Abstract
Objective
To evaluate whether the progression of individual motor features was influenced by early deep brain stimulation (DBS), a post hoc analysis of Unified Parkinson's Disease Rating Scale–III (UPDRS-III) score (after a 7-day washout) was conducted from the 2-year DBS in early Parkinson disease (PD) pilot trial dataset.
Methods
The prospective pilot trial enrolled patients with PD aged 50–75 years, treated with PD medications for 6 months–4 years, and no history of dyskinesia or other motor fluctuations, who were randomized to receive optimal drug therapy (ODT) or DBS plus ODT (DBS + ODT). At baseline and 6, 12, 18, and 24 months, all patients stopped all PD therapy for 1 week (medication and stimulation, if applicable). UPDRS-III “off” item scores were compared between the ODT and DBS + ODT groups (n = 28); items with significant between-group differences were analyzed further.
Results
UPDRS-III “off” rest tremor score change from baseline to 24 months was worse in patients receiving ODT vs DBS + ODT (p = 0.002). Rest tremor slopes from baseline to 24 months favored DBS + ODT both “off” and “on” therapy (p < 0.001, p = 0.003, respectively). More ODT patients developed new rest tremor in previously unaffected limbs than those receiving DBS + ODT (p = 0.001).
Conclusions
These results suggest the possibility that DBS in early PD may slow rest tremor progression. Future investigation in a larger cohort is needed, and these findings will be tested in the Food and Drug Administration–approved, phase III, pivotal, multicenter clinical trial evaluating DBS in early PD.
Classification of evidence
This study provides Class II evidence that for patients with early PD, DBS may slow the progression of rest tremor.
Deep brain stimulation (DBS) is an effective adjunctive therapy for mid-stage and advanced stage Parkinson disease (PD), improving motor symptoms and quality of life while reducing medication-associated complications.1,2 The demonstrated efficacy of DBS in later PD stages and preclinical data supporting its earlier application3–5 warrant clinical trials to explore whether DBS applied in very early stage PD could extend or even enhance its benefits. Vanderbilt University completed a prospective, randomized, controlled clinical trial investigating subthalamic nucleus (STN) DBS in early PD.6 This pilot trial was designed to evaluate safety and tolerability of DBS in early PD, and the Food and Drug Administration (FDA) limited enrollment to 30 patients. The trial met its primary safety endpoint,6 and the FDA has approved the conduct of a pivotal, multicenter trial of DBS in early PD (IDE G050016). A key design feature of the pilot was implementation of week-long washouts of all PD therapy (medication and stimulation, if applicable) every 6 months during the 2-year trial. This 7-day washout was conducted in order capture the underlying state of early motor symptoms. A secondary analysis of the Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) total motor examination “off” score favored DBS plus optimal drug therapy (ODT) over ODT but did not reach statistical significance.6 This study's objective was to evaluate whether progression of individual motor features was influenced by early DBS.
Methods
Standard protocol approvals, registrations, and patient consents
The DBS in early PD pilot was a prospective, randomized, controlled, single-blind clinical trial (NCT00282152) that was approved by the FDA (IDEG050016) and Vanderbilt institutional review board (IRB040797).
Patients
Thirty patients with idiopathic PD aged 50–75 years, taking PD medications for 6 months–4 years at enrollment, Hoehn & Yahr II “off” medication, and without history of dyskinesia or motor fluctuations were enrolled in the study. For this analysis, 2 patients were excluded: 1 ODT patient dropped out after baseline; 1 DBS + ODT patient was discovered after the trial concluded to have not met the inclusion criteria for the duration of medication treatment.
Study procedures
The pilot study design and methods were previously described.6 Patients were admitted to the clinical research center for a week-long inpatient washout at baseline and every 6 months during the 2-year trial. At baseline, the UPDRS-III examination was videotaped on day 1 “on” therapy (“on” medication), and patients then discontinued PD therapy for 1 week. On day 8, the UPDRS-III examination was videotaped again “off” therapy (“off” medication). After baseline evaluations, patients were then randomized 1:1 to receive ODT or bilateral STN DBS + ODT.7 All follow-up visits (6, 12, 18, and 24 months) were also videotaped on day 1 “on” therapy (“on” medication and stimulation, if applicable) and on day 8 “off” therapy (“off” medications and stimulation, if applicable). After the study concluded, all videotapes were de-identified with respect to treatment assignment, follow-up duration, and “on”/“off” status and scored in random order by an independent, blinded neurologist certified in scoring the UPDRS.6 The UPDRS-III total score reported includes all motor examination items except rigidity, which cannot be assessed by videotape. DBS + ODT patients did not begin active treatment until ∼6 weeks after baseline assessments in order to allow time for preoperative planning and postoperative healing.6
Medication and stimulation management
Patients' medication and stimulation adjustments were made by their treating neurologist (not the principal investigator). Monopolar stimulation with case positive and the optimal contact negative was utilized for all patients (model 3389 leads; Medtronic, Minneapolis, MN).7 Patients were initially programmed per standard of care for DBS, and the optimal contact was titrated to efficacy alongside medication adjustments 4 weeks after surgery.6 DBS and medications could then be adjusted by the patient's treating neurologist as needed over the trial. Stimulation pulse width and frequency were fixed at 60 μs and 130 Hz, respectively. Levodopa equivalent daily dose (LEDD)8 and voltages are reported as mean ± SD.
Patient satisfaction survey
A survey was conducted to collect information regarding patient experiences and satisfaction participating in the trial. Twenty-seven respondents completed the written survey (n = 14 ODT, n = 13 DBS + ODT), which addressed their decision to participate, experience with the expanded informed consent process, study procedures, and poststudy reflections. DBS + ODT patients completed additional questions designed to explore their DBS experience.
Statistical analysis
This analysis was conducted to provide Class II evidence of the effect of DBS on the progression of individual motor features of early stage PD. Patients were analyzed in the treatment group to which they were randomized. Statistical analyses were performed using IBM SPSS Statistics 23.0 (IBM, Armonk, NY), STATA 13.1 (StataCorp LP, College Station, TX), and SAS 9.3 (SAS Institute, Inc., Cary, NC). p Values comparing the 2 groups on changes from baseline to 24 months in UPDRS-III “off” items (i.e., individual motor examination items) were determined from Wilcoxon rank-sum tests; using a Bonferroni multiple comparisons correction,9 p < 0.0038 (0.05/13 items) was considered statistically significant.
A multiple linear regression model with generalized estimating equations and robust variance estimation10 was used to assess the magnitude and statistical significance of the between-treatment group difference in item trends (difference in slopes) over 24 months. The model adjusted for baseline score and accounted for within-patient correlations in the repeated measures (changes in score from baseline to 6, 12, 18, and 24 months); exchangeable correlations within patients were assumed. Separate models for score change were fit for the “off” and “on” treatment states. Each model included a binary term for treatment group, a continuous term for time, and a term for the treatment group by time interaction, as well as a baseline score term. The p value for the difference in the DBS + ODT vs ODT score trends was determined from a 2 degrees of freedom χ2 test of the null hypothesis that both the treatment and treatment by time interaction terms were zero.
A Cox proportional hazards model11 with a binary term for treatment group was used to estimate the between-group hazard ratio for the time to at least a 2-point worsening in UPDRS-III “off” rest tremor score. A 2-point worsening of the rest tremor item was selected because the minimum clinically important difference for the entire UPDRS-III (combination of all motor items) is 2.3.12 The p value for difference between the treatment groups for risk of at least 2-point worsening was determined from the log-rank test.
The number of limbs affected by rest tremor (face, right hand, left hand, right leg, left leg) was evaluated for each patient at baseline and 24 months, and mean change from baseline to 24 months was compared between groups using a 2-sample t test with equal variances. Each patient was also evaluated for rest tremor development in previously unaffected limbs. De novo development was defined per limb as having no baseline rest tremor present (question 20 UPDRS-III “off” item score = 0) and a non-zero 24-month rest tremor item severity score. Patients with rest tremor onset in at least one limb at 24 months were categorized as having developed rest tremor de novo. Fisher exact test was used to assess the difference between treatment groups in the risk of de novo rest tremor development (development vs no development). p < 0.05 Was considered to be significant.
Data availability
The individual de-identified participant data and related study documents are not being publicly shared at this time as they are currently being used for the development of a proprietary, multicenter, phase III, pivotal clinical trial (IDE G050016).
Results
Baseline characteristics
Twenty-eight out of the 30 patients enrolled in the pilot trial were included in this analysis (1 ODT patient dropped out after baseline; 1 DBS + ODT protocol violation). At baseline, the groups were similar in sex, age, Hoehn & Yahr, medication duration, UPDRS-III “off” score, and PD phenotype distribution (table). The DBS + ODT group had lower LEDD and worse “on” scores for total UPDRS and UPDRS-III at baseline, but these differences did not reach statistical significance in this small study.
Table.
Baseline characteristics

UPDRS-III motor items “off” therapy
Mean UPDRS-III total “off” scores worsened for both groups from baseline to 24 months (figure 1, A, C). Figure 1B shows mean baseline and 24-month scores for individual UPDRS-III “off” items, and change scores from baseline to 24 months were compared between groups (figure 1D). Rest tremor “off” change scores were 3.1 points better for the DBS + ODT group than the ODT group, and this was the only UPDRS-III item that reached statistical significance after adjusting for multiple comparisons (p = 0.002).
Figure 1. Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) motor examination “off” item scores.
Mean ± SEM UPDRS-III scores after a 7-day washout (day 8) by treatment group (n = 14, optimal drug therapy [ODT]; n = 13, deep brain stimulation [DBS] + ODT) and mean ± SEM changes in scores from baseline to 24 months. (A) UPDRS-III total score (excludes rigidity) at baseline and 24 months. (B) UPDRS-III item scores at baseline and 24 months. (C) Change in UPDRS-III total score (excludes rigidity) from baseline to 24 months. (D) Change in UPDRS-III item scores from baseline to 24 months. Δ = ODT minus DBS + ODT difference in change from baseline to 24 months; p values determined from Wilcoxon rank-sum test; *p < 0.0038 (0.05/13) was considered significant for this analysis.
Rest tremor “off” therapy
Mean rest tremor “off” score worsened 3.2 points for the ODT group over 2 years, with minimal change (+0.2 points) in the DBS + ODT group (figure 2A). The difference in rest tremor “off” score slopes favored DBS + ODT (DBS + ODT vs ODT = −2.0 points/y; p < 0.001). A Cox proportional hazards model revealed that the risk of at least 2-point worsening in rest tremor “off” score was 2.6 times greater in the ODT group compared to the DBS + ODT group (figure 2B; hazard ratio [HR], ODT vs DBS + ODT = 2.6; 95% confidence interval [CI] 0.8–8.3; log-rank p = 0.07). Seventy-one percent of the ODT group (10/14) and 31% of the DBS + ODT group (4/13) experienced at least a 2-point worsening in their rest tremor “off” score by 24 months (figure 2B). In a sensitivity analysis, the Cox proportional hazards model was repeated including the DBS + ODT patient with a protocol violation, which yielded similar results (HR, ODT vs DBS + ODT = 2.8; 95% CI 0.9–9.1; log-rank p = 0.047).
Figure 2. Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) motor examination “off” rest tremor.
UPDRS-III rest tremor score after 7-day washout (day 8) by treatment group (n = 14, optimal drug therapy [ODT]; n = 13, deep brain stimulation [DBS] + ODT). (A) Mean ± SEM rest tremor “off” score at baseline and 6, 12, 18, and 24 months by treatment group, with regression lines showing the trend in score over time for each group. The p value for the DBS + ODT vs ODT difference in rest tremor “off” score trends (difference in slopes = −1.97 points/y) is determined from a 2 degrees of freedom χ2 test from analysis of covariance of the change in score from baseline, adjusting for the baseline score and within-patient correlations and was significant at the 0.01 level (p < 0.001, exchangeable correlations). Model for change in rest tremor “off” score over time: change in rest tremor “off” score = 1.93 × time (y) + 1.04 × DBS −1.97 × DBS × time (y) − 0.05 × Baseline rest tremor “off” score − 0.53. (B) Time to reach at least 2-point worsening in rest tremor score “off” therapy. The hazard ratio (HR) and 95% confidence interval (CI) were derived from a Cox regression model with treatment group as the single model variable; the p value was determined by the log-rank test.
De novo rest tremor development
The mean number of limbs per patient affected by rest tremor at baseline was 1.4 ± 0.8 and 1.6 ± 1.3 for the ODT and DBS + ODT groups, respectively (figure 3A). The mean number of limbs per patient affected by rest tremor at 24 months doubled in the ODT group (2.8 ± 1.3) and decreased slightly in the DBS + ODT group (1.5 ± 1.3), with the change from baseline to 24 months favoring DBS + ODT (p = 0.001). Furthermore, 86% of ODT patients developed rest tremor in previously unaffected limbs from baseline to 24 months (12/14), compared to 46% of patients receiving DBS + ODT (figure 3B; 6/13; odds ratio 7.0, 95% CI 1.10–45.45, p = 0.046). Seven DBS + ODT patients did not develop rest tremor in any previously unaffected limb, and 4 of those patients had rest tremor present in a limb at baseline that resolved by 24 months. Of note, rest tremor disappeared from all affected limbs for 1 DBS + ODT patient (rest tremor item score: baseline = 4, 24 months = 0).
Figure 3. De novo development of rest tremor.
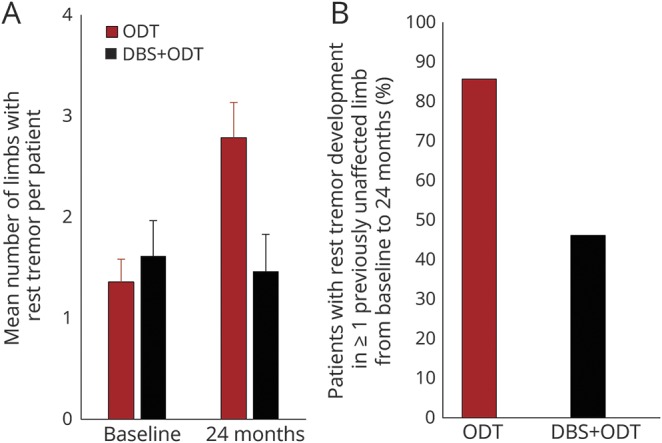
(A) Mean ± SEM number of limbs affected by rest tremor at baseline and 24 months. Difference between groups in change from baseline to 24 months, p = 0.001. p Value determined by the 2-sample t test with equal variances. (B) Proportion of patients who developed rest tremor in previously unaffected limbs from baseline to 24 months. Optimal drug therapy [ODT] n = 14, deep brain stimulation [DBS] + ODT n = 13. Odds ratio 7.0, 95% confidence interval 1.10–45.45, p = 0.046. p Value determined using the Fisher exact test was used to assess the difference between the ODT and DBS + ODT groups in the risk of de novo development of rest tremor (development vs no development). p < 0.05 was considered significant for this analysis.
Rest tremor “on” therapy
Mean rest tremor scores were also analyzed “on” treatment (figure 4A). At baseline, the DBS + ODT group was 1.3 points worse than the ODT group, and in this small study, this difference was not significant (p = 0.18). The DBS + ODT group mean rest tremor “on” score improved over the 2-year period (−1.5 points), while the ODT group mean score worsened (+0.9 points). The difference in rest tremor “on” change scorefavored the DBS + ODT group (difference in slopes = −0.74 points/y; p = 0.003).
Figure 4. Unified Parkinson's Disease Rating Scale, part III (UPDRS-III) motor examination “on” rest tremor and treatment use.

(A) Mean ± SEM UPDRS-III rest tremor score “on” therapy (day 1) by treatment group (n = 13, optimal drug therapy [ODT]; n = 14, deep brain stimulation [DBS] + ODT) over time with regression lines showing the trend in score over time for each group. The p value for the DBS + ODT vs ODT difference in rest tremor “on” score trends (difference in slopes = −0.74 points/year) is determined from a 2 degrees of freedom χ2 test from analysis of covariance of the change in score from baseline, adjusting for the baseline score and within-patient correlations and was significant at the 0.01 level (p = 0.003, exchangeable correlations). Model for change in rest tremor “on” score over time: change in rest tremor “on” score = 0.58 × time (y) − 0.90 × DBS − 0.74 × DBS × time (years) − 0.34 × baseline rest tremor “on” score + 0.84. (B) Mean ± SEM levodopa equivalent daily dose (LEDD) for ODT (n = 14) and DBS + ODT (n = 14) over the 24-month study period. (C) Stimulation measures (rate 130 Hz, pulse width 60 ms). Mean ± SEM stimulation amplitude for the DBS + ODT group (n = 14). Amplitude shown as average of left and right leads. V = voltage. Amplitude range = 1.0–2.4 V.6
Treatment measures
The DBS + ODT group took less medication on average at each visit than the ODT group (figure 4B). Mean stimulation voltage was modest over the 2-year period, beginning at 1.6 ± 0.2 V at 6 months and increasing to 1.9 ± 0.3 V at 24 months (figure 4C).
Patient survey responses
A patient satisfaction survey was conducted after the trial concluded. DBS + ODT patients were asked the open-ended question, “What has been the greatest benefit of undergoing DBS surgery?” Responses were categorized, and 6/13 patients treated with DBS + ODT identified tremor improvement as the greatest DBS benefit. Additional responses were related to quality of life improvement (n = 2), symptom reduction (n = 2), PD progression slowed (n = 1), research to help patients with PD sooner (n = 1), and making friends with PD (n = 1).
Discussion
This study was conducted to evaluate changes in individual UPDRS-III motor examination items collected following a 7-day therapeutic washout in the pilot trial of DBS in early PD. Rest tremor was identified as a motor feature that may be slowed by early DBS. As expected in early PD, mean rest tremor “off” score for the ODT group progressively worsened, whereas there was virtually no worsening for the DBS + ODT group. Rest tremor development or spread to previously unaffected limbs occurred in the majority of ODT patients (86%) compared to only 46% of DBS + ODT patients. The spread of motor features from limbs affected at diagnosis to previously asymptomatic areas is a hallmark feature of PD. These data suggest that patients with early PD treated with ODT alone are 7 times more likely to develop rest tremor in previously unaffected limbs after 2 years than patients treated with DBS + ODT. Furthermore, rest tremor “on” treatment improved in early DBS + ODT patients compared to ODT patients, similar to the benefit expected with STN-DBS in more advanced PD stages.13 Taken together, these findings suggest that DBS in early PD not only improves rest tremor in the treated state but that early STN stimulation may also slow rest tremor progression.
Tremor is highly variable and is often not the single overriding symptom associated with disability and the need for long-term care in advanced PD. There are even reports of tremor disappearing late in the disease.14 Nevertheless, tremor is a highly visible feature of PD,15 is often distressing, and evokes feelings of embarrassment and stigma for patients.16,17 While the importance of PD tremor is sometimes downplayed,17 up to 75% of patients are affected by this cardinal motor feature.18 Tremor is especially distressing in the early stages of the disease due to challenges faced in vocational and social settings.19,20 The importance of tremor control in early PD is also reflected by DBS patients in this study, with nearly half responding that the greatest benefit of undergoing surgery was management of their tremor.
These results should be interpreted with caution due to limitations of this study including its post hoc comparisons, small sample size, and open-label design. This single-blind UPDRS-III subanalysis excludes rigidity, which cannot be evaluated by videorecording, and the effect of early DBS on the 2-year progression of this cardinal feature therefore remains unknown. Medication-refractory tremor is known to respond well to STN-DBS21; however, this was not assessed during the pilot trial. In addition, without an objective PD biomarker to monitor disease progression, clinical trials such as this one must utilize currently available clinical scales. For the pilot, single-blind UPDRS-III scores were assessed after a 7-day washout to evaluate underlying motor symptoms. While the UPDRS-III is a validated evaluation method, there is a subjective element to this clinical assessment, and, in lieu of a biomarker, future investigations may benefit from objective data collection technology. The open-label pilot study was also vulnerable to potential placebo/lessebo effects.22 However, UPDRS-III videos were rated after the study concluded by an independent neurologist blinded to treatment, “on”/“off” status, and study visit chronology.6
This study's design also restricts distinction between benefits due to DBS surgery (implantation + stimulation) vs stimulation alone. Microlesion-induced motor symptom improvement after surgery is well-documented,23 but this phenomenon is reported to resolve within weeks to months after surgery and likely correlates with resolving edema.24 A recent neuroimaging study indicates that microlesion benefits are not only transient (deteriorate within 1 month) but they also occur through a different mechanism (brainstem-mediated), distinguishable from that of STN stimulation (thalamocortical-mediated).25 That study also found that clinical microlesion signs had no effect at 1 year postsurgery. In one advanced PD study where microlesion benefit was reported to last up to 6 months, the effect was not specific to tremor, with improvements also observed in bradykinesia and rigidity.26 Nevertheless, this study cannot rule out influence of a microlesion effect, and future investigations of DBS in PD should include designs to better understand microlesioning effects and their correlation with long-term clinical benefit. Preoperative and postoperative baseline assessments are included in the study design for the FDA-approved pivotal trial of DBS in early PD.
The pilot's 7-day washout produced one of the most robust longitudinal “off” datasets collected in a prospective, randomized PD trial. While PD medication washout durations are highly variable,27,28 it is important to note that ODT patients received more medications on average than the DBS + ODT group throughout this study.6,29 Therefore, any lasting benefit of medications after the washout is expected to favor ODT. Although lack of tremor progression with DBS in advanced PD reported by others may suggest long-lasting stimulation effects,30 STN-DBS washout times are reported to be much faster than for medications, with 90% of motor symptoms worsening within 2 hours of discontinuing STN-DBS31 and resting-state beta-band local field potentials becoming stable within seconds after STN-DBS withdrawal.32 While long-lasting stimulation benefit remains possible, it is less likely given reports of the rapid return of tremor within minutes after stopping stimulation—much faster than other motor signs.31,33
Another important point is that the STN-DBS effect on rest tremor in this study was achieved with modest stimulation. Therefore, the effect could potentially have been more pronounced if stimulation measures were maximized. While typical stimulation voltage for advanced PD ranges from 2.5 to 3.5 V,34 patients in this early PD trial were managed at much lower voltages (mean 1.6–1.9 V). Treating neurologists in this study were instructed to program per standard of care for DBS such that stimulation was titrated to clinical efficacy alongside medication adjustments.7 Future studies could consider trial designs focused on higher initial amplitude of stimulation to determine if greater benefit could be achieved with increased stimulation.
Tremor pathophysiology is well-established to be distinct from other PD motor features, notable for pronounced cerebellar influence and lack of correlation with striatal dopaminergic depletion. Tremor progression does not correlate with other motor symptoms,35 and patients with tremor-dominant PD typically have a better prognosis and slower disability progression than non-tremor-dominant patients.36 Ultimately, the identification of rest tremor as a feature that may progress more slowly after STN-DBS in early PD could further reflect the unique, still poorly understood circuitry and pathophysiology of this cardinal motor feature. Although there is not yet a clear mechanism for rest tremor in PD,37,38 there is increasing evidence for subcortical basal ganglia and cerebellar interactions that may be influenced by early STN-DBS.39,40 It is worth noting that leg agility and bradykinesia scores from baseline to 24 months favored ODT over DBS + ODT by 1.2 and 0.8 points, respectively, although these differences were not statistically significant in this small study after accounting for multiple item comparisons. A larger cohort is needed to adequately investigate potential beneficial or harmful effects on motor progression resulting from early DBS.
The DBS in early PD pilot trial suggests that STN stimulation may slow rest tremor progression. More investigation is needed in a larger cohort, and the FDA has approved the conduct of a multicenter, pivotal clinical trial testing STN-DBS in early PD.
Acknowledgment
The authors acknowledge the support and guidance of Professor Alim-Louis Benabid, M.D., Ph.D., emeritus professor of biophysics at the Joseph Fourier University in Grenoble, France, and chairman of the board of the Edmond J. Safra Biomedical Research Center at Clinatec, in our efforts to determine if DBS therapy might slow Parkinson's disease progression, if applied in the earliest stages of the disease.
Glossary
- CI
confidence interval
- DBS
deep brain stimulation
- FDA
Food and Drug Administration
- HR
hazard ratio
- LEDD
levodopa equivalent daily dose
- ODT
optimal drug therapy
- PD
Parkinson disease
- STN
subthalamic nucleus
- UPDRS-III
Unified Parkinson's Disease Rating Scale, part III
Footnotes
See page e495
CME Course: NPub.org/cmelist
Authors contributions
M.L.H.: research project: conception, organization, execution; statistical analysis: review and critique; manuscript preparation: writing of the first draft. M.R.D.: research project: conception; statistical analysis: design and review and critique; manuscript preparation: review and critique. M.T.: statistical analysis: design, execution, review, and critique; manuscript preparation: review and critique. L.E.H.: research project: organization; statistical analysis: review and critique; manuscript preparation: review and critique. J.L.O.: statistical analysis: review and critique; manuscript preparation: review and critique. A.L.M.: research project: execution; manuscript preparation: review and critique. A.D.C.: research project: organization; manuscript preparation: review and critique. P.E.K.: research project: execution; manuscript preparation: review and critique. T.L.D.: research project: execution; manuscript preparation: review and critique. F.T.P.: research project: execution; manuscript preparation: review and critique. P.H.: research project: execution; manuscript preparation: review and critique. K.C.: research project: execution; manuscript preparation: review and critique. L.T.D.: statistical analysis: design, execution, review, and critique; manuscript preparation: review and critique. A.L.S.: statistical analysis: design, execution, review, and critique; manuscript preparation: review and critique. D.M.S.: statistical analysis: design, review, and critique; manuscript preparation: review and critique. J.T.: statistical analysis: design, execution, review, and critique; manuscript preparation: review and critique. D.C.: research project: conception, organization, execution; statistical analysis: review and critique; manuscript preparation: review and critique.
Study funding
This work was supported by Vanderbilt CTSA grant UL1TR000445 from the National Center for Advancing Translational Sciences (NCATS), NCATS/NIH award UL1TR000011, NIH R01EB006136, Medtronic, Inc, the Michael J. Fox Foundation for Parkinson's Research, the American Parkinson Disease Foundation, and the Parkinson's Foundation. This study received partial support from Medtronic, Inc., which manufactures the DBS system. This work was also supported by a generous gift from Phyllis G. Heard, and her children, Elizabeth Heard, and Tony Heard.
Disclosure
M. Hacker, M. DeLong, M. Turchan, and L. Heusinkveld report no disclosures relevant to the manuscript. J. Ostrem has done consulting work for and receives fellowship grants from Medtronic. A. Molinari and A. Currie report no disclosures relevant to the manuscript. P. Konrad receives research funding and is on the speaker's bureau for Medtronic. T. Davis reports no disclosures relevant to the manuscript. F. Phibbs has done consulting work for Medtronic. P. Hedera, K. Cannard, L. Drye, A. Sternberg, D. Shade, and J. Tonascia report no disclosures relevant to the manuscript. D. Charles receives income from Medtronic for consulting and education services, and Vanderbilt University receives income from Medtronic for educational programs led by David Charles. Go to Neurology.org/N for full disclosures.
References
- 1.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 2.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- 3.Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 2007;130:2129–2145. [DOI] [PubMed] [Google Scholar]
- 4.Spieles-Engemann AL, Steece-Collier K, Behbehani MM, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis 2011;1:123–136. [PMC free article] [PubMed] [Google Scholar]
- 5.Musacchio T, Rebenstorff M, Fluri F, et al. Subthalamic nucleus deep brain stimulation is neuroprotective in the A53T α-synuclein Parkinson's disease rat model. Ann Neurol 2017;81:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles D, Konrad PE, Neimat JS, et al. Subthalamic nucleus deep brain stimulation in early stage Parkinson's disease. Park Relat Disord 2014;20:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles D, Tolleson C, Davis TL, et al. Pilot study assessing the feasibility of applying bilateral deep brain stimulation in very early stages of Parkinson's disease: study design and rationale. J Parkinsons Dis 2012;2:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa does equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2685. [DOI] [PubMed] [Google Scholar]
- 9.Papaioannou T, Hsu JC. Multiple comparisons: theory and methods. Biometrics 1997;53:1561–1562. [Google Scholar]
- 10.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 11.Cox DR. Regression models and life tables (with discussion). J R Stat Soc Ser B (Statistical Methodol) 1972;34:187–220. [Google Scholar]
- 12.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the Unified Parkinson's Disease Rating Scale. Arch Neurol 2010;67:64–70. [DOI] [PubMed] [Google Scholar]
- 13.Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson's disease. Handb Clin Neurol 2013;116:107–128. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Blankson S, et al. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 1993;50:140–148. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED. More time with tremor: the experience of essential tremor versus Parkinson's disease patients. Mov Disord Clin Pract 2016;3:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijhof G. Parkinson's disease as a problem of shame in public appearance. Sociol Health Illn 1995;17:193–205. [Google Scholar]
- 17.Hariz GM, Limousin P, Hamberg K. “DBS means everything: for some time”: patients' perspectives on daily life with deep brain stimulation for Parkinson's disease. J Parkinsons Dis 2016;6:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budzianowska A, Honczarenko K. Assessment of rest tremor in Parkinson's disease. Neurol Neurochir Pol 2008;42:12–21. [PubMed] [Google Scholar]
- 19.Murphy R, Tubridy N, Kevelighan H, et al. Parkinson's disease: how is employment affected? Ir J Med Sci 2013;182:415–419. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann R, Deuschl G, Hornig A, et al. Tremors in Parkinson's disease. Clin Neuropharmacol 1994;17:303–314. [PubMed] [Google Scholar]
- 21.Kumar R, Lozano AM, Kim YJ, et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease completion of diaries. Neurology 1998;3:850–855. [DOI] [PubMed] [Google Scholar]
- 22.Mestre TA, Shah P, Marras C, et al. Another face of placebo: the lessebo effect in Parkinson disease: meta-analyses. Neurology 2014;82:1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltête D, Derrey S, Chastan N, et al. Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Mov Disord 2008;23:1047–1050. [DOI] [PubMed] [Google Scholar]
- 24.Jech R, Mueller K, Urgošík D, et al. The subthalamic microlesion story in Parkinson's disease: electrode insertion-related motor improvement with relative cortico-subcortical hypoactivation in fMRI. PLoS One 2012;7:e49056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holiga Š, Mueller K, Möller Harald E, et al. Resting-state functional magnetic resonance imaging of the subthalamic microlesion and stimulation effects in Parkinson's disease: indications of a principal role of the brainstem: central to Parkinson's disease. NeuroImage Clin 2015;9:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann JM, Foote KD, Garvan CW, et al. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. J Neurol Neurosurg Psychiatry 2009;80:794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller T. Drug therapy in patients with Parkinson's disease. Transl Neurodegener 2012;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olanow CW, Hauser RA, Gauger L, et al. The effect of deprenyl and levodopa on the pregression of Parkinson's disease. Ann Neurol 1995;38:771–777. [DOI] [PubMed] [Google Scholar]
- 29.Hacker ML, Currie AD, Molinari AL, et al. Subthalamic nucleus deep brain stimulation may reduce medication costs in early stage Parkinson's disease. J Parkinsons Dis 2016;6:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castrioto A, Lozano AM, Poon YY, et al. Ten-year outcome of subthalamic stimulation in Parkinson disease. Arch Neurol 2011;68:1550–1556. [DOI] [PubMed] [Google Scholar]
- 31.Temperli P, Ghika J, Villemure JG, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 2003;60:78–81. [DOI] [PubMed] [Google Scholar]
- 32.Trager MH, Koop MM, Velisar A, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson's disease. Neurobiol Dis 2016;96:22–30. [DOI] [PubMed] [Google Scholar]
- 33.Blahak C, Bäzner H, Capelle HH, et al. Rapid response of Parkinsonian tremor to STN-DBS changes: direct modulation of oscillatory basal ganglia activity? Mov Disord 2009;24:1221–1225. [DOI] [PubMed] [Google Scholar]
- 34.Groiss SJ, Wojtecki L, Südmeyer M, et al. Review: deep brain stimulation in Parkinson's disease. Ther Adv Neurol Disord 2009;2:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Bennett DA, Gilley DW, et al. Progression of parkinsonian signs in Alzheimer's disease. Neurology 2000;54:1284–1289. [DOI] [PubMed] [Google Scholar]
- 36.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. What predicts mortality in Parkinson disease? A prospective population-based long-term study. Neurology 2010;75:1270–1276. [DOI] [PubMed] [Google Scholar]
- 37.Helmich RC, Janssen MJ, Oyen WJ, et al. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor 2011;69:269–281. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Lao-Kaim NP, Jacopo P, et al. Pallidal dopaminergic denervation and rest tremor in early Parkinson's disease: PPMI cohort analysis. Parkinsonism Relat Disord Epub 2018. Feb 24. [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Sternad D, Corcos DM, et al. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 2007;35:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helmich RC, Hallett M, Deuschl G, et al. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 2012;135:3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson's Disease Rating Scale: comparison with the Unified Parkinson's Disease Rating Scale. Mov Disord 2013;28:2012–2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The individual de-identified participant data and related study documents are not being publicly shared at this time as they are currently being used for the development of a proprietary, multicenter, phase III, pivotal clinical trial (IDE G050016).




