Abstract
Ventricular trabeculation and compaction are two essential morphogenetic events for generating a functionally competent ventricular wall. A significant reduction in trabeculation is usually associated with hypoplastic wall and ventricular compact zone deficiencies, which commonly leads to embryonic heart failure and early embryonic lethality. In contrast, the arrest of ventricular wall compaction (noncompaction) is believed to be causative to the left ventricular noncompaction (LVNC), a genetically heterogeneous disorder and the third most common cardiomyopathy among pediatric patients. After critically reviewing recent findings from genetically engineered mouse models, we suggest a model which proposes that defects in myofibrillogenesis and polarization in trabecular cardiomyocytes underlies the common pathogenic mechanism for ventricular noncompaction.
Introduction
Left ventricular noncompaction (LVNC, OMIM604169) is a rare and unique type of cardiomyopathy which has gained increasing attention in the past decade [1–3]. LVNC was initially described in 1926 as spongy myocardium [4,5]. The incidence among 0–10 year olds was around 0.12 per 100,000/year [6]. In the pediatric population, LVNC is the third most common cardiomyopathy after dilated cardiomyopathy and hypertrophic cardiomyopathy [7–9]. Its prevalence was recently estimated at 0.05% in adults [10]. An article by Oechslin and Jenni presented a thorough clinical review of LVNC, including the diagnostic criteria, the epidemiology, the clinical presentation, outcome and management, the use of cardiac magnetic resonance imaging (MRI) to overcome the potential misdiagnosis by commonly used echocardiography, as well as the genetic heterogeneity of LVNC [3]. Despite these important clinical observations over the past 25 years, the underlying molecular mechanism(s) which give rise to LVNC are still largely unknown. LVNC was classified as one of five inherited cardiomyopathies by American Heart Association in 2006, and as an unclassified cardiomyopathy by the European Heart Association in 2008. This conflict of view in part reflects the poor genotype-phenotype association in LVNC patients and the limited understanding of the molecular control of normal compaction during ventricular myocardial development. Here, we describe the normal compaction process in the developing ventricular wall. This is followed by a description of genetic variants associated with LVNC in humans, as well as mouse models exhibiting noncompaction phenotypes. We then propose a working model wherein defects in cell polarity and/or myofibrillagenesis can provide a unifying mechanism for the genesis of LVNC,
The development of ventricular wall
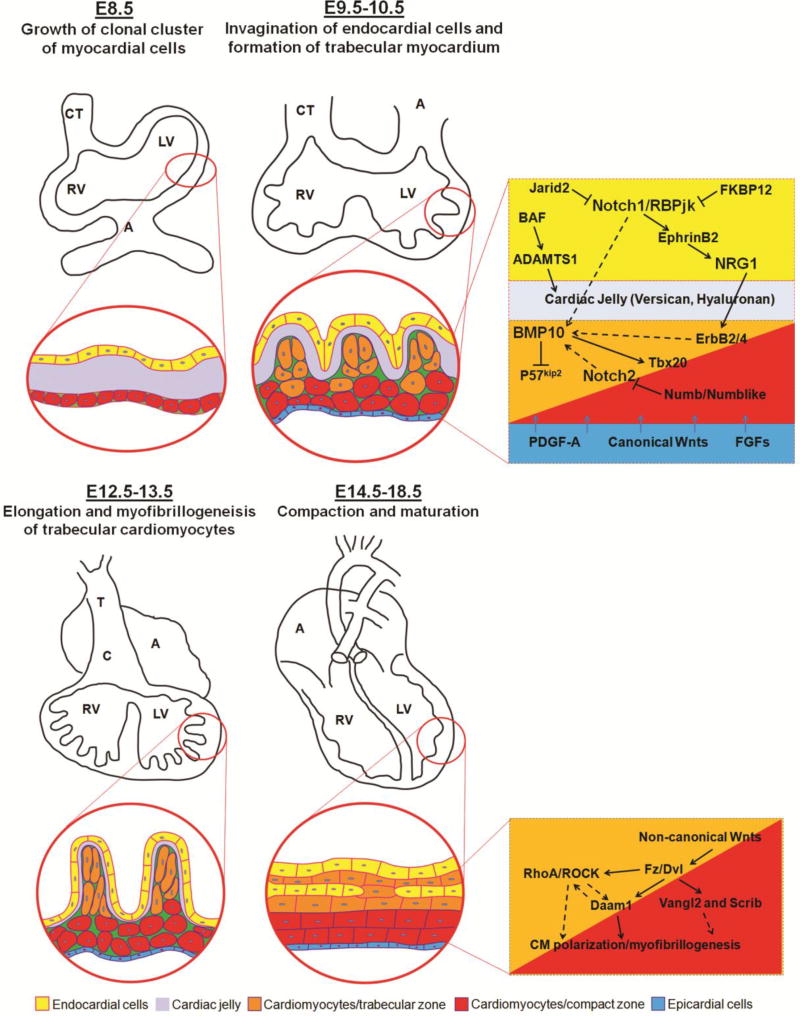
During mammalian heart development, the ventricles undergo a series of morphogenetic events to create a functionally competent ventricular wall [11–13], including trabeculation and compaction [14]. The trabeculation and compaction can be viewed as a 4-step process and involved with multiple signaling from endocardial-, myocardial, and epicardial-derived signals (Figure 1). The first step involves the formation of a single cell layer of myocardium at an early developmental stage. Following induction via adjacent endoderm (e.g., E8.5 in mouse embryos), the lateral mesoderm gives rise to an early tubular heart, which is composed of one cell layer of myocardium and one cell layer of endocardium lining the lumen, with extracellular matrix (cardiac jelly) in between the two cell layers [15,16]. The second step involves the formation of the trabeculated myocardium at early midgestation stage (e.g., E9.5–E10.5 in mouse embryos). After the looping, as the myocardium thickens, cardiomyocytes in specific regions along the inner wall of the heart form sheet-like protrusions into the lumen to give rise to the trabecular myocardium. The outside layer of myocardium remains as the base for forming the compact myocardium. Ventricular trabeculation has been suggested to facilitate oxygen and nutrient exchange in the heart muscle and to enhance heart muscle force generation to match the increasing blood and oxygen demand in developing embryos [17]. The third step involves myocardial compaction at late midgestation stage (e.g., E12.5-E13.5 in mouse embryos). As development proceeds, the trabecular myocardium collapses towards the myocardial wall in a process termed compaction, which contributes to forming a thicker, compact ventricular wall. The majority of trabeculae thus become incorporated into the compacted wall after E14.5 in mouse embryos [17,18]. The fourth step is the formation of a mature and multilayered spiral myocardium during late fetal and neonatal stage [19]. Unlike the skeletal muscle, the ventricular myocardium consists of aggregated myocytes within a three-dimensional mesh. A series of elegant studies by Anderson and colleagues demonstrated that myocytes in the subendocardial and subepicardial layers of the myocardium form helical sheets with reciprocal angulation, whereas the myocytes at the middle layer form a circular sheet [20–27]. This unique myocardial structure is believed to be essential for normal contractile function of the heart. The mechanism by which regulates this morphogenic process is unknown. Whether ventricular trabeculation and compaction is part of this dynamic process requires further investigation.
Figure 1.
The development and growth of ventricular wall. The interactive regulation of endocardial-, myocardial-, and epicardial-derived growth factors and signaling networks is critical to the ventricular wall growth, trabeculation and compaction.
Genetics of LVNC
LVNC presents as at least eight different forms, depending on the nature of associated phenotypes [10]: 1) a mild form with normal left ventricular size, wall thickness, systolic function and diastolic function; 2) in association with dilated cardiomyopathy; 3) in association with hypertrophic cardiomyopathy; 4) in association with hypertrophic/dilated-mixed cardiomyopathy; 5) in association with restrictive cardiomyopathy; 6) biventricular noncompaction; 7) in association with early onset of arrhythmia; and 8) in association with congenital heart defects. Given this vast etiologic heterogeneity, it is not surprising that the genetics for LVNC is heterogeneous, with many different causative genes as well as multiple disease-causing mutations in each gene.
Tafazzin (TAZ/G4.5) is the first known disease-causing genetic mutation for LVNC in association of Barth syndrome. LVNC is one of several clinical hallmarks of Barth syndrome. Tafazzin catalyzes cardiolipin biosynthesis [28,29]. Normal cardiolipin is critical to mitochondrial bioenergetic function. Subsequently, genetic screening of many LVNC patients identified a handful of genetic mutations associated with LVNC, such as β-myosin heavy chain (βMHC/MYH7), myosin binding protein C (MYBPC3), α-cardiac actin (ACTC1), cardiac troponin T (TNNT2), cardiac troponin I (TNNI3), myosin light chains (MYL2, and MYL3), α-6 dystrobrevin, lamin A/C (LMNA), ZASP/LBD3, and dystrophin [30,31]. Interestingly, most of these genes are part of myofibril structure and are associated with contractile function. In addition, mutations in genes encoding the voltage-gated sodium channel (SCN5A) and the potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel (HCN4) were also associated with LVNC [32,33]. These genes are known to be involved in other forms of inherited cardiomyopathies. It is puzzling how these genetic variants could lead to ventricular noncompaction in the developing hearts. One report suggested that the presence or absence of a sarcomere gene mutation in LVNC does not predict the LVNC clinical phenotype [34], further questioning whether a specific genetic etiology and pathogenic pathway/s are existed for LVNC. A mutation in gene encoding mindbomb homolog 1 (MIB1) is found in association with a familial case of LVNC. MIB1 is an E3 ubiquitin ligase that regulates the endocytosis of the Notch ligands Delta and Jagged. Notch mediated signaling is believed to be involved in many morphogenetic events, and thus may be a missing link to explain the disease-causing mechanism. In addition, the recent advances in the use of genetic modified mouse to create LVNC disease models have generated much hope to finally understand the etiology of LVNC.
The controversial interpretation of the role of cardiomyocyte proliferation in the genesis of the LVNC phenotype
In the past decade, efforts were made to use genetic technology to model LVNC in mice (summarized in Table 1). A good example was the work by Phoon and his colleagues, used an inducible shRNA transgenic approach to knock-down tafazzin in mice (TAZKD) and studied the pathogenesis of Barth syndrome and LVNC [35]. As expected, Tafazzin knockdown led to altered cardiolipin and mitochondrial ultrastructure, as well as prenatal and perinatal lethality due to altered cardiac function [35]. The defects of TAZKD hearts mimicked some clinical phenotype of ventricular noncompaction, which appeared as early as E13.5 and thus providing an animal model for Barth syndrome and LVNC [35]. Another example is the myocardial specific knockout of Mib1 recapitulates the clinical hallmarks of LVNC [36], confirming the notion that Notch signaling is a critical contributor to the pathogenesis of LVNC. However, many other mouse models harboring mutations in genes associated with LVNC in humans failed to produce LVNC phenotype. For example, a missense mutation at codon 96 (from GAG to AAG) in exon 10 of the cardiac troponin T gene (TNNT2) is found to associate with a familial case of LVNC. Transgenic mice expressing this mutation in the heart exhibit altered cardiac function and cardiomyopathy, but histological analyses have excluded LVNC phenotype [37]. This discrepancy could be multiple; one likely cause is due to the experimental system with the transgenic overexpression, which may not mimic pathogenic pathway. Many mouse models represent the severe form of LVNC with various CHD. These mutant mice die at embryonic stage, suggesting that these genes are critical to cardiogenesis and that mutations in these genes in human likely lead to premature death in utero. Nevertheless, the detailed molecular analyses on these mouse models have helped us in understanding the potential pathogenic pathways contributing to LVNC.
Table 1.
Summary of mouse models for LVNC
| Mouse model | Gene function and mutant mouse phenotype |
|---|---|
|
| |
| Fkbp1a Loss-of-function [43] | Play a role in immunoregulation and basic cellular processes involving protein folding and trafficking. |
| Fkbp1a-deficient mice have severe dilated cardiomyopathy, ventricular septal defects and LVNC. | |
|
| |
| Jarid2 (Jumonji) Loss-of-function [44] | Nuclear protein and transcriptional regulator, and necessary for mouse embryogenesis. |
| The jmj homozygous mouse embryos showed heart malformations, including the ventriclar septal defect, noncompaction of the ventricular wall, double-outlet right ventricle, and dilated atria. | |
|
| |
| PBP/TRAP220 Loss-of-function [45] | Functions as a nuclear receptor coactivator, working with co-activators to direct transcriptional initiation by the RNA polymerase II apparatus. |
| PBP null mutation mice have noncompaction of the ventricular myocardium and resultant dilatation and cause embryonic lethality at E11.5. | |
|
| |
| Pegl(Mest) Loss-of-function [46] | Mesoderm specific, encodes a member of the alpha/beta hydrolase superfamily, has isoform-specific imprinting, and plays a role in development. |
| Peg1 knock out mice have a subtle alteration in the pattern of trabeculation: an increase in thickness and reduction in density of the compact myocardium. | |
|
| |
| TACE (ADAM17) Loss-of-function [47] | Member of a family of transmembrane and secreted metalloendopeptidases. Involving cell-cell and cell-matrix interactions, including fertilization, muscle development, and neurogenesis. |
| TACE null mutation die at birth, phenotypes include the failure of eyelid fusion, hair and skin defects, abnormalities of lung development, markedly enlarged fetal hearts with increased myocardial trabeculation and reduced compaction at late gestation. | |
|
| |
| Scrib/Vangl2 Loss-of-function [48] | Both involved in PCP signaling and was shown to be involved in synaptic function, neuroblast differentiation, and epithelial polarization. |
| Crc mice (Scrib mutant) develop heart malformations, cardiac misalignment defects and ventricular noncompaction. Double heterozygosity of both Scrib and Vangl2 can cause cardiac defects similar to those found in homozygous mutants for each gene but without other major defects. | |
|
| |
| Vangl2 Loss-of-function [49] | A membrane protein involved in the regulation of planar cell polarity, especially in the stereociliary bundles of the cochlea. |
| Lp mice (Vangl2 mutant) develop double outlet right ventricle with perimembranous ventricular septal defects and aortic arch defects, and the defects are caused by mutations in the Vangl2 gene, mutant ventricles have characteristics of noncompaction. | |
|
| |
| PTPN11(SHP2) Q79R Gain-of-function mutation [50] | Regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. |
| SHP2 (Q79R) transgenic mice selectively activated the ERK 1/2 pathway and cause aberrant cardiac architecture during cardiac development, including ventricular noncompaction. | |
|
| |
| FRNK Gain-of-function mutation [51] | Cytoplasmic protein tyrosine kinase found in the focal adhesions that form between cells growing in the presence of extracellular matrix. |
| Cardiac-specific over-expression of FRNK 5 lead to a severe ventricular noncompaction defect associated with reduced cardiomyocyte proliferation. | |
|
| |
| cTNT(E96K) Gain-of-function mutation [37] | The protein located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration. cTNT (E96K) is linked to LVNC. |
| cTNT mutant mice displayed an impaired left ventricular function and induction of marker genes of heart failure. Left ventricular non-compaction was not observed. | |
|
| |
| Gbe1(E609X) Loss-of-function [52] | Encodes a glycogen branching enzyme that adds short glucosyl chains in α-1,6 glycosidic links to the glycogen molecule to yield a branched polymer with increased water solubility and glycogen synthetic activity. |
| The ENU-induced Gbe1 mutant mice exhibit abnormal cardiac development, including hypertrabeculation and noncompaction of the ventricular wall. Poor ventricular function in late gestation and ultimately cause heart failure, fetal hydrops and embryonic lethality. | |
|
| |
| Daaml Loss-of-function [42] | A potential non-canonical Wnt/PCP signaling effector, involved in cell polarity and actin polarization. |
| Daam1-deficient mice exhibit embryonic and neonatal lethality and suffer multiple cardiac defects, including ventricular noncompaction, double outlet right ventricles and ventricular septal defects. | |
|
| |
| YWHAE Loss-of-function [53] | Ywhae belongs to the 14-3-3 family of proteins which mediate signal transduction by binding to phosphoserine-containing proteins, also called 14-3-3ε |
| Ywhae-deletion led to ventricular non-compaction and selective reduction in compact myocardium thickness. | |
|
| |
| Tafazzin (TAZ) Loss-of-function [35] | Located in mitochondria, involved in altering a fat (lipid) called cardiolipin, which plays critical roles in the mitochondrial inner membrane. |
| TAZ knockdown (TAZKD) mice died within the neonatal period, mutant hearts show myocardial thinning, hypertrabeculation and noncompaction, and defective ventricular septation. | |
|
| |
| Mib1 Loss-of-function [36] | Encodes protein functions as an E3 ubiquitin ligase, positively regulates Notch signaling by ubiquitinating the Notch receptors, thereby facilitating their endocytosis. |
| Targeted inactivation of Mib1 in mouse myocardium causes LVNC, reduced ventricular Notch1 activity, expansion of compact myocardium to proliferative, immature trabeculae and abnormal expression of cardiac development and disease genes. | |
|
| |
| Nkx2-5(R52G) DNA binding domain missense mutation [54] | Plays critical roles in regulating cardiac-specific gene expression essential for cardiomyocyte differentiation and heart development. |
| Nkx2-5+/R52G and cardiomyocyte conditional knockout mice demonstrated noncompaction and hypertrabeculation, along with diverse cardiac anomalies, including atrioventricular septal defects, Ebstein malformation of the tricuspid valve, perimembranous and muscular ventricular septal defects, and cardiac conduction system defects. | |
|
| |
| BrafQ241R Gain-of-function [55] | Plays important role in signaling pathway known as the RAS/MAPK pathway and its mediated cellular function. |
| BrafQ241R/+ mice manifested embryonic/neonatal lethality, showing liver necrosis, edema, craniofacial abnormalities, and multiple heart defects, including cardiomegaly, enlarged cardiac valves, ventricular noncompaction and ventricular septal defects. | |
|
| |
| Caszl Loss-of-function [56] | Encodes a zinc finger transcription factor, function as a tumor suppressor, and single nucleotide polymorphisms in this gene are associated with blood pressure variation. |
| Casz1 deletion in mice resulted in abnormal heart development including hypoplasia of myocardium, cardiac noncompaction, ventricular septal defect, and disorganized morphology. | |
|
| |
| SLC25A5/ANT2 Loss-of-function [57] | Functions as a major component in the mitochondrial permeability-transition pore complex that promotes the exchange of mitochondrial ATP with cytosolic ADP. |
| Ant2-null embryos showed E14.5 lethality with cardiac developmental failure, immature cardiomyocytes, cardiomyocyte hyperliferation, and cardiac failure due to hypertrabeculation/noncompaction. | |
|
| |
| S1pr1 Loss-of-function [58] | Structurally similar to G protein-coupled receptors and is highly expressed in endothelial cells. It has an important role in regulating endothelial cell cytoskeletal structure, migration, capillary-like network formation and vascular maturation. |
| S1pr1 conditional knockout showed ventricular noncompaction and ventricular septal defects and perinatal lethality in majority of mutants. | |
|
| |
| NUMB/NUMBL Loss-of-function [59] | Plays a role in the determination of cell fates during development. Its primary function in cell differentiation is as an inhibitor of Notch signaling which is essential for maintaining self-renewal potential in stem and progenitor cells. |
| Loss of NUMB or both NUMB and NUMBL in cardiomyocytes results in ventricular noncompaction phenotypes. | |
|
| |
| Rlf Loss-of-function [60] | Encodes a Zinc finger protein, acts as an epigenetic modifier maintaining low levels of DNA methylation at CpG island and enhancers across the genome. |
| Two independent Rlf ENU mutant lines showed heart defects resembling LVNC at E11.5–E 14.5. | |
|
| |
| Lrp2 Loss-of-function [61] | Critical for the reuptake of numerous ligands, including lipoproteins, sterols, vitamin-binding proteins, and hormones. |
| Lrp2 knockout mice display a range of severe cardiovascular abnormalities, including aortic arch anomalies, common arterial trunk (persistent truncus arteriosus) with coronary artery anomalies, ventricular septal defects, overriding of the tricuspid valve and marked thinning of the ventricular myocardium. | |
|
| |
| Slc39a8 Loss-of-function [62] | Encodes a transmembrane protein that acts as a transporter of several divalent cations, including manganese (Mn), zinc (Zn), cadmium (Cd), and iron (Fe). |
| Slc39a8-null mouse embryos do not survive embryogenesis and exhibit a LVNC phenotype. | |
|
| |
| DtnaN49S Gain-of-function [63] | Dystrobrevin alpha belongs to the dystrobrevin subfamily and the dystrophin family. This protein is a component of the dystrophin-associated protein complex (DPC). |
| Multiple trabeculation and a higher ratio of noncompacted to compacted myocardial layer were found in the Myh6-DtnaN49S transgenic mice, and with left ventricular dilation and systolic dysfunction. | |
|
| |
| SRC-1 and -3 Loss-of-function [64] | Encode steroid receptor coactivator-1 (SRC-1) and SRC-3, transcriptional coactivators for nuclear hormone receptors and certain other transcription factors that regulate many genes in development and organ function. |
| Ablation of SRC-1/3 in the myocardial lineage resulted in prominent trabeculae, deep intertabecular recesses and thin ventricular wall and septum. | |
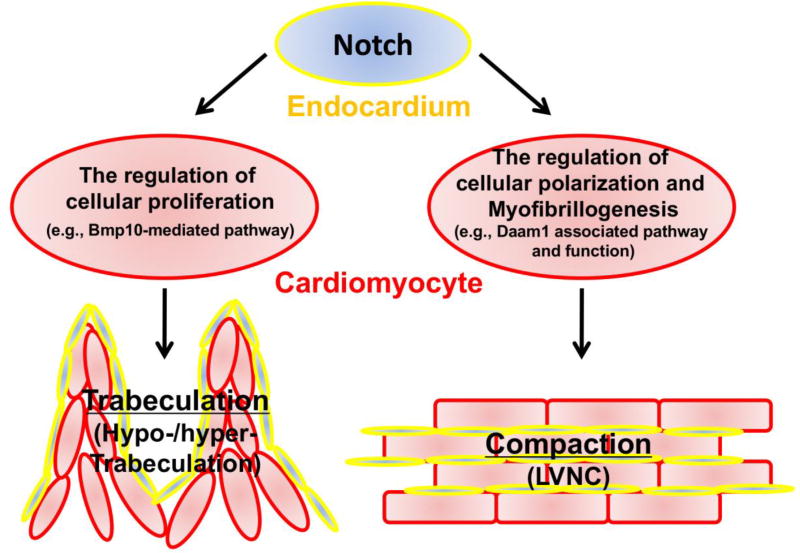
Surveying the literature reveals that some mice develop LVNC due to altered signaling in Notch signaling via either endocardial-mediated pathway or myocardial-mediated pathway, including Fkbp1a-deficient mouse models, Numb/Numblike-deficient mouse models, Jarid2 (Jumonji)-deficient mouse model, and Mib1-deficient mouse model. Two commonly known features of these LVNC models are that the altered Bmp10 expression and altered cardiomyocyte proliferation. However, reduced level of Bmp10 expression and a concomitant reduction in cardiomyocyte proliferation is observed in Mib1-deficient hearts, whereas increased level of Bmp10 expression and a concomitant increase in cardiomyocyte proliferation are seen in other mutant models. Currently, it is rathe controversial if up- or down-regulation of cardiomyocyte proliferation is the key factor in contributing to the LVNC pathogenic pathway. The likely answer to the debate is that neither the up- nor down-regulation of cell proliferation is directly relevant to the pathogenesis of LVNC.
Clues from Daam1-deficient mice suggest a common molecular etiology for LVNC
Disheveled-associated activator of morphogenesis 1 (Daam1) is a potentially important effector for noncanonical Wnt pathway signaling, especially the Wnt/Planar cell pplarity (PCP) signaling. The key features for PCP signaling include the control of cellular alignment and orientation in polarized tissues [38,39]. Wnt/PCP signaling has an essential function in embryonic patterning and organogenesis, more specifically in the regulation of gastrulation, neurulation, and hair cell polarity within the inner ear in mouse and within the lateral line in zebrafish [38,39]. Cardiomyocytes are not epithelial cells and do not have a classic PCP phenotype. However mature cardiomyocytes are highly polarized with the majority of cell-cell junction proteins located at intercalated disc, joining cell end to end (i.e., distal-proximal axis), whereas the attachment to the extracellular matrix are on their lateral surface [40,41]. Interestingly, the immature cardiomyocytes at early embryonic hearts are mostly polygonal or spherical and thus lack an overt PCP phenotype [40,41]. Daam1-deficient mice develop LVNC, double outlet ventricle (DORV), and ventricular septal defect (VSD); the majority of these mice die in utero [42]. Most importantly, Daam1-deficient hearts have normal cardiomyocyte proliferation and Bmp10 expression, indicating that altered cell cycle activity is not required for ventricular noncompaction phenotype [42]. In contrast, cardiomyocyte myofibrillogenesis, sarcomere formation, cardiomyocyte polarization, cell-cell alignment, and cell-cell junction are altered in these mice, raising the possibility that defects in cardiomyocyte myofibrillogenesis and polarization during ventricular development is the common pathogenic pathway of LVNC. Supporting this hypothesis, Fkbp1a-deficient hearts have defects in cardiomyocyte myofibrillogenesis and polarity. Further support comes from the observation that the majority of genetic variants associated with LVNC in humans are in genes encoding sarcomeric proteins, related binding proteins, and cytoskeletal proteins, which clearly can affect the myofibrillogenesis and cellular polarity in cardiomyocytes. It is now important to evaluate all reported LVNC mouse models (Table-1) and to determine whether defects in myofibrillogenesis and cardiomyocyte polarity are common in all these models (Figure 2).
Figure 2.
Hypothetical model for different signaling pathways in regulating ventricular trabeculation and compaction.
Acknowledgments
Funding: The work is funded by NIHP01HL134599 to W.S. and by the Riley Children’s foundation to W.S.
Footnotes
Compliance with Ethical Standards:
Ethical approval: This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Conflict of Interest: Authors declare that they have no conflict of interest.
References
- 1.Towbin JA. Left ventricular noncompaction: a new form of heart failure. Heart Fail Clin. 2010;6:453–469. viii. doi: 10.1016/j.hfc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446–1456. doi: 10.1093/eurheartj/ehq508. [DOI] [PubMed] [Google Scholar]
- 4.Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S, Anderson RH. The morphological spectrum of ventricular noncompaction. Cardiology in the young. 2005;15:345–364. doi: 10.1017/S1047951105000752. [DOI] [PubMed] [Google Scholar]
- 5.Grant RT. An unusual anomaly of the coronary vessels in the malformed heart of a child. heart. 1926;13:273–283. [Google Scholar]
- 6.Lee TM, Hsu DT, Kantor P, Towbin JA, Ware SM, Colan SD, Chung WK, Jefferies JL, Rossano JW, Castleberry CD, Addonizio LJ, Lal AK, Lamour JM, Miller EM, Thrush PT, Czachor JD, Razoky H, Hill A, Lipshultz SE. Pediatric Cardiomyopathies. Circ Res. 2017;121:855–873. doi: 10.1161/CIRCRESAHA.116.309386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114:2671–2678. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- 8.Nugent AW, Daubeney PE, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–1338. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- 9.Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, Wilkinson LC, Davis AM, Kahler SG, Chow CW, Wilkinson JL, Weintraub RG. The epidemiology of childhood cardiomyopathy in Australia. The New England journal of medicine. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 10.Towbin JA, Jefferies JL. Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ Res. 2017;121:838–854. doi: 10.1161/CIRCRESAHA.117.310987. [DOI] [PubMed] [Google Scholar]
- 11.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 12.Taber LA. Mechanical aspects of cardiac development. Prog Biophys Mol Biol. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 13.Moorman AF, Christoffels VM, Anderson RH, van den Hoff MJ. The heart-forming fields: one or multiple? Philos Trans R Soc Lond B Biol Sci. 2007;362:1257–1265. doi: 10.1098/rstb.2007.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Brutsaert DL, Andries LJ. The endocardial endothelium. The American journal of physiology. 1992;263:H985–1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- 16.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 17.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Risebro CA, Riley PR. Formation of the ventricles. The Scientific World Journal. 2006;6:1862–1880. doi: 10.1100/tsw.2006.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taber LA. Mechanical aspects of cardiac development. Progress in biophysics and molecular biology. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 20.Dorri F, Niederer PF, Redmann K, Lunkenheimer PP, Cryer CW, Anderson RH. An analysis of the spatial arrangement of the myocardial aggregates making up the wall of the left ventricle. Eur J Cardiothorac Surg. 2007;31:430–437. doi: 10.1016/j.ejcts.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Lunkenheimer PP, Redmann K, Kling N, Jiang X, Rothaus K, Cryer CW, Wubbeling F, Niederer P, Heitz PU, Ho SY, Anderson RH. Three-dimensional architecture of the left ventricular myocardium. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:565–578. doi: 10.1002/ar.a.20326. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RH, Ho SY, Sanchez-Quintana D, Redmann K, Lunkenheimer PP. Heuristic problems in defining the three-dimensional arrangement of the ventricular myocytes. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:579–586. doi: 10.1002/ar.a.20330. [DOI] [PubMed] [Google Scholar]
- 23.Lunkenheimer PP, Redmann K, Westermann P, Rothaus K, Cryer CW, Niederer P, Anderson RH. The myocardium and its fibrous matrix working in concert as a spatially netted mesh: a critical review of the purported tertiary structure of the ventricular mass. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S41–49. doi: 10.1016/j.ejcts.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 24.Schmid P, Lunkenheimer PP, Redmann K, Rothaus K, Jiang X, Cryer CW, Jaermann T, Niederer P, Boesiger P, Anderson RH. Statistical analysis of the angle of intrusion of porcine ventricular myocytes from epicardium to endocardium using diffusion tensor magnetic resonance imaging. Anat Rec (Hoboken) 2007;290:1413–1423. doi: 10.1002/ar.20604. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RH, Sanchez-Quintana D, Redmann K, Lunkenheimer PP. How are the myocytes aggregated so as to make up the ventricular mass? Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:76–86. doi: 10.1053/j.pcsu.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RH, Sanchez-Quintana D, Niederer P, Lunkenheimer PP. Structural-functional correlates of the 3-dimensional arrangement of the myocytes making up the ventricular walls. J Thorac Cardiovasc Surg. 2008;136:10–18. doi: 10.1016/j.jtcvs.2007.09.083. [DOI] [PubMed] [Google Scholar]
- 27.Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. The Journal of biological chemistry. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M. A Drosophila model of Barth syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11584–11588. doi: 10.1073/pnas.0603242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, Greutmann M, Hurlimann D, Yegitbasi M, Pons L, Gramlich M, Drenckhahn JD, Heuser A, Berger F, Jenni R, Thierfelder L. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117:2893–2901. doi: 10.1161/CIRCULATIONAHA.107.746164. [DOI] [PubMed] [Google Scholar]
- 31.Xing Y, Ichida F, Matsuoka T, Isobe T, Ikemoto Y, Higaki T, Tsuji T, Haneda N, Kuwabara A, Chen R, Futatani T, Tsubata S, Watanabe S, Watanabe K, Hirono K, Uese K, Miyawaki T, Bowles KR, Bowles NE, Towbin JA. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab. 2006;88:71–77. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer PA, Schroter J, Greiner S, Haas J, Yampolsky P, Mereles D, Buss SJ, Seyler C, Bruehl C, Draguhn A, Koenen M, Meder B, Katus HA, Thomas D. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol. 2014;64:757–767. doi: 10.1016/j.jacc.2014.06.1155. [DOI] [PubMed] [Google Scholar]
- 33.Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, Postma AV, van der Bilt IA, Baars MJ, van Haelst PL, Caliskan K, Hoedemaekers YM, Le Scouarnec S, Redon R, Pinto YM, Christiaans I, Wilde AA, Bezzina CR. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol. 2014;64:745–756. doi: 10.1016/j.jacc.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Probst S, Oechslin E, Schuler P, Greutmann M, Boye P, Knirsch W, Berger F, Thierfelder L, Jenni R, Klaassen S. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet. 2011;4:367–374. doi: 10.1161/CIRCGENETICS.110.959270. [DOI] [PubMed] [Google Scholar]
- 35.Phoon CK, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N, Ren M. Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. Journal of the American Heart Association. 2012;1 doi: 10.1161/JAHA.111.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luxan G, Casanova JC, Martinez-Poveda B, Prados B, D'Amato G, MacGrogan D, Gonzalez-Rajal A, Dobarro D, Torroja C, Martinez F, Izquierdo-Garcia JL, Fernandez-Friera L, Sabater-Molina M, Kong YY, Pizarro G, Ibanez B, Medrano C, Garcia-Pavia P, Gimeno JR, Monserrat L, Jimenez-Borreguero LJ, de la Pompa JL. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med. 2013;19:193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- 37.Luedde M, Ehlermann P, Weichenhan D, Will R, Zeller R, Rupp S, Muller A, Steen H, Ivandic BT, Ulmer HE, Kern M, Katus HA, Frey N. Severe familial left ventricular non-compaction cardiomyopathy due to a novel troponin T (TNNT2) mutation. Cardiovascular research. 2010;86:452–460. doi: 10.1093/cvr/cvq009. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 39.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Developmental biology. 2006;289:430–441. doi: 10.1016/j.ydbio.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 41.Henderson DJ, Chaudhry B. Getting to the heart of planar cell polarity signaling. Birth Defects Res A Clin Mol Teratol. 2011;91:460–467. doi: 10.1002/bdra.20792. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, Field LJ, Atkinson SJ, Shou W. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, Mathews LM, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y, Song AJ, Baker R, Micales B, Conway SJ, Lyons GE. Jumonji, a nuclear protein that is necessary for normal heart development. Circ Res. 2000;86:932–938. doi: 10.1161/01.res.86.9.932. [DOI] [PubMed] [Google Scholar]
- 45.Crawford SE, Qi C, Misra P, Stellmach V, Rao MS, Engel JD, Zhu Y, Reddy JK. Defects of the heart, eye, and megakaryocytes in peroxisome proliferator activator receptor-binding protein (PBP) null embryos implicate GATA family of transcription factors. The Journal of biological chemistry. 2002;277:3585–3592. doi: 10.1074/jbc.M107995200. [DOI] [PubMed] [Google Scholar]
- 46.King T, Bland Y, Webb S, Barton S, Brown NA. Expression of Peg1 (Mest) in the developing mouse heart: involvement in trabeculation. Dev Dyn. 2002;225:212–215. doi: 10.1002/dvdy.10142. [DOI] [PubMed] [Google Scholar]
- 47.Shi W, Chen H, Sun J, Buckley S, Zhao J, Anderson KD, Williams RG, Warburton D. TACE is required for fetal murine cardiac development and modeling. Developmental biology. 2003;261:371–380. doi: 10.1016/s0012-1606(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 48.Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, Anderson RH, Copp AJ, Chaudhry B, Henderson DJ. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ Res. 2007;101:137–145. doi: 10.1161/CIRCRESAHA.106.142406. [DOI] [PubMed] [Google Scholar]
- 49.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW, 2nd, Robbins J. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiMichele LA, Hakim ZS, Sayers RL, Rojas M, Schwartz RJ, Mack CP, Taylor JM. Transient expression of FRNK reveals stage-specific requirement for focal adhesion kinase activity in cardiac growth. Circ Res. 2009;104:1201–1208. doi: 10.1161/CIRCRESAHA.109.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YC, Chang CJ, Bali D, Chen YT, Yan YT. Glycogen-branching enzyme deficiency leads to abnormal cardiac development: novel insights into glycogen storage disease IV. Hum Mol Genet. 2011;20:455–465. doi: 10.1093/hmg/ddq492. [DOI] [PubMed] [Google Scholar]
- 53.Kosaka Y, Cieslik KA, Li L, Lezin G, Maguire CT, Saijoh Y, Toyo-oka K, Gambello MJ, Vatta M, Wynshaw-Boris A, Baldini A, Yost HJ, Brunelli L. 14-3-3epsilon plays a role in cardiac ventricular compaction by regulating the cardiomyocyte cell cycle. Mol Cell Biol. 2012;32:5089–5102. doi: 10.1128/MCB.00829-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashraf H, Pradhan L, Chang EI, Terada R, Ryan NJ, Briggs LE, Chowdhury R, Zarate MA, Sugi Y, Nam HJ, Benson DW, Anderson RH, Kasahara H. A mouse model of human congenital heart disease: high incidence of diverse cardiac anomalies and ventricular noncompaction produced by heterozygous Nkx2-5 homeodomain missense mutation. Circ Cardiovasc Genet. 2014;7:423–433. doi: 10.1161/CIRCGENETICS.113.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue S, Moriya M, Watanabe Y, Miyagawa-Tomita S, Niihori T, Oba D, Ono M, Kure S, Ogura T, Matsubara Y, Aoki Y. New BRAF knockin mice provide a pathogenetic mechanism of developmental defects and a therapeutic approach in cardio-facio-cutaneous syndrome. Hum Mol Genet. 2014;23:6553–6566. doi: 10.1093/hmg/ddu376. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Li W, Ma X, Ding N, Spallotta F, Southon E, Tessarollo L, Gaetano C, Mukouyama YS, Thiele CJ. Essential role of the zinc finger transcription factor Casz1 for mammalian cardiac morphogenesis and development. J Biol Chem. 2014;289:29801–29816. doi: 10.1074/jbc.M114.570416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kokoszka JE, Waymire KG, Flierl A, Sweeney KM, Angelin A, MacGregor GR, Wallace DC. Deficiency in the mouse mitochondrial adenine nucleotide translocator isoform 2 gene is associated with cardiac noncompaction. Biochim Biophys Acta. 2016;1857:1203–1212. doi: 10.1016/j.bbabio.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clay H, Wilsbacher LD, Wilson SJ, Duong DN, McDonald M, Lam I, Park KE, Chun J, Coughlin SR. Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev Biol. 2016;418:157–165. doi: 10.1016/j.ydbio.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirai M, Arita Y, McGlade CJ, Lee KF, Chen J, Evans SM. Adaptor proteins NUMB and NUMBL promote cell cycle withdrawal by targeting ERBB2 for degradation. J Clin Invest. 2017;127:569–582. doi: 10.1172/JCI91081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourke LM, Del Monte-Nieto G, Outhwaite JE, Bharti V, Pollock PM, Simmons DG, Adam A, Hur SS, Maghzal GJ, Whitelaw E, Stocker R, Suter CM, Harvey RP, Harten SK. Loss of Rearranged L-Myc Fusion (RLF) results in defects in heart development in the mouse. Differentiation. 2017;94:8–20. doi: 10.1016/j.diff.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Baardman ME, Zwier MV, Wisse LJ, Gittenberger-de Groot AC, Kerstjens-Frederikse WS, Hofstra RM, Jurdzinski A, Hierck BP, Jongbloed MR, Berger RM, Plosch T, DeRuiter MC. Common arterial trunk and ventricular non-compaction in Lrp2 knockout mice indicate a crucial role of LRP2 in cardiac development. Dis Model Mech. 2016;9:413–425. doi: 10.1242/dmm.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin W, Li D, Cheng L, Li L, Liu F, Hand NJ, Epstein JA, Rader DJ. Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. J Clin Invest. 2018;128:826–833. doi: 10.1172/JCI96993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Q, Shen Y, Liu X, Yu X, Yuan P, Wan R, Liu X, Peng X, He W, Pu J, Hong K. Phenotype and Functional Analyses in a Transgenic Mouse Model of Left Ventricular Noncompaction Caused by a DTNA Mutation. Int Heart J. 2017;58:939–947. doi: 10.1536/ihj.16-019. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Qin L, Liu Z, Liao L, Martin JF, Xu J. Knockout of SRC-1 and SRC-3 in Mice Decreases Cardiomyocyte Proliferation and Causes a Noncompaction Cardiomyopathy Phenotype. Int J Biol Sci. 2015;11:1056–1072. doi: 10.7150/ijbs.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]