Abstract
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are commonly comorbid, share genetic liability, and often exhibit overlapping cognitive impairments. Clarification of shared and distinct cognitive effects while considering comorbid symptoms across disorders has been lacking. In the current study, children ages 7-15 years assigned to three diagnostic groups: ADHD (n = 509), ASD (n = 97), and controls (n = 301) completed measures spanning the cognitive domains of attention/arousal, working memory, set-shifting, inhibition, and response variability. Specific processes contributing to response variability were examined using a drift diffusion model, which separately quantified drift rate (i.e., efficiency of information processing), boundary separation (i.e., speed-accuracy trade-offs), and non-decision time. Children with ADHD and ASD were impaired on attention/arousal, processing speed, working memory, and response inhibition, but did not differ from controls on measures of delayed reward discounting, set-shifting, or interference control. Overall, impairments in the ASD group were not attributable to ADHD symptoms using either continuous symptom measures or latent categorical grouping approaches. Similarly, impairments in the ADHD group were not attributable to ASD symptoms. When specific RT parameters were considered, children with ADHD and ASD shared impairments in drift rate. However, children with ASD were uniquely characterized by a wider boundary separation. Findings suggest a combination of overlapping and unique patterns of cognitive impairment for children with ASD as compared to those with ADHD, particularly when the processes underlying reaction time measures are considered separately.
Keywords: Attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders (ASD), executive function, neuropsychology, reaction time variability, diffusion model
There is increasing recognition that many psychiatric symptom dimensions are shared across existing diagnostic boundaries, and the extent to which this indicates shared liability or etiology across disorders remains a major question (Insel et al., 2010; Sanislow et al., 2010). Despite conceptually distinct surface phenotypes, ADHD and ASD share overlapping features (Ronald, Larsson, Anckarsäter, & Lichtenstein, 2014) often enough to warrant clinical recognition in the DSM-5, co-occur at rates well above chance (Musser et al., 2014; Rommelse, Geurts, Franke, Buitelaar, & Hartman, 2011), and have overlapping genetic correlates (Rommelse, Franke, Geurts, Hartman, & Buitelaar, 2010; Ronald, Simonoff, Kuntsi, Asherson, & Plomin, 2008), suggesting they may be different manifestations of a shared liability. Crucially, for the focus of the current investigation, ADHD and ASD may also share some common cognitive impairments that can both confound and help clarify their clinical and phenomenological relationship.
Executive Function in ASD and ADHD
Multiple cognitive domains have been proposed as relevant in ADHD and, to a lesser extent, in ASD, including: 1) response inhibition (Logan, 1994; Nigg, 2000); 2) interference control; 3) working memory (Baddeley, 1996; De Luca et al., 2003; Engle, 2002; Kane, Conway, Hambrick, & Engle, 2007; Unsworth & Engle, 2007), 4) set-shifting and 5) reward valuation, particularly delayed reward discounting (Demurie, Roeyers, Baeyens, & Sonuga-Barke, 2012; Hulst et al., 2017; Mitchell, 1999; Sagvolden, Aase, Zeiner, & Berger, 1998).
Although there is heterogeneity in the ADHD population (Fair, Bathula, Nikolas, & Nigg, 2012; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005), at the group level relatively consistent impairments have been found in individuals with ADHD in each of these domains (Huang-Pollock, Karalunas, Tam, & Moore, 2012; Kofler et al., 2013; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Deficits in several aspects of executive function have also been found in individuals with ASD relative to controls, particularly in set-shifting (Hill, 2004; Ozonoff & Jensen, 1999; Sanders, Johnson, Garavan, Gill, & Gallagher, 2008).
Head-to-head comparisons of children with ASD and ADHD have yielded conflicting results as to whether executive function impairments and differences in reward processing are more pronounced in ADHD (Happe, Booth, Charlton, & Hughes, 2006; Nyden et al., 2010) or in ASD (Geurts, Verte, Oosterlaan, Roeyers, & Sergeant, 2004; Pennington & Ozonoff, 1996). Individuals with ADHD may have greater difficulty with response inhibition and delayed reward discounting, whereas those with ASD may have more trouble with set-shifting and working memory (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Demurie et al., 2012; Gargaro, Rinehart, Bradshaw, Tonge, & Sheppard, 2011; Happe et al., 2006; Hulst et al., 2017; Ozonoff & Jensen, 1999; Sinzig, Bruning, Morsch, & Lehmkuhl, 2008). However, other studies also suggest that individuals with ADHD and ASD perform similarly (Geurts et al., 2004; Raymaekers, Antrop, van der Meere, Wiersema, & Roeyers, 2007; Semrud-Clikeman, Walkowiak, Wilkinson, & Butcher, 2010).
An obvious confound is that many children with ASD also experience clinically-significant ADHD symptoms, yet very few studies historically take this into account. One hypothesis is that the observed cognitive impairments in ASD may be related to comorbid ADHD symptoms rather than shared genetic liability or core mechanisms. Several studies have compared children with ASD and comorbid ADHD to those with ASD-only. However, this approach is limited because comparing categorical groups based on DSM diagnostic criteria does not account for sub-threshold but non-trivial ADHD symptoms that are often present in children with ASD. Some evidence using continuous symptom measures supports that impairments in response inhibition (Corbett et al., 2009; Yerys, Kenworthy, Jankowski, Strang, & Wallace, 2013) and arousal (Corbett et al., 2009) in ASD may be driven by comorbid ADHD symptoms, whereas ASD symptoms may contribute unique variance to processing speed (Yerys et al., 2013). However, studies are small and have included tasks with emotional face stimuli (Yerys et al., 2013), which may be uniquely related to ASD traits in ways that non-emotional and non-face stimuli are not. Thus, it remains unclear whether the executive function problems attributed to ASD are truly due to shared liability or simply reflect comorbid ADHD symptoms.
Beyond Executive Function? Reaction Time Measures in ASD and ADHD
In addition to traditional measures of executive functioning, reaction time (RT) measures are of increasing interest in both ADHD and ASD as a window into the intersection of bottom-up information processing and cognitive control (Karalunas, Geurts, Konrad, Bender, & Nigg, 2014; Kofler et al., 2013; Rommelse et al., 2011; van der Meer et al., 2012). Increased RT variability in ADHD is well-established. In ASD, although RT variability has received theoretical attention as a putative trans-diagnostic phenotype (e.g., Rommelse et al., 2011), the empirical evidence for increased RT variability is less clear. A recent meta-analysis suggests that RT variablity in ASD may be driven by comorbid ADHD but highlights the need for additional studies, particularly those that account dimensionally for ADHD symptoms in the ASD group (Karalunas et al., 2014).
Another question is the extent to which RT impairments are driven by the same cognitive processes in both ADHD and ASD. RT speed and variability reflect the influence of multiple interacting processes (Ratcliff, 2002) that traditional RT measures, such as mean and standard deviation, do not differentiate. The drift diffusion model provides one approach for separately quantifying the processes affecting the final RT and has been applied in the ADHD literature to clarify mechanisms of RT impairment. Briefly, the drift diffusion model (Ratcliff & Rouder, 2016) assumes that how quickly a person responds is related both to the conservativeness of the criteria they have set for responding (called boundary separation, related to speed-accuracy trade-offs) and to the rate at which information is accumulated in favor of one of the response criterion (called drift rate). Processes not directly related to the response decision, such as stimulus encoding and motor response, are modeled by non-decision time (Ratcliff, 2006).
In ADHD, RT impairments appear to be primarily driven by slow drift rates (i.e., slow, inefficient information processing) and to a lesser extent fast non-decision times, but are unrelated to differences in boundary separation (Huang-Pollock et al., 2012; Karalunas et al., 2014; Karalunas & Huang-Pollock, 2013). The only study to-date applying this model in ASD found that children with ASD have wider boundary separations and slower non-decisions times than typically-developing children, but no differences in drift rates (Pirrone, Dickinson, Gomez, Stafford, & Milne, 2017). Results suggest possibly unique mechanisms driving RT differences, but need to be confirmed in larger samples and direct comparisons between children with ASD and ADHD taking into account comorbid symptoms are needed.
Summary
The shared and unique cognitive characteristics of ADHD and ASD remain insufficiently characterized. Here, we examine overlapping and distinct neuropsychological profiles of children with ADHD and ASD and evaluate the effects of dimensional symptom measures on neuropsychological profiles for both groups. Based on the previous literature, we hypothesized that the ASD group would show greater impairment than the ADHD group on set-shifting and working memory, but would have similar or less impairment than children with ADHD on measures of arousal, inhibitory control, and reward discounting. We did not expect ASD symptoms to account for cognitive impairments in ADHD, but sought to clarify whether ADHD symptoms accounted for cognitive impairments in ASD. We further hypothesized that the ADHD and ASD groups would be equally impaired on measures of response variability but that these differences would be driven by different mechanisms: slow drift rate in ADHD and wide boundary separations in ASD.
Method
Overview of Procedure
All procedures were approved by the Oregon Health & Science University's Institutional Review Board. Parents provided written informed consent and children provided written assent. Participants were ages 7-15 years assigned to three diagnostic groups using screening and diagnostic procedures described below. The final sample included 509 children ADHD, 97 children with ASD, and 301 typically-developing controls. Table 1 shows participant demographics. Study data were collected and managed, in part, using REDCap electronic data capture tools hosted at OHSU. It provides a secure web-based and intuitive interface and export capabilities (Harris et al., 2009).
Table 1.
Participant characteristics.
| Control | ASD | ADHD | P | Effect Size (d)a | Posthoc | |
|---|---|---|---|---|---|---|
| N | 301 | 97 | 509 | -- | -- | |
| Sex (Male:Female) | 146:155 | 84:13 | 359:150 | <.001 | -- | ADHD>C; ASD>C; ASD>ADHD |
| Age in years (SD) | 9.48 (1.59) | 11.34 (2.49) | 9.72 (1.59) | <.001 | .89, .15, .78 | ASD>C; ASD>ADHD |
| Estimated IQ (SD) | 115.28 (12.75) | 99.53 (16.37) | 108.27 (13.88) | <.001 | 1.07, .52, .58 | ADHD<C; ASD<C; ASD<ADHD |
| ADHD-RS IA (SD) | 44.73 (7.32) | 63.54 (13.20) | 72.17 (12.07) | <.001 | 1.76,2.75, .68 | ADHD>C; ASD>C; ASD<ADHD |
| ADHD-RS HI (SD) | 45.11 (7.20) | 60.07 (12.77) | 67.59 (14.51) | <.001 | 1.44, 1.96, .55 | ADHD>C; ASD>C; ASD<ADHD |
| ADHD-RS Total (SD) | 44.61 (7.18) | 62.82 (12.37) | 71.48 (12.61) | <.001 | 1.81, 2.62, .69 | ADHD>C; ASD>C; ASD<ADHD |
| SRS-2 Total (SD)+ | 45.54 (6.46) | 77.93 (11.38) | 55.38 (9.51) | <.001 | 3.50,1.21,2.15 | ASD>ADHD>C |
Note. p-values indicate three-group significance test. ASD = Autism spectrum disorder; ADHD = Attention-deficit/hyperactivity disorder; IA = Inattention; HI = Hyperactive-Impulsive. ADHD-RS and SRS-2 scores reported are age- and sex-normed T-scores.
These values reflect the Cohen's d value for the Control/ASD, Control/ADHD, and ASD/ADHD comparison, respectively. Effects sizes presented are the absolute value of the Cohen's d value.
Participant recruitment: ADHD and typically-developing controls
ADHD and control children in the current study were part of an ongoing longitudinal study. Only the data from the first year of the study are included here to match available data for the ASD sample. Participants were recruited via mailings to commercial mailing lists and public advertisements. Families participated in a multi-gated procedure that included an initial phone screen. Parents of children who remained eligible upon completion of the initial phone screen were invited to complete the ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 1998), Conner's Rating Scale, 3rd edition (Conners, 2008), and the Strengths and Difficulties Questionnaire (Goodman, 2001), and an in-person semi-structured diagnostic interview (Kiddie Schedule for Affective Disorders and Schizophrenia, KSADS; Puig-Antich & Ryan, 1986), while the child completed IQ screening and brief academic achievement testing.
A best estimate DSM-IV diagnosis was established by a multi-disciplinary diagnostic team. Blind to one another's ratings and to the subsequent cognitive test scores, they formed a diagnostic opinion based on all available information. Their agreement rate was excellent (ADHD diagnosis kappa =.88). Disagreements were conferenced and consensus reached. Cases where consensus was not readily achieved were excluded. Specific symptom counts for determining diagnosis followed DSM-IV cutoffs and are detailed in Supplemental materials.
Participant recruitment: ASD
ASD youth were recruited in a more targeted fashion. Children who met criteria for DSM-IV diagnosis of either Autistic Disorder, Asperger's Disorder, or Pervasive Developmental Disorder Not Otherwise Specified were recruited through the University's Autism Clinic in the Child Development and Rehabilitation Center, community support groups and outreach, and targeted mailings based on charted ICD-9 codes. After passing initial screening criteria, participants in the ASD group were administered the Autism Diagnostic Observation Schedule (Lord, Rutter, DiLavore, & Risi, 2001) and parents completed the Autism Diagnostic Interview–Revised (Rutter, Le Couteur, & Lord, 2003). Parents also completed the Social Responsiveness Scale, 2nd edition (SRS-2, Constantino & Gruber, 2012), Children's Communication Checklist (Bishop, 2003), and a detailed developmental history questionnaire. Parents had the option to provide any existing documentation regarding a previous ASD diagnosis including IEP's, chart records, and psychological evaluations. A multi-disciplinary diagnostic team that included three licensed clinicians then utilized all of the aforementioned materials to determine a consensus diagnosis.
Exclusionary criteria
Children in all three diagnostic groups were excluded if they: were prescribed long-acting psychotropic medications; had neurological impairment, seizure history, head injury with loss of consciousness, other major medical conditions, or substance abuse; had a prior diagnosis of intellectual disability, or psychosis; were currently experiencing a major depressive episode; or had estimated IQ <70. Other comorbidities were assessed by the multi-disciplinary teams, but were not exclusionary in any of the diagnostic groups except that children in the ADHD and Control samples were excluded if they had a parent-reported history of ASD diagnosis or if the multi-disciplinary team identified a diagnosis of ASD.
Stimulant medication
Children with ASD or ADHD taking stimulant medications were included in the study but were required to be off medication for 24 (for short-acting preparations) to 48 hours (for long-acting preparations) prior to testing
Measures of Cognitive Function
A description of each cognitive task is below. Additional details of tasks, data cleaning and validity criteria, and rates of missing data are detailed in the Supplemental materials.
Working memory
Children completed a computerized version of a spatial working memory task identical to the spatial span task from the CANTAB (De Luca et al., 2003). Forward and backwards conditions were both administered. Children also completed the familiar Digit Span task from the WISC-IV, including both forward and backward conditions.
Set-shifting
The Trailmaking Test (Delis, Kaplan, & Kramer, 2001) from the Delis-Kaplan Executive Function System (D-KEFS) was used as a measure of set-shifting. The final dependent variable was the residual of Condition 4 (switching) score after partialing out performance on the number- (Condition 2) and letter- (Condition 3) sequencing conditions.
Interference control
Condition 3 of the Color-Word test (Delis et al., 2001) from the D-KEFS was used as a measure of interference control. The final dependent variable was the residual Condition 3 (inhibition) score after partialing out performance on the color naming (Condition 1) and word reading (Condition 2) control conditions.
Attention/Arousal
A computerized Identical Pairs CPT (CPT-IP), similar to the A-X CPT design used by Nigg et al. (1996) was used to assess attention/arousal. Children viewed a series of four-digit numbers displayed one at a time in pseudo-random order for 200ms followed by a 1500ms blank screen. When two identical numbers appear back-to-back, the child pushed the response button. Target frequency was 20%, with an additional 20% “catch” trials (only one digit off— “hard” discriminations), and 60% “stim” or “non-target” trials (more than one digit different— “easy” discriminations). The task included a total of 300 stimuli and required about 10 minutes to complete. The primary outcome measures were signal detection accuracy (d-prime [d′]) on “catch” and “stim” trials.
Processing speed
The control conditions from the DKEFS (Delis et al., 2001) Trailmaking (Conditions 2 and 3) and Color Word (Conditions 1 and 2) tests were used as measures of processing speed.
Response inhibition
Response inhibition was evaluated based on a tracking version of the stop task (Logan, 1994; Nigg, 1999). On 75% of trials (“go” trials), children were asked to indicate with a key press whether an “X” or an “O” appeared in the center of the screen. On 25% of trials (“stop” trials), an auditory tone presented after the stimulus indicated that the child should not respond. After 32 practice trials, children completed 8 blocks of 64 trials.
Response time and response variability
Mean and standard deviation of RT on correct go trials from the stop task were calculated for each child. In addition, diffusion model parameters were estimated from the trial-by-trial RT time series using the Fast-dm modeling technique and the downloadable program from the author's website (Voss & Voss, 2007), and as previously published (Huang-Pollock et al., 2012; Karalunas & Huang-Pollock, 2013). Smaller absolute values of drift rate indicate slower/less efficient information processing. Smaller values of boundary separation indicate greater speed-accuracy trade-offs, and smaller non-decision times indicate faster non-decision time.
Delayed Reward Valuation
Delayed reward valuation was assessed using a computerized delay discounting questionnaire, based on the task described by Mitchell (1999) and Wilson, Mitchell, Musser, Schmitt, and Nigg (2011). Children were presented a series of 91 questions in which they chose between (1) a varying amount of hypothetical money now ($0–$10.50 in $0.50 increments) and (2) a hypothetical $10.00 after a varying delay (7, 30, 90, or 180 days; e.g., $6.50 now or $10 in 30 days). Specific answers had no effect on the overall duration of the task; however, children's response time affected task length because the next question was not presented until the previous question was answered. The task took approximately 12 minutes. Valuation of delayed rewards was quantified as the discounting gradient (k) because prior publication indicated this best differentiates ADHD and control performance (Mitchell, Wilson, & Karalunas, 2015). Values were natural-log transformed to address non-normality.
Data Analytic Plan
All analyses were conducted in Mplus v. 7.4 using the robust maximum likelihood estimator and full-information maximum likelihood methods to handle missing data. The nesting of children within families was handled using Mplus' cluster command.
Data reduction
In order to take full advantage of the multiple assessments of cognitive processes, confirmatory factor analysis (CFA, 1000 random starts and 50 integration points) was applied to create latent variables for: 1) working memory indicated by Spatial Span backward and forward and digit span forward and backward; 2) an attention/arousal indicated by d' on “catch” and “stim trials” for the CPT and the drift rate RT parameter; and 3) processing speed indicated by scores on Condition 1 and Condition 2 of Color Word and Condition 2 and Condition 3 of Trails. The measurement model fit the data well, χ2 (39) = 164.70, p = .00, CFI = .96, TLI = .95, RMSEA = .06. Latent variables were used in between-group comparisons along with single indicators for response inhibition, interference control, switching, response variability, and delayed reward discounting for which there were not the multiple measures necessary to create latent variables. Single indicators for each diffusion model parameter were also compared.
Role of ADHD and ASD symptoms in EF impairment
To assess the role of ADHD symptoms in ASD cognitive impairment and ASD features in ADHD cognitive impairment, two approaches were used: 1) covarying continuous dimensions and 2) comparing empirically-derived groups based on parent ratings. For our analyses using continuous dimensions, we first compared all diagnostic groups to each other. We then repeated the ASD-control group comparisons covarying: a) inattention and b) hyperactivity-impulsivity using T-scores from parent report on the well-validated ADHD Rating Scale (DuPaul et al., 1998). Because the ADHD group is necessarily defined by their ADHD symptoms, analyses covarying ADHD symptoms included only the ASD and typically-developing control participants.
Continuous ASD features (we avoid using the word symptoms because the SRS-2 is not a symptom checklist) based on parent ratings on the SRS-2 were available for a subset of ADHD and control participants in from the full sample (ADHD= 177, Control= 137). Children with and without SRS-2 did not differ in age, IQ, ADHD symptom severity, or sex distribution (all p > .10). Using this subsample, we repeated the ADHD-control group comparisons covarying the total T-score from the SRS-2 (Constantino & Gruber, 2012). Because the ASD group is necessarily defined by their ASD features, analyses covarying ASD features included only the ADHD and typically-developing control participants.
For our analyses comparing empirically-derived groups based on parent ratings, we next conducted a latent profile analysis in the ASD sample using the age- and gender normed T-scores from the ADHD-RS (inattention, hyperactivity-impulsivity, total) to determine whether there were distinct ASD subgroups characterized by varying levels of ADHD symptoms. We repeated between-group comparisons using these empirically-derived groups. A latent profile analysis was also applied in the ADHD sample using T-scores from all subscales on the SRS-2 and between-group comparisons repeated using these empirically defined ADHD groups.
Covariates
Age was used as a covariate in all between-groups analyses. Groups also differed in IQ, however, covarying for characteristics (such as IQ) that differ between groups but are representative of the underlying populations can violate statistical assumptions and result in comparison at levels of the covariate that do not exist in the actual population (Dennis et al., 2009). For this reason, we report analyses without IQ as a covariate. For completeness, Table 2 shows changes in patterns of significance when IQ is covaried, which are minimal.
Table 2. Neuropsychological tasks by diagnostic group.
| Cognitive Measure | Control | ASD | ADHD | Posthoc covarying Age | Effect Size (β)a | Posthoc covarying Age & IQ |
|---|---|---|---|---|---|---|
| Delay Discounting | -3.90 (2.08) | -4.15 (2.80) | -3.64 (2.61) | --- | .01, .07, .02 | --- |
| Arousal | .19 (.49) | .19 (.52) | -.14 (.47) | ASD=ADHD>C | .22, .37, .11 | ASD>ADHD>C |
| Speed | -1.47(5.22) | 1.80 (9.81) | .54 (6.88) | ASD>ADHD>C | .46, .24, .28 | ASD>ADHD>C |
| Working Memory | .44(1.10) | -.20 (1.38) | -.22 (1.10) | ASD<ADHD<C | .48, .40, .18 | ASD=ADHD<C |
| Switching | 126.35(51.80) | 139.03 (64.38) | 145.18(60.43) | ADHD>C | .18, .00, .13 | ADHD>C |
| Interference Control | 85.30 (24.68) | 91.04 (50.81) | 99.49 (31.76) | ADHD>C | .14, .10, .15 | ADHD>C |
| Response Inhibition | 248.55 (78.84) | 302.52 (122.26) | 291.56 (115.06) | ASD=ADHD>C | .33, .21, .10 | ASD=ADHD>C |
| RT Variability | 214.22 (72.05) | 249.68 (106.21) | 259.97 (92.86) | ASD>ADHD>C | .36, .28, .13 | ASD>ADHD>C |
| Drift Rate | 3.15 (.73) | 3.00 (.89) | 2.72 (.76) | ASD=ADHD<C | .29, .30, .04 | ASD=ADHD<C |
| Boundary Separation | 1.55 (.33) | 1.71 (.34) | 1.56 (.30) | ASD>C=ADHD | .21, .00, .18 | ASD>ADHD=C |
| Non-decision Time | .47 (.13) | .45 (.15) | .44 (.11) | ADHD<C | .07, .12, .02 | ADHD<C |
Note: ASD = Autism spectrum disorder; ADHD= Attention-deficit/hyperactivity disorder. Raw means for each group are reported.
Post-hoc and effect sizes reflect findings of the final model including covariates.
These values reflect the absolute value of the standardized regression coefficient for the Control/ASD, Control/ADHD, and ASD/ADHD comparison, respectively.
Multiple Comparisons
A false discovery rate correction for multiple comparisons was applied (Benjamini & Hochberg, 1995). Uncorrected p-values are reported in the text for ease of comparison with other studies; however, a corrected p-value threshold of p = .042 was used for interpreting all results (McDonald, 2014).
Results
Means and SDs of cognitive measures are presented in Table 2 for diagnostic groups and in Table S2 for the empirically-derived subgroups. The 95% confidence intervals for the between-group effect sizes, which are generally in the small to medium range, are provided in Table S3 and S4. Neuropsychological profiles are depicted in Figures 1-3.
Figure 1.
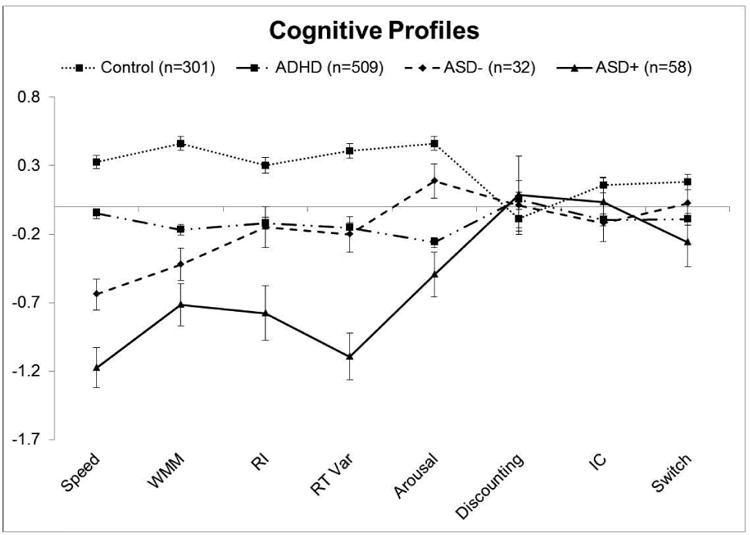
Cognitive profiles for ADHD, control, ASD+ADHD, ASD-ADHD groups. Z-scores are plotted so that all variables are represented on the same scale. More negative scores indicate worse performance for all measures.
Figure 3.
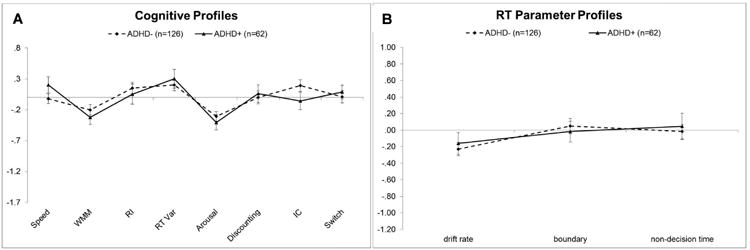
A) Cognitive and B) Reaction time parameters in the ADHD+ASD and ADHD-ASD groups. Z-scores are plotted so that all variables are represented on the same scale. More negative scores indicate worse performance on cognitive profile measures and factors contributing to slower performance for RTs (i.e., less efficient information processing, wider boundary separation, and slower non-decision times). The scales are intentionally kept the same as in Figures 1 and 2 to facilitate visualization of group differences.
Executive Functioning
Continuous symptoms
As expected, children with ADHD performed more poorly than typically-developing controls on all executive function measures (all p < .001). However, there were no reliable differences in delayed reward discounting (p = .067). The pattern of results remained the same when controlling for ASD features as measured on the SRS-2, suggesting ASD features did not account for cognitive impairments in the ADHD group.
When comparing children with ASD to typically-developing children, the ASD group had lower arousal, slower processing speed, lower working memory, and worse response inhibition (all p < .001). There were no reliable group differences on tests of interference control, switching, or delayed reward discounting (all p > .156). The patterns of significance all remained the same when controlling for inattention and hyperactivity-impulsivity symptoms, suggesting ADHD symptoms did not account for cognitive impairments in the ASD group.
When directly comparing children with ASD and ADHD, children with ASD had slower speed and worse working memory than children with ADHD (all p < .001). There were no reliable difference between children with ADHD and ASD on measures of arousal, response inhibition, interference control, or switching (all p > .05).
Empirically-defined groups
Children with ASD were split into empirically-defined groups based on their ADHD symptoms. The best-fitting LPA model1 indicated two ASD groups: one with ADHD symptoms in the non-clinical range similar to the typically-developing controls and a second group with both inattention and hyperactivity-impulsivity scores in the clinical range similar to the ADHD sample (see Table S1). Groups were heuristically labeled ASD-ADHD and ASD+ADHD.
When comparing executive function profiles using these empirically-defined ASD groups, the pattern of results was similar to analyses using continuous symptoms. Both the ASD-ADHD and ASD+ADHD groups had slower processing speed, worse working memory, and worse response inhibition than typically-developing controls (all p < .01). Although both ASD groups were impaired relative to typically-developing controls, children in the ASD+ADHD group were more impaired on measures of processing speed and response inhibition (all p < .02) than their ASD-ADHD counterparts. The two ASD subgroups did not differ in degree of working memory impairment (p = .12).
When compared to children with ADHD-only, both ASD groups had slower processing speed than the ADHD group (p < .001). The ASD+ADHD group also had lower working memory (p < .001) and worse response inhibition (p = .01) than children with ADHD-only, while the ASD-ADHD group performed similarly to children with ADHD-only on these measures (all p > .12). Overall, results indicate the cognitive impairments in ASD are not attributable to comorbid ADHD symptoms.
In contrast to results using continuous symptom dimensions, which suggested that arousal impairments in ASD were unrelated to ADHD symptoms, results from the categorical grouping approach suggested that arousal impairments in ASD were dependent on ADHD symptom profile. Results indicated that the ASD+ADHD and ASD-ADHD groups significantly differed from each other in arousal (p < .001). The ASD+ADHD group performed similarly to children with ADHD on arousal measures (p = .14) and were significantly worse than controls (p < .001). In contrast, the ASD-ADHD performed similarly to typically-developing children (p = .33), and had significantly higher arousal than children with ADHD (p < .001).
The two ASD subgroups did not differ from a) children with ADHD, b) controls, or c) each other on delay reward discounting, interference control, or set-shifting (p > .12).
Children with ADHD were also split into empirically-defined groups based on their ASD features. The best-fitting LPA model2 indicated two ADHD groups: one with ASD symptoms in the non-clinical range similar to the typically-developing controls and a second group with elevated SRS-2 scores across all domains. Groups were heuristically labeled ADHD-ASD and ADHD+ASD, although we note that SRS scores in the ADHD+ASD group were significantly lower than in the ASD group, consistent with our exclusion of children with an ASD diagnosis (see Table S1). The ADHD subgroups did not reliably differ from each other on any of the cognitive measures (all p > .075).
Reaction time parameters
Continuous symptoms
As expected, children with ADHD exhibited more reaction time variability than their typically-developing peers (p = .007). When parameters accounting for RT performance were considered separately, children with ADHD had slower drift rates and faster non-decision times than typically-developing children (all p < .001), but groups did not reliably differ in boundary separation (p = .950). The patterns of significance all remained the same when controlling for ASD features.
Children with ASD also exhibited more RT variability than typically-developing controls. The ASD group had slower drift rates than typically-developing controls (p < .001), similar to impairments observed in ADHD. However, children with ASD also had wider boundary separation (p < .001) and did not reliably differ from typically-developing controls in non-decision time (p = .275). The patterns of significance for ASD-control comparisons remained the same when controlling for inattention and hyperactivity-impulsivity symptoms.
When directly compared to children with ADHD, children with ASD had wider boundary separations (p < .001), but did not reliably differ in either drift rates or non-decision times.
Empirically-defined groups
When children with ASD were split into empirically-defined groups based on their ADHD symptoms, the pattern of results was similar. Children in the ASD-ADHD and ASD+ADHD groups both exhibited greater reaction time variability than typically-developing controls. When RT parameters were considered separately, both ASD groups had slower drift rates (all p < .011) and wider boundary separations (all p < .004) than typically-developing controls. The ASD+ADHD group was more impaired than their ASD-ADHD counterparts on measures of overall RT variability and drift rate (all p < .001), but the two ASD groups did not reliably differ from each other in boundary separation (p = .75).
When compared to children with ADHD-only, the ASD+ADHD group was more variable overall and had slower drift rates (all p < .001), while the ASD-ADHD group did not reliability differ from the ADHD-only group (all p < .15). The ASD+ADHD group also had faster non-decision time than typically-developing controls, children with ADHD, or their ASD-ADHD counterparts (all p < .03), while the ASD-ADHD group had non-decision times similar to typically-developing controls (p = .338). Wider boundary separation again appeared to be specifically related to ASD. The ASD+ADHD and ASD-ADHD groups both had wider boundary separations than typically-developing controls or children with ADHD (all p < .005).
When children with ADHD were split into empirically-defined groups based on their ASD symptoms, the ADHD subgroups did not reliably differ from each other on any of the reaction time measures (all p > .402).
Discussion
The current study examined shared and distinct cognitive impairments in children with ADHD and ASD using both continuous symptom measures and empirically-defined categorical groups. Several cognitive impairments in ASD, including in processing speed, working memory, and response inhibition, were independent of ADHD symptoms. Similarly, impairments in ADHD were independent of ASD symptoms. Results support a model in which impairments in these areas are shared across disorders and are not attributable to comorbid symptom profiles.
Equally important, however, when specific measures contributing to RT performance were considered, children with ADHD and ASD had partially distinct profiles. Slow drift rates occurred in both clinical groups, suggesting that this is a shared liability marker similar to many of the executive functioning measures. In contrast, wider boundary separations, which are related to emphasizing accuracy over speed, were specific to the ASD group. Children with ASD who had elevated ADHD symptoms and those with normative ADHD symptom levels both had equally wide boundary separations. This is the first study to directly compare children with ASD and ADHD on these differentiated RT parameters. Wider boundary separation is not associated with ADHD (Karalunas et al., 2014; van Mourik, Oosterlaan, & Sergeant, 2005), but has been found in ASD (as compared to typical development) in the only other study applying a diffusion model approach in this population (Pirrone et al., 2017). Pirrone et al., did not find slower drift rates in ASD, as we did here, but the sample included only a small sample of adults and used a perceptual discrimination task, either of which may account for differences in results. Overall, results confirm some specificity in features contributing to RT differences in ASD and ADHD. Put another way, although both groups are characterized by slower and more variable RTs on fast tasks, this is for partly distinct reasons. Wide boundary separation may be a specific feature of the ASD cognitive profile that could be used to differentiate diagnostic groups.
How might the present findings inform our conceptualization of these two disorders? ASD and ADHD are sometimes hypothesized to be different manifestations of a shared liability (van der Meer et al., 2012), and cognitive impairments have been proposed as indicators of this shared risk. The current results are consistent with the proposition that many cognitive deficits in ASD and ADHD reflect shared liability and are not simply accounted for by comorbid symptoms. Impairments in working memory, response inhibition, and speed of processing remained present in ASD even after accounting for continuous measures of ADHD symptoms. They occurred in children with ASD who had elevated ADHD symptoms, as well as in children with normative ADHD symptom levels. Although prior research and theory has focused on the role of ADHD symptoms in driving ASD cognitive impairments, we also confirm that comorbid ASD symptoms do not account for these cognitive performance deficits in ADHD. This further strengthens the argument that these impairments reflect shared liability and not comorbid symptom profiles. However, our results suggest important amendments to the shared liability proposal for RT measures (Rommelse et al., 2011). At least some RT differences in ASD are driven by distinct processes from those which drive the similar surface RT findings in ADHD.
Prior studies using continuous symptoms dimensions have similarly found that ADHD symptoms cannot explain ASD-related impairments in working memory (Corbett et al., 2009) and that there may be distinct contributions of ASD symptoms to RT measures (Yerys et al., 2013). However, current results are partially in contrast to findings that continuous ADHD symptoms predict response inhibition in both ASD and ADHD (Corbett et al., 2009; Yerys et al., 2013). Differences may be due to small samples (n∼20/group) in prior studies, use of different stimuli, or differences in statistical approach. At least one prior study comparing children with ADHD and ASD has identified ADHD-specific differences in brain connectivity in regions often associated with response inhibition (Di Martino et al., 2013). This finding would not be predicted based on the shared cognitive impairment seen here; however, cognitive performance was not measured or related brain connectivity in the prior study and brain connectivity was not considered here. Future work combining these approaches will be important.
Two cognitive markers may also be specifically associated with ADHD symptoms but not ASD. Faster non-decision times, possibly related to a type of motor impulsivity, and low arousal occurred only in children with high levels of ADHD symptoms, but not in typically-developing controls or those with ASD but without ADHD symptoms. These impairments were unrelated to ASD symptoms in the ADHD group. Here, results were less clear cut, however, in that differences were only found using categorical grouping approaches. Corbett et al. (2009), albeit in an small exploratory analysis, similarly found that the relationship of arousal measures to ADHD symptoms depended on whether categorical or continuous symptom measures were used. Additional studies using both categorical and continuous approaches are needed to clarify the specificity of these impairments to ADHD versus ASD. It may be that these effects are more dependent than other domains on how symptoms are viewed.
Clinically, recent review of the National Survey of Children's Health data found that ASD diagnosis is often delayed when ADHD symptoms are also present, and these delays are independent of ASD severity (Miodovnik, Harstad, Sideridis, & Huntington, 2015). Thus, when comorbid ADHD symptoms are present, children with ASD may miss early intervention opportunities because of diagnostic uncertainty. Unique RT markers for ASD may offer one way to facilitate improved diagnosis for children with comorbid symptoms.
It is also clinically relevant, however, that effects for cognitive measures were generally in the small to medium range, suggesting substantial overlap of diagnostic groups with typical development. ADHD and ASD are both heterogeneous, and small to medium effects are consistent with prior research suggesting that only a subset of children with these disorders experience cognitive impairments. Future studies with sample sizes adequate to consider within-group heterogeneity in cognitive functioning in children with ASD will be important for determining how much of the ASD population these findings represent or whether they may represent definable subgroups, such as we have suggested for ADHD (Fair et al., 2012; Nigg et al., 2005).
Several other caveats are also notable. Severity effects were observed for many measures. Even in cases where ADHD-only, ASD-ADHD, and ASD+ADHD were all impaired relative to typically-developing controls, the ASD+ADHD group was often the most impaired. This likely reflects the greater number of overall symptoms in this group, rather than a unique or distinct mechanism. Similar severity effects are often observed within ADHD, where greater symptom severity is associated with more cognitive impairment.
Cognitive abilities develop rapidly over middle childhood and adolescence (Best & Miller, 2010; Karalunas et al., 2017; Miller, Loya, & Hinshaw, 2013) and whether the ASD and ADHD groups show distinct trajectories of cognitive development is unable to be evaluated in this cross-sectional study. Additionally, although our overall sample size was sufficient to handle the missing data observed, missing data was non-trivial in the ASD group. Children with ASD who completed all tasks were older than the children who did not, and so additional studies are needed to confirm that results are similar in younger samples as well.
Although there was variation within the sample, at the group-level the children with ASD included in the study had average IQ scores. This could have resulted in under-estimation of cognitive effects and limits generalizability of results to children with ASD and intellectual disability. Exclusion of children on non-stimulant medications may also reduce generalizability of results, particularly for the ASD sample because of possible limited efficacy and potential for greater side effects in this diagnostic group (Davis & Kollins, 2012).
Although children with ADHD were impaired on measures of interference control and switching, children with ASD were not, which stands in contrast to prior work suggesting that switching, in particular, may be a primary cognitive impairment in ASD. This may be related to the ASD sample being relatively high functioning, although this did not preclude finding impairments on many other cognitive measures. Alternatively, our approach of partialing out basic speed to isolate interference control and switching may account for this difference.
In addition, none of the groups here differed on measures of delayed reward discounting. Recent work from our group found that ADHD-related differences in delayed reward discounting on this task may be largest in early childhood, partially normalizing at the group-level by early adolescence (Karalunas et al., 2017). Our ASD sample was older than the other groups, which may have contributed to the lack of positive findings here. Alternatively, use of a task with real (rather than hypothetical) rewards may have better elicited group differences. These are both directions for future research.
Summary
Overall, current results indicate that cognitive impairments in working memory, response inhibition, and processing speed, as well as slow drift rates, may reflect shared liability in ADHD and ASD. In contrast, a conservative response style may be a unique marker of ASD pathology that helps partially differentiate the two disorders at the level of cognitive endophenotypes.
Supplementary Material
Figure 2.
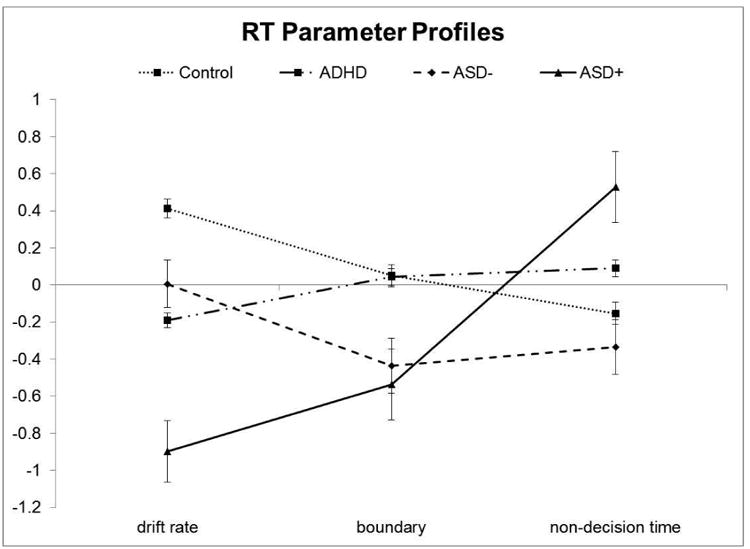
Reaction time parameter profiles in the ADHD, control, ASD+ADHD, ASD-ADHD groups. Z-scores are plotted so that all variables are represented on the same scale. More negative scores indicate factors contributing to slower performance (i.e., less efficient information processing, wider boundary separation, and slower non-decision times).
Acknowledgments
This project was supported by Simons Foundation #177894 and by National Institutes of Health R01 MH096773 (Fair), R00 MH091238 (Fair), R01 MH086654 (Nigg), R37 MH059105 13 (Nigg), and K23 MH108656 (Karalunas). We thank Corinne Stevens, Libby Nousen, and Andrew Bontemps for assistance with data collection and preparation. We gratefully acknowledge the participants and their caregivers, as well as the graduate students, staff, and research assistants whose remarkable dedication has made this research possible.
Footnotes
AIC = 2000.6, Sample Size Adjusted BIC = 1994.0, Vuong-Lo-Mendell-Rubin Likelihood Ratio Test for 1 versus 2 classes p < .001; Vuong-Lo-Mendell-Rubin Likelihood Ratio Test for 2 versus 3 classes p = .199
AIC = 7794.79, Sample Size Adjusted BIC = 7856.28, Vuong-Lo-Mendell-Rubin Likelihood Ratio Test for 1 versus 2 classes p = .047; Vuong-Lo-Mendell-Rubin Likelihood Ratio Test for 2 versus 3 classes p = .521
References
- Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences U S A. 1996;93(24):13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995:289–300. [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Development. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. The Children's Communication Checklist, Second Edition (CCC-2) London: The Psychological Corporation; 2003. [Google Scholar]
- Conners CK. The Conners 3rd Edition (Conners 3) North Tonawanda, NJ: Multi-Health System; 2008. [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale, Second Edition (SRS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2):210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NO, Kollins SH. Treatment for co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics. 2012;9(3):518–530. doi: 10.1007/s13311-012-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca De, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: development of executive function over the lifespan. Journal of Clinical Experimental Neuropsychology. 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Pscyhological Corporation; 2001. [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Developmental science. 2012;15(6):791–800. doi: 10.1111/j.1467-7687.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Milham MP. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G, Power T, Anastopoulos A, Reid R. ADHD Rating Scale IV: Checklists, Norms, and Clinical Interpretation. NY, NY: Guilford Press; 1998. [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11(1):19–23. [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences of the USA. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM. Autism and ADHD: how far have we come in the comorbidity debate? Neuroscience & Biobehavioral Reviews. 2011;35(5):1081–1088. doi: 10.1016/j.neubiorev.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45(4):836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Happe F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain and cognition. 2006;61(1):25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in cognitive sciences. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnorm Psychol. 2012;121(2):360–371. doi: 10.1037/a0027205. doi:doi:2012-06943-001 [pii] 10.1037/a0027205 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst BM, Zeeuw P, Bos DJ, Rijks Y, Neggers SF, Durston S. Children with ADHD symptoms show decreased activity in ventral striatum during the anticipation of reward, irrespective of ADHD diagnosis. Journal of Child Psychology and Psychiatry. 2017;58(2):206–214. doi: 10.1111/jcpp.12643. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway A, Hambrick DZ, Engle RW. Variation in working memory capacity as variation in executive attention and control. Variation in Working Memory. 2007:21–48. [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: Reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. Journal of Child Psychology and Psychiatry. 2014;55(6):685–710. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Dieckmann N, Tipsord J, Mitchell SH, Nigg JT. Heterogeneity in development of aspects of working memory predicts longitudinal ADHD symptom change. Journal of Abnormal Psychology. 2017 doi: 10.1037/abn0000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL. Integrating impairments in reaction time and executive function using a diffusion model framework. Journal of Abnormal Child Psychology. 2013;41(5):837–850. doi: 10.1007/s10802-013-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Logan G. On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 2001. [Google Scholar]
- McDonald JH. Handbook of Biological Statistics. 3rd. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- Miller M, Loya F, Hinshaw SP. Executive functions in girls with and without childhood ADHD: developmental trajectories and associations with symptom change. Journal of Child Psychology and Psychiatry. 2013;54(9):1005–1015. doi: 10.1111/jcpp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Harstad E, Sideridis G, Huntington N. Timing of the diagnosis of attention-deficit/hyperactivity disorder and autism spectrum disorder. Pediatrics. 2015;136(4):e830–e837. doi: 10.1542/peds.2015-1502. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Wilson VB, Karalunas SL. Comparing hyperbolic, delay-amount sensitivity and present-bias models of delay discounting. Behavioural Processes. 2015;114:52–62. doi: 10.1016/j.beproc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, et al. Nigg JT. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. Journal of Child Psychology and Psychiatry. 2014;55(7):819–827. doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal Heterogeneity in Attention-Deficit/Hyperactivity Disorder: Do We Need Neuropsychologically Impaired Subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Hinshaw SP, Halperin JM. Continuous performance test in boys with attention deficit hyperactivity disorder: Methylphenidate dose response and relations with observed behaviors. Journal of Clinical Child Psychology. 1996;25(3):330–340. [Google Scholar]
- Nyden A, Niklasson L, Stahlberg O, Anckarsater H, Wentz E, Rastam M, Gillberg C. Adults with autism spectrum disorders and ADHD neuropsychological aspects. Res Dev Disabil. 2010;31(6):1659–1668. doi: 10.1016/j.ridd.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 1999;29(2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. Special Issue: Annual research review. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pirrone A, Dickinson A, Gomez R, Stafford T, Milne E. Understanding perceptual judgment in autism spectrum disorder using the drift diffusion model. Neuropsychology. 2017;31(2):173. doi: 10.1037/neu0000320. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. Kiddie schedule for affective disorders and schizophrenia. Pittsburgh, PA: Western Psychiatric Institute; 1986. [Google Scholar]
- Ratcliff R. A diffusion model account of response time and accuracy in a brightness discrimination task: fitting real data and failing to fit fake but plausible data. Psychonomic Bulletin & Review. 2002;9(2):278–291. doi: 10.3758/BF03196283. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Modeling response signal and response time data. Cognitive Psychology. 2006;53(3):195–237. doi: 10.1016/j.cogpsych.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Rouder JN. Modeling Response Times for Two-Choice Decisions. Psychological Science. 2016;9(5):347–356. doi: 10.1111/1467-9280.00067. [DOI] [Google Scholar]
- Raymaekers R, Antrop I, van der Meere JJ, Wiersema JR, Roeyers H. HFA and ADHD: a direct comparison on state regulation and response inhibition. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):418–427. doi: 10.1080/13803390600737990. [DOI] [PubMed] [Google Scholar]
- Rommelse N, Geurts H, Franke B, Buitelaar J, Hartman C. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience & Biobehavioral Reviews. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European child & adolescent psychiatry. 2010;19(3):281–295. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Larsson H, Anckarsäter H, Lichtenstein P. Symptoms of autism and ADHD: A Swedish twin study examining their overlap. Journal of Abnormal Psychology. 2014;123(2):440. doi: 10.1037/a0036088. [DOI] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behavioural and brain research. 1998;94(1):61–71. doi: 10.1016/S0166-4328(97)00170-8. [DOI] [PubMed] [Google Scholar]
- Sanders J, Johnson KA, Garavan H, Gill M, Gallagher L. A review of neuropsychological and neuroimaging research in autistic spectrum disorders: Attention, inhibition and cognitive flexibility. Research in Autism Spectrum Disorders. 2008;2(1):1–16. [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, et al. Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of abnormal psychology. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Butcher B. Executive functioning in children with Asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of Autism and Developmental Disorders. 2010;40(8):1017–1027. doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Bruning N, Morsch D, Lehmkuhl G. Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatrica. 2008;20(4):207–215. doi: 10.1111/j.1601-5215.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychological review. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- van der Meer JM, Oerlemans AM, van Steijn DJ, Lappenschaar MG, de Sonneville LM, Buitelaar JK, Rommelse NN. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1160–1172 e1163. doi: 10.1016/j.jaac.2012.08.024. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: a meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry. 2005;46(2):150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Voss A, Voss J. Fast-dm: a free program for efficient diffusion model analysis. Behavior Research Methods. 2007;39(4):767–775. doi: 10.1037/0033-295x.85.2.5910.3758/bf03192967. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle A, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: application in young children. Journal of Child Psychology and Psychiatry. 2011;52(3):256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Kenworthy L, Jankowski KF, Strang J, Wallace GL. Separate components of emotional go/no-go performance relate to autism versus attention symptoms in children with autism. Neuropsychology. 2013;27(5):537. doi: 10.1037/a0033615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


