) was used as a reference in the digestibility studies. Two commercial enzymes, both described by the producers to be efficient in hydrolyzing feathers, were compared in experiment 1. Cibenza IDN900 was kindly donated by Novus International, Inc. (St. Charles, MO). The product contains sodium sulfate, dried Bacillus licheniformis fermentation solubles, mineral oil, and natural flavor. The producer stated a minimum enzyme activity of 1.1 mkat/g. NovoProD was kindly donated by Novozymes, Bagsværd, Denmark. This product is a non-specific protease, and contains subtilisin initially obtained from B. subtilis. We found an activity of 12.6 μkat/g at pH 7.5 with casein. Na2SO3 was obtained from BDH Prolabo (VWR International, Pty Ltd., Tingalpa, Australia) and porcine pepsin (activity 167 μkat/g with casein) was obtained from Sigma (Sigma-Aldrich Co. LLC., St. Louis, MO).
Design of Experiment 1
Response surface methodology was used to map the effects of a set of hydrolysis factor levels (NaOH, Na2SO3, enzymes, pressure-thermic treatment time) on solubility and IVPD of the products. A central composite face-centered design with 4 factors and 3 levels of each factor was applied to study second-order response surfaces (Table 1). A total of 27 runs were conducted in 2 replicates. The measured effects included solubility, IVPD in solubilized and residual fractions, and total IVPD was calculated. The treatments were performed on 3 subsequent days, where day 1 comprised the runs with boiling for 30 min, day 2 autoclaving (2.4 bar, 133°C) for 60 min, and day 3 autoclaving (2.4 bar, 133°C) for 120 min.
Table 1.
Coded, actual, and observed levels in the central composite design matrix of experiment 1 including 4 factors and 3 levels of each factor applied to study second-order response surfaces on pH, DM solubility, and IVPD.
| Coded level | Actual level | Observed level | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVPD | |||||||||||||
| Run | χ1 | χ2 | χ3 | χ4 | NaOH, % (v/v) | Na2SO3,% (v/v) | Enzyme, % (v/v)1 | AC time, min2 | pH | DM solubility, g/kg of DM | Solubilized fraction, g/kg of DM | Residual fraction, g/kg of DM | Total, g/kg of DM |
| 1 | –1 | –1 | –1 | –1 | 0 | 0 | 0 | 0 | 6.3 | 30 | 735 | 158 | 169 |
| 2 | 1 | –1 | –1 | –1 | 0.5 | 0 | 0 | 0 | 12.4 | 577 | 850 | 395 | 654 |
| 3 | –1 | 1 | –1 | –1 | 0 | 0.25 | 0 | 0 | 7.4 | 62 | 846 | 294 | 328 |
| 4 | 1 | 1 | –1 | –1 | 0.5 | 0.25 | 0 | 0 | 12.5 | 588 | 877 | 384 | 679 |
| 5 | –1 | –1 | 1 | –1 | 0 | 0 | 1 | 0 | 7.0 | 40 | 808 | 215 | 232 |
| 6 | 1 | –1 | 1 | –1 | 0.5 | 0 | 1 | 0 | 12.4 | 650 | 860 | 446 | 710 |
| 7 | –1 | 1 | 1 | –1 | 0 | 0.25 | 1 | 0 | 7.4 | 121 | 853 | 258 | 331 |
| 8 | 1 | 1 | 1 | –1 | 0.5 | 0.25 | 1 | 0 | 12.5 | 597 | 887 | 389 | 693 |
| 9 | –1 | –1 | –1 | 1 | 0 | 0 | 0 | 120 | 6.8 | 136 | 963 | 658 | 692 |
| 10 | 1 | –1 | –1 | 1 | 0.5 | 0 | 0 | 120 | 9.9 | 953 | 920 | 632 | 889 |
| 11 | –1 | 1 | –1 | 1 | 0 | 0.25 | 0 | 120 | 7.0 | 230 | 939 | 890 | 899 |
| 12 | 1 | 1 | –1 | 1 | 0.5 | 0.25 | 0 | 120 | 9.9 | 993 | 922 | 682 | 925 |
| 13 | –1 | –1 | 1 | 1 | 0 | 0 | 1 | 120 | 6.8 | 138 | 970 | 751 | 758 |
| 14 | 1 | –1 | 1 | 1 | 0.5 | 0 | 1 | 120 | 9.9 | 960 | 919 | 678 | 897 |
| 15 | –1 | 1 | 1 | 1 | 0 | 0.25 | 1 | 120 | 7.2 | 271 | 949 | 875 | 894 |
| 16 | 1 | 1 | 1 | 1 | 0.5 | 0.25 | 1 | 120 | 9.9 | 1024 | 915 | 630 | 947 |
| 17 | –1 | 0 | 0 | 0 | 0 | 0.125 | 0.5 | 60 | 6.9 | 142 | 919 | 841 | 834 |
| 18 | 1 | 0 | 0 | 0 | 0.5 | 0.125 | 0.5 | 60 | 10.2 | 971 | 899 | 618 | 886 |
| 19 | 0 | –1 | 0 | 0 | 0.25 | 0 | 0.5 | 60 | 9.7 | 755 | 961 | 695 | 877 |
| 20 | 0 | 1 | 0 | 0 | 0.25 | 0.25 | 0.5 | 60 | 9.8 | 860 | 947 | 724 | 916 |
| 21 | 0 | 0 | –1 | 0 | 0.25 | 0.125 | 0 | 60 | 9.7 | 800 | 945 | 705 | 889 |
| 22 | 0 | 0 | 1 | 0 | 0.25 | 0.125 | 1 | 60 | 9.8 | 820 | 947 | 686 | 897 |
| 23 | 0 | 0 | 0 | –1 | 0.25 | 0.125 | 0.5 | 0 | 12.0 | 208 | 820 | 286 | 394 |
| 24 | 0 | 0 | 0 | 1 | 0.25 | 0.125 | 0.5 | 120 | 9.5 | 872 | 941 | 735 | 901 |
| 25 | 0 | 0 | 0 | 0 | 0.25 | 0.125 | 0.5 | 60 | 9.8 | 819 | 940 | 693 | 890 |
| 26 | 0 | 0 | 0 | 0 | 0.25 | 0.125 | 0.5 | 60 | 9.7 | 809 | 952 | 699 | 894 |
| 27 | 0 | 0 | 0 | 0 | 0.25 | 0.125 | 0.5 | 60 | 9.8 | 821 | 927 | 729 | 878 |
| N-5 | 0 | 0 | 1 | 0 | 6.1 | 56 | 842 | 156 | 189 | ||||
| N-13 | 0 | 0 | 1 | 120 | 6.6 | 121 | 993 | 639 | 658 | ||||
| N-16 | 0.5 | 0.25 | 1 | 120 | 10.0 | 992 | 913 | 642 | 915 | ||||
| N-25 | 0.25 | 0.125 | 0.5 | 60 | 9.7 | 785 | 940 | 709 | 869 | ||||
1Cibenza IDN900 in runs 1 to 27; NovoProD in runs N-5 to N-25; runs N-5 to N-25 were not used in modeling.
2AC = autoclaving, 2.4 bar, 133°C.
IVPD, in vitro protein digestibility.
Chemical Hydrolysis
Twenty grams of milled feathers were placed in 1,000 mL plastic bottles and mixed with pre-heated (80°C) stock solutions of NaOH, Na2SO3, and de-ionized water adding up to 400 mL according to the experimental plan. The bottles were placed in a water bath heated to 80°C for 60 min. During the treatment, the temperature in the bottles varied between 71 and 75°C. Thereafter, the bottles were chilled in an ice bath to 55 to 58°C to prepare for the enzymatic treatment step.
Enzymatic Hydrolysis
Cibenza IDN900 or NovoProD were dissolved in water with continuous steering overnight to a stock solution of 2%. The enzyme solution and de-ionized water were added to the bottles, resulting in enzyme concentrations of 0, 0.5, and 1.0% (w enzyme/w feather). The bottles were continuously agitated in a shaker (INFORS HT Ecotron, Infors AG, Bottmingen, Switzerland) at 55°C with 100 rpm for 60 min, before heating or autoclaving.
Pressure-thermic Hydrolysis
In the treatments without autoclaving, enzyme activity was stopped by heating the bottles in boiling water for 30 min. After 10 min the temperature in the bottles was 70°C. Autoclaving was conducted in a steam sterilizer (GE 6610, Getinge Sterilization AB, Gothenburg, Sweden) for 60 or 120 min at 133°C and 2.4 bar.
After heating or autoclaving, the bottles were chilled in an ice bath prior to separation of solubilized and residual fractions by centrifugation. Four of the runs with Cibenza IDN900 (runs 5, 13, 16, 25) were also conducted with NovoProD (runs N-5, N-13, N-16, N-25) to compare the effects of these enzymes.
Design of Experiment 2
The model for total IVPD achieved in experiment 1 was used to design treatments with predicted total IVPD of 900 g/kg of DM (Table 2). We aimed to develop feather meals with high digestibility compared to commercial feather meals. However, maximizing the total IVPD implies a risk of degrading AA. Solubility, IVPD, and concentrations of N, C, Na, S, and ash were measured in solubilized and residual material with the aim to detect the fraction with highest feed value.
Table 2.
Hydrolysis treatments in experiment 2 with a predicted total IVPD of 900 g/kg of DM (n = 2).
| Treatment | NaOH, % | Na2SO3, % | AC time, min1 |
|---|---|---|---|
| 1 | 0.528 | 0.000 | 30 |
| 2 | 0.343 | 0.000 | 60 |
| 3 | 0.274 | 0.000 | 90 |
| 4 | 0.000 | 0.693 | 30 |
| 5 | 0.000 | 0.357 | 60 |
| 6 | 0.000 | 0.210 | 90 |
| 7 | 0.210 | 0.219 | 60 |
1Autoclaving at 2.4 bar, 133°C.
IVPD, in vitro protein digestibility.
Adding enzyme had no effect on solubility or digestibility in experiment 1 and was therefore not included in experiment 2. Thirty grams of unmilled and unsterilized feathers were placed in 1,000 mL plastic bottles and mixed with pre-heated (80°C) stock solutions of NaOH, Na2SO3, and de-ionized water adding up to 400 mL. The bottles were placed in a water bath heated to 85°C for 60 min. After the chemical treatment, the bottles were autoclaved for 30, 60, or 90 min at 133°C and 2.4 bar and thereafter chilled in an ice bath to 40 to 45°C before centrifugation and separation.
Separation of Solubilized and Residual Fractions
In both experiments, solubilized and residual fractions were separated by centrifugation (Sorvall RC 12BP, Thermo Fisher Scientific Inc., Waltham, MA; 3,963 × g for 15 min, ambient temperature) and the liquid phase was poured into separate containers. The pH in the liquid fraction was measured (Knick pH-Meter 766, Calimatic, Knick Elektronische Messgeräte GmbH & Co. KG, Berlin, Germany) and both fractions were freeze dried (Christ Epsilon 2–25 DS, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) and weighed. The solubilized fraction was crushed with a spoon and stored in tight plastic bags at –20°C until analysis. The residual fraction was ground through a 0.5-mm screen (Fritsch Pulverisette 14, Idar-Oberstein, Germany) and stored in tight plastic bags at –20°C until analysis. Separation of soluble and non-soluble fractions is denoted fractionation in the following.
Analytical Measurements
In vitro pepsin digestibility was analyzed according to the AOAC method 971.09 (AOAC International, 2012) with some modifications. Briefly, 2 parallels of 0.5 g were incubated in a 2 mg pepsin/mL solution for 16 h at 40°C in 2 M hydrochloric acid (HCl). After digestion, samples were centrifuged (3,230 × g for 20 min) and separated. Both fractions (solubilized and residual) were then dried at 55°C overnight. The remaining moisture content in the samples was determined gravimetrically after drying at 105°C until constant weight of samples was achieved (typically 24 h). Ash content was determined after heating dry samples at 590°C for 12 h. Total N and C were determined by CHNS-O elemental combustion system (ECS 4010, Costech Analytical Technologies Inc., Valencia, CA) in 4 parallels (experiment 1 only). Concentrations of Na were analyzed by inductively coupled plasma atomic emission spectrophotometry after dry ashing according to the AOAC method 999.11, and S by inductively coupled plasma atomic emission spectrophotometry after microwave oven digestion under pressure according to the AOAC method 991.10 (AOAC International, 2012). Fat content was analyzed by acid hydrolysis (Soxtec System, Foss Analytical, Denmark). The AA concentrations in freeze-dried ground samples were analyzed by a high-performance liquid chromatography system (Agilent Infinity 1260, Agilent Technologies, Santa Clara, CA) coupled to an online post-column derivatization module (Pinnacle PCX, Pickering laboratories, Mountain View, CA), using nynhydrin (Trione) as a derivatizing reagent and a Na+ exchange column (4.6 × 110 mm, 5 μm). Amounts of AA, taurine, and ammonium (NH4+) were quantified from standard curves. Prior to the analysis, the samples were hydrolyzed in 6 M hydrochloric acid containing 0.4% merkaptoethanol for 24 h at 110°C. Glutamine and asparagine were converted to glutamic and aspartic acid. Cysteine was quantified in its dipeptide form, cystine, but cysteine and its oxidation products could not be detected. The samples were filtered by microfilter, the pH was adjusted to 2.2, and the samples were further diluted with a citrate buffer (pH 2.2) for the high-performance liquid chromatography analysis. Tryptophan was analyzed, but the method was not optimized for that AA.
Calculations
Dry matter solubility was calculated as DM yield of solubilized feather hydrolysates divided by the initial sample weight (DM). For calculation of N and C solubility, the weights were multiplied with the concentrations of N or C. The IVPD was calculated as weight of the initial sample subtracted the weight of the residual fraction after dissolution in pepsin and HCl, divided by weight of the initial feather sample. Total IVPD was calculated as sum of IVPD for the solubilized and the residual fractions from feather hydrolysis, weighted by the proportions of solubilized and residual fractions measured in the hydrolysis step. Recovery of a specific AA was calculated as: Yield of solubilized feather hydrolysates multiplied with concentration of the AA in the solubilized fraction added the yield of residual feather hydrolysates multiplied with the concentration of the AA in the residual fraction, divided by initial feather weight multiplied with the concentration of total AA. Dietary protein quality was assessed by determining its chemical score, i.e., the ratio of the limiting AA in the tested feed (g/16 g of N) divided by the AA in ideal protein for the specific animal (g/16 g of N), multiplied with 100.
Statistical Analysis
In experiment 1, the results were modeled using multiple linear regression procedures of the MODDE statistical software (version 11.0.1, Umetrics AB, Umeå, Sweden). The replicated center-point experiments (runs 25, 26, and 27) were used to estimate the replicative error. For each measured characteristic (solubility, pH, IVPD of solubilized and residual fraction), and calculated total IVPD, a complete model with all linear, interaction, and quadratic terms was first developed. Then, statistically insignificant terms were removed to maximize the level of prediction (Q2) and the goodness of fit (R2). The model was considered good if Q2 > 0.5 and the difference between R2 and Q2 < 0.2 to 0.3 (Eriksson et al., 2008). The quadratic model of the system is presented in Eq. 1:
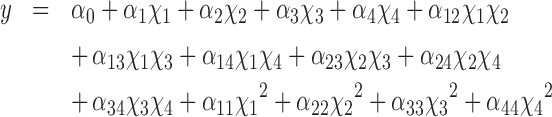 |
(1) |
where y is the predicted response; α0 is a constant coefficient (intercept); α1, α2, α3, and α4 are linear effects; α12, α13, α14, α23, α24, and α34 are interaction effects; and α11, α22, α33, and α44 are quadratic effects, whereas χ1, χ2, χ3, and χ4 are the independent variables NaOH concentration (%), Na2SO3 concentration (%), enzyme concentration (%), and autoclaving duration (min).
In experiment 2, solubility, total IVPD, and recovery of AA (Eq. 2), and chemical composition and proportions of AA (Eq. 3) were analyzed using the mixed model procedure in SAS (SAS Institute Inc., 2016).
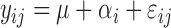 |
(2) |
where y was the individual dependent variable (n = 14); μ was the average of all observations; α was the fixed effect of treatment (i = 1 to 7); and εij was the random residual error, assumed to be independent and N(0, σ2).
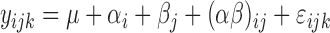 |
(3) |
where y was the individual dependent variable (n = 28); μ was the average of all observations; α was the fixed effect of treatment (i = 1 to 7); β was the fixed effect of fraction separation (i = 1, 2; where 1 = solubilized fraction, 2 = residual fraction); (αβ) was the interaction of the fixed effects; and εij was the random residual error, assumed to be independent and N (0, σ2).
Statistical significance of differences between means was tested with the Tukey–Kramer test (P < 0.05).
RESULTS
Experiment 1
Dry matter solubility and pH of feathers were affected by NaOH and autoclaving time (Table 3). Enzyme or Na2SO3 had no effect. The enzyme activity of Cibenza IDN900 measured with casein in a 10-min incubation at 30°C gave 170 nkat/g at pH 7.5, 310 nkat/g at pH 9.0, and 220 nkat/g at pH 12.8, which was low compared to a minimum of 1.1 mkat/g stated by the producer.
Table 3.
Effects of the hydrolysis factors NaOH and Na2SO3 concentrations, proteolytic enzyme (E)1 concentration, and autoclaving (AC) time on chicken feather solubility, pH, and IVPD expressed as corresponding unscaled coefficients in the models for the selected responses in experiment 1.
| IVPD | |||||
|---|---|---|---|---|---|
| Factor | Dry matter solubility | pH | Solubilized fraction | Residual fraction | Total |
| Constant | 2.234 | 7.169 | 78.60 | 17.58 | 19.54 |
| E2 | NS3 | NS | NS | NS | NS |
| NaOH | 240.9 | 22.26 | 28.61 | 44.15 | 91.76 |
| Na2SO3 | NS | NS | –17.61 | 52.11 | 57.91 |
| AC | 0.7393 | –0.02668 | 0.3179 | 1.107 | 1.194 |
| NaOH × NaOH | –258.7 | –23.24 | –35.45 | NS | NS |
| Na2SO3 × Na2SO3 | NS | NS | 148.1 | NS | NS |
| AC × AC | –0.004942 | 0.0002076 | –0.001404 | –0.005286 | –0.006066 |
| NaOH × Na2SO3 | NS | NS | NS | –120.1 | –101.2 |
| NaOH × AC | 0.4158 | –0.04117 | –0.1568 | –0.5173 | –0.5253 |
| Na2SO3 × AC | NS | NS | –0.2139 | NS | NS |
| R2 | 0.956 | 0.979 | 0.924 | 0.962 | 0.974 |
| Q2 | 0.907 | 0.958 | 0.810 | 0.932 | 0.957 |
1Cibenza IDN900.
2Effects of E × E, E × NaOH, E × Na2SO3 and E × AC had effect on any of the variables.
3Not significant effects, P > 0.05.
IVPD, in vitro protein digestibility.
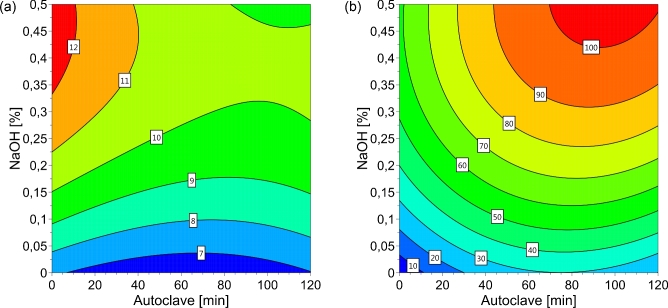
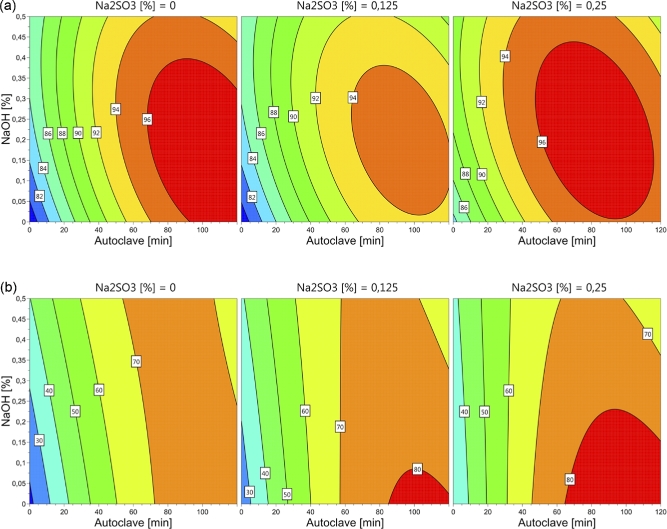
The IVPD of the solubilized and residual fractions, and the total IVPD were affected by NaOH, Na2SO3, and autoclaving time. The contour plots illustrate the effects on pH, DM solubility, and IVPD (Figures 1 to 3). Application of the enzyme Cibenza IDN900 had no effect on DM solubility, pH, or IVPD of solubilized, residual, or combined fractions. NovoProD resulted in low values for DM solubility and IVPD similar to those of Cibenza IDN900. Consequently, the enzymatic treatment step was excluded from the models.
Figure 1.
Contour plot for pH (a) and dry matter solubility (%) (b) in chicken feather hydrolysates as affected by NaOH concentration and autoclaving time.
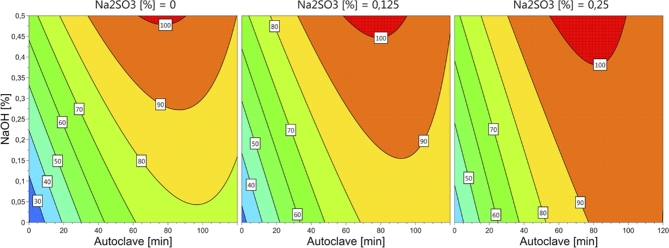
Figure 3.
Contour plot for total in vitro pepsin digestibility (solubilized and residual fractions combined) (%) of chicken feather hydrolysates as affected by concentrations of NaOH, Na2SO3 and autoclaving time.
Figure 2.
Contour plot for in vitro pepsin digestibility (%) in solubilized (a) and residual (b) chicken feather fractions as affected by concentrations of NaOH, Na2SO3 and autoclaving time.
Total IVPD increased with increasing concentrations of both NaOH and Na2SO3. For autoclaving time the model indicated increase in total IVPD between 80 and 100 min, but a rapid reduction of IVPD for longer autoclaving times. None of the studied factors alone was enough to achieve a total IVPD of 900 g/kg of DM, but the model indicates that this could be achieved by combining the factors NaOH, Na2SO3, and autoclaving time. The predicted solubility varied significantly among the treatments with a predicted total IVPD of 900 g/kg of DM. The model for total IVPD was used to design experiment 2.
Experiment 2
The average total IVPD across all treatments was 863 g/kg of DM (SEM 16.8, P = 0.18), which was slightly lower than predicted, and considerably higher than the IVPD of the untreated feathers and the commercial feather meal (Tables 4 and 5). The solubility of DM was considerably higher (P <0.001) for treatments including NaOH alone (on average 817 g/kg of DM) than for Na2SO3 alone (on average 198 g/kg of DM). A similar effect was found for the solubilities of N (P <0.001) and C (P <0.001). An interaction between hydrolysis treatment and fractionation was observed for IVPD. For treatments including only NaOH the IVPD values were higher (P <0.001) in solubilized than in residual fractions, whereas no differences were found between fractions of the Na2SO3 treatments.
Table 4.
Chemical composition, solubility of N, C, and DM for hydrolysis of chicken feathers, and sum of IVPD in solubilized and residual feather fractions in experiment 2 (n = 2).
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|---|
| AC time, min1 | 30 | 60 | 90 | 30 | 60 | 90 | 60 | ||
| NaOH, % | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | ||
| Na2SO3, % | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | ||
| Solubility, g/kg of DM | |||||||||
| DM | 903a | 815b | 735c | 238e | 194f | 162f | 620d | 5.6 | <0.001 |
| N | 785a | 728a,b | 677b | 132d | 134d | 120d | 535c | 10.2 | <0.001 |
| C | 858a | 750b | 713b | 146d | 143d | 123d | 562c | 8.3 | <0.001 |
| Total IVPD, g/kg of DM | 853 | 873 | 893 | 866 | 870 | 871 | 813 | 16.8 | 0.18 |
| Na, g/kg of DM | 49.7a | 31.9c | 25.3d | 36.3b | 19.9e | 12.2f | 32.1c | 0.71 | <0.001 |
a-fMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1Autoclaving at 2.4 bar, 133°C.
IVPD, in vitro protein digestibility.
Table 5.
In vitro pepsin digestibility and chemical composition in feathers, commercial feather meal, and solubilized and residual feather fractions in experiment 2 (n = 2).
| Treatment | Feathers1 | Feather meal2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC time, min4 | – | – | 30 | 60 | 90 | 30 | 60 | 90 | 60 | H2 | F3 | H × F | |||||||||
| NaOH, % | – | – | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | ||||||||||||
| Na2SO3, % | – | – | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | ||||||||||||
| Fraction | – | S | R | S | R | S | R | S | R | S | R | S | R | S | R | ||||||
| Item, g/kg of DM | |||||||||||||||||||||
| IVPD | 156 | 549 | 865a,b | 673e | 903a,b | 704e | 938a | 746c,d,e | 858a,b | 868a,b | 829b,c,d | 880a,b | 838a,b,c | 878a,b | 860a,b | 732e,d | 18.1 | <0.001 | <0.001 | <0.001 | |
| N | 151 | 128 | 131c,d | 95h | 135b,c,d | 119e,f | 139a,b,c,d | 132c,d | 83i | 142a,b,c | 104h,g | 146a,b | 112f,g | 148a | 130d,e | 140a,b,c,d | 2.1 | <0.001 | <0.001 | <0.001 | |
| C | 495 | 461 | 470c,d | 514a,b | 456d | 529a | 481b,c,d | 511a,b | 305f | 503a,b,c | 365e | 519a | 375e | 507a,b | 449d | 499a,b,c | 6.3 | <0.001 | <0.001 | <0.001 | |
| Na | <0.250 | 0.746 | 51.2c | 27.1f | 35.1e | 14.2g | 30.2e,f | 9.6h,g,i | 116.1a | 12.6h,g | 69.1b | 7.6h,i | 52.3c | 3.9i | 42.2d | 13.8h,g | 1.12 | <0.001 | <0.001 | <0.001 | |
| S | 18.9 | 14.9 | 20.0e | 12.8e | 20.2e | 15.2e | 21.0e | 15.8e | 110.8a | 21.8e | 83.4b | 20.2e | 72.1c | 17.7e | 34.8d | 18.4e | 1.79 | <0.001 | <0.001 | <0.001 | |
| Ash | 5.8 | 13.5 | 285a,b | 103d,e | 210b,c,d | 57e | 208b,c,d | 35e | 364a | 43e | 213b,c,d | 23e | 171c,d | 13e | 248b,c | 40e | 16.8 | <0.001 | <0.001 | 0.005 | |
a-iMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1Untreated cut feathers.
2Commercial feather meal, GoldMehl FM., GePro, Diepholz, Germany.
3H = effect of hydrolysis treatment, F = effect of fractionation, H × F = interaction.
4Autoclaving at 2.4 bar, 133°C.
IVPD, in vitro protein digestibility.
When feathers were treated with NaOH, higher concentrations of N were found in the solubilized than in the residual fractions, but for Na2SO3-treated samples the N concentrations were higher in the residual fractions and the highest value was found in the residual fraction of treatment 6 (0% NaOH, 0.21% Na2SO3, 90 min autoclaving, interaction P ≤ 0.001). Concentrations of C were higher in residual than solubilized fractions, but differences were higher for Na2SO3 than for NaOH (interaction P ≤ 0.001). Untreated feathers and commercial feather meal had low concentrations of Na. After hydrolysis, the Na concentrations were increased by the additives and the largest values were found in the solubilized fractions, with the highest concentration found in treatment 4 with 116.1 g Na/kg of DM. Concentrations of S and ash were higher (P ≤ 0.001) in solubilized than residual fractions. Na2SO3 contributed with additional S to the hydrolysates. Treatment 7 (0.21% NaOH, 0.22% Na2SO3, 60 min autoclaving) resulted in intermediary values with regard to solubility and chemical composition.
Untreated feathers had a total AA concentration of 971 g/kg of DM with the prevailing AA being serine, glutamic acid + glutamine, leucine, proline, valine, and cystine (Table 6). The AA composition of commercial feather meal was comparable to that of the untreated feathers except for the proportion of cystine, which was considerably lower. Calculated for combined solubilized and residual fractions, the treatments with only NaOH had higher proportions of leucine, isoleucine, phenylalanine, valine, asparagine/aspartic acid, glutamine/glutamic acid, hydroxylysine, and proline compared to the treatments with only Na2SO3. For threonine, arginine, cystine, serine, taurine, and tyrosine the proportions were higher for the Na2SO3 treatments. Proportions of cystine were low compared to untreated feathers, but also compared to the commercial feather meal. The highest proportion of cystine was found in treatment 6, but even there the recovery of cystine was only 377 g/kg (Supplementary Table S1). Decreasing Na2SO3 concentration and at the same time increasing autoclaving time increased the recovery of cystine. Substantial losses were found for lysine (mean recovery: 722 g/kg) and for threonine, serine, tyrosine, and arginine when treated with NaOH (mean recovery: 596 g/kg), but losses were small when treated with Na2SO3 treatments (908 g/kg). Recovery rates close to 100% were found for glutamine/glutamic acid, proline, and glycine. For 30-min autoclaving, the proportions of cystine, glutamine/glutamic acid, proline, and serine were higher than for 90 min. Total AA concentrations were higher (P = 0.004) and NH4+ concentrations lower (P < 0.001) for Na2SO3 than for NaOH treatments.
Table 6.
Amino acid (AA) composition and NH4+ concentration in chicken feathers, commercial feather meal, and chicken feather hydrolysates (solubilized and residual fractions combined) in experiment 2 (n = 2).
| Treatment | Feathers1 | Feather meal2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC time, min3 | – | – | 30 | 60 | 90 | 30 | 60 | 90 | 60 | ||
| NaOH, % | – | – | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | ||
| Na2SO3, % | – | – | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | ||
| AA, g/kg of total AA | |||||||||||
| Essential AA | |||||||||||
| His | 7.5 | 8.1 | 8.5 | 9.1 | 8.0 | 8.6 | 8.9 | 7.6 | 9.2 | 0.58 | 0.54 |
| Lys | 23.4 | 20.2 | 22.4 | 21.0 | 17.8 | 20.6 | 19.8 | 18.2 | 18.0 | 1.30 | 0.24 |
| Leu | 87.5 | 86.4 | 97.0a | 92.3b | 91.6b | 84.1c | 84.3c | 83.4c | 93.5a,b | 0.74 | <0.001 |
| Ile | 49.7 | 51.0 | 56.0 | 54.2 | 53.6 | 52.4 | 52.6 | 52.5 | 56.1 | 0.68 | 0.03 |
| Met | 7.1 | 5.6 | 7.2 | 7.7 | 6.2 | 8.7 | 6.9 | 6.3 | 7.3 | 0.82 | 0.46 |
| Phe | 47.5 | 51.2 | 56.6a | 53.7a,b | 53.3b | 48.9c | 49.1c | 48.3c | 55.0a,b | 0.56 | <0.001 |
| Trp | 3.8 | 7.4 | 8.6 | 8.2 | 6.4 | 9.2 | 8.1 | 7.9 | 6.4 | 1.04 | 0.17 |
| Val | 75.5 | 78.2 | 91.8a | 85.5a,b | 84.0b,c | 78.5c | 77.7c | 79.9b,c | 86.5a,b | 1.15 | 0.001 |
| Thr | 34.5 | 28.8 | 20.0d | 23.7b,c | 25.2b,c,d | 30.9a,b | 30.4a,b | 32.2a | 28.2a,b,c | 1.06 | 0.002 |
| Non-essential AA | |||||||||||
| Asx (Asn+Asp) | 59.7 | 66.8 | 74.0a | 70.3a,b | 72.5a | 64.7b,c | 63.3c | 61.5c | 63.8b,c | 1.20 | 0.001 |
| Glx (Glu+Gln) | 94.5 | 102.1 | 128.4a | 121.6a,b | 121.6a,b | 119.7a,b | 116.9b | 112.7b | 112.0b | 1.86 | 0.008 |
| Arg | 70.3 | 71.6 | 43.0c | 57.8b | 62.3a,b | 69.2a | 70.9a | 73.0a | 66.7a,b | 1.84 | <0.001 |
| Ala | 56.9 | 57.7 | 64.0a,b | 62.4a,b | 60.4a,b | 53.1b | 55.1b | 53.6b | 67.4a | 1.87 | 0.009 |
| Cys4 | 75.3 | 42.7 | 7.3d | 10.5c,d | 9.2c,d | 10.7c,d | 19.3b | 29.6a | 17.2b,c | 1.43 | <0.001 |
| Gly | 71.3 | 73.1 | 97.6 | 90.4 | 89.5 | 75.7 | 80.8 | 82.9 | 77.4 | 4.30 | 0.08 |
| Hyl | 0.0 | 0.0 | 3.7a | 2.2a,b | 1.8a,b | 0.0b | 0.0b | 0.0b | 1.7a,b | 0.62 | 0.03 |
| Pro | 85.0 | 95.7 | 119.6a | 115.0a,b | 110.5b,c | 106.1c,d | 103.3c,d | 101.8d | 108.3b,c,d | 1.47 | 0.001 |
| Ser | 119.7 | 127.0 | 71.2d | 91.5c | 102.4b | 126.0a | 122.5a | 120.6a | 99.1b | 1.31 | <0.001 |
| Tau | 0.0 | 0.0 | 0.0b | 0.0b | 0.0b | 1.1a | 0.3b | 0.0b | 0.0b | 0.12 | 0.004 |
| Tyr | 30.9 | 26.4 | 23.1b | 22.8b | 23.6a,b | 31.7a | 29.9a,b | 28.0a,b | 26.3a,b | 1.42 | 0.02 |
| Total AA, g/kg of DM | 971 | 866 | 761c | 804b,c | 826b,c | 822b,c | 888a,b | 946a | 788b,c | 18.0 | 0.004 |
| NH4+, g/kg of DM | 14.4 | 13.4 | 16.7a | 15.3a,b | 14.8b,c | 11.9d | 13.4c,d | 14.6b,c | 13.9b,c | 0.29 | <0.001 |
a-dMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1Untreated cut feathers.
2Commercial feather meal, GoldMehl FM., GePro, Diepholz, Germany.
3Autoclaving at 2.4 bar, 133°C.
4Analyzed as dipeptide cystine.
The concentrations of total AA ranged from 513 to 831 g/kg of DM in solubilized and from 541 to 1004 g/kg of DM in residual fractions (Table 7). Interactions of hydrolysis treatment and fractionation were found for all AA except for isoleucine, tryptophan, threonine, hydroxylysine, and proline.
Table 7.
Amino acid (AA) composition and NH4+ concentration in solubilized and residual feather fractions in experiment 2 (n = 2).
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC time, min2 | 30 | 60 | 90 | 30 | 60 | 90 | 60 | H2 | F3 | H × F | ||||||||
| NaOH, % | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | |||||||||||
| Na2SO3, % | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | |||||||||||
| Fraction | S | R | S | R | S | R | S | R | S | R | S | R | S | R | ||||
| AA, g/kg of total AA | ||||||||||||||||||
| Essential AA | ||||||||||||||||||
| His | 8.4c,d,e | 11.3abc | 9.0b,c,d,e | 9.4a,b,c,d,e | 8.1c,d,e | 7.8d,e | 11.9a,b | 8.0c,d,e | 12.5a | 8.3c,d,e | 11.8a,b | 7.1e | 10.9a,b,c,d | 6.5e | 0.60 | 0.02 | <0.001 | <0.001 |
| Lys | 21.7c,d,e | 38.9a | 19.5c,d,e | 30.7b | 16.0e,f | 23.8c | 8.2g | 22.6c,d | 11.0f,g | 21.3c,d,e | 12.0f,g | 19.0c,d,e | 16.6d,e,f | 20.2c,d,e | 1.08 | <0.001 | <0.001 | <0.001 |
| Leu | 97.5a | 86.1b | 93.4a | 85.3b | 93.3a | 85.9b | 85.0b | 84.0b | 84.9b | 84.2b | 87.7b | 82.8b | 97.7b | 86.8b | 0.89 | <0.001 | <0.001 | <0.001 |
| Ile | 56.4a,b,c,d | 48.3e | 54.6a,b,c,d | 51.3d,e | 54.4a,b,c,d | 51.1d,e | 57.1a,b | 51.6c,d,e | 56.9a,b,c | 51.9b,c,d,e | 58.3a | 51.7b,c,d,e | 57.6a | 53.7a,b,c,d | 0.94 | 0.03 | <0.001 | 0.18 |
| Met | 7.1c,d | 10.3a,b,c | 7.8b,c,d | 7.5b,c,d | 6.4c,d | 5.6d | 12.7a | 8.0b,c,d | 12.4a | 6.0d | 11.2a,b | 5.6d | 8.2b,c,d | 5.8d | 0.73 | 0.001 | <0.001 | <0.001 |
| Phe | 57.0a | 49.8c,d | 54.5a,b | 48.6c,d | 54.1a,b | 50.5b,c,d | 50.4b,c,d | 48.7c,d | 52.5b,c | 48.5c,d | 51.3b,c,d | 47.9d | 57.8a | 50.6b,c,d | 0.79 | <0.001 | <0.001 | 0.02 |
| Trp | 8.6a,b | 10.0a,b | 8.5a,b | 5.9a,b | 6.2a,b | 7.1a,b | 12.3a | 8.7a,b | 7.7a,b | 8.2a,b | 7.2a,b | 8.1a,b | 7.0a,b | 5.5b | 1.18 | 0.03 | 0.37 | 0.24 |
| Val | 92.1a | 84.8a,b | 85.8a,b | 83.2b,c | 84.4a,b | 82.8b,c | 71.0e | 79.8b,c,d | 71.8e,d | 78.7b,c,d,e | 75.6c,d,e | 80.5b,c | 86.4a,b | 86.9a,b | 1.43 | <0.001 | 0.097 | 0.001 |
| Thr | 19.9d | 23.0c,d | 23.4b,c,d | 25.8a,b,c,d | 23.3b,c,d | 31.4a,b,c | 27.0a,b,c,d | 31.6a,b,c | 26.3a,b,c,d | 31.1a,b,c | 31.8a,b | 32.2a | 26.0a,b,c,d | 32.0a,b | 1.53 | <0.001 | <0.001 | 0.3 |
| Non-essential | ||||||||||||||||||
| Asx (Asn+Asp) | 74.2a,b | 70.3a,b,c,d,e | 71.6a,b,c,d | 61.4d,e,f | 75.4a | 63.3c,d,e,f | 73.2a,b,c | 63.3c,d,e,f | 80.8a | 60.4e,f | 75.6a | 59.6f | 64.8b,c,d,e,f | 62.1d,e,f | 1.86 | 0.008 | <0.001 | 0.004 |
| Glx (Glu+Gln) | 127.5b,c | 148.2a | 120.4b,c,d,e | 129.7b | 120.7b,c,d,e | 124.0b,c,d | 116.3c,d,e | 120.3b,c,d,e | 127.4b,c | 115.1d,e,f | 119.1b,c,d,e | 111.8e,f | 103.5f | 125.4b,c,d | 2.07 | <0.001 | <0.001 | <0.001 |
| Arg | 42.8e | 48.5d,e | 56.5c,d | 66.7a,b | 59.7b,c | 70.9a | 70.5a | 69.0a,b | 67.8a,b | 71.4a | 65.5a,b,c | 74.0a | 65.0a,b,c | 69.3a,b | 1.71 | <0.001 | <0.001 | 0.03 |
| Ala | 64.3a,b | 57.8b | 63.1b | 58.1b | 62.5b | 53.5b | 57.1b | 52.5b | 55.0b | 55.1b | 63.7a,b | 52.3b | 77.0a | 52.3b | 2.43 | 0.01 | <0.001 | 0.006 |
| Cys3 | 7.3d | 8.5c,d | 10.0b,c,d | 14.1b,c,d | 9.7b,c,d | 7.8c,d | 10.4b,c,d | 10.7b,c,d | 7.3d | 21.3a,b,c | 17.1b,c,d | 31.3a | 22.7a,b | 8.6c,d | 2.42 | <0.001 | 0.07 | <0.001 |
| Gly | 98.3a | 82.7a,b | 92.2a,b | 78.4a,b | 92.1a,b | 80.7a,b | 81.3a,b | 74.8b | 81.2a,b | 80.6a,b | 72.6b | 84.3a,b | 76.2b | 79.2a,b | 3.83 | 0.03 | 0.04 | 0.03 |
| Hyl | 3.7a | 3.9a | 2.3a,b | 1.3a,b | 1.7a,b | 2.3a,b | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 1.7a,b | 1.6a,b | 0.54 | 0.001 | 0.91 | 0.84 |
| Pro | 10.6a,b | 11.0a | 10.2a,b | 11.0a | 10.4a,b | 10.2a,b | 10.2a,b | 9.8a,b | 10.2a,b | 9.6a,b | 10.5a,b | 9.2b | 10.8a | 10.3a,b | 0.26 | 0.01 | 0.08 | 0.02 |
| Ser | 71.5f | 64.2f | 92.0d,e | 88.6e | 101.7c,d | 104.6c | 133.6a | 124.8a,b | 124.3a,b | 122.2b | 120.0b | 120.6b | 93.5d,e | 107.8c | 1.94 | <0.001 | 0.55 | <0.001 |
| Tau | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 7.8a | 0.0b | 2.3b | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 0.59 | <0.001 | <0.001 | <0.001 |
| Tyr | 22.6c,d | 33.5a | 21.8d | 29.1a,b,c | 21.9d | 29.1a,b,c | 30.2a,b | 31.9a,b | 30.3a,b | 29.8a,b | 26.3b,c,d | 28.3a,b,c,d | 26.3b,c,d | 26.5a,b,c,d | 1.25 | 0.003 | <0.001 | 0.004 |
| Total AA, g/kg of DM | 776e,d | 541h,i | 828c,d | 676e,f,g | 831c,d | 810c,d | 513i | 914a,b,c | 614g,h | 950a,b | 665f,g | 1,004a | 753d,e,f | 851b,c,d | 19.7 | <0.001 | <0.001 | <0.001 |
| NH4+, g/kg of DM | 17.0b | 11.4f,g | 16.0b | 11.4f,g | 15.5b,c | 12.5e,f | 9.9g | 12.5e,f | 14.3c,d | 13.2d,e | 19.4a | 13.6d,e | 14.2c,d | 13.3e,d | 0.29 | <0.001 | <0.001 | <0.001 |
a-iMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1H = effect of hydrolysis treatment, F = effect of fractionation, H × F = interaction.
2Autoclaving at 2.4 bar, 133°C.
3Analyzed as dipeptide cystine.
Isoleucine and asparagine/aspartic acid were generally more associated with the solubilized fractions than residual fractions, whereas lysine was more associated with the residual fractions. Histidine was associated with residual fractions for NaOH treatments, and with residual fractions for Na2SO3 treatments. Leucine and phenylalanine were associated with the solubilized fractions and tyrosine and arginine with the residual fractions for NaOH treatments, but for these AA no differences were found between fractions for Na2SO3 treatments. Methionine was associated with the solubilized and cystine with the residual fractions for Na2SO3 treatments, but no differences between fractions were found for NaOH treatments. Valine and hydroxylysine had higher proportions for NaOH than Na2SO3 treatments with no differences between fractions. The concentration of total AA was higher in solubilized than in residual fractions when treated with NaOH, but for Na2SO3 treatments, it was higher in residual than in solubilized fractions. Autoclaving time of 90 min compared to 30 min increased proportions of threonine. Proportions of serine increased for NaOH treatments but decreased for Na2SO3 treatments when autoclaving time increased from 30 to 90 min. Proportions of arginine increased with autoclaving time for NaOH treatments, but not for Na2SO3 treatments.
The chemical scores showed that lysine and histidine were the first and second limiting essential AA in combined soluble and residual fractions, assessed as feed for Atlantic salmon in the growth interval from 1.4 to 2.8 kg body weight (Table 8). For treatments 5 and 6 only lysine and histidine had scores below 100, which was also the case for the commercial feather meal. For the treatments 3 to 5 AA had a chemical score below 100. The chemical scores of the separated fractions showed that the residual fractions of treatment 5 and 6 had only 2 scores below 100; however, the solubilized fractions of treatments 6 and 7 had chemical scores close to 100 for tryptophan and are therefore similar to the residual fractions of treatments 5 and 6 (Table 9).
Table 8.
Chemical score of essential AA in chicken feathers, commercial feather meal, and chicken feather hydrolysates (solubilized and residual fractions combined) in ideal proteins for Atlantic salmon (Salmo salar L.) in experiment 2 (n = 2).
| Treatment | Feather1 | Feather meal2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC time, min3 | - | - | 30 | 60 | 90 | 30 | 60 | 90 | 60 | ||
| NaOH, % | - | - | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | ||
| Na2SO3, % | - | - | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | ||
| Chemical score4 | |||||||||||
| Lys | 45 | 41 | 40 | 38 | 32 | 40 | 39 | 37 | 32 | 1.9 | 0.09 |
| Met+Cys5 | 287 | 198 | 52c | 67b,c | 56c | 75b,c | 102b | 145a | 87b,c | 6.6 | <0.001 |
| Thr | 146 | 140 | 85c | 104c | 109b,c | 142a,b | 141a,b | 154a | 120a,b,c | 6.2 | 0.002 |
| Trp | 82 | 116 | 119 | 116 | 90 | 137 | 121 | 123 | 87 | 15.9 | 0.08 |
| Val | 313 | 347 | 356 | 341 | 332 | 328 | 330 | 350 | 335 | 12.5 | 0.65 |
| Ile | 228 | 248 | 238 | 237 | 232 | 240 | 244 | 252 | 238 | 6.1 | 0.45 |
| Leu | 221 | 235 | 230 | 226 | 222 | 216 | 219 | 224 | 222 | 5.9 | 0.69 |
| Phe+Tyr | 142 | 144 | 129 | 128 | 127 | 141 | 140 | 140 | 132 | 5.2 | 0.32 |
| His | 49 | 50 | 46 | 50 | 44 | 50 | 53 | 47 | 49 | 3.2 | 0.58 |
| Arg | 155 | 166 | 87d | 121c | 129c | 151a,b | 157a | 167a | 135b,c | 3.0 | <0.001 |
a-dMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1Untreated cut feathers.
2Commercial feather meal, GoldMehl FM., GePro, Diepholz, Germany.
3Autoclaving at 2.4 bar, 133°C.
4Chemical scores = AA in test feed [g/16 g N]/AA in ideal protein [g/16 g N] × 100. A value of 100 indicates that the level of a particular AA within the feed protein is identical to the dietary AA requirement level for Atlantic salmon (1.4 to 2.8 kg body weight; Rollin et al., 2003). The lowest value in each column indicates the first limiting AA.
5Analyzed as dipeptide cystine.
AA, amino acid.
Table 9.
Chemical score of essential AA in chicken feather hydrolysates in solubilized (S) and residual (R) fractions in ideal proteins for Atlantic salmon (Salmo salar L.) in experiment 2 (n = 2).
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | SEM | P-value1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC time, min2 | 30 | 60 | 90 | 30 | 60 | 90 | 60 | H | F | H × F | ||||||||
| NaOH, % | 0.53 | 0.34 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | |||||||||||
| Na2SO3, % | 0.00 | 0.00 | 0.00 | 0.69 | 0.36 | 0.21 | 0.22 | |||||||||||
| Fraction | S | R | S | R | S | R | S | R | S | R | S | R | S | R | ||||
| Chemical score3 | ||||||||||||||||||
| Lys | 39c | 67a | 36c,d | 53b | 29d,e | 44b,c | 15f | 44b,c | 20e,f | 42c | 22e,f | 39c | 29d,e | 37c,d | 1.7 | <0.001 | <0.001 | <0.001 |
| Met+Cys4 | 52c | 64b,c | 66b,c | 74b,c | 59b,c | 50c | 86b,c | 73b,c | 73b,c | 107a,b | 102a,b,c | 151a | 108a,b | 53c | 9.2 | <0.001 | 0.43 | 0.002 |
| Thr | 85e | 94e | 104c,d,e | 105b,c,d,e | 100d,e | 138a,b,c,d | 119a,b,c,d,e | 145a,b | 116a,b,c,d,e | 145a,b,c | 136a,b,c,d | 157a | 108b,c,d,e | 140a,b,c,d | 7.3 | <0.001 | <0.001 | 0.24 |
| Trp | 118a,b | 131a,b | 122a,b | 78b | 86a,b | 102a,b | 174a | 130a,b | 111a,b | 123a,b | 99a,b | 127a,b | 93a,b | 77a,b | 16.9 | 0.02 | 0.56 | 0.18 |
| Val | 358a | 316a,b | 346a,b | 310a,b | 331a,b | 332a,b | 287b | 336a,b | 290b | 337a,b | 295a,b | 359a | 328a,b | 347a,b | 11.5 | 0.20 | 0.03 | 0.002 |
| Ile | 240a,b | 197c | 241a,b | 209b,c | 234a,b,c | 225a,b,c | 252a | 238a,b | 252a | 243a,b | 249a | 252a | 239a,b | 235a,b,c | 6.9 | 0.003 | 0.001 | 0.06 |
| Leu | 232a | 196b | 231a | 195b | 225a,b | 212a,b | 211a,b | 217a,b | 210a,b | 221a,b | 210a,b | 226a,b | 228a,b | 213a,b | 5.8 | 0.84 | 0.009 | 0.002 |
| Phe+Tyr | 129 | 130 | 129 | 121 | 125 | 134 | 136 | 142 | 140 | 140 | 127 | 142 | 134 | 129 | 4.9 | 0.08 | 0.34 | 0.32 |
| His | 45d,e | 59a,b,c,d | 51b,c,d,e | 49c,d,e | 44d,e | 43d,e | 67a,b | 47c,d,e | 71a | 49b,c,d,e | 64a,b,c | 44d,e | 57a,b,c,d | 36e | 3.1 | 0.002 | <0.001 | <0.001 |
| Arg | 87g | 95g | 119f | 130e,f,d | 123f | 149b,c,d | 149b,c,d | 152b,c | 143b,c,d,e | 160a,b | 134c,d,e,f | 173a | 129e,f | 145b,c,d,e | 3.4 | <0.001 | <0.001 | 0.003 |
a-gMeans within a row with different superscripts differ (Tukey-Kramer test, P < 0.05).
1H = effect of hydrolysis treatment, F = effect of fractionation, H × F = interaction.
2Autoclaving at 2.4 bar, 133°C.
3Chemical scores = AA in test feed [g/16 g N]/AA in ideal protein [g/16 g N] × 100. A value of 100 indicates that the level of a particular AA within the feed protein is identical to the dietary AA requirement level for Atlantic salmon (1.4 to 2.8 kg body weight; Rollin et al., 2003). The lowest value in each column indicates the first limiting AA.
4Analyzed as dipeptide cystine.
AA, amino acid.
DISCUSSION
Enzyme Treatment
Novus International claims that Cibenza IND900 improves nutritional value of feather meal, lowers heat requirements of the rendering process, and supports feather meal profitability and environmental sustainability (NOVUS International, 2013). However, in the present study the enzyme did not affect DM solubility or IVPD when used alone or in combination with other treatments. A possible explanation may be the low activity of the enzyme measured in casein. It is not clear why the activity was low.
Positive effects of proteolytic enzymes have been claimed in several studies. Papadopoulos (1986) reported positive effect on IVPD of proteolytic enzyme treatment (Maxatase, Gist-Brodcades NV, Delft) of feathers hydrolyzed by pressure-thermic treatment. However, adding NH4+ to maintain pH at 8.5 may have confounded the effects of the enzyme in that study. Mokrejs et al. (2011) found that increasing enzyme (Savinase, EC 3.4.21. 62, Ultra 16 L, Novozymes A/S Bagsvaerd, Denmark) concentration from 1 to 5% in a 2-stage hydrolysis of degreased feathers, using 0.3% potassium hydroxide and enzyme treatment (62°C for 4 h), increased feather solubilization from about 705 to 785 g/kg. Kim et al. (2002) found that INSTA-PRO enzyme (INSTA-PRO International, Des Moines, IA 50,322) treatment for 24 h after NaOH treatment (1.0 N for 2 h at 37°C) increased N solubility, but not IVPD. This enzyme product includes B. subtilis fermentation extract and Na2SO3 that may both have contributed to the observed solubilization. In other studies, supernatants from keratinolytic bacteria or bacteria cultures have been used, which may contain mixture of several enzymes. Grazziotin et al. (2006) used supernatants or whole culture of the keratinolytic bacterium Vibrio sp. strain kr2 to hydrolyze autoclaved and hammer milled feathers. After cultivation, the whole culture and the supernatants were autoclaved. Supernatant culture hydrolysate (985 g/kg) had higher IVPD than whole culture hydrolysate (834 g/kg) and a commercial feather meal (578 g/kg). It is not clear if the autoclaving after cultivation had a confounding effect on digestibility. Tiwary and Gupta (2012) studied the effects of different enzyme concentrations produced by B. licheniformis ER-15 on solubilization and IVPD of pressure-thermic treated chicken feathers. Increasing the enzyme concentration to 20 μkat degraded the feathers completely within 12 h (at 50°C, pH = 8), but IVPD increased only from 670 to 734 g/kg of protein. In summary, these studies show varying effects of keratinolytic enzymes.
Lange et al. (2016) hypothesized that isolated keratinolytic enzymes are not able to degrade keratin, but a combination of fungal keratinases, endoprotease (S8 protease family (Rawlings et al., 2016; MEROPS, 2017)), exoproteases (M28), and oligopeptidase (M3) is needed for keratin degradation, and for bacterial keratinases M28 may be substituted by a bacterial exopeptidase with similar function. In addition, Onygena corvina AA11/lytic polysaccharide monooxygenases, disulfide reductase, cysteine dioxygenase, and sulfite can act in synergy with a combination of proteases. The results of the present study support the hypothesis of Lange et al. (2016) that keratinolytic enzymes alone are not able to hydrolyze feathers.
Alkali and Pressure-thermic Treatments
Increased IVPD with prolonged autoclaving time is in accordance with Steiner et al. (1983). The feather keratin solubilizing effect of NaOH in experiments 1 and 2 is in accordance with Mukesh Kumar et al. (2012) and studies using other alkaline solutions (Ferrer et al., 1999; Coward-Kelly et al., 2006; Mokrejs et al., 2011). Both alkali Na2SO3 and heat treatments are known to cleave disulfide bonds (Florence, 1980; Thannhauser et al., 1984; Chojnacka et al., 2011). The results from experiment 1 indicate different mechanisms of action. Sodium hydroxide affected both solubility and IVPD, whereas Na2SO3 only affected IVPD. This result has implications for the composition and quality of the produced feather meal.
Amino Acid Composition
The low recovery rates of cystine were most likely caused by cleavage of disulfide bonds resulting in the formation of cysteine and cysteic acid, which were not analyzed. However, oxidation of dipeptide cystine may also have occurred during sample preparation prior to analysis, and may have led to an underestimation of cysteine proportions. Cystine was also the AA with the highest losses in the study of Moritz and Latshaw (2001) where losses increased with pressure during pressure-thermic treatment. The results from the present study indicate that NaOH degrades several AA and that decreasing Na2SO3 concentration and increasing autoclaving time reduces losses, while total IVPD is kept constant.
Excluding Na2SO3 from the treatment required inclusion of NaOH (over 0.25%) and autoclaving with the corresponding autoclaving time to reach a total IVPD of 900 g/kg of DM. Treatment 6, with no NaOH and the longest autoclaving time, had the best recovery rate of cystine and sum of all AA. This indicates that the way of action differs for the applied reagents. Hence, high digestibility and low losses of AA may be achieved by application of Na2SO3 and autoclaving time of 60 or 90 min (treatments 5 and 6).
Separating solubilized from residual fractions allows for production of fractions with different AA composition, depending on hydrolysis treatment. The residual fractions from Na2SO3 treatments (treatments 4, 5, and 6) had a higher nutritional value when high contents of cystine and lysine are requested. The solubilized fractions with higher proportions of histidine, isoleucine, and methionine may be interesting for cat feed, with high requirements of these AA. The chemical scores for Atlantic salmon of the combined fractions confirm that treatments 5 and 6 are the best treatments where the residual fractions have slightly higher scores than the solubilized fractions. However, differences between combined fractions and separated fractions were insignificant, and therefore the AA composition alone does not warrant separation.
Evaluation of Feather Treatments in a Commercial Process
Aiming for a total IVPD lower than 90% in experiment 2 would have resulted in more gentle treatments with lower inclusion level of additives and shorter autoclaving time and thus processing with lower costs. Applying more gentle treatments may affect AA composition and most likely lower losses of AA may occur. According to the model in experiment 1, a total IVPD of 750 g/kg of DM could have been obtained by autoclaving for 80 min without any addition of additives. In a commercial process, the use of water must be lowered significantly to reduce processing and drying expenses. This requires further investigation. Lower inclusion levels of sodium salts would also decrease levels of undesired Na+ in the hydrolysates. Despite the association of Na+ with the solubilized fractions, the concentrations in residual fractions were also high and significantly higher than in the commercial feather meal. Addition of salts may have led to slightly overestimated IVPD values, because it can be assumed that Na+ and SO32− were associated with the soluble fraction in the IVPD analysis. The IVPD of all treatments was considerably higher compared to the commercial feather meal, and correction for added salts alone can most likely not explain the increase in digestibility. The feed industry requests feedstuffs with a digestibility over 700 g/kg of DM and thus the experimental treatments may result in an added value compared to the tested commercial feather meal. However, in vivo measurements are necessary for a more reliable evaluation of the digestibility. Na and S from the added salts contributed heavily to the ash content in the hydrolysates. With regard to Na+ and ash concentrations the residual fractions are preferred as a feed component. Higher Na concentration can be easily accepted in salmon feed compared with feed for terrestrial animals, but increased salt content will in any case lower the feed value due to dilution of the feed.
CONCLUSIONS
The present study confirmed that NaOH, Na2SO3, and autoclaving time affect solubility, IVPD, and AA composition in chicken feather hydrolysates. Under the conditions in experiment 1, the tested proteolytic enzymes alone or in combination with the above treatments did not have any effects on these response variables. A total IVPD of 900 g/kg of DM can be achieved by different combinations of NaOH, Na2SO3, and autoclaving. Adding low concentrations of Na2SO3 combined with an autoclaving time of 60 to 90 min results in hydrolysates with high IVPD and low losses of AA. For this hydrolysis treatment, lysine and histidine are the only limiting essential AA compared to ideal protein for Atlantic salmon. Separation of solubilized and residual fractions is most likely not feasible due to small differences between the fractions in chemical scores for the most relevant treatments (treatments 5 and 6) studied here. A limiting factor for the use of hydrolyzed chicken feathers as a feed component is the high concentration of Na+ and ash. Fractionation can be used in treatments with low solubility to keep ash concentration of the residual fraction low. If fractionation is not an option, treatments resulting in slightly lower IVPD may be considered to further improve AA composition and lower salt content.
SUPPLEMENTARY DATA
Supplementary data are available at Poultry Science online.
Supplementary Table S1. Apparent recovery of AA and NH4+ in chicken feather hydrolysates in the sum of solubilized and residual fractions in experiment 2 (n = 2)
ACKNOWLEDGMENTS
This work was funded by The Research Council of Norway as part of the research project “Total utilization of raw materials in the supply chain for food with a bio-economical perspective (CYCLE)” (http://cycleweb.no/) [225349/E40, 2013]. The authors thank Nortura SA for supplying raw materials. The authors thank Riitta Alander (VTT) and Panu Lahtinen (VTT) for skillful technical assistance and feather millings.
REFERENCES
- AOAC International 2012. Official methods of analysis of AOAC International. Gaithersburg, MD, USA.
- Bandegan A., Kiarie E., Payne R. L., Crow G. H., Guenter W., Nyachoti C. M.. 2010. Standardized ileal amino acid digestibility in dry-extruded expelled soybean meal, extruded canola seed-pea, feather meal, and poultry by-product meal for broiler chickens. Poult. Sci. 89:2626–2633. [DOI] [PubMed] [Google Scholar]
- Chojnacka K ., Górecka H., Michalak I., Górecki H.. 2011. A review: valorization of keratinous materials. Waste Biomass Valor 2:317–321. [Google Scholar]
- Coward-Kelly G., Chang V. S., Agbogbo F. K., Holtzapple M. T.. 2006. Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1. Chicken feathers. Bioresour. Technol. 97:1337–1343. [DOI] [PubMed] [Google Scholar]
- EC 2013. Commission Regulation (EC) 56/2013 of 16 January 2013 amending Annexes I and IV to Regulation (EC) 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform enc. EU.
- Elmayergi H. H., Smith R. E.. 1971Influence of growth of Streptomyces fradiae on pepsin-HCl digestibility and methionine content of feather meal. Can. J. Microbiol. 17:1067–1072. [DOI] [PubMed] [Google Scholar]
- Eriksson L., Johansson E., Kettaneh-Wold N., Wikström C., Wold S.. 2008. Design of Experiments: Principles and Applications. 3rd ed Umetrics AB, Umeå, Sweden. [Google Scholar]
- FAOSTAT 2017. No Title. Food Agric. Organ. United Nations, Rome, Italy. Accessed May 1, 2017. http://faostat3.fao.org/faostat-gateway/go/to/home/E.
- Ferrer A., Byers F. M., de Ferrer B. S., Dale B. E., Ricke S. C.. 1999. Increasing nutrient availability of feather meal for ruminants and non-ruminants using an ammonia pressurisation/depressurisation process. J. Sci. Food Agric. 79:828–832. [Google Scholar]
- Florence T. M. 1980. Degradation of protein disulphide bonds in dilute alkali. Biochem. J. 189:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glem-Hansen N. 1980. The requirements for sulphur containing amino acids of mink during the growth period. Acta Agric. Scand. 30:349–356. [Google Scholar]
- Grazziotin A., Pimentel F. A., de Jong E. V., Brandelli A.. 2006. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 126:135–144. [Google Scholar]
- Gupta R., Rajput R., Sharma R., Gupta N.. 2013. Biotechnological applications and prospective market of microbial keratinases. Appl. Microbiol. Biotechnol. 97:9931–9940. [DOI] [PubMed] [Google Scholar]
- Gupta R., Ramnani P.. 2006. Microbial keratinases and their prospective applications: an overview. Appl. Microbiol. Biotechnol. 70:21–33. [DOI] [PubMed] [Google Scholar]
- Kim W. K. K., Lorenz E. S. S., Patterson P. H. H.. 2002. Effect of enzymatic and chemical treatments on feather solubility and digestibility. Poult. Sci. 81:95–98. [DOI] [PubMed] [Google Scholar]
- Łaba W., Szczekała K. B.. 2013. Keratinolytic proteases in biodegradation of pretreated feathers. Polish J. Environ. Stud. 22:1101–1109. [Google Scholar]
- Lange L., Huang Y., Busk P. K.. 2016. Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 100:2083–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasekan A., Abu Bakar F., Hashim D.. 2013. Potential of chicken by-products as sources of useful biological resources. Waste Manage. 33:552–565. [DOI] [PubMed] [Google Scholar]
- MEROPS 2017. Families of proteolytic enzymes. May 1, 2017. http://merops.sanger.ac.uk/cgi-bin/family_index?type=P. Accessed May 1, 2017.
- Mokrejs P., Svoboda P., Hrncirik J., Janacova D., Vasek V.. 2011. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag. Res. 29:260–267. [DOI] [PubMed] [Google Scholar]
- Moritz J. S., Latshaw J. D.. 2001. Indicators of nutritional value of hydrolyzed feather meal. Poult. Sci. 80:79–86. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Rogers Q. R.. 1978. Arginine: an essential amino acid for the cat. J. Nutr. 108:1944–1953. [DOI] [PubMed] [Google Scholar]
- Mukesh Kumar D. J., Lavanya S., Priya P., Immaculate Nancy Rebecca A., Balakumaran M. D., Kalaichelvan P. T.. 2012. Production of feather protein concentrate from feathers by in vitro enzymatic treatment, its biochemical characterization and antioxidant nature. Middle-East J. Sci. Res. 11:881–886. [Google Scholar]
- NOVUS International 2013. CIBENZA IND900, data sheet.:2 https://na38.salesforce.com/sfc/p/#E0000000Xz5h/a/E0000000LAYN/XLWNUGhoPmIjn5dqb1bmeECfiSWdvRTaPOICpeL5KUQ. Accessed May 1, 2017.
- Owens C. M., Alvarado C., Sams A. R.. 2001. Poultry Meat Processing. Sams AR, ed. CRC Press, Boca Raton, FL. [Google Scholar]
- Papadopoulos M. C. 1986. The effect of enzymatic treatment on amino acid content and nitrogen characteristics of feather meal. Anim. Feed Sci. Technol. 16:151–156. [Google Scholar]
- Papadopoulos M. C., El Boushy A. R., Roodbeen A. E., Ketelaars E. H.. 1986. Effects of processing time and moisture content on amino acid composition and nitrogen characteristics of feather meal. Anim. Feed Sci. Technol. 14:279–290. [Google Scholar]
- Pedersen M. B., Yu S., Plumstead P., Dalsgaard S.. 2012. Comparison of four feed proteases for improvement of nutritive value of poultry feather meal. J. Anim. Sci. 90:350–352. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J., Robert F.. 2016. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 44:D343–D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin X., Mambrini M., Abboudi T., Larondelle Y., Kaushik S. J.. 2003. The optimum dietary indispensable amino acid pattern for growing Atlantic salmon (Salmo salar L.) fry. Br. J. Nutr. 90:865. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2016. https://www.sas.com/en_us/home.html. Accessed May 1, 2017.
- Steiner R. J. R., Kellems R. O. R., Church D. C.. 1983. Feather and hair meals for ruminants. IV. Effects of chemical treatments of feathers and processing time on digestibility. J. Anim. Sci. 57:495–502. [Google Scholar]
- Swisher K. 2012. Market Report: Industry savors record prices and growing global demand. Placerville, CA.
- Thannhauser T. W., Konishi Y., Scheraga H. A.. 1984. Sensitive quantitative analysis of disulfide bonds in polypeptides and proteins. Anal. Biochem. 138:181–188. [DOI] [PubMed] [Google Scholar]
- Tiwary E., Gupta R.. 2012. Rapid conversion of chicken feather to feather meal using dimeric keratinase from Bacillus licheniformis ER-15. J. Bioproces. Biotechniq. 2:1–5. [Google Scholar]
- Wang X., Parsons C. M.. 1997. Effect of processing systems on protein quality of feather meals and hog hair meals. Poult. Sci. 76:491–496. [DOI] [PubMed] [Google Scholar]
- Yokote Y., Kubo Y., Takahashi R., Ikeda T., Akahane K., Tsuboi M.. 2007. Structural details of a fowl feather elucidated by using polarized raman microspectroscopy. BCSJ 80:1148–1156. [Google Scholar]
- Zhang Y., Yang R., Zhao W.. 2014. Improving digestibility of feather meal by steam flash explosion. J. Agric. Food Chem. 62:2745–2751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.