Abstract
Over a lifetime, humans build relationships with family, friends, and partners that are critically important for our mental and physical health. Unlike commonly used laboratory mice and rats, Microtine rodents provide a unique model to study the neurobiology underlying pair bonding and the selective attachments that form between adults. Comparisons between monogamous prairie voles and the closely related but nonmonogamous meadow and montane voles have revealed that brain-region-specific neuropeptide receptor patterning modulates social behavior between and within species. In particular, diversity in vasopressin 1a receptor (V1aR) distribution has been linked to individual and species differences in monogamy-related behaviors such as partner preference, mate guarding, and space use. Given the importance of differential receptor expression for regulating social behavior, a critical question has emerged: What are the genetic and epigenetic mechanisms that underlie brain-region-specific receptor patterns? This review will summarize what is known about how the vasopressin (AVP)-V1aR axis regulates social behaviors via signaling in discrete brain regions. From this work, we propose that brain-region-specific regulatory mechanisms facilitate robust evolvability of V1aR expression to generate diverse sociobehavioral traits. Translationally, we provide a perspective on how these studies have contributed to our understanding of human social behaviors and how brain-region-specific regulatory mechanisms might be harnessed for targeted therapies to treat social deficits in psychiatric disorders such as depression, complicated grief, and autism spectrum disorder.
Keywords: Prairie vole, vasopressin, epigenetics, regulatory mechanisms, social behavior, AVP, V1aR, microsatellite, evolvability, pair bonding, monogamy
INTRODUCTION
A key aspect of human nature is the selective social bonds that we form with a variety of relationship partners, including family, friends, and romantic partners. These relationships are crucial for our mental and physical health, yet the selective nature of these bonds is not recapitulated in commonly used animal models such as mice and rats.1–8 Instead, monogamous prairie voles (Microtus ochrogaster) provide a laboratory-amenable species for studying the neural and molecular basis of selective social attachment between adults9–12 (Figure 1a). This animal model was identified when Lowell Getz, an ecologist studying rodent population cycles in the grasslands of the United States, noticed that he repeatedly caught the same two prairie voles in his field study traps.13 Subsequently, Sue Carter and colleagues established that voles could be bred and manipulated in a laboratory setting, similar to mice and rats. To characterize monogamous behaviors in prairie voles, Carter and colleagues developed behavioral assays that quantified the suite of behavioral changes induced by pair bond formation such as partner preference, mate guarding, and biparental care.14
Figure 1.
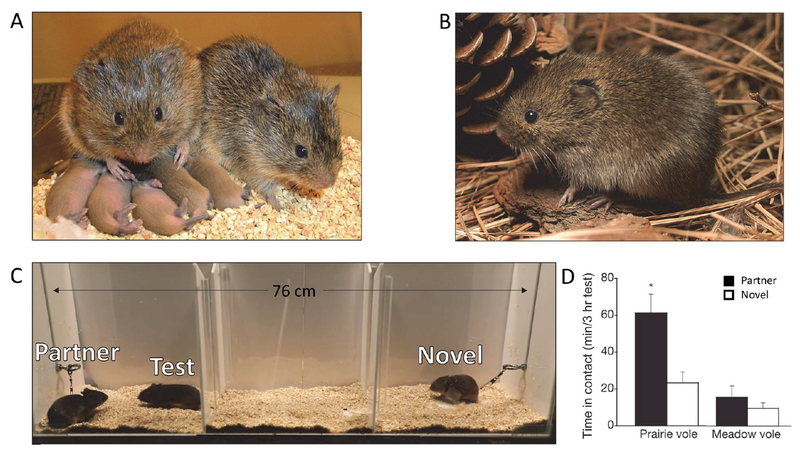
Monogamous prairie voles and nonmonogamous meadow voles provide comparative models for understanding pair bonding and attachment behaviors in adults. (A) A prairie vole pair with offspring. Photo credit: Todd Ahern. (B) A solitary meadow vole. Photo credit: John White. (C) The partner preference test provides a proxy measure for pair bond formation and expression. A test vole is allowed free range of a three-chambered apparatus with their partner and a novel, opposite-sex vole tethered on opposite ends. After 3 h, a pair-bonded test vole will spend the majority of their time huddled with partner over the stranger. Photo credit: Paul Muhlrad. (D) Quantification of partner preference behavior for prairie and meadow voles. Adapted from ref 64 with permission from Macmillan Publishers Ltd., copyright 2004.
In particular, the partner preference test has been extensively used to delineate the neurochemistry involved in forming a partner bond. In a partner preference test, the partner vole and a novel, opposite-sex individual are tethered at opposite ends of a three-chambered apparatus. The test vole can freely explore the apparatus for 3 h, and the amount of time interacting with each tethered individual is recorded. Pair-bonded voles will spend the majority of their time huddled with their partner while an unbonded vole will not show a preference for either individual (Figure 1c). Using this test, Carter and colleagues demonstrated that mating facilitates partner preference, enabling future genetic and pharmacological gain and loss of function experiments to ask whether partner preference can be induced in the absence of mating or blocked in animals that had mated.14,15 For instance, intracerebroventricular (i.c.v.) infusion of oxytocin or arginine—vasopressin (AVP) induces bond formation in sexually naïve animals while blocking signaling at their respective receptors inhibits preference in mated animals.16–19 These and other experiments have shown that signaling initiated by oxytocin, dopamine, endogenous opioids, and AVP are required for partner preference and other component behaviors of monogamy. This work is extensively reviewed elsewhere.20–28
To identify the species-specific characteristics of these neuromodulatory systems that explain differences in social attachment behaviors, researchers compared prairie voles with the closely related but nonmonogamous meadow voles (Microtus pennsylvanicus) or montane voles (Microtus montanus)29,30 (Figure 1b). In stark contrast to prairie voles, meadow and montane voles are asocial in the partner preference test and will spend the majority of their time in the middle, neutral chamber (Figure 1d). Over the last three decades, substantial evidence supports the overall hypothesis that species and individual differences in partner preference and other social behaviors are influenced by species-specific patterning and densities of neuromodulator receptors.31–35
In this review, we focus on how the density and distribution of vasopressin receptor (V1aR) directly modulates multiple component behaviors of monogamy. We summarize the work that delineates the role of V1aR signaling in specific brain regions to modulate the component behaviors that underlie monogamy in prairie voles and discuss the regulatory mechanisms that drive diversity in receptor expression patterns across and within species. From this work, we propose that species-specific social behaviors have evolved due to the brain-region-specific mechanisms underlying V1aR expression. Finally, we offer a perspective on how this has enriched our understanding of the molecular basis of selective attachments in humans and how brain-region-specific manipulations of V1aR, or similar receptors, might be harnessed for targeted therapies in a variety of psychiatric disorders.
AVP-V1AR AXIS REGULATES SOCIAL BEHAVIORS VIA SIGNALING IN DISCRETE BRAIN REGIONS
AVP and V1aR are essential for modulating species-typical male social behaviors across vertebrates, including social attachment, aggression, and parental care.23,36–52 AVP acts on 3 receptors, two subtypes of V1 receptor, V1a and V1b, and the V2 receptor. AVP also has low affinity for oxytocin receptors, resulting in potential cross-reactivity.53 The experiments reviewed here focus on the AVP-V1aR axis as this receptor has the widest distribution in the brain and has been implicated in male social behaviors across diverse taxa.24
In male prairie voles, AVP mediates preference formation and mate guarding, but it was not initially clear if this species-typical social behavior was due to bulk release of AVP or species-specific signaling activity. To test if AVP release explained differences in social behavior across species, montane voles and sexually naïve prairie voles were administered an i.c.v. infusion of AVP by minipump. In sexually naïve male prairie voles, this treatment was sufficient to induce a partner preference and aggression toward an intruder.52 However, in the nonmonogamous montane vole, exogenous AVP did not induce partner preference.55 These results indicated that bulk AVP release alone is not responsible for species differences in social behaviors.
To test whether AVP signaling via V1aR modulates pair-bonding, a V1aR antagonist was infused i.c.v. in male prairie voles. Post infusion, these males cohabitated and mated with a receptive female for 24 h.52 Unlike their control counterparts, males treated with the antagonist failed to demonstrate partner preference and did not display mating-induced increases in aggression.52 These experiments confirm the importance of intact AVP-V1aR signaling for modulating multiple monogamous behaviors.
Given the key role of V1aR in partner preference, Insel, Wang, and colleagues compared V1aR expression and patterning across monogamous and nonmonogamous vole species56,57 (Figures 2a and b). They identified striking differences in the distribution and density of V1aR in monogamous and polygamous vole species. The direction of receptor density differences varied across brain regions; for instance, higher V1aR was detected in the ventral pallidum (referred to as the diagonal band in earlier publications) in monogamous prairie voles, while in promiscuous montane voles, higher levels were observed in the lateral septum.57 With multiple differences in V1aR patterning throughout the brain, researchers initially focused on regions that also had robust activation following mating using an immediate early gene, c-fos, as a proxy for neuronal activity.58–62 V1aR-expressing brain regions that had increased c-fos expression following mating in prairie voles included the medial dorsal thalamus, medial amygdala, and the ventral pallidum. A V1aR antagonist was administered by local injection into each of these brain regions. Of these, only bilateral blockade of V1aR in the ventral pallidum inhibited partner preference.62 This result suggests that AVP-V1aR signaling modulates species-typical social behaviors in a brain region dependent manner.
Figure 2.
Brain-region-specific V1aR densities modulate different social behaviors. Autoradiograms of V1aR densities in prairie voles (A) and meadow voles (B). The lateral septum (LS) and ventral pallidum (VP) are two brain regions implicated in pair bonding. Adapted from ref 63 with permission from Wiley-Liss, Inc., copyright 2004. (C) Autoradiogram of V1aR density in the VP of meadow voles after viral mediated gene transfer. Adapted from ref 64 with permission from Macmillan Publishers Ltd., copyright 2004. (D) Increasing VP V1aR to levels comparable with prairie voles increased the proportion of time meadow voles spent with their partner such that they display a partner preference. Adapted from ref 64 with permission from Macmillan Publishers Ltd., copyright 2004. (E) Increased V1aR density in the anterior hypothalamus (AH) of a pair bonded versus sexually naïve male prairie voles, quantified in (F). Scale bar = 1 mm. Adapted with permission from ref 66.
The importance of the ventral pallidum for regulating partner preference prompted further functional experiments to determine how V1aR contributes to differences in social behaviors between monogamous and nonmonogamous vole species.52,54,55 A series of gain of function experiments demonstrated that ventral pallidum V1aR modulates partner preference. Using adeno-associated viral (AAV) vector-mediated gene delivery, the V1aR gene was injected directly in the ventral pallidum of male prairie voles. This treatment nearly doubled the amount of V1aR in the ventral pallidum and, even in the absence of mating, these males demonstrated partner preference and more general affiliative behaviors than their control counterparts.55 When AAV-mediated gene transfer was used to increase ventral pallidal V1aR density of meadow voles to levels typically observed in prairie voles, this nonmonogamous species exhibited partner preference64 (Figures 2c and d). Loss of function experiments complement these results. RNA interference (RNAi) is a technique used to degrade a specific target mRNA, resulting in decreased levels of the associated gene product. A cocktail of AAV-packaged short hairpin RNAs targeting avpr1a was injected into the ventral pallidum of prairie voles, resulting in an approximately 30% maximal reduction in V1aR expression. The RNAi males with the lowest V1aR densities demonstrated impaired partner preference.65 Together, these functional manipulations demonstrate that the levels of V1aR in the ventral pallidum are a key driver for attachment behaviors irrespective of endogenous AVP levels. These results are parsimonious with a model in which selection for the expression of a single gene, avpr1a, in a single brain region, led to the ability to form a partner preference.
V1aR signaling is also crucial for modulating the onset of selective aggression following pair bonding. In bonded male prairie voles, the experience-dependent onset of selective aggression toward conspecifics occurs as a result of increased levels of V1aR in the anterior hypothalamus following bond formation66 (Figures 2e and f). In gain of function experiments, overexpression of V1aR using AAVs or injection of a V1aR agonist into the anterior hypothalamus of sexually naïve males induced aggression toward novel females. In contrast, local infusion of a V1aR antagonist into the same brain region reduced aggression in pair bonded males.66 Together, these results indicate that experience-dependent changes in anterior hypothalamus V1aR levels modulate aggressive behaviors in bonded male prairie voles and that the mechanistic basis of AVP-modulated aggression is independent of that underlying partner preference.
Another distinguishing feature of social monogamy, paternal care, is also modulated by AVP-V1aR signaling.25 Pharmacological manipulations of AVP-V1aR signaling in the lateral septum of male prairie voles modulate behaviors such as grooming, crouching over, and retrieving pups. When AVP was injected into the lateral septum of sexually naïve male prairie voles, these animals increased parental behaviors such as crouching over and contacting pups. In contrast, when a V1aR antagonist was administered into the lateral septum of bonded male prairie voles, these animals spent less time performing parental behaviors.67 Neither of these treatments affected pup retrieval. This result suggests that the component behaviors underlying monogamy and parental behavior may have distinct biological underpinnings. The dissociated paternal phenotypes seen in prairie voles are paralleled by recent genetic studies of paternal behaviors in a wild mouse species (Peromyscus polionotus).68
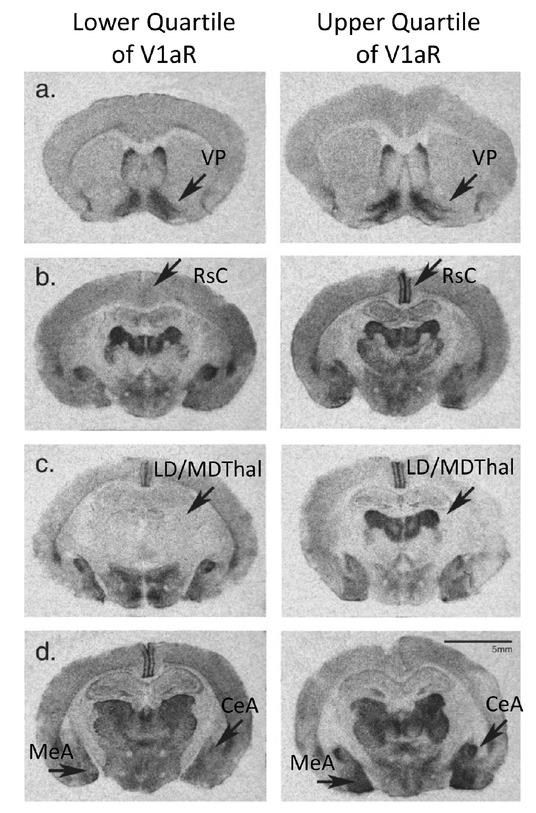
In addition to the notable differences in social behaviors between vole species, there is a spectrum of attachment-related phenotypes among individual prairie voles. This raises the question: are individual behavioral differences regulated by the same mechanisms as species differences? Similar to the pioneering studies across vole species, initial investigations of V1aR patterning within a semiwild population of prairie voles revealed that there is as much variation in V1aR densities between individuals as between vole species69 (Figure 3). Despite this tremendous diversity in V1aR in most brain regions, the ventral pallidum had the lowest individual variance. In addition, within an individual, brain regions with shared developmental origins had similar V1aR levels. In a population of laboratory-reared prairie voles, there was considerably less variation in the V1aR densities between individuals, but brain region covariance was retained and, as initially observed in wild populations, the highest correlation in levels were observed across regions with shared developmental origins.70 These results highlight the tremendous individual variation in V1aR expression across prairie voles and provide insights into the ontogeny of expression pattern variability.
Figure 3.
Individual variation in V1aR densities within a population of semiwild prairie voles. Autoradiograms of the lower (L) and upper (R) quarter of V1aR densities within a prairie vole population. Panels: (a) ventral pallidum (VP), (b) retrosplenial cortex (RsC), (c) laterodorsal (LD) and mediodorsal thalamus (MDThal), and (d) central (CeA) and medial amygdala (MeA). Scale = 5 mm. Adapted from ref 69 with permission from Wiley-Liss, Inc., copyright 2003.
Seminaturalistic field studies support the idea that variation in social behaviors correlates with individual differences in V1aR patterning. In a naturalistic setting, bonded male prairie voles can adopt distinct strategies for space use where “resident” males have small home ranges while “wanderer” males have large home ranges that overlap multiple resident territories. As result of their large home ranges, wandering males have more opportunities to interact with females and engage in extra-pair copulations. This variation in sexual fidelity may be advantageous at certain population densities, which can fluctuate across generations in wild rodents. The V1aR densities in resident and wanderer male prairie voles were examined. In brain regions implicated in social monogamy, such as the ventral pallidum and lateral septum, there was little variation in V1aR densities between individuals. However, in brain regions implicated in spatial memory, such as the retrosplenial cortex, resident males had nearly sixfold more V1aR expression than wanderer males.71 While further functional studies are needed to determine the causal role of V1aR in the retrosplenial cortex, these results suggest that, similar to pair bonding and selective aggression, space use and sexual fidelity may be independently regulated via V1aR signaling in a distinct brain region.
Together, functional and correlative evidence supports the hypothesis that AVP signaling via V1aR coordinates multiple component behaviors of monogamy, from partner preference to aggressive mate guarding and space use. These studies provide a working model in which signaling of AVP-V1aR within different brain regions drive specific, dissociable social behaviors. The region-specific tuning of V1aR levels between brain regions provides a flexible system; component behaviors can be modified and selected for without disrupting all aspects of pair bonding. This leads to a critical question: what regulatory mechanisms enable the independent tuning of V1aR levels in a brain-region-specific manner? The answer to this question has important implications for understanding the origins and organization of complex human social behaviors.
REGULATORY MECHANISMS FOR V1AR PATTERNING
AVP modulates multiple species-specific social behaviors. The high conservation of AVP and, to a lesser extent, V1aR suggests that modulation of social behaviors is more likely attributed to the evolution of different expression patterns rather than mutations in protein-coding sequences.24 The regulatory code that maintains diverse expression patterns of V1aR throughout the brain has not been fully elucidated, but we posit that brain-wide patterns of V1aR are determined by multiple, potentially modular, cis-regulatory elements. In this model, expression patterning is the combined result of independent genetic regulatory mechanisms. Brain-region-specific variation in patterning can therefore be achieved via genetic and epigenetic changes that affect one or a subset of these regulatory mechanisms. Such a modular regulatory system provides a mechanism for natural selection to independently sculpt different AVP-dependent social behaviors.
Initial experiments asked whether the regulatory elements required for prairie vole-like V1aR patterning existed proximal to the coding region. To do this, transgenic mice were made by randomly inserting a prairie vole V1aR minigene that included the coding region and intron, with flanking 2.2 kb upstream and 2.4 kb downstream sequences. Out of four transgenic lines, one partially recapitulated the prairie vole-like V1aR patterning and showed increased affiliative behaviors after i.c.v. infusion of exogenous AVP.72 This prompted Young and colleagues to ask what sequence differences existed between monogamous and nonmonogamous vole species within the minigene region. They identified a complex microsatellite in the 5′ flanking region of the avpr1a gene consisting of di- and tetranucleotide repeats interspersed with nonrepetitive sequence. In prairie and pine voles, this microsatellite is roughly 700 bp long but is nearly nonexistent in meadow and montane voles72 (Figure 4a). Throughout evolution, microsatellites undergo repeated expansions and contractions due to slippage of the DNA polymerase during replication. As a result, microsatellites located within regulatory regions can alter chromosome architecture or produce novel enhancers or promoters.73,74 The striking difference in microsatellite length between species provided an attractive candidate for explaining the inter- and intraspecies differences in V1aR patterning and, by extension, social behaviors. Below we provide an overview of the extensive research examining this genetic element, the conclusion of which is that while there is substantial evidence that cis-regulatory variation shapes V1aR patterns, the avpr1a microsatellite is not a sole or even primary driver of V1aR pattern diversity or behavior.
Figure 4.
Role of the avpr1a microsatellite in regulating expression levels of V1aR. (A) In the 5′ flanking region of avpr1a, the nonmonogamous meadow vole has a nearly nonexistent microsatellite, while prairie voles have a polymorphic microsatellite-containing element. Reprinted/adapted with permission from Springer Nature: Springer. Animal Models of Behavior Genetics by J.C. Gewirtz, Y.-K. Kim (eds.), copyright 2016. (B) There exists a diversity of avpr1a microsatellite alleles within a population of semiwild prairie voles ranging from 711 bp to 760 bp. (C) In vitro luciferase reporter assays indicate that the length of the microsatellite correlates with expression of V1aR. (D) Clockwise from top left, audioradiograms of transgenic mice with the meadow vole microsatellite, the short prairie vole microsatellite, or the long prairie vole microsatellite, with quantification of V1aR density in different brain region. Brain regions: ventral pallidum (VP), lateral septum (LS), paraventricular nucleus of the thalamus (PVthal), central amygdala (CeA), and dentate gyrus (DG). Reprinted/adapted by permission from Springer Nature: Springer. Animal Models of Behavior Genetics by J.C. Gewirtz, Y.-K. Kim (eds.), copyright 2016. (A) and (D) reprinted from ref 75 with permission from AAAS, 2005. (B) and (C) reprinted from ref 76 with permission from AAAS, 2005.
The correlation between microsatellite length and monogamous behaviors prompted the question: can avpr1a microsatellite length regulate avpr1a expression to produce the spectrum of monogamous behaviors observed in a population? Multiple microsatellite alleles were identified within a population of semiwild prairie voles with the most common lengths between 721 and 745 bp (Figure 4b). An in vitro luciferase assay demonstrated that a “long” microsatellite of 746 bp had 2-fold higher expression over a “short” microsatellite of 727 bp (Figure 4c). This result was found to be cell-type dependent but provided a promising indication that the expression level of V1aR may be dependent on microsatellite length. To determine if the microsatellite length was also correlated with monogamous behaviors, laboratory-reared prairie voles were bred to be homozygous for either the long or short microsatellite allele.76 A subset of parental behaviors correlated with allele length such that long allele males exhibited increased pup licking and grooming. However, no other parental behaviors were significantly different between the two populations. In a partner preference test of males cohoused with a receptive female for 18 h, long allele males demonstrated a partner preference, while the short allele males did not.76 These correlative results were promising, but one limitation of this study is that the subtle phenotype differences could also be attributed to unknown genetic variants linked to either the long or short microsatellite allele.
Donaldson and Young addressed this limitation and tested the direct contribution of the microsatellite using genome editing in mice. They generated three mouse lines in which the mouse avpr1a 5′ flanking region was replaced with corresponding sequence from prairie voles. These lines were identical except for the insertion of different avpr1a microsatellites, originating from the meadow vole or long or short alleles from prairie voles. The resulting transgenic mouse lines showed that, overall, the 5′ flanking region plays a minimal role in driving species-typical receptor patterns; the transgenic mice had V1aR patterns much more similar to that of mice than of prairie voles. However, patterning of V1aR across the lines recapitulated individual and species differences in specific brain regions, namely the hippocampus, thalamus, and amygdala77 (Figure 4d). These results suggest that microsatellite diversity may modulate V1aR levels in discrete brain regions, but that it is not the primary driver of brain-wide variation in V1aR expression patterns across or within species. Instead, these studies suggest that a combination of proximal and distal regulatory elements coordinate to produce diversity in brain-wide V1aR patterning.
Field studies also indicate that the effects of the avpr1a microsatellite cannot fully explain the vast diversity in V1aR patterns or AVP-mediated sociobehavioral traits. A comparative study of 62 Microtus species did not find a correlation between microsatellite length and social organization.78 The same lack of association was found for an analogous region in the primate avpr1a gene.79 Further, while some field studies have found associations between microsatellite length and V1aR levels in some brain regions, a review of the existing lab and field studies identified inconsistencies in the association between microsatellite length and receptor expression in specific brain regions (see Table 2 in ref 81).71,80,81 Together, these results suggest that cis-regulatory variation is an important driver of V1aR expression diversity, but the inconsistent associations between region-specific V1aR levels and microsatellite length and the nuanced effects in transgenic mice have led to the conclusion that the microsatellite is not a primary source of V1aR patterning.
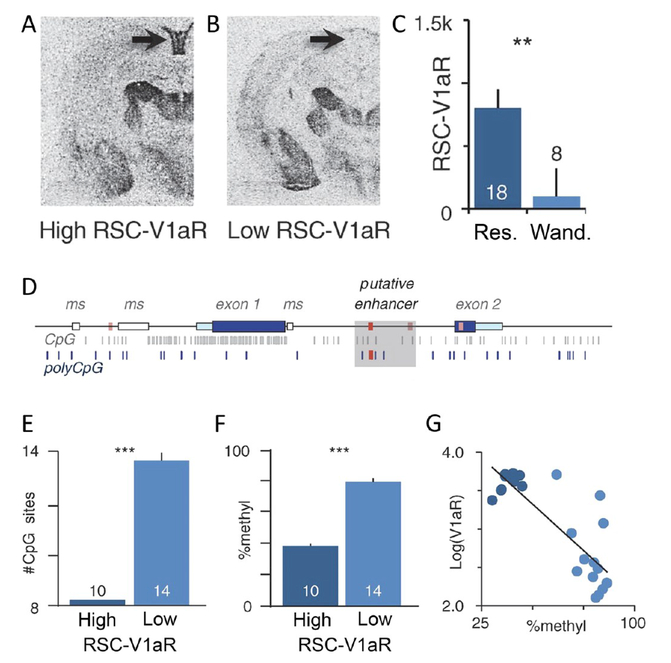
Determining that the microsatellite is not a master regulator of V1aR expression prompted the search for additional cis-regulatory mechanisms that may influence behavioral diversity. Phelps and colleagues searched for regulatory elements that could account for the known correlation between V1aR abundance in the retrosplenial cortex and a resident or wanderer mating strategy in male prairie voles71 (Figures 5a–c). They found that a particular avpr1a SNP haplotype in male prairie voles was strongly associated with V1aR density in the retrosplenial cortex and with differences in space and, by extension, sexual fidelity82 (Figure 5d). Additionally, the SNP haplotype was correlated with increased H3K4me1 marks and CpG site abundance, both of which are epigenetic indicators of active transcription (Figures 5e–g). These results led to a model in which SNPs potentially interacted with specific epigenetic alterations to produce individual differences in retrosplenial V1aR levels and mating strategies.
Figure 5.
Variation in retrosplenial cortex V1aR levels of male prairie voles are correlated with genetic variation in the avpr1a locus, epigenetic modifications, and differences in space use and fidelity. (A, B) Representative autoradiogram of a male prairie vole with high (A) and low (B) V1aR density in the retrosplenial cortex (RSC). (C) Quantification of the association of RSC-V1aR density and fidelity/space use strategy in male prairie voles. Males with a resident (res.) strategy have higher RSC-V1aR levels, while males a wanderer (wand.) strategy have with low RSC-V1aR levels. (D) avpr1a gene locus with genetic variants and epigenetic markers. Red bars are the SNPs associated with RSC-V1aR abundance. Fixed CpG sites are in gray with polymorphic CpG sites in blue. The shaded gray box indicates a putative intronic enhancer. (E) Males with high RSC-V1aR have fewer CpG sites in the intron and (F) lower levels of enhancer methylation. (G) RSC-V1aR abundance is correlated with enhancer methylation. Bars are means ± SE ***P < 0.001. From Okhovat, M. et al., Science. 2013. Reprinted with permission from AAAS.82
The role of epigenetic marks has also been independently studied in the context of pair bonding. Epigenetics changes can alter transcription independent of DNA sequence. Therefore, epigenetics can potentially fine-tune gene expression in response to different experiences, such as mating. When a histone deacetylase inhibitor was infused i.c.v. into female prairie voles that had been cohoused with a male for 6 h in the absence of mating, they displayed a partner preference and increased avpr1a mRNA levels in the nucleus accumbens.83 However, the use of a deacetylase inhibitor to manipulate chromatin marks has genome-wide effects and may alter the transcription of many genes. Specifically, Wang et al. also observed increased levels of oxytocin receptor in animals receiving the inhibitor. Therefore, future experiments designed to selectively modify histone marks only at the avpr1a locus and its relevant enhancers are needed to determine how epigenetic changes affect region and cell-type-specific gene expression and behavior.
To date, experimental evidence suggests that brain-region-specific regulation of avpr1a requires the coordination of proximal and distal regulatory elements including SNP haplotypes, microsatellite elements, and epigenetic regulation. Of note, none of the regulatory mechanisms identified to date can account for how V1aR is regulated in the ventral pallidum, a brain region essential for partner preference formation. Future work using advanced sequencing techniques, such as ATAC-seq and 4C-seq performed on pallidal tissue in prairie and meadow voles is needed to delineate the contributions of distal regulatory elements.84,85 In addition, advanced genome and epigenome editing techniques provide a powerful approach for functionally interrogating candidate regulatory differences. Ultimately, a combination of neuromolecular techniques is needed to fully elucidate the diverse regulatory elements for V1aR expression across species and individuals.
COMBINATORIAL GENE REGULATION FOR BRAIN REGION SPECIFIC EXPRESSION
Signaling via the AVP-V1aR axis in different brain regions enables independent control of distinct social behavior traits. For example, AVP modulates preference formation via signaling in the ventral pallidum, selective aggression via the hypothalamus and, potentially, space use strategies via the retrosplenial cortex. This evidence suggests a model where a single, highly conserved ligand can act on brain-region-specific receptor populations to coordinate a suite of social behaviors.
Current evidence suggests that the regulatory mechanisms for V1aR expression are brain-region-specific. The avpr1a microsatellite influences V1aR levels in hippocampal, amygdala, and thalamic brain regions while genetic variants, in combination with epigenetic mechanisms, appear to modulate V1aR levels in the retrosplenial cortex and parts of the thalamus. However, none of these proximal regulatory mechanisms can explain V1aR densities in the prefrontal cortex or the ventral pallidum, two brain regions crucial for pair bond formation. The full suite of regulatory mechanisms for V1aR expression therefore likely encapsulates both proximal and distal regulatory elements that range from DNA variants to altered binding of regulatory proteins to epigenetic modifications. We propose that genetic and epigenetic diversity synergistically creates a code for brain-region and cell-type-specific V1aR expression. This facilitates the evolvability of species-typical social behaviors. By uncoupling the mechanisms that guide V1aR expression in different brain regions, one behavior can be maintained while another is independently selected for or against. The result is a remarkable diversity of social behaviors across and within species.86
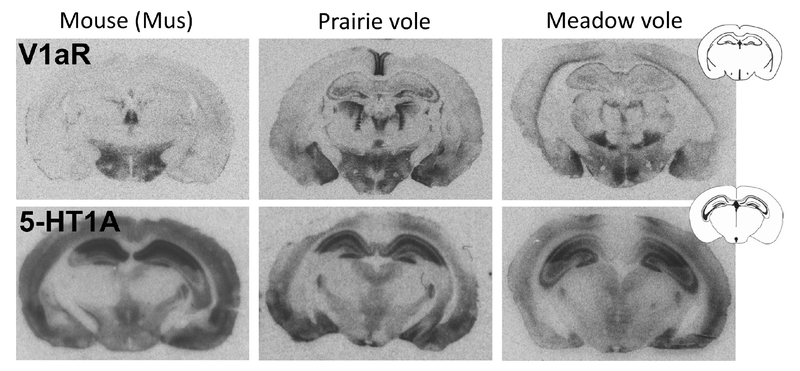
An open question is the extent to which this framework applies to other neuromodulatory systems. Other behaviorally relevant neuromodulators, such as a serotonin, exhibit much more highly conserved patterns of receptor expression across species (Figure 6). However, unlike nonapeptides, the serotonin system has 14 identified receptors in humans.87 This suggests that modifications that specialize receptor gene function may be more important than patterning diversity. Future research incorporating cross-disciplinary approaches is needed to characterize the full suite of regulatory mechanisms underlying neuromodulatory and social behavior diversity.
Figure 6.
Receptor autoradiograms for V1aR and 5-HT1a in three rodent species: mouse (Mus musculus), prairie vole, and meadow vole. V1aR patterns show remarkable diversity across species (upper row), while spatial patterns of 5-HT1A tend to be conserved (bottom).
TRANSLATIONAL RELEVANCE
The basic science underlying attachment in prairie voles has translational validity in humans. Tissue accessibility and experimental approaches remain limited in humans. However, intranasal AVP or V1aR antagonists have probed the effects of AVP-V1aR signaling on social behavior, and genetic association studies have examined correlations between variation in the AVPR1A gene and social traits. These initial studies, a subset of which are detailed below, suggest that signaling through the AVP-V1aR axis impacts human social behavior and may have relevance for treating the social deficits observed in autism spectrum disorders. Supporting this, the V1aR antagonist, balovaptan, was recently granted Breakthrough Therapy designation by the Food and Drug Administration and is proceeding to phase II testing.88
Noninvasive intranasal delivery of neuropeptides or their antagonists remains an attractive therapeutic. Studies in nonhuman primates suggest that intranasal delivery of AVP crosses the blood brain barrier, providing validity to this route of administration for future therapeutics.89 The behavioral effects of intranasal AVP further support its potential therapeutic utility, albeit with careful consideration of sexually dimorphic effects. For instance, when men were administered intranasal AVP, they perceived a friendly facial expression more negatively while the same treatment in women shifted perception of neutral facial expressions to friendly.90 In a separate study, subjects that had received intranasal AVP were presented with images of happy, angry, or neutral human faces. The next day they were presented with a mixture of known and unknown images and asked to remember if they had already seen each image. Those that had administered AVP were more likely to remember the happy and angry faces, thus suggesting that AVP facilitates the encoding of salient social cues.91 While promising, these studies were conducted in individuals that did not have diagnosed social deficits, such as those observed in autism. To test the validity of manipulating the AVP-V1aR axis as a therapy for social deficits, a small group of 10 adult males diagnosed with autism spectrum disorder received intravascular injections of the V1aR antagonist, RG7713.92 The participants displayed nominal positive effects on social cognition (effect size = 0.8) as measured by an increase in biological motion orienting when free viewing videos of two people interacting. However, the authors point out that these behavioral changes were quantitatively small, and when they aggregated effects across traits into an overall performance score, the antagonist did not significantly improve performance. The authors also acknowledge that their study was not appropriately powered, which remains an ongoing challenge in interpreting similar intranasal studies.92,93 In addition to the bolavaptan phase II trial mentioned previously, an ongoing clinical trial in children is testing the validity of intranasal AVP for treating the social symptoms observed in high functioning forms of autism.94 Together, these studies suggest that noninvasive manipulation of the AVP-V1aR axis could be harnessed to positively alter social behavior.
Gene association studies have found nuanced correlations between genotype and human social behaviors, a few of which are highlighted here. Humans and other primates have a microsatellite element, termed RS3, upstream of the AVPR1A transcription start site that is similar to but not homologous with the repetitive region identified in voles.79 A study of 552 same-sex Swedish twins and their partners found that, in men but not women, the V1aR microsatellite allele 334 was strongly correlated with a lower Partner Bonding Scale score in a dose dependent manner (heterozygous p < 0.001 and Cohen’s d = 0.27, homozygous p < 0.0004 and Cohen’s d = 0.38).95 Additionally, males homozygous for the 334 allele were twice as likely to have discussed divorce in the past year (34 vs 15%) and less likely to be married to their partner when compared to males that did not have the allele (32 vs 17%). While intriguing, these results represent a single study in a relatively homogeneous genetic population, and additional investigation is needed to replicate this finding and begin to understand potential mechanisms.
One important consideration that has received relatively little attention is the potential sensitive periods and developmental trajectories in which the AVP-V1aR system may impact complex social behaviors in humans.96,97 For instance, the relationship between age, RS3 genotype, and altruism is not straightforward. In children, the most common RS3 allele (327 bp) was associated with lower giving behavior, while in adults, RS3 alleles of similar length were found to be positively associated with altruism. The authors of the study suggest that this apparent contradiction reflects the known maturation of giving behaviors.96
To date, the association between the AVPR1A microsatellite allele and the risk or severity of autism is not clear. There is evidence both supporting and refuting the predictive power of microsatellite length.97,98 Similar to voles, other cis-regulatory variation may be more important than RS3 for explaining human sociobehavioral diversity and autistic phenotypes. Additionally, these studies may be confounded in their inclusion of diverse types of autism spectrum disorders and a lack of examination of intermediate phenotypes. For instance, autism is characterized by stereotypies, developmental delays, and social deficits, each of which can differ in severity; based on available evidence, one would not necessarily expect the AVP-V1aR axis to be important for the first two traits.
A final line of evidence suggests a generally conserved role of the AVP-V1aR axis in primate social behavior. In our evolutionary cousin, the chimpanzee, RS3 is found within a larger duplicated region that exists as duplicate and single allelic variants. A number of studies have now investigated the relationship between this duplicated region and social traits in captive chimpanzees.99–101 Similar to work in voles and humans, the results of these gene-association studies are sexually dimorphic. Like humans, chimpanzees will follow the gaze or a pointed finger of another individual (including a human), a trait referred to as receptive joint attention. Males heterozygous for the duplication are better at receptive joint attention, and genetic variation at this locus can explain 25% of the variance in attention scores. This study augments initial work that showed that heterozygous males have significantly higher dominance scores and lower conscientiousness.99 However, these results have not been confirmed in wild populations.
While dysfunctional social behavior is a hallmark of multiple psychiatric and neurodevelopmental disorders, the presentation of these social deficits is highly diverse. These deficits can include social anhedonia, social anxiety, impaired ability to form attachments, and overattachment. The biological mechanisms of different forms of sociobehavioral dysfunction may be nonoverlapping. As a result, these disorders may require distinct treatments. To achieve customized therapies that can target specific V1aR receptor subpopulations within the human brain, there is a pressing need to delineate the factors that contribute to brain-region-specific regulation of neuromodulatory systems as they relate to diverse aspects of social behavior.
CONCLUSIONS
Comparative studies between vole species provide an excellent model system to study the biological basis for complex social behaviors. These studies support a model in which brain-region-specific V1aR patterning and densities modulate different facets of social behavior, from affiliation to aggression. We propose that independent regulatory control of different V1aR subpopulations within the brain facilitates the evolvability of social traits. Although the extent of variation in the expression of human AVPR1A remains unknown, delineating the mechanisms that underlie brain-region-specific expression of V1aR has important implications for identifying how the AVP-V1aR axis may be disrupted in disease states as well as for providing opportunities to develop novel therapeutics.
Acknowledgments
Funding
The authors report the following funding sources: Whitehall Foundation Grant, T32 GM008759, R00 MH102352, CCTSI CNS Pilot award.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).The St. Petersburg U. S. A. O. R. T. (2008) THE EFFECTS OF EARLY SOCIAL-EMOTIONAL AND RELATIONSHIP EXPERIENCE ON THE DEVELOPMENT OF YOUNG ORPHANAGE CHILDREN. Monogr. Soc. Res. Child Dev 73, vii–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kaunonen M, Tarkka MT, Paunonen M, and Laippala P (1999) Grief and social support after the death of a spouse. J. Adv. Nurs 30, 1304–11. [DOI] [PubMed] [Google Scholar]
- (3).Heim C, Shugart M, Craighead WE, and Nemeroff CB (2010) Neurobiological and psychiatric consequences of child abuse and neglect. Dev. Psychobiol 52, 671–90. [DOI] [PubMed] [Google Scholar]
- (4).Stroebe M, Stroebe W, and Abakoumkin G (2005) The broken heart: suicidal ideation in bereavement. Am. J. Psychiatry 162, 2178–80. [DOI] [PubMed] [Google Scholar]
- (5).Southwick SM, Vythilingam M, and Charney DS (2005) The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annu. Rev. Clin. Psychol 1, 255–291. [DOI] [PubMed] [Google Scholar]
- (6).Kikusui T, Winslow JT, and Mori Y (2006) Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc, B 361, 2215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Reblin M, and Uchino BN (2008) Social and emotional support and its implication for health. Curr. Opin Psychiatry 21, 201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Yang YC, Boen C, Gerken K, Li T, Schorpp K, and Harris KM (2016) Social relationships and physiological determinants of longevity across the human life span. Proc. Natl. Acad. Sci. U. S. A 113, 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kleiman D (1977) Monogamy in mammals. Q. Rev. Biol 52, 39–69. [DOI] [PubMed] [Google Scholar]
- (10).Carter CS, DeVries AC, and Getz LL (1995) Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev 19, 303–14. [DOI] [PubMed] [Google Scholar]
- (11).Insel TR, and Young LJ (2001) The neurobiology of attachment. Nat. Rev. Neurosci 2, 129–36. [DOI] [PubMed] [Google Scholar]
- (12).Young LJ, and Wang Z (2004) The neurobiology of pair bonding. Nat. Neurosci 7, 1048–54. [DOI] [PubMed] [Google Scholar]
- (13).Getz LL, Carter CS, and Gavish L (1981) The mating system of the prairie vole Microtus ochrogaster: Field and laboratory evidence for pair bonding. Behav Ecol Sociobiol 8, 189–194. [Google Scholar]
- (14).Williams JR, Catania KC, and Carter CS (1992) Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav 26, 339–349. [DOI] [PubMed] [Google Scholar]
- (15).Williams JR, Carter CS, and Insel T (1992) Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann. N. Y. Acad. Sci 652, 487–9. [DOI] [PubMed] [Google Scholar]
- (16).Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, and Young LJ (2016) (3) Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav 79, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Winslow JT, Hastings N, Carter CS, Harbaugh CR, and Insel TR (1993) A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–8. [DOI] [PubMed] [Google Scholar]
- (18).Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, and Young LJ (2009) Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162, 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Insel TR, and Hulihan TJ (1995) A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav. Neurosci 109, 782. [DOI] [PubMed] [Google Scholar]
- (20).Young LJ, Murphy Young AZ, and Hammock EA (2005) Anatomy and neurochemistry of the pair bond. J Comp Neurol 493 (1), 51–7. [DOI] [PubMed] [Google Scholar]
- (21).Curtis JT, Liu Y, Aragona BJ, and Wang Z (2006) Dopamine and monogamy. Brain Res 1126, 76–90. [DOI] [PubMed] [Google Scholar]
- (22).Ross HE, and Young LJ (2009) Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol 30, 534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bielsky IF, and Young LJ (2004) Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–74. [DOI] [PubMed] [Google Scholar]
- (24).Donaldson ZR, and Young LJ (2008) Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–4. [DOI] [PubMed] [Google Scholar]
- (25).Carter CS, DeVries AC, and Getz LL (1995) Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev 19, 303–314. [DOI] [PubMed] [Google Scholar]
- (26).Young LJ, and Wang Z (2004) The neurobiology of pair bonding. Nat. Neurosci 7, 1048–54. [DOI] [PubMed] [Google Scholar]
- (27).Aragona BJ, and Wang Z (2004) The Prairie Vole (Microtus ochrogaster): An Animal Model for Behavioral Neuroendocrine Research on Pair Bonding. ILAR J 45, 35–45. [DOI] [PubMed] [Google Scholar]
- (28).Tickerhoof M, and Smith AS (2017) Vasopressinergic Neurocircuitry Regulating Social Attachment in a Monogamous Species . Front. Endocrinol 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Madison DM. (1980) Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav. Ecol Sociobiol 7, 65–71. [Google Scholar]
- (30).McGuire B, and Novak M (1984) A comparison of maternal behavior in the meadow vole, prairie vole, and pine vole. Anim. Behav 32, 1132–1141. [Google Scholar]
- (31).Anacker AM, and Beery AK (2013) Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci 7, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Inoue K, Burkett JP, and Young LJ (2013) Neuroanatomical distribution of mu-opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non-monogamous meadow voles (Microtus pennsylvanicus). Neuroscience 244, 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Beery AK, and Zucker I (2010) Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–73. [DOI] [PubMed] [Google Scholar]
- (34).Matthews TJ, Williams DA, and Schweiger L (2013) Social motivation and residential style in prairie and meadow voles. Open Behav. Sci J 7, 16–23. [Google Scholar]
- (35).Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, and Young LJ (2009) Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci 29, 1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, and Young LJ (2003) Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur. J. Neurosci 18, 403–11. [DOI] [PubMed] [Google Scholar]
- (37).van Wimersma Greidanus TB, van Ree JM, and de Wied D (1983) Vasopressin and memory. Pharmacol. Ther 20, 437. [DOI] [PubMed] [Google Scholar]
- (38).van Wimersma Greidanus TB, and Maigret C (1996) The role of limbic vasopressin and oxytocin in social recognition. Brain Res 713, 153. [DOI] [PubMed] [Google Scholar]
- (39).Goodson JL, and Bass AH (2001) Social behavior functions and related anatomical characteristics ofvasotocin/vasopressin systems in vertebrates. Brain Res. Rev 35, 246. [DOI] [PubMed] [Google Scholar]
- (40).De Vries GJ, and Panzica GC (2006) Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138, 947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Dantzer R, Koob G, Bluthe R, and Le Moal M (1988) Septal vasopressin modulates social memory in male rats. Brain Res 457, 143–147. [DOI] [PubMed] [Google Scholar]
- (42).Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, and Crawley JN (2008) Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav. Brain Res 187, 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Bielsky IF, Hu S-B, Szegda KL, Westphal H, and Young LJ (2004) Profound Impairment in Social Recognition and Reduction in Anxiety in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 29, 483–493. [DOI] [PubMed] [Google Scholar]
- (44).Johnson ZV, and Young LJ (2017) (5) Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience . Neurosci. Biobehav. Rev. 76 (Part A), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, and Hoekstra HE (2010) Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol. Biol. Evol 27, 1269–1278. [DOI] [PubMed] [Google Scholar]
- (46).Phelps SM (2010) From endophenotypes to evolution: social attachment, sexual fidelity and the avpr1a locus. Curr. Opin. Neurobiol 20, 795–802. [DOI] [PubMed] [Google Scholar]
- (47).Everts HGJ, and Koolhaas JM (1999) Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav. Brain Res 99, 7. [DOI] [PubMed] [Google Scholar]
- (48).Le Moal M, Dantzer R, Michaud B, and Koob GF (1987) Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci. Lett 77, 353. [DOI] [PubMed] [Google Scholar]
- (49).Campbell P, Ophir AG, and Phelps SM (2009) Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol 516, 321–333. [DOI] [PubMed] [Google Scholar]
- (50).Bosch OJ, and Neumann ID (2008) Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. U. S. A 105, 17139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Bosch OJ, and Neumann ID (2012) Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav 61, 293–303. [DOI] [PubMed] [Google Scholar]
- (52).Winslow JT, Hastings N, Carter CS, Harbaugh CR, and Insel TR (1993) A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–8. [DOI] [PubMed] [Google Scholar]
- (53).Birnbaumer M (2000) Vasopressin Receptors. Trends Endocrinol. Metab 11, 406–410. [DOI] [PubMed] [Google Scholar]
- (54).King L, and Young L (2016) Oxytocin, Vasopressin, and Diversity in Social Behavior In Molecular Neuroendocrinology: From Genome to Physiology, John Wiley & Sons, Ltd., Chichester, UK. [Google Scholar]
- (55).Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, and Young LJ (2001) Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J. Neurosci 21, 7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Wang Z, Young LJ, Liu Y, and Insel TR (1997) Species differences in vasopressin receptor binding are evident early in development: Comparative anatomic studies in prairie and montane voles. J. Comp. Neurol 378, 535–546. [PubMed] [Google Scholar]
- (57).Insel TR, Wang ZX, and Ferris CF (1994) Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci 14, 5381–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Curtis JT, and Wang Z (2003) Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster). Physiol. Behav 80, 95–101. [DOI] [PubMed] [Google Scholar]
- (59).Robertson HA (1992) Immediate-early genes, neuronal plasticity, and memory. Biochem. Cell Biol 70, 729–37. [DOI] [PubMed] [Google Scholar]
- (60).Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L, Hawrylycz MJ, Rockland K, Seung HS, and Osten P (2015) (12) Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep 10, 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Sheng M, and Greenberg ME (1990) The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–85. [DOI] [PubMed] [Google Scholar]
- (62).Lim MM, and Young LJ (2004) Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. [DOI] [PubMed] [Google Scholar]
- (63).Lim MM, Hammock EA, and Young LJ (2004) The role of vasopressin in the genetic and neural regulation of monogamy. J. Neuroendocrinol 16 (4), 325–32. [DOI] [PubMed] [Google Scholar]
- (64).Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, and Young LJ (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–7. [DOI] [PubMed] [Google Scholar]
- (65).Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, and Young LJ (2013) Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm. Behav 63, 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Gobrogge KL, Liu Y, Young LJ, and Wang Z (2009) Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. U. S. A 106, 19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Wang Z, Ferris CF, and De Vries GJ (1994) Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. U. S. A 91, 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C, and Hoekstra HE (2017) The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Phelps SM, and Young LJ (2003) Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J. Comp. Neurol 466, 564–76. [DOI] [PubMed] [Google Scholar]
- (70).Hammock EA, Lim MM, Nair HP, and Young LJ (2005) Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes, Brain Behav 4, 289–301. [DOI] [PubMed] [Google Scholar]
- (71).Ophir AG, Wolff JO, and Phelps SM (2008) Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. U. S. A 105, 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Young LJ, Nilsen R, Waymire KG, MacGregor GR, and Insel TR (1999) Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–8. [DOI] [PubMed] [Google Scholar]
- (73).Young L, and Hammock EA (2007) On switches and knobs, microsatellites and monogamy. Trends Genet 23, 209–212. [DOI] [PubMed] [Google Scholar]
- (74).Fondon JW III, Hammock EA, Hannan AJ, and King D (2008) Simple sequence repeates: genetic modulators of brain function and behavior. Trends Neurosci 31, 328–334. [DOI] [PubMed] [Google Scholar]
- (75).Donaldson ZR, and Young LJ Chapter 4: The Neurobiology and Genetics of Affiliation and Social Bonding in Animal Models In Animal Models of Behavior Genetics; Gewirtz JC, and Kim Y-K, Eds; Springer Science+Business Media: New York, 2016; pp. 101–134. [Google Scholar]
- (76).Hammock EA, and Young LJ (2005) Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308, 1630–1634. [DOI] [PubMed] [Google Scholar]
- (77).Donaldson ZR, and Young LJ (2013) The relative contribution of proximal 5′ flanking sequence and microsatellite variation on brain vasopressin 1a receptor (Avpr1a) gene expression and behavior. PLoS Genet 9, e1003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Fink S, Excoffier L, and Heckel G (2006) Mammalian monogamy is not controlled by a single gene. Proc. Natl. Acad. Sci. U. S. A 103, 10956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Donaldson ZR, Kondrashov FA, Putnam A, Bai Y, Stoinski TL, Hammock EAD, and Young LJ (2008) Evolution of a behavior-linked microsatellite-containing element in the 5′ flanking region of the primate AVPR1A gene. BMC Evol Biol 8, 180–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Solomon NG, Richmond AR, Harding PA, Fries A, Jacquemin S, Schaefer RL, Lucia KE, and Keane B (2009) Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Mol. Ecol 18, 4680–4695. [DOI] [PubMed] [Google Scholar]
- (81).Ophir AG, Campbell P, Hanna K, and Phelps SM (2008) Field tests of cis-regulatory variation at the prairie vole avpr1a locus: association with V1aR abundance but not sexual or social fidelity. Horm. Behav 54, 694–702. [DOI] [PubMed] [Google Scholar]
- (82).Okhovat M, Berrio A, Wallace G, Ophir AG, and Phelps SM (2015) Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350, 1371–1374. [DOI] [PubMed] [Google Scholar]
- (83).Wang H, Duclot F, Liu Y, Wang Z, and Kabbaj M (2013) (February 6) Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat. Neurosci 16, 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Buenrostro J, Wu B, Chang HY, and Greenleaf W (2015) ATAC-seq: A method for assaying chromatin accessibility genomewide. Curr. Protoc. Mol. Biol, 21–29.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).van de Werken HJG, Landan G, Holwerda SJB, Hoichman M, Klous P, Chachik R, Splinter E, Valdes-Quezada C, Oz Y, Bouwman B, Verstegen MJAM, de Wit E, Tanay A, and de Laat W (2012) Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat. Methods 9, 969–972. [DOI] [PubMed] [Google Scholar]
- (86).O’Connell LA, and Hofmann HA (2012) Evolution of a vertebrate social decision-making network. Science 336, 1154–7. [DOI] [PubMed] [Google Scholar]
- (87).Bockaert J, Claeysen S, Dumuis A, and Marin P (2010) Classification and Signaling Characteristics of 5-HT Receptors. Handbook of Behavioral Neuroscience 21, 103–121. [Google Scholar]
- (88).Roche. (2018) FDA grants Breakthrough Therapy Designation for Roche’s balovaptan in autism spectrum disorder. Investor Update, Roche, Basel. [Google Scholar]
- (89).Born J, Lange T, Kern W, McGregor GP, Bickel U, and Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci 5, 514–16. [DOI] [PubMed] [Google Scholar]
- (90).Thompson RR, George K, Walton JC, Orr SP, and Benson J (2006) Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. U. S. A 103, 7889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Guastella AJ, Kenyon A, Alvares GA, Carson D, and Hickie IB (2010) Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol. Psychiatry 67, 1220–1222. [DOI] [PubMed] [Google Scholar]
- (92).Umbricht D, del Vaelle Rubido M, Hollander E, McCracken JT, Shic F, Scahill L, Noeldeke J, Boak L, Khwaja O, Squassante L, Grundschober C, Kletzl H, and Fontoura P (2017) A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagnoist (RG7713) in high-functioning adults with Autsim Spectrum Disorder. Neuropsychopharmacology 42, 1914–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Walum H, Waldman ID, and Young LJ (2016) Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry 79 (3), 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Harden A, and Parker K Intranasal vasopressin treatment in children with autsim. Clinical Trial. 2018 [Google Scholar]
- (95).Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, and Lichtenstein P (2008) Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc. Natl. Acad. Sci. U. S. A 105, 14153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Avinun R, Israel S, Shalev I, Gritsenko I, Bornstein G, Ebstein R, and Knafo A (2011) AVPR1A variant associated with preschoolers’ lower altruistic behavior. PLoS One 6, e25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Bachner-Melman R, and Ebstein RP (2014) The role of oxytocin and vasopressin in emotional and social behaviors. Handbook of Clinical Neurology 124, 53–68. [DOI] [PubMed] [Google Scholar]
- (98).Ebstein RP, Knafo A, Mankuta D, Chew SH, and Lai PS (2012) The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm. Behav 61, 359–79. [DOI] [PubMed] [Google Scholar]
- (99).Hopkins WD, Donaldson ZR, and Young LJ (2012) A polymorphic indel containing the RS3 microsatellite in the 5′ flanking region of the vasopressin Via receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav 11, 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, and Young LJ (20i5) Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes). Sci. Rep 4, 3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Latzman RD, Hopkins WD, Keebaugh AC, and Young LJ (2014) Personality in chimpanzees (Pan troglodytes): exploring the hierarchical structure and associations with the vasopressin V1A receptor gene. PLoS One 9, e95741. [DOI] [PMC free article] [PubMed] [Google Scholar]