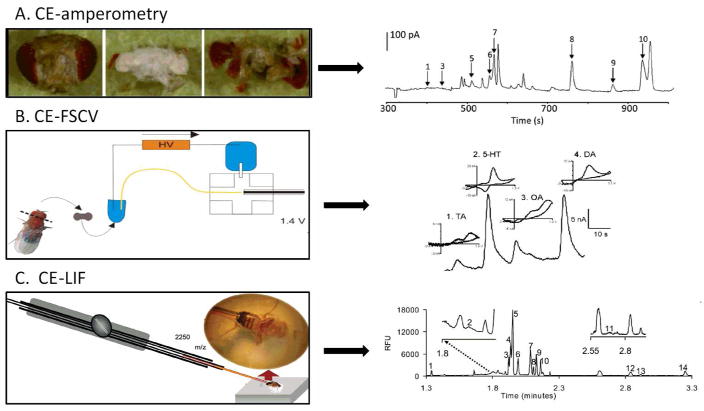
Abstract
Drosophila melanogaster, the fruit fly, is an important, simple model organism for studying the effects of genetic mutations on neuronal activity and behavior. Biologists use Drosophila for neuroscience studies because of its genetic tractability, complex behaviors, well-known and simple neuroanatomy, and many orthologs to human genes. Neurochemical measurements in Drosophila are challenging due to the small size of the central nervous system. Recently, methods have been developed to measure real-time neurotransmitter release and clearance in both larvae and adults using electrochemistry. These studies have characterized dopamine, serotonin, and octopamine release in both wild type and genetic mutant flies. Tissue content measurements are also important, and separations are predominantly used. Capillary electrophoresis, with either electrochemical, laser-induced fluorescence, or mass spectrometry detection, facilitates tissue content measurements from single, isolated Drosophila brains or small samples of hemolymph. Neurochemical studies in Drosophila have revealed that flies have functioning transporters and autoreceptors, that their metabolism is different than in mammals, and that flies have regional, life stage, and sex differences in neurotransmission. Future studies will develop smaller electrodes, expand optical imaging techniques, explore physiological stimulations, and use advanced genetics to target single neuron release or study neurochemical changes in models of human diseases.
Keywords: Drosophila, Dopamine, Octopamine, Serotonin, Optogenetics, Glutamate, Voltammetry, Capillary Electrophoresis
Graphical Abstract
1. Introduction
The complex neural circuitry and vast number of cells within the mammalian brain pose serious challenges to our understanding of behavior, brain development, and neurological disease. Simpler model systems, such as Drosophila, C. elegans, or zebrafish provide tractable research models for understanding neurological processes, and Drosophila in particular are especially attractive because they are easy to genetically alter and are capable of generating complex behaviors.1 Similar to zebrafish and C. elegans, Drosophila has a short life cycle and large brood size; however, zebrafish have comparatively few mutants available whereas Drosophila and C.elegans have large libraries of transgenics/mutants available to researchers.2, 3 Additionally, Drosophila, unlike C. elegans, have a central nervous system which makes the fly model advantageous to study comparable behaviors found in mammals, like aggression, sensory processing, and arousal.4 Flies use the regular canon of neurotransmitters (dopamine, GABA, glutamate, acetylcholine, serotonin, etc.) to communicate between neurons, though flies do possess their own norepinephrine equivalent, octopamine. Furthermore, many pathological features found in human neurodegenerative diseases, like Parkinson’s and Alzheimer’s, can be found in Drosophila disease models.5, 6 Given that over half of Drosophila coding sequences have homologs in the human genome, and that approximately 75% of human disease genes have a counterpart in the fly genome, neurological studies in flies should yield insights into the workings and diseases of the human brain.7, 8
1.1 Organization of the Drosophila nervous system
The ability to connect neuronal activity to behavior requires a model organism that develops quickly, is genetically pliable, and has a simple neuronal network. Drosophila matures in approximately ten days, moving through embryo, larval, and pupal stages to become a fertile adult (Fig. 1A).9 The larval central nervous system contains 2,000 neurons, as compared to the 100,000 found in the adult stage, and is organized into the ventral nerve cord (VNC) and the protocerebrum (Fig. 1).10 Three-dimensional reconstructions of the much larger adult brain have revealed 41 anatomical regions and 58 interregional tracts.11 Like mammalian systems, the Drosophila brain utilizes neurotransmitters to communicate between neurons, and neurotransmitters vary by region. In Drosophila, dopamine is essential for male courtship behavior, controlling movement, sleep, and learning.12–15 Immunohistochemistry has detected about 282 dopamine neurons in the adult brain, located in 18 scattered clusters.16 Octopamine, a derivative of tyrosine like dopamine, is likewise restricted to a limited number of neurons (~150) in the adult brain and regulates aggression and reward in the olfactory system.17–19 Ten clusters of serotonin neurons exist to mediate behaviors like place memory and aggression.20, 21 Though dopamine, octopamine, and serotonin are only found in a few hundred neurons, each exerts a profound impact on fly behavior.
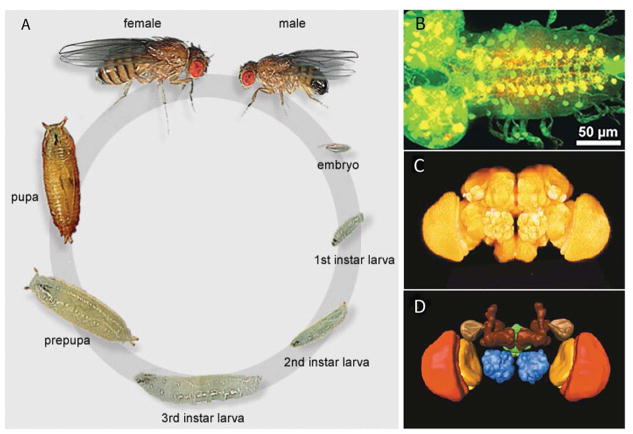
Figure 1.
(A) Drosophila melanogaster develops in approximately 10 days, moving through embryo, three larval, pupal, and adult stages. (B) Ddc-GAL4 drives GFP expression (green) in dopamine and serotonin neurons of the larval brain. Antibody detecting serotonin (red) present; overlap of cells expressing GFP and serotonin appears yellow. (C) Three-dimensional imaging of the adult brain reveals a few distinct anatomical regions. (D) Anatomical regions within the brain were labeled. Dark brown: mushroom body; blue: antennal lobe; green: central complex; red: medulla; orange: lobula. Figures reprinted from ref [6] and [27], with permission of Elsevier.
1.2 Genetic tools in Drosophila
Many genetic tools have been developed that allow researchers to explore the genes and neurons necessary for a functioning brain. The GAL4/UAS (upstream activating sequence) system has proven foundational for targeting gene expression.22 The GAL4 transgene encodes a transcriptional activator capable of binding to the upstream activating DNA sequence and driving expression of any downstream gene. Expression of GAL4 is dependent on cell-specific enhancer elements; thus, any gene introduced downstream of the UAS element is activated only in the same cells defined by the GAL4 enhancer (Fig. 2A). Because the GAL4/UAS method has great versatility, large collections of stocks have been created that drive GAL4 in different tissues and developmental stages.23–25 Reporter genes, like green fluorescent protein (GFP), have been used to map anatomical structures and neural networks. To understand specific gene function, libraries of fly strains have been created that use RNAi to target and knockdown any gene of interest.26, 27
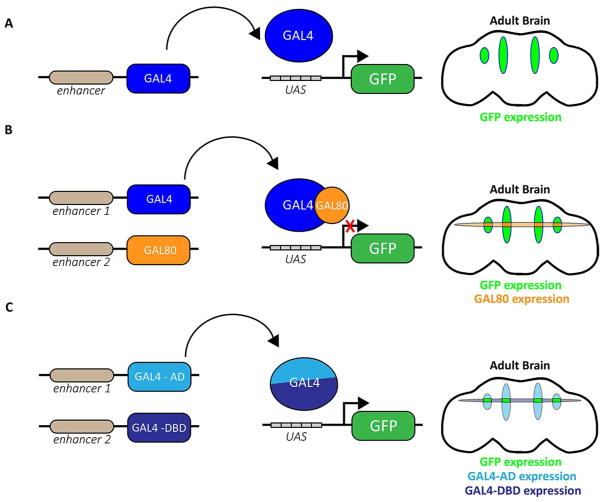
Figure 2.
(A) In flies carrying both the GAL4 (blue) and the UAS constructs, GAL4 will drive expression of the transgene. In this example, GAL4 drives expression of transgenic GFP in four regions of the adult brain. (B) GAL80 (orange) represses GAL4 transcriptional activity, and in cells expressing both GAL4 (blue) and GAL80, transgenic GFP expression will be restricted. (C) With split-GAL4, transgenic GFP (green) will only be expressed in the limited number of cells expressing both the GAL4 activating domain (GAL4-AD, light blue) and the GAL4 DNA-binding domain (GAL4-DBD, dark blue).
Use of a simple GAL4 driver line with GFP reporter often labels too many neurons, and additional genetic modifiers are required to refine GAL4 activity. The GAL80 protein antagonizes GAL4 transcriptional activity, and its addition in Drosophila restricts GAL4 to fewer sets of cells (Fig. 2B). However, further transcriptional elements must be employed to limit transgene expression to a narrower subset of cells. In the split-GAL4 system, the DNA-binding domain and the activation domain of the GAL4 transcriptional activator are each fused to a leucine zipper motif and expressed independently by two different ‘hemi’ drivers (Fig. 2C).28, 29 Functionality of the GAL4 protein is restored only in cells expressing both protein domains, which occurs in the overlapping expression pattern of the two ‘hemi’ drivers. The use of the split-GAL4 system has allowed the mapping of restricted neuronal subsets important in behaviors such as sleep and visual memory. 30, 31
1.3 Developing Drosophila as a tool for understanding neurochemistry
The imaging and genetic tools developed so far in Drosophila have been able to identify neurotransmitters, neuronal clusters, and genes important for brain activity and disease pathology. However, real challenges exist in measuring neurotransmitter in Drosophila as the brain is roughly 54 μm3 in size, or about 8 nL.32 This review highlights the neurochemical techniques that have been developed for real-time neurotransmitter measurements and tissue content studies in Drosophila and highlights the biological impacts of these neurochemical measurements. Finally, we offer a perspective on future challenges in the field.
2. Real-Time Neurochemical Measurements
Electrochemical methods, such as constant-potential amperometry, chronoamperometry, and fast-scan cyclic voltammetry (FSCV), are popular tools to monitor real-time changes of neurotransmitters in the brain and can be performed with sub-second temporal resolution. FSCV scans through voltages, provides 100 ms time resolution, and a cyclic voltammogram that identifies the analyte, while amperometry is less selective, applying a constant voltage, but provides high temporal resolution and is best for measuring quantal size. Chronoamperometry steps to different voltages, so it provides a ratio of oxidation/reduction peak to aid in analyte detection, but is not as selective as FSCV. Carbon-fiber microelectrodes (CFMEs) are commonly used for measuring neurochemicals because of their biocompatibility, sensitivity, favorable electrochemical properties, and small size, typically less than 10 Fm.33 CFMEs measure from the extracellular space, so they are best for measuring volume transmission, and the background subtraction of many techniques makes it more appropriate for measuring fast neurotransmitters including dopamine, serotonine, histamine, and norephinephrine, as well as rapid neuromodulators, like hydrogen peroxide and adenosine.34–38 Because there are no stereotaxic coordinates or brain atlas for flies, initial experiments are often performed in flies that that express GFP in the desired region, which provides a target for electrode implantation.
2.1 Electrochemical measurements of uptake
The first real-time neurochemical measurements in Drosophila were performed by the Ewing group, which measured uptake of exogenously applied dopamine in vivo in adult flies.39 Dopamine was applied to the protocerebral anterior medial (PAM) region and clearance time by the dopamine transporter (DAT) determined (Fig. 3). Peak concentration after cocaine application, a DAT inhibitor, was significantly larger in fumin, a DAT knockout, than wild type demonstrating large doses of cocaine are an effective blocker of DAT. The Venton group also studied dopamine clearance in Drosophila larval ventral nerve cords (VNC) ex vivo.40 The entire central nervous system (CNS) of the larva was removed, a CFME implanted from one end, and a picrosptritzing pipette containing dopamine from the other end. While uptake measurements can be made in any fly without genetic modifications, reproducibility is challenging because exact placement of the pipette with respect to the electrode is difficult.
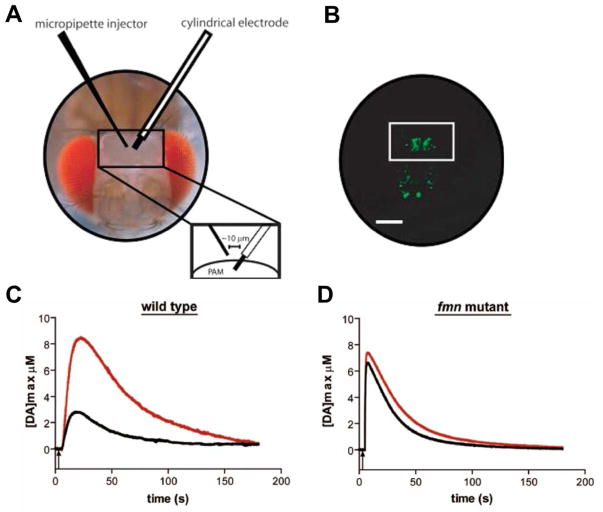
Figure 3.
In vivo measurement of exogenously applied dopamine. (A) Adult fly head after microsurgery exposing PAM neurons (scale bar = 100 μm). (B) Fluorescence image of microsugeried fly head showing GPF expressed in dopaminergic neurons and white box highlights PAM neurons. (C) Representative wild type (WT) data of exogenously applied dopamine trace before (black line) after (red line) the brain was exposed to 1.0 mM cocaine. (D) Exogenously applied dopamine in fumin mutant flies, where dopamine concentration is significantly increased compared to WT. Figures modified from ref [39], with permission of American Chemical Society.
2.2 Optogenetically Stimulated Neurotransmitter Release
In rodents, electrical stimulation is commonly used to activate select neurons by applying a stimulation at the cell bodies and measuring at the terminals. While Drosophila neurons can be activated by electrical stimulation, their brain is too small to stimulate specific neurons. Thus, activation of specific cells in Drosophila is typically performed with optogenetic, a combination of optical and genetic tools, that uses a light sensitive protein to activate neurons in genetically defined cells with millisecond precision.41 Light stimulation opens ion channels, allowing cations such as sodium and calcium to enter the cell, depolarize the membrane, and cause neuronal firing and exocytosis.42 In mammals, channels are typically expressed using a viral vector, such as an adeno-associated virus, to deliver the transgene into target cells.43 However, in Drosophila, the GAL4/UAS system is used to make stable lines that express the channels in specific neurons.
The Venton group pioneered using optogenetic stimulation and FSCV to measure real-time changes in monoamines in the Drosophila larval VNC.44 First, Channelrhodopsin-2 (ChR2), a blue light sensitive channel, was expressed specifically in serotonin neurons containing the synthesis enzyme tryptophan hydroxylase (Tph). Regulation of the serotonin system in Drosophila was analogous to mammals; short term release was maintained primarily by uptake while long term release was maintained by synthesis.45 Similarly, ChR2-mediated stimulation of dopamine was measured in larval dopamine cells expressing tyrosine hydroxylase (TH).46 Dopamine release was decreased by reserpine, which blocks loading of neurotransmitters into vesicles, proving it was vesicular. Optogenetic stimulation also caused pH shifts, similar to mammalian systems where pH shifts are observed after electrical stimulation. 47
The Ewing group developed ChR2 optical stimulation to amperometrically monitor octopamine release in the Drosophila larval neuromuscular junction (NMJ).48 The NMJ was carefully dissected using the fillet preparation and individual octopamine terminals visualized with mCherry (promoter: Tdc2-GAL4), a red fluorescent marker. Optogenetic stimulation caused spikes of release, which were analyzed to determine the number of octopamine molecules released per vesicle. Incubation with tyramine, an octopamine precursor, dramatically increased octopamine release. Complementary electrophysiological measurements at the NMJ have likewise highlighted the importance of octopamine in eliciting muscle depolarization. Application of 10 mM octopamine evoked an excitatory junction potential, a response that was diminished in a mutant for an octopamine receptor, octb2R, or the octopamine synthesizing enzyme tbh.49
Blue light stimulation suffers from a number of limitations, including limited penetration depth, strong absorbance by endogenous chromophores,50 and the photoelectric effect, which causes an error at the electrode during FSCV.51 Moreover, to achieve functional levels of ChR2, a homozygous transgenic line was necessary, with two copies of the channel.52 To circumvent these problems, a red light activated channel, CsChrimson, was employed (Fig. 4). The lower energy of red light did not produce stimulation artifacts at the electrode,51 provided better tissue penetration,53 and required only a single copy of CsChrimson. Different brain regions were studied, including the larval protocerebrum, which develops into the adult protocerebrum that controls olfactory learning and memory, and the VNC, analogous to the vertebrate spinal cord, which contains clusters of motor neurons that are innervated in a highly organized manner.54 More optogenetically-stimulated dopamine release was detected in the larval VNC than in the protocerebrum, demonstrating strong neurotransmission in the motor neurons.51 Using CsChrimson, dopamine release was detected with a single stimulation pulse (4 ms) in both the VNC and protocerebrum,51 whereas at least 10 stimulation pulses were needed with ChR2.55 Octopamine release and uptake in Drosophila larval VNC were also studied,56 and octopamine release was vesicular and stimulation frequency dependent, similar to dopamine and serotonin release.
Figure 4.
CsChrimson stimulated dopamine release from Drosophila larvae. (A) CFME in protocerebrum. Scale bar is 50 μm (top). Concentration trace of CsChrimson evoked dopamine release in the larval protocerebrum (2s continuous stimulation) with cyclic voltammogram (CV) of released dopamine (bottom). (B) CFME in larval VNC. Scale bar is 50 μm (top). Concentration trace and CV of CsChrimson evoked dopamine release in the larval VNC (2s continuous stimulation) (bottom). Figures modified from reference [51], with permission of John Wiley and Sons.
2.3 Chemically Stimulated Neurotransmitter Release
An alternative to inserting a light-activated channel is to express a chemically-sensitive channel in specific Drosophila neurons. P2X2, a ligand-gated cation channel, is a mammalian ATP receptor that is not encoded in Drosophila.57 P2X2 is activated by ATP, causing membrane depolarization and neuronal firing, but not apoptosis. The amount of dopamine released using ATP/P2X2 stimulation was approximately 400 nM, smaller than with CsChrimson stimulation.58 Pharmacological manipulation of dopamine synthesis and uptake demonstrated both release and uptake are important for replenishing the dopamine releasable pool.
Another option is to use pharmacological agents to evoke endogenous release without expressing any exogenous channel. For example, nicotine and acetylcholine activate nicotinic acetylcholine receptors (nAChRs) to evoke neurotransmitter release.59 Fuenzalida-Uribe et al. studied nicotine stimulated octopamine release in adult Drosophila brains using high-speed chronoamperometry.60 As 5 mM nicotine was pressure ejected 1 mm from the brain surface, the efflux of octopamine was recorded in the mid-ventral side of an isolated adult brain. Octopamine was important for nicotine-mediated startle reflex. In the larval VNC, nAChR agonists, such as acetylcholine, nicotine, and neonicotinoids insecticides, stimulated dopamine release.61 Neonicotinoid-stimulated dopamine release was lowered in Drosophila nAChRs subunits mutants, EMS1, α1 subunit mutant and EMS2, β2 subunit mutant which are known to resistant to neonicotinoids insecticides. The benefit of using nAChR stimulation is that no genetic manipulation is required; thus, any fly can be tested.
2.4 Biological insights gained from real-time measurements of neurotransmitters
While many of the studies of Drosophila neurochemistry have developed new techniques, these studies are already providing new insight into the dynamics of neurotransmitter release and the genes that regulate neurotransmission. Early electrochemical studies proved the similarity of Drosophila and mammalian system, establishing that monoamine release is due to exocytosis44 and clearance due to uptake via transporters.62 These results are critical for establishing Drosophila as a good model system for studying neurotransmitter release and uptake. Here, we highlight some of the biological insights gained from real-time neurotransmitter studies.
2.4.1 Psychostimulant and antidepressant activity
Monoamine transporters regulate extracellular levels and are targets for psychostimulants and antidepressants. Pharmacology is slightly different in the fly, where psychostimulants like cocaine are potent inhibitors of the serotonin transporter (SERT, Ki=464 nM) as well as dopamine transporter (DAT, Ki=2660 nM),63 their main target in mammals. Antidepressants given to humans, like selective serotonin reuptake inhibitors (SSRIs), often target SERT. The uptake kinetics for Drosophila DAT were determined using optogenetic stimulation and Michaelis-Menten modeling.40 The maximum uptake rate (Vmax) was higher in the protocerebrum than the VNC but the affinity of dopamine (Km) for DAT was similar in each region.51 For SERT, Vmax and Km in the larval VNC were similar to mammalian values.44 In adult fumin flies, higher dopamine concentrations were detected because they lack functional DAT.62 Similarly, in fumin larvae, clearance rates of dopamine were slower.40 Methylphenidate, a drug used to treat attention deficit disorder and narcolepsy,64 inhibits DAT with a similar binding affinity to cocaine but has a longer half-life.65 Methylphenidate is therefore less addictive and is a proposed treatment for cocaine addiction. The clearance rate of dopamine was significantly slower after oral administration of methylphenidate and methylphenidate-treatment reduced the effects of cocaine.66 Future studies can examine how genetic mutations in the transporter affect the actions of uptake inhibitors, which could be important for understanding human variations in response to drugs such as SSRIs.
2.4.2 Autoreceptor regulation
Another important finding from real-time neurotransmitter measurements in Drosophila was that flies have functioning D2 autoreceptors, similar to mammals.67 Mammalian D2 dopamine receptors (D2R) are presynaptically expressed and act as autoreceptors, regulating dopamine release. Drosophila D2-like receptors have homologous amino acid sequences to mammals,68 but their cellular localization and ability to regulate dopamine release was not known. D2Rs are associated with locomotion, learning, and grooming69 and the D2R agonist bromocriptine improves locomotion in Parkinson flies.70 Bromocriptine also significantly decreased optogenetically stimulated dopamine in the larval VNC and D2R antagonists, such as flupenthixol and butaclamol, had the opposite effect, increasing release.67 Thus, D2R functions as an autoreceptor. Autoreceptors are critically important for regulating dopamine and serotonin release and are the targets of many human drugs, including antipsychotics, so Drosophila could be a good model system to study how genetic mutations in D2Rs affect the efficacy of drugs.
2.4.3 Octopamine function
Most studies have focused on dopamine or serotonin, as they also signal in mammals. However, octopamine, a homologous neurotransmitter for the vertebrate transmitter norepinephrine, is also important because it modulates neuromuscular transmission and locomotion. Octopamine was released from Type II varicosities in the neuromuscular junction, on average about 22,700 molecules per vesicle.48 In the larval VNC, octopaminergic neurons were located mostly in the middle of the abdominal section and octopamine release was vesicular and stimulation frequency dependent.56 The half-life for octopamine was faster than serotonin, which is interesting because no octopamine transporter has been identified and there is no evidence that other transporters, such as SERT, are involved in octopamine clearance. Measurements in adults have also revealed that octopamine is important for a nicotine-induced startle response, which was not observed in flies deficient in octopamine.60
3. Tissue Content Measurements
Tissue content studies that quantify multiple neurotransmitters and their metabolites provide another critical piece of information about neurotransmission. Typically, high-performance liquid chromatography with electrochemical detection (HPLC-EC) has been used for separation and quantification of neurotransmitters in Drosophila 71 but samples are typically pooled due to high mass detection limits.71, 72 Capillary electrophoresis (CE) is a popular separation method for neurotransmitters and metabolites, that separates charged molecules according to their charge and size.73 Neutral molecules can also be separated by adding in a pseudostationary phase, such as sodium dodecyl sulfate (SDS), in a technique called micellar electrokinetic chromatography (MEKC).74 The advantages of CE are high resolution, low sample volume requirement (nL to fL compared to FL for HPLC), and high sensitivity. 75 In this section, we will focus on capillary electrophoresis studies coupled to electrochemical detection (EC), laser induced fluorescence (LIF), and mass spectrometry (MS) for neurochemical content analysis in Drosophila.
3.1 Capillary Electrophoresis – Electrochemical Detection
The Ewing group pioneered using CE coupled with EC detection to identify neurotransmitters in Drosophila. They employed MEKC coupled with amperometric electrochemical detection and identified tyramine, serotonin, dopamine, and the dopamine precursor L-DOPA.76 Further optimization of the run buffer allowed 14 neurochemicals to be separated, including N-acetylated metabolites for the first time.77 Inactive mutants (iav1), defective in locomotor activity, had lower tyramine, octopamine, and N-acetyloctopamine content than Canton S wild types, which is biologically important because octopamine controls locomotion and is in the neuromuscular junction.72
To ease the burdens of dissection of multiple brains, many studies are performed on whole heads; however cuticle and eye pigments interfered with separations in head homogenates and thus isolating brains is better.74 The Drosophila brain was further dissected into three different brain regions: optic lobes, central brain, and posterior superiormedial protocerebrum (PPM1) region. The amount of dopamine was higher in the PPM1 than in the central brain or optic lobes.78 Sample preparation was an important consideration for tissue content experiments, as enzymes might break down neurotransmitters after tissue collection. An alternative freeze-drying method facilitated better dissections and preserved neurotransmitters so they would not degrade.79 In freeze-dried dissected brains, contamination peaks from the eye pigment did not occur (Fig. 5A).
Figure 5.
Neurochemical tissue content using different separations. (A) Image of a freeze-dried adult Drosophila head (left), extracted brain (center), and the cuticle (right). Electropherogram of freeze-dried dissected 15 brain homogenates with 10 Identified peaks (right) (1) dopamine, (2) salsolinol, (3) N-acetyloctopamine, (4) octopamine,(5) N-acetylserotonin, (6) N-acetyltyramine, (7) N-acetyldopamine, (8) L-DOPA, (9) catechol, and (10) tyramine. (B) Schematic diagram of CE-FSCV instrument (left). Electropherogram separation of tyramine, serotonin, octopamine, and dopamine in a single brain (Right). Cyclic voltammograms of each analyte are labeled at the corresponding peak. (C) Schematic diagram of a Drosophila hemolymph sampling setup (left). Electropherogram for fluorescamine labeled amino acids of wildtype adult fly hemolymph (right). (1) arginine, (2) citrulline, (3) tyrosine, (4) histidine, (5) glutamine, (6) asparagine and threonine, (7) alanine and serine, (8) taurine, (9) lysine, (10) glycine, (11) cysteine, (12) glutamate, (13) aspartate, and (14) unknown. Panel A modified from ref. [79], Panel B from [80], and Panel C from [85], all with permission from the American Chemical Society.
Amperometric detection lacks chemical selectivity and therefore identifying peaks relies on comparing migration times with an internal or external standard. CE with FSCV detection overcomes the problem of identification, as the cyclic voltammogram gives a signature of the analyte (Fig. 5B). Although FSCV is not as sensitive as amperometry, field–amplified sample injection can be applied by diluting the sample with acetonitrile, which enhances loading of sample into the capillary. Thus, Drosophila content was determined in a single larval CNS.80 Levels of octopamine and tyramine differed by life stage (Fig. 5B).81 Further studies optimized detection of histamine, carcinine, and dopamine from different tissue types including brain, eyes, and cuticle.82
3.2 Capillary Electrophoresis – Laser Induced Fluorescence
CE with laser induced fluorescence (CE-LIF) detection has high sensitivity and has been used to analyze microdialysis samples from vertebrates.83, 84 While LIF is sensitive, most neurochemicals are not fluorescent and thus must be derivatized. The Shippy group used fluorescamine derivitization and CE-LIF to measure amino acid content in Drosophila hemolymph, a fluid which is analogous to the blood in vertebrates (Fig. 5C).85 Hemolymph samples are extremely low volume (50–300 nL in larvae) so a homemade sampling probe was developed to pierce a single larval body cavity with tubing and cover the sample with oil to prevent evaporation.86 Amino acids were quantified in control Oregon-R flies and genderblind (gb) mutants, where a cysteine-glutamate transporter mutant causes bisexual behavior. A nanoliter sampling technique was developed to collect 25 nL of hemolymph from a dorsal abdominal incision of adult Drosophila (Fig. 5C). Thiols such as glutathione and cysteine were present in the mM range in hemolymph.87 CE-LIF is a good method for these volume limited samples.
3.3 Capillary Electrophoresis – Mass Spectrometry
CE coupled with mass spectrometry (MS) is powerful because it can detect low concentrations in a volume limited sample, while providing a mass to charge ratio to identify compounds. Electrospray ionization (ESI) interfaces well with the flowing stream of CE, although care must be taken to ground the CE voltage before the ionization.88 Some common CE separation buffers, such as TES and borate, as well as SDS surfactants, are non-volatile and cause severe ion suppression with ESI.89 CE-MS was used to determine the concentration of neurotransmitters, metabolites, and drugs in adult Drosophila treated with methylphenidate, commonly called Ritalin.90 The amount of tyramine and octopamine increased after methylphenidate, but there were no changes in N-acetyl metabolites. As CE-MS grows and commercial instruments become more available, more applications will likely be developed for neurotransmitter tissue content measurements.
3.4 Biological insights gained from separations studies
Tissue content studies provide important insight into levels of neurotransmitters and drugs. For example, ratios of different neurotransmitters and metabolites have been determined and N-acetyl metabolites were identified for the first time, confirming monoamine metabolism is different in flies than mammals.77 Studies of hemolymph measured circulating amino acid levels and revealed that genetic mutations can dramatically alter these levels.86 Separations are also the only method to quantify how much drug gets to the brain when it is fed orally, and studies have confirmed about 2% of the oral dose reaches the brain.66 Here, we highlight some of the biological insights gained from these techniques.
3.4.1 Sex, regional, and life stage differences in neurotransmitter content
Tissue content studies have demonstrated that neurotransmitters vary by sex, region, and life stage. Dopamine tissue content was higher in Drosophila females,81 similar to rodent models, where evoked dopamine released was significantly higher in females.91 In adults, tissue content varied by brain region78, with 25-fold higher dopamine content in the PPM1 region, rich in dopamine neurons, compared to the central brain. When looking at neurotransmitter differences during development, dopamine and serotonin did not change but pupae had large amounts of tyramine but no octopamine.81 This finding is consistent with octopamine being important for locomotion and feeding since pupae are immobile and do not eat. Additionally, tyramine is a synthetic precursor to octopamine, thus tyramine levels may build up if octopamine is not being synthesized.
3.4.2 Histamine metabolism
Histamine is a transmitter in Drosophila, found in both the eyes and brain, that regulates visual transduction.92 Histamine is metabolized by β-alanylation, forming carcinine by N-β-alanyl-dopamine synthase (Ebony) which also converts dopamine to a β-alanyl dopamine (BADA). β-alanyl-dopamine hydrolase (Tan) converts carcinine and BADA back to histamine and dopamine. Histamine content was higher in the eyes compared to the brain and cuticle, but there were no significant differences in carcinine content.82 However, carcinine content was higher in tan3 mutant flies, especially in the eyes. Tan and Ebony are more highly expressed in the eye than the brain so these differences correspond to gene expression. Overall, these studies provided insight into histamine and dopamine metabolism.
3.4.3 Correlating drug and tissue content
Measuring the drugs that reach a brain in Drosophila is quite challenging and CE-MS allows both drug levels and neurotransmitter content to be correlated. The tissue content data validated that different feeding dosages changes the amount of methyphenidate in fly brains.90 Methylphenidate increased neurotransmitter levels with dose until the effect saturated after feeding around 20 mM. In addition, a hydroxylated metabolite of methylphenidate was identified, a different metabolism pathway than in mammals.
4. Future Directions for Neurotransmitter Detection in Drosophila
Research on neurochemical monitoring in Drosophila started as an analytical challenge, as there were not good existing methods to measure in the small brain of Drosophila. Even today, there are still several methodological challenges to be overcome to improve the use of Drosophila for studies of neurotransmission. In addition, there are biological challenges and advantages of Drosophila that can be harnessed to make it even more of a valuable model organism for neurotransmitter measurements. In this section, we address some of the future challenges and directions of Drosophila neurotransmitter research.
4.1 Challenge 1: Measurements in discrete regions
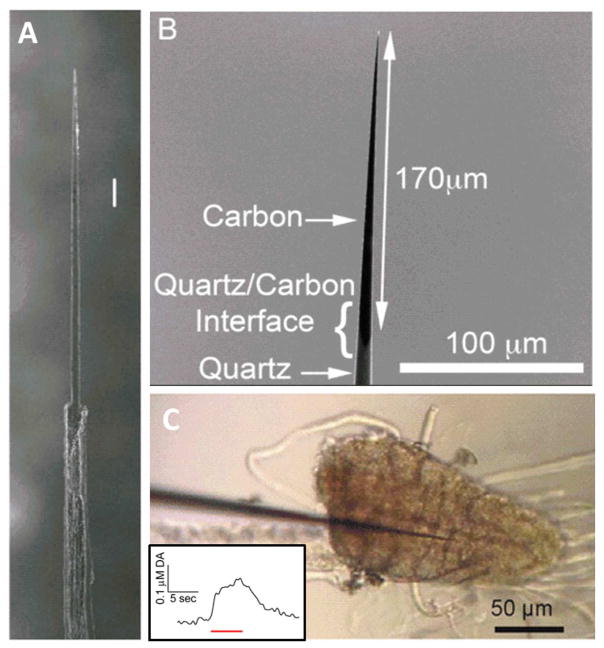
While CFMEs have been the standard electrochemical tool, the size of their tips is large compared to specific Drosophila regions. For example, the mushroom bodies of the adult brain have discrete parts including the vertical and medial lobes, calyx, and peduncle. Ideally, the electrode is localized in one discrete area to determine how different clusters of neurons control behaviors. These areas are only about 10 μm wide and the typical 7 μm diameter electrode is likely to destroy or displace much of the tissue. In addition, while octopamine have been measured in the central complex, it too is made up a fan shaped and ellipsoid body, and the ellipsoid body also has a diameter that is on the scale of 10 microns.60 Therefore, there is a push to develop smaller nanoelectrodes. One strategy is to etch carbon fibers, with a tip diameter less than a micron, that are insulated to form a disk electrode.93 Electrodes can be electrochemically or flame etched (Fig. 6A),94, 95 but both methods require much skill to obtain the perfect size tip. Etched electrodes have been used to measure exocytosis in a single synapses in cultured neurons.95 An alternative strategy is to use carbon nanopipettes, where carbon is grown on the inside of a pipette that is then etched. The Venton lab demonstrated CNPEs, with 200–400 nm tips, for larval VNC dopamine measurements (Fig. 6B–C).96 The challenge with all nanoelectrodes is that current scales with surface area in electrochemistry; thus, smaller electrodes give smaller signals and electrode development strategies are needed, such as the use of nanoparticles, to increase sensitivity. The ultimate goal is small, sensitive electrode localize in discrete regions or measure from a single neuron.
Figure 6.
Nanometer scale carbon electrodes. (A) Scanning electron microscopy (SEM) image of a flame-etched carbon electrode. Scale bar is 100 nm, tip is 1 μm. (B) SEM of carbon nanopipette electrode (CNPE, 50 nm tip diameter). (C) CNPE inserted into Drosophila VNC neuropils. Insert is dopamine after a 5 s continuous red light stimulation. Panel A is reprinted from ref. [94] and Panel B and C are reprinted and modified from ref. [96], both with permission of the American Chemical Society.
Genetic tools are also useful to reduce the number of active neurons to probe the response of single neurons or discrete regions. The split-GAL4 system separates the transcriptional GAL4 activator into two genes, both of which need to be expressed by hemi-drivers to drive UAS-gene activity. The split-GAL4 system has been used in genetic screens to pinpoint single neurons important for behaviors such as walking and sleep30, 97, 98. Thus, using the split-GAL4 system to drive light-activated channels would facilitate accurate, real-time measurement of neurotransmitter release from specific regions or even single neurons. In Drosophila, the exact number and distribution of neurons is conserved, which makes it possible to manipulate a single neuron reproducibly. With the co-expression of light-activated channels and RNAi-elements, which knockdown genes of interest, studies could tease out how genes regulate neuronal activity, neurotransmitter release, and behavior.
4.2 Challenge 2: In vivo measurements of physiologically-stimulated release
The first study of dopamine uptake in flies was actually performed in vivo,39 but all endogenous release experiments have been performed ex vivo with the brain removed from the fly. In vivo, flies can be prepared in a “fly collar”39 that immobilizes their heads but allows some behaviors. Several challenges are presented by in vivo preparations. First, the entire brain, as well as individual structures in Drosophila, are encased in a glial sheath,96 which is tough and must be removed to implant electrodes. While collagenase can be used to soften the tissue, it might also destroy neuronal connections and so is best avoided. Second, the brain can dry out, and there needs to be an electrical connection to a reference electrode, so a drop of solution is maintained on the head. Third, optogenetic studies might be challenging, as light needs to penetrate into the neurons. However, the red light that activates CsChrimson can penetrate to suitable depths for in vivo studies.99
Drosophila is a good model system for studying complex behaviors and in particular sensory responses.100, 101 Several studies have made use of the immobilized flies to measure flight behavior and identify important neural circuitry upon odor or visual stimulations.101-103 A future direction would be to use these head-fixed in vivo experiments to understand how physiological stimuli elicit neurotransmitter release.
4.3 Challenge 3: Expanding the toolbox to other neurotransmitters/neurochemicals
The most used tools for Drosophila neurotransmitter measurements are electrochemical measurements and separations, which are useful but have limitations. Direct electrochemistry is limited to electroactive species, or a biosensor can be constructed to turn non-electroactive molecules into electroactive products.104 For separations, electrochemical detection is similarly limited to electroactive species and fluorescence detection requires molecules that can be easily tagged (typically amines).75, 83 Mass spectrometry detection better identifies analytes but for electrospray, buffers and salts increase the surface tension of droplets and reduce the volatility, decreasing sensitivity; thus, buffer optimization is critical and more volatile buffers are preferred. Other techniques being developed, such as optical sensors and mass spectrometry imaging, have the potential to detect other analytes and multiple analytes simultaneously.
Optical methods have been recently developed for monitoring neurotransmission. Fluorescent dyes have been synthesized to detect neurotransmitters in the synapse,105, 106 but they have not yet been applied to Drosophila. These dyes, called Neurosensors, have been developed for dopamine and norepinephrine106, and there is another class of dyes that respond to zinc, glutamate, and the pH shift of exocytosis to monitor only neurotransmitters in the synapse107. Another approach is genetically encoded biosensors, that use modified proteins that change fluorescence when neurotransmitter binds.108 Genetically-encoded biosensors have been made for glutamate, the most abundant excitatory neurotransmitter 109. These genetically-encoded biosensors provide a unique understanding of the neurotransmitter available at the synapse, which is complementary to the information about neurotransmitter overflow in the extracellular space that is typically measured with microelectrodes. Synaptic ATP levels have been monitored in real time in Drosophila and C. elegans. Genetically-encoded biosensors have rapid temporal resolution and high spatial resolution, but are best used in ex vivo preparations, which pairs well with whole brain ex vivo Drosophila preparations. More genetically encoded sensors are being developed and protein engineering performed to expand the available colors to facilitate multi-analyte measurements110.
Another possible method to understand more about neurotransmission is mass spectrometry imaging. Mass spectrometry provides the molecular mass of the compounds it detects, and MS/MS techniques are even more powerful to fragment and identify molecular structures. Matrix-assisted laser desorption ionization (MALDI) imaging has been used for Drosophila, mostly to identify lipids in the brain111. These lipids are important for controlling exocytosis and thus understanding them is crucial to understanding the process of neurotransmission. Secondary ion mass spectrometry (SIMS) imaging has also been used to identify the spatial distribution of lipids, eye pigments, and diacylglycerides. Mass spectrometry provides good spatial resolution, but is typically performed on fixed tissue112.
4.4 Challenge 4: Studies of disease models related to human health
Many Drosophila models have been made of human diseases; Drosophila models of Alzheimer’s, Parkinson disease, and restless leg syndrome exist that have similar phenotypes to higher order model systems.5, 6, 113 For the example of Parkinson disease, several genetic mutation models have been made, with mutations in parkin or pink1 genes that mimic mammalian mutants. Recent evidence in pink1 mutants using dopamine clearance and tissue content measurements, as well as behavioral and genetic studies, suggests there are early changes in DAT expression and dopamine levels during early adulthood in these Parkinson models114. Genetic modifications of Drosophila are relatively easy, and models can be made with either permanent or inducible gene inactivation.115 The advantage of Drosophila is the well mapped anatomy; for example, if you want to study cell death during a neurodegenerative disease, you can easily track exactly which populations die. By expanding neurotransmitter measurements into these disease models, we will better understand exactly how disease phenotypes affect neurotransmission. Both real-time measurements and tissue content measurements are key to pinpointing how neurotransmission malfunctions during diseases.
6. Conclusions
Drosophila is an important model system for understanding neurotransmitter changes because it has a high homology to mammals, relatively complex behaviors, and a well-defined anatomy. Real-time measurements of neurotransmitters can be performed in Drosophila with electrochemistry and separation methods are important for understanding tissue content. Overall, the initial studies of neurotransmission in Drosophila have shown it is similar to mammals, regulated by autoreceptors and transporters, and thus it will be an important model system for understanding monoamine regulation and dysfunction in disease.
Acknowledgments
Drosophila work in the Venton lab is funded by NIH R01MH085159.
Footnotes
Author Contributions: M.S. wrote sections 2 and 3 and part of the introduction. J.M.C. wrote parts of section 1 and 4 and made figure 2. B.J.V wrote section 4 and supervised the work.
References
- 1.Sokolowski MB. Drosophila: genetics meets behaviour. Nature Reviews Genetics. 2001;2:879–890. doi: 10.1038/35098592. [DOI] [PubMed] [Google Scholar]
- 2.Johnson TE. Advantages and disadvantages of Caenorhabditis elegans for aging research. Experimental gerontology. 2003;38:1329–1332. doi: 10.1016/j.exger.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends in neurosciences. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tissenbaum HA. Using C. elegans for aging research. Invertebrate reproduction & development. 2015;59:59–63. doi: 10.1080/07924259.2014.940470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusing K, Voigt A, Schulz JB. Drosophila melanogaster as a model organism for Alzheimer’s disease. Molecular neurodegeneration. 2013;8:35. doi: 10.1186/1750-1326-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo M. Drosophila as a model to study mitochondrial dysfunction in Parkinson’s disease. Cold Spring Harbor perspectives in medicine. 2012;2:a009944. doi: 10.1101/cshperspect.a009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin GM, Yandell MD, Wortman JR, Gabor GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nature Reviews Genetics. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 9.Weigmann K, Klapper R, Strasser T, Rickert C, Technau G, Jackle H, Janning W, Klambt C. FlyMove–a new way to look at development of Drosophila. Trends in Genetics. 2003;19:310–311. doi: 10.1016/S0168-9525(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 10.Nassif C, Noveen A, Hartenstein V. Early development of the Drosophila brain: III. The pattern of neuropile founder tracts during the larval period. Journal of Comparative Neurology. 2003;455:417–434. doi: 10.1002/cne.10482. [DOI] [PubMed] [Google Scholar]
- 11.Chiang A-S, Lin C-Y, Chuang C-C, Chang H-M, Hsieh C-H, Yeh C-W, Shih C-T, Wu J-J, Wang G-T, Chen Y-C. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Current Biology. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, Ferveur J-F, Guo A. Increased dopamine level enhances male–male courtship in Drosophila. Journal of Neuroscience. 2008;28:5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iche-Torres M, Cassar M, Strauss R, Preat T. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proceedings of the National Academy of Sciences. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, Kume K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nature neuroscience. 2012;15:1516–1523. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- 15.Van Swinderen B, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proceedings of the Royal Society of London B: Biological Sciences. 2011 doi: 10.1098/rspb.2010.2564. rspb20102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in neural circuits. 2009;3 doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. Journal of Comparative Neurology. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nature neuroscience. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 19.Vogt K, Schnaitmann C, Dylla KV, Knapek S, Aso Y, Rubin GM, Tanimoto H. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife. 2014;3:e02395. doi: 10.7554/eLife.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PloS one. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitaraman D, Zars M, LaFerriere H, Chen Y-C, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proceedings of the National Academy of Sciences. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR. Tools for neuroanatomy and neurogenetics in Drosophila. Proceedings of the National Academy of Sciences. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenett A, Rubin GM, Ngo T-T, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J. A GAL4-driver line resource for Drosophila neurobiology. Cell reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi S, Ito K, Sado Y, Taniguchi M, Akimoto A, Takeuchi H, Aigaki T, Matsuzaki F, Nakagoshi H, Tanimura T. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. genesis. 2002;34:58–61. doi: 10.1002/gene.10137. [DOI] [PubMed] [Google Scholar]
- 26.Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 27.Ni J-Q, Zhou R, Czech B, Liu L-P, Holderbaum L, Yang-Zhou D, Shim H-S, Tao R, Handler D, Karpowicz P. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nature methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitaraman D, Aso Y, Rubin GM, Nitabach MN. Control of sleep by dopaminergic inputs to the Drosophila mushroom body. Frontiers in neural circuits. 2015;9 doi: 10.3389/fncir.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogt K, Aso Y, Hige T, Knapek S, Ichinose T, Friedrich AB, Turner GC, Rubin GM, Tanimoto H. Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. Elife. 2016;5:e14009. doi: 10.7554/eLife.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rein K, Zockler M, Mader MT, Grubel C, Heisenberg M. The Drosophila standard brain. Current Biology. 2002;12:227–231. doi: 10.1016/s0960-9822(02)00656-5. [DOI] [PubMed] [Google Scholar]
- 33.Huffman ML, Venton BJ. Carbon-fiber microelectrodes for in vivo applications. Analyst. 2009;134:18–24. doi: 10.1039/b807563h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen MD, Venton BJ. Fast-scan cyclic voltammetry for the characterization of rapid adenosine release. Computational and structural biotechnology journal. 2015;13:47–54. doi: 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical chemistry. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 36.Palij P, Stamford JA. Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry: 1. Characterisation of evoked noradrenaline efflux and uptake from nerve terminals in the bed nucleus of stria terminalis, pars ventralis. Brain research. 1992;587:137–146. doi: 10.1016/0006-8993(92)91438-k. [DOI] [PubMed] [Google Scholar]
- 37.Pihel K, Hsieh S, Jorgenson JW, Wightman RM. Electrochemical detection of histamine and 5-hydroxytryptamine at isolated mast cells. Analytical chemistry. 1995;67:4514–4521. doi: 10.1021/ac00120a014. [DOI] [PubMed] [Google Scholar]
- 38.Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, Sombers LA. Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Analytical chemistry. 2010;82:5205–5210. doi: 10.1021/ac100536s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makos MA, Kim Y-C, Han K-A, Heien ML, Ewing AG. In vivo electrochemical measurements of exogenously applied dopamine in Drosophila melanogaster. Analytical chemistry. 2009;81:1848–1854. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vickrey TL, Xiao N, Venton BJ. Kinetics of the dopamine transporter in Drosophila larva. ACS chemical neuroscience. 2013;4:832–837. doi: 10.1021/cn400019q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nature neuroscience. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nature neuroscience. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, De Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature protocols. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borue X, Cooper S, Hirsh J, Condron B, Venton BJ. Quantitative evaluation of serotonin release and clearance in Drosophila. Journal of neuroscience methods. 2009;179:300–308. doi: 10.1016/j.jneumeth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borue X, Condron B, Venton BJ. Both synthesis and reuptake are critical for replenishing the releasable serotonin pool in Drosophila. Journal of neurochemistry. 2010;113:188–199. doi: 10.1111/j.1471-4159.2010.06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vickrey TL, Condron B, Venton BJ. Detection of endogenous dopamine changes in Drosophila melanogaster using fast-scan cyclic voltammetry. Analytical chemistry. 2009;81:9306–9313. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makos MA, Omiatek DM, Ewing AG, Heien ML. Development and characterization of a voltammetric carbon-fiber microelectrode pH sensor. Langmuir. 2010;26:10386–10391. doi: 10.1021/la100134r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majdi S, Berglund EC, Dunevall J, Oleinick AI, Amatore C, Krantz DE, Ewing AG. Electrochemical measurements of optogenetically stimulated quantal amine release from single nerve cell varicosities in Drosophila larvae. Angewandte Chemie. 2015;127:13813–13816. doi: 10.1002/anie.201506743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, Budnik V. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nature neuroscience. 2011;14:190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nature neuroscience. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Privman E, Venton BJ. Comparison of dopamine kinetics in the larval Drosophila ventral nerve cord and protocerebrum with improved optogenetic stimulation. Journal of neurochemistry. 2015;135:695–704. doi: 10.1111/jnc.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng H, Madisen L. Mouse transgenic approaches in optogenetics. Progress in brain research. 2012;196:193. doi: 10.1016/B978-0-444-59426-6.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z. Independent optical excitation of distinct neural populations. Nature methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohsaka H, Guertin AP, Nose A. Neural circuits underlying fly larval locomotion. Current pharmaceutical design. 2017;23:1722–1733. doi: 10.2174/1381612822666161208120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao N, Privman E, Venton BJ. Optogenetic control of serotonin and dopamine release in Drosophila larvae. ACS chemical neuroscience. 2014;5:666–673. doi: 10.1021/cn500044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyakurel P, Privman Champaloux E, Venton BJ. Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord. ACS chemical neuroscience. 2016;7:1112–1119. doi: 10.1021/acschemneuro.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. Journal of neurophysiology. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao N, Venton BJ. Characterization of dopamine releasable and reserve pools in Drosophila larvae using ATP/P2X2-mediated stimulation. Journal of neurochemistry. 2015;134:445–454. doi: 10.1111/jnc.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. α7 and non-α7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. European Journal of Neuroscience. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 60.Fuenzalida-Uribe N, Meza RC, Hoffmann HA, Varas R, Campusano JM. nAChR-induced octopamine release mediates the effect of nicotine on a startle response in Drosophila melanogaster. Journal of neurochemistry. 2013;125:281–290. doi: 10.1111/jnc.12161. [DOI] [PubMed] [Google Scholar]
- 61.Pyakurel P, Shin M, Venton BJ. Nicotinic acetylcholine receptor (nAChR) mediated dopamine release in larval Drosophila melanogaster. Neurochemistry International. 2018 doi: 10.1016/j.neuint.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makos MA, Han K-A, Heien ML, Ewing AG. Using in vivo electrochemistry to study the physiological effects of cocaine and other stimulants on the Drosophila melanogaster dopamine transporter. ACS chemical neuroscience. 2009;1:74–83. doi: 10.1021/cn900017w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Molecular pharmacology. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 64.Carrey NJ, Wiggins DM, Milin RP. Pharmacological treatment of psychiatric disorders in children and adolescents. Drugs. 1996;51:750–759. doi: 10.2165/00003495-199651050-00004. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R. Is methylphenidate like cocaine?: Studies on their pharmacokinetics and distribution in the human brain. Archives of general psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 66.Berglund EC, Makos MA, Keighron JD, Phan N, Heien ML, Ewing AG. Oral administration of methylphenidate blocks the effect of cocaine on uptake at the Drosophila dopamine transporter. ACS chemical neuroscience. 2013;4:566–574. doi: 10.1021/cn3002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vickrey TL, Venton BJ. Drosophila Dopamine2-like receptors function as autoreceptors. ACS chemical neuroscience. 2011;2:723–729. doi: 10.1021/cn200057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proceedings of the National Academy of Sciences. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proceedings of the national academy of sciences. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draper I, Kurshan PT, McBride E, Jackson FR, Kopin AS. Locomotor activity is regulated by D2-like receptors in Drosophila: An anatomic and functional analysis. Developmental neurobiology. 2007;67:378–393. doi: 10.1002/dneu.20355. [DOI] [PubMed] [Google Scholar]
- 71.Hardie SL, Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. Journal of neuroscience methods. 2006;153:243–249. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Chen A, Ng F, Lebestky T, Grygoruk A, Djapri C, Lawal HO, Zaveri HA, Mehanzel F, Najibi R, Seidman G. Dispensable, redundant, complementary, and cooperative roles of dopamine, octopamine, and serotonin in Drosophila melanogaster. Genetics. 2013;193:159–176. doi: 10.1534/genetics.112.142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kostal V, Katzenmeyer J, Arriaga EA. Capillary electrophoresis in bioanalysis. Analytical chemistry. 2008;80:4533–4550. doi: 10.1021/ac8007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuklinski NJ, Berglund EC, Ewing AG. Micellar capillary electrophoresis–Electrochemical detection of neurochemicals from Drosophila. Journal of separation science. 2010;33:388–393. doi: 10.1002/jssc.200900634. [DOI] [PubMed] [Google Scholar]
- 75.Altria KD. Capillary electrophoresis guidebook: principles, operation, and applications. Vol. 52. Springer Science & Business Media; 1996. [Google Scholar]
- 76.Ream PJ, Suljak SW, Ewing AG, Han K-A. Micellar Electrokinetic Capillary Chromatography-Electrochemical Detection for Analysis of Biogenic Amines in Drosophila m elanogaster. Analytical chemistry. 2003;75:3972–3978. doi: 10.1021/ac034219i. [DOI] [PubMed] [Google Scholar]
- 77.Paxon TL, Powell PR, Lee H-G, Han K-A, Ewing AG. Microcolumn separation of amine metabolites in the fruit fly. Analytical chemistry. 2005;77:5349–5355. doi: 10.1021/ac050474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuklinski NJ, Berglund EC, Engelbrektsson J, Ewing AG. Biogenic Amines in Microdissected Brain Regions of Drosophila melanogaster Measured with Micellar Electrokinetic Capillary Chromatography · Electrochemical Detection. Analytical chemistry. 2010;82:7729–7735. doi: 10.1021/ac101603d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berglund EC, Kuklinski NJ, Karagunduz E, Ucar K, Hanrieder, Ewing AG. Freeze-Drying as Sample Preparation for Micellar Electrokinetic Capillary Chromatography–Electrochemical Separations of Neurochemicals in Drosophila Brains. Analytical chemistry. 2013;85:2841–2846. doi: 10.1021/ac303377x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang H, Vickrey TL, Venton BJ. Analysis of biogenic amines in a single Drosophila larva brain by capillary electrophoresis with fast-scan cyclic voltammetry detection. Analytical chemistry. 2011;83:2258–2264. doi: 10.1021/ac103092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Denno ME, Privman E, Venton BJ. Analysis of neurotransmitter tissue content of Drosophila melanogaster in different life stages. ACS chemical neuroscience. 2014;6:117–123. doi: 10.1021/cn500261e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denno ME, Privman E, Borman RP, Wolin DC, Venton BJ. Quantification of histamine and carcinine in Drosophila melanogaster tissues. ACS chemical neuroscience. 2016;7:407–414. doi: 10.1021/acschemneuro.5b00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergquist J, Vona MJ, Stiller C–O, O’Connor WT, Falkenberg T, Ekman R. Capillary electrophoresis with laser-induced fluorescence detection: a sensitive method for monitoring extracellular concentrations of amino acids in the periaqueductal grey matter. Journal of neuroscience methods. 1996;65:33–42. doi: 10.1016/0165-0270(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 84.Kaul S, Faiman MD, Lunte CE. Determination of GABA, glutamate and carbamathione in brain microdialysis samples by capillary electrophoresis with fluorescence detection. Electrophoresis. 2011;32:284–291. doi: 10.1002/elps.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piyankarage SC, Featherstone DE, Shippy SA. Nanoliter hemolymph sampling and analysis of individual adult Drosophila melanogaster. Analytical chemistry. 2012;84:4460–4466. doi: 10.1021/ac3002319. [DOI] [PubMed] [Google Scholar]
- 86.Piyankarage SC, Augustin H, Grosjean Y, Featherstone DE, Shippy SA. Hemolymph amino acid analysis of individual Drosophila larvae. Analytical chemistry. 2008;80:1201–1207. doi: 10.1021/ac701785z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borra S, Featherstone DE, Shippy SA. Total cysteine and glutathione determination in hemolymph of individual adult D. melanogaster. Analytica chimica acta. 2015;853:660–667. doi: 10.1016/j.aca.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Risley EMJM. Electrospray Ionization Interface Development for Capillary. Capillary Electrophoresis-Mass Spectrometry (CE-MS): Principles and Applications. 2016:7. [Google Scholar]
- 89.Whatley H. Clinical and Forensic Applications of Capillary Electrophoresis. Springer; 2001. Basic principles and modes of capillary electrophoresis; pp. 21–58. [Google Scholar]
- 90.Phan NT, Hanrieder, Berglund EC, Ewing AG. Capillary Electrophoresis–Mass Spectrometry-Based Detection of Drugs and Neurotransmitters in Drosophila Brain. Analytical chemistry. 2013;85:8448–8454. doi: 10.1021/ac401920v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walker Q, Rooney M, Wightman R, Kuhn C. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 1999;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- 92.Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 93.Strein TG, Ewing AG. Characterization of submicron-sized carbon electrodes insulated with a phenol-allylphenol copolymer. Analytical Chemistry. 1992;64:1368–1373. [Google Scholar]
- 94.Strand AM, Venton BJ. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Analytical chemistry. 2008;80:3708–3715. doi: 10.1021/ac8001275. [DOI] [PubMed] [Google Scholar]
- 95.Li YT, Zhang SH, Wang L, Xiao RR, Liu W, Zhang XW, Zhou Z, Amatore C, Huang WH. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angewandte Chemie International Edition. 2014;53:12456–12460. doi: 10.1002/anie.201404744. [DOI] [PubMed] [Google Scholar]
- 96.Rees HR, Anderson SE, Privman E, Bau HH, Venton BJ. Carbon nanopipette electrodes for dopamine detection in Drosophila. Analytical chemistry. 2015;87:3849–3855. doi: 10.1021/ac504596y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal control of Drosophila walking direction. Science. 2014;344:97–101. doi: 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- 98.Sitaraman D, Aso Y, Jin X, Chen N, Felix M, Rubin GM, Nitabach MN. Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Current Biology. 2015;25:2915–2927. doi: 10.1016/j.cub.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wietek J, Prigge M. Enhancing channelrhodopsins: an overview. Optogenetics: Methods and Protocols. 2016:141–165. doi: 10.1007/978-1-4939-3512-3_10. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto S, Seto ES. Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Experimental animals. 2014;63:107–119. doi: 10.1538/expanim.63.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reiser MB, Dickinson MH. A modular display system for insect behavioral neuroscience. Journal of neuroscience methods. 2008;167:127–139. doi: 10.1016/j.jneumeth.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Wasserman SM, Aptekar JW, Lu P, Nguyen J, Wang AL, Keles MF, Grygoruk A, Krantz DE, Larsen C, Frye MA. Olfactory neuromodulation of motion vision circuitry in Drosophila. Current Biology. 2015;25:467–472. doi: 10.1016/j.cub.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aptekar JW, Keleş MF, Lu PM, Zolotova NM, Frye MA. Neurons forming optic glomeruli compute figure–ground discriminations in Drosophila. Journal of Neuroscience. 2015;35:7587–7599. doi: 10.1523/JNEUROSCI.0652-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ganesana M, Lee ST, Wang Y, Venton BJ. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Analytical chemistry. 2016;89:314–341. doi: 10.1021/acs.analchem.6b04278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hettie KS, Glass TE. Turn-On Near-Infrared Fluorescent Sensor for Selectively Imaging Serotonin. ACS chemical neuroscience. 2015;7:21–25. doi: 10.1021/acschemneuro.5b00235. [DOI] [PubMed] [Google Scholar]
- 106.Hettie KS, Liu X, Gillis KD, Glass TE. Selective catecholamine recognition with NeuroSensor 521: a fluorescent sensor for the visualization of norepinephrine in fixed and live cells. ACS chemical neuroscience. 2013;4:918–923. doi: 10.1021/cn300227m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin C, Huo F, Cooley NP, Spencer D, Bartholomew K, Barnes CL, Glass TE. A Two-Input Fluorescent Logic Gate for Glutamate and Zinc. ACS Chemical Neuroscience. 2017 doi: 10.1021/acschemneuro.6b00420. [DOI] [PubMed] [Google Scholar]
- 108.Liang R, Broussard GJ, Tian L. Imaging chemical neurotransmission with genetically encoded fluorescent sensors. ACS chemical neuroscience. 2015;6:84–93. doi: 10.1021/cn500280k. [DOI] [PubMed] [Google Scholar]
- 109.Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T-W, Bargmann CI. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nature methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akerboom J, Calderon NC, Tian L, Wabnig S, Prigge M, Tolo J, Gordus A, Orger MB, Severi KE, Macklin JJ. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in molecular neuroscience. 2013;6 doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niehoff A-C, Kettling H, Pirkl A, Chiang YN, Dreisewerd K, Yew JY. Analysis of Drosophila lipids by matrix-assisted laser desorption/ionization mass spectrometric imaging. Analytical chemistry. 2014;86:11086–11092. doi: 10.1021/ac503171f. [DOI] [PubMed] [Google Scholar]
- 112.Phan NT, Fletcher JS, Sjovall P, Ewing AG. ToF-SIMS imaging of lipids and lipid related compounds in Drosophila brain. Surface and Interface Analysis. 2014;46:123–126. doi: 10.1002/sia.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freeman A, Pranski E, Miller RD, Radmard S, Bernhard D, Jinnah H, Betarbet R, Rye DB, Sanyal S. Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Current Biology. 2012;22:1142–1148. doi: 10.1016/j.cub.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molina-Mateo D, Fuenzalida-Uribe N, Hidalgo S, Molina-Fernandez C, Abarca J, Zarate RV, Escandon M, Figueroa R, Tevy MF, Campusano JM. Characterization of a presymptomatic stage in a Drosophila Parkinson’s disease model: Unveiling dopaminergic compensatory mechanisms. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2017;1863:2882–2890. doi: 10.1016/j.bbadis.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 115.Roman G, Endo K, Zong L, Davis RL. P {Switch}, a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proceedings of the national academy of sciences. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]