Abstract
Purpose
To evaluate the relationship of cardiorespiratory fitness (CRF) with cardiovascular disease (CVD) risk factors and a biomarker of endothelial dysfunction (e-selectin) among Hispanic/Latino youth.
Methods
The study included 1380 Hispanic/Latino youths (8-16 years old) from the Hispanic Children Health Study/Study Of Latino Youth (HCHS/SOL Youth) that enrolled from four cities (Bronx, Chicago, Miami, and San Diego). CRF was assessed by a 3-min step test that uses post-exercise heart rate to estimate maximal oxygen uptake. Regression models assessed differences in cardiometabolic markers across quartiles of CRF, adjusting for potential confounders.
Results
CRF was higher among boys (mean: 57.6 ml/kg/min, 95% CI: 56.8, 58.4) compared to girls (mean: 54.7 ml/kg/min, 95% CI: 53.9, 55.5). Higher levels of CRF were associated with more favorable levels of cardiometabolic, inflammation, and endothelial dysfunction factors (p-values<0.001) and independently of physical activity and sedentary time. Compared to the lowest quartile of CRF, the odds of having ≥2 CVD risk factors was lower at higher quartiles of CRF, after adjustment for potential confounders.
Conclusions
Among Hispanic/Latino youth, CRF appears to be a strong protective factor for endothelial dysfunction and cardiometabolic risk factors. Strategies to improve CRF may be a useful approach for improving cardiovascular health in youth.
INTRODUCTION
The epidemic of obesity has underscored the need to identify modifiable factors that could reduce the metabolic abnormalities linked to excess weight. This is particularly important for Hispanic/Latino youth who have high rates of obesity(1). In adults, higher cardiorespiratory fitness (CRF) is associated with lower risk of CVD and mortality (2–6). Evidence suggests that higher CRF levels in youth may be cardio-protective later in life as these are associated with lower BMI and inflammatory markers (7–10). Furthermore, higher levels of CRF in adolescence C predict lower risk of myocardial infarction and premature death (11, 12). Yet, the role of CRF in subclinical cardiovascular disease during adolescence, such as endothelial dysfunction, has been less studied. Several biomarkers may be used as noninvasive measures of endothelial function (13). Selectins are molecules that assist in attracting white blood cells to the lining of the blood vessel at localized sites of inflammation. In particular, e-selectin is a cellular adhesion molecule that is produced in response to inflammation (signaled by IL-1 and TNF-alpha). In addition, plasminogen activator inhibitor-1 (PAI-1) inhibits the degradation of blood clots and has been associated with endothelial dysfunction (13, 14). In youth, markers of endothelial dysfunction are related to obesity and insulin resistance (15), suggesting that alterations of the endothelial function may represent an early manifestation of the atherosclerotic process.
Since prior studies suggest that exercise may improve markers of endothelial dysfunction in youth (16, 17), in this study we examined the role of CRF levels on cardiometabolic risk factors and endothelial dysfunction in a sample of Hispanic/Latino youth, a population that has been largely understudied and is at high risk for metabolic and cardiovascular disease. Because we have identified sex differences in the distribution of cardiometabolic risk factors (1, 18), we also examined whether effects varied by sex. In addition, as prior studies indicate an inverse association between BMI and CRF levels (19, 20), we also tested for interaction by obesity status.
MATERIALS AND METHODS
Study population
The Hispanic Community Health Study/ Study of Latinos (HCHS/SOL) is a population-based cohort study of 16,415 Hispanic/Latino adults (ages 18–74 years) who were selected using a two-stage probability sampling design from four US communities (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA). SOL Youth is an ancillary study to HCHS/SOL that enrolled a subset of the offspring of HCHS/SOL participants from the same four field centers. HCHS/SOL enrolled participants form 9,872 households. From these, we identified about 7,350 households that likely had at least one child potentially eligible for SOL Youth. Between 2012 and 2014, 6,741 households were screened (92%) by phone using a standardized script; the screening identified 1,777 eligible youth between the ages of 8-16 years, of whom 1,466 were enrolled (participation rate of 82%). Of these 1,466 youths, we excluded participants who did not provide a fasting blood sample or for whom fasting status was unknown (n=24, 1.8%), and an additional n=55 (3.8%) who did not complete the cardiorespiratory fitness test. Lastly, we excluded seven participants whose BMI or waist circumference were extreme outliers, yielding an analytic sample of n=1,380. Details about the methodology and protocols of HCHS/SOL and SOL Youth are published elsewhere (21, 22). The study was conducted with approval from the institutional review boards of each of the institutions involved. Written informed consent and assent were obtained from parent/caregivers and their children, respectively.
Measures
Examinations took place at each of the field center research clinics. Staff was centrally trained on each of the study procedures and followed a standardized protocol. Study questionnaires were interviewer-administered.
Cardiorespiratory fitness (CRF)
A submaximal test was used to assess CRF in the study. Youths were asked to perform a 3-minute step test using a height-appropriate bench. Resting and post-exercise heart rates were obtained assessing the heart beats over 15 seconds. This method has been validated for children 6-18 years old (23). VO2max was estimated using published methods [VO2_MAX = 105.3959 – (1.643756 × 15-seconds Post Exercise Heart Rate)] (23–25).
Adiposity Measures
Weight and percentage body fat were obtained using a Tanita Body Composition Analyzer TBF-300A, which applied a bioelectrical impedance method. Height and waist circumference were measured three times per participant and rounded to the nearest centimeter according to a standard protocol. An average of three measurements was used in the study for each participant (26). BMI was calculated as weight (kg) divided by height squared (m2) and BMI z-scores were derived following CDC guidelines (27). Obesity was defined as BMI≥95th percentile of the sex-specific age-standardized BMI using CDC Growth Charts (28).
Blood pressure
Blood pressure was measured after 5 minutes of sitting rest using an OMRON HEM −907XL sphygmomanometer (Omron Healthcare Co. Ltd., Kyoto, Japan). The mean of the second and third measurements was used for analysis. National Heart, Lung, and Blood Institute blood pressure level tables were used to calculate age-, sex-, and height-specific diastolic and systolic blood pressure percentiles (29).
Laboratory measurements
All blood specimens were drawn in the morning under fasting conditions (at least 10 hours), processed on site, and stored at −70°C. The University of Minnesota’s Advanced Research and Diagnostic Laboratory performed all laboratory assays using standard methods. Measures of cardiometabolic factors included fasting glucose, HbA1c, insulin, lipid panel (total cholesterol, LDL-C, HDL-c, and triglycerides). Markers of inflammation included High-sensitivity C-reactive protein (hs-CRP),PAI-1, and adiponectin. Endothelial dysfunction was assessed by e-selectin.
Number of cardiovascular disease risk (CVD) factors
An index of overall cardiovascular health was calculated by summing the presence of the following eight risk factors: obesity (BMI ≥95th percentile); SBP or DBP ≥90th percentile, fasting glucose ≥100 mg/dL, HbA1c ≥5.7%; total cholesterol ≥200 mg/dL, LDL-c ≥130 mg/dL, triglycerides ≥100 mg/dL for 8-9 year-olds and ≥130 mg/dL for 10-16 year-olds or HDL-c <40 mg/dL. Meeting one of each of these thresholds added to the count of numbers of CVD risk factors (1). The correlation coefficients between factors ranged from 0.01 to 0.92. The Cronbach alpha coefficient was moderate/low (0.49).
Self-reported and measured covariates
Age, sex, place of birth, and Hispanic/Latino background were self-reported by the participant. The participant’s parent or legal guardian reported annual household income and highest level of educational attainment. Time spent in sedentary behavior and moderate-to-vigorous physical activity (MVPA) was assessed using accelerometers (Actical model 198-0200-03; Respironics Co. Inc., Bend, Oregon) worn for 7 days =. Non-wear time was defined as consecutive zero counts for at least 90 minutes, allowing for short time intervals with nonzero counts lasting up to 2 minutes if no counts were detected during both the 30 minutes upstream and downstream from that interval (30). Data were summarized as the average time spent sedentary and in MVPA on adherent days (at least 8 hours of wear time) for youth with at least three adherent days according to the following cut points: sedentary (<18 counts/15-second epoch) and moderate or vigorous (>440 counts/15 seconds) (31).
Statistical analysis
All reported values are weighted to the sampling frame and account for clustering within sampling units and households. Sampling weights were also non-response adjusted, trimmed and calibrated by age and sex distributions of Hispanic/Latinos in the target areas according to the 2010 U.S. Population Census. P-values corresponding to Wald F-statistics and 95% confidence intervals were computed using variance estimates derived from Taylor series linearization methods using SUDAAN release 11.0.1 (RTI International, Research Triangle Park, NC). All tests of significance were two-sided using a threshold of 5%.
Missing values of accelerometer measures (n=338), parent education (n=3), household income (n=44), and place of birth (n=11) were imputed using 10 imputed data sets. Imputation models included all variables in analytical models, as well survey design variables, hours of accelerometer wear time, and inclusion of a weekend day in accelerometer measurement. Primary analyses were performed on the 10 imputed data sets and carried out using SUDAAN survey procedures with proper variance estimation. Results were combined using Rubin’s rule accounting for uncertainty of the imputation in SAS.
Multiple linear regression models were used to estimate the association of CRF with cardiometabolic risk factors and makers of endothelial function, with CRF as a continuous variable and also in quartiles. Mean (95% CI) levels of cardiometabolic risk factors, markers of endothelial function, and accelerometer variables were estimated within quartiles of fitness as predicted marginal means, derived from linear regression models adjusted for age and sex. Regression coefficients for differences in cardiometabolic risk factors and markers of endothelial function were estimated from models that were further adjusted for a series of additional potential confounders. Initial models adjusted for sociodemographic variables determined a priori as potential confounders including age, sex, field center, Hispanic/Latino background, place of birth, annual household income, parent educational attainment, time per day spent in physical activity and sedentary behavior, which are closely linked with fitness. Then, in order to examine the effect of fitness levels beyond adiposity, final models further adjusted for z-score of waist circumference, which was previously reported as a stronger correlate of cardiovascular disease risk factors than BMI in Hispanic youth (32). Similarly, prevalence ratios for a 3-level CVD risk factors (1, 2+, relative to 0) were derived from multinomial logistic regression models. Heterogeneity in the associations was examined across levels of age, sex, and obesity status using two-way interaction terms.
RESULTS
The study included 699 girls and 681 boys between the ages of 8 and 16 (mean age 12.2 years). Fifty-four percent were 8-12 years old, 23% were between 13-14 years and 24% were 15-16 years old. The majority (78%) of youth were born within the US 50 states. Youth of Mexican heritage comprised 47.6% of the sample. The sample was predominantly of low socioeconomic status; 51% of youth were living in families reporting an annual household income ≤$20,000 and 38% of their caregivers did not graduate from high school. Boys had higher levels of CRF than girls (57.6 vs. 54.7, p-value <0.0001). CRF levels also varied by Hispanic background. (Table 1). Time spent at MVPA was higher at higher quartiles of CRF (Figure 1). Conversely, youth at lower quartiles of CRF spent more time in sedentary behaviors.
Table 1.
Mean cardiorespiratory fitness of Hispanic/Latino youth by socio-demographic characteristics. HCHS/SOL Youth (n=1,380)
| Sociodemographic characteristics | Cardiorespiratory fitness,* ml/kg/min
|
P |
|---|---|---|
| Mean (95% CI)* | ||
| Age group | 0.8023 | |
| 8 – 12 years | 56.2 (55.4, 56.9) | |
| 13 – 14 years | 56.5 (55.3, 57.7) | |
| 15 – 16 years | 55.9 (54.3, 57.4) | |
| Sex | <0.0001 | |
| Girls | 54.7 (53.9, 55.5) | |
| Boys | 57.6 (56.8, 58.4) | |
| Nativity | 0.1763 | |
| Foreign born (includes Puerto Rico) | 55.3 (53.8, 56.8) | |
| Born in US 50 states | 56.4 (55.7, 57.1) | |
| Hispanic/Latino background | 0.0497 | |
| Mexican | 57.0 (56.1, 57.9) | |
| Central American | 56.6 (54.6, 58.7) | |
| Cuban | 56.7 (54.9, 58.5) | |
| Dominican | 54.1 (52.4, 55.7) | |
| Puerto Rican | 54.5 (52.3, 56.7) | |
| South American | 55.7 (53.4, 58.0) | |
| Other/>1 | 55.9 (54.2, 57.6) | |
| Annual household income | 0.2136 | |
| ≤$20,000 | 55.7 (54.8, 56.6) | |
| $20,001 - $40,000 | 56.9 (55.7, 58.1) | |
| >$40,000 | 56.9 (55.2, 58.5) | |
| Parent educational attainment | 0.3383 | |
| No H.S. Diploma/GED | 56.3 (55.3, 57.3) | |
| High school diploma/GED | 55.5 (54.6, 56.5) | |
| > H.S. diploma/GED | 56.6 (55.4, 57.8) |
Values are non-response adjusted and weighted to the age and sex distribution of the target population.
Figure 1.
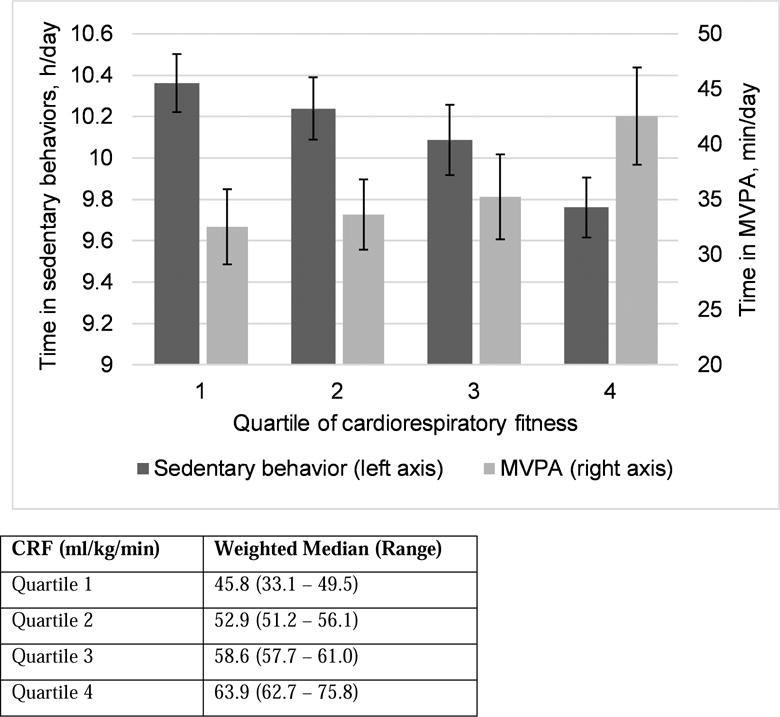
Age and sex adjusted means of accelerometer-based physical activity and sedentary behavior across quartiles of cardiorespiratory fitness,* HCHS/SOL Youth (n=1,380)
Cardiorespiratory fitness, adiposity, and cardiometabolic risk factors
CRF was inversely associated with measures of adiposity in multivariate models (all p-values<0.0001) (Table 2). Compared to the lowest quartile of CRF, each increasing quartile of CRF was associated with lower BMI-z score, waist circumference, and percent body fat. These associations were independent of time spent in MVPA and sedentary behaviors.
Table 2.
Multiple linear regression (β; 95% Confidence Interval) for the association of cardiorespiratory fitness (CRF) with adiposity and cardiometabolic risk factors, HCHS/SOL Youth (n=1380)*
| CRF | Quartiles of CRF | ||||
|---|---|---|---|---|---|
|
| |||||
| Each 10mL/kg/min | p-value | Quartile 2 | Quartile 3 | Quartile 4 | |
| Adiposity | |||||
|
| |||||
| BMI, z-score | −0.48 (−0.60, −0.36) | <0.0001 | −0.38 (−0.60, −0.16) | −0.66 (−0.88, −0.44) | −0.97 (−1.21, −0.73) |
| WC, z-score | −0.48 (−0.57, −0.39) | <0.0001 | −0.45 (−0.65, −0.25) | −0.71 (−0.91, −0.52) | −0.92 (−1.11, −0.73) |
| BF, % | −5.35 (−6.34, −4.35) | <0.0001 | −5.1 (−7.1, −3.0) | −8.4 (−10.3, −6.4) | −10.8 (−12.8, −8.8) |
|
| |||||
| Blood Pressure | |||||
|
| |||||
| SBP, percentile | |||||
| Model 1 | −3.78 (−5.96, −1.60) | 0.0007 | −0.1 (−4.6, 4.3) | −2.6 (−7.6, 2.4) | −9.5 (−13.9, −5.2) |
| Model 2† | −3.36 (−5.84, −0.88) | 0.0081 | 0.3 (−4.2, 4.8) | −2.0 (−7.3, 3.2) | −8.8 (−13.7, −3.8) |
| DBP, percentile | |||||
| Model 1 | −5.93 (−7.97, −3.88) | <0.0001 | −3.6 (−7.8, 0.6) | −8.6 (−12.7, −4.4) | −12.0 (−16.1, −8.0) |
| Model 2† | −4.45 (−6.57, −2.32) | <0.0001 | −2.2 (−6.4, 2.0) | −6.3 (−10.5, −2.1) | −9.1 (−13.3, −4.8) |
|
| |||||
| Glycemic markers | |||||
|
| |||||
| FPG, mg/dL | |||||
| Model 1 | −0.60 (−1.22, 0.02) | 0.0596 | −0.7 (−1.9, 0.4) | −1.1 (−2.3, 0.1) | −1.3 (−2.7, 0.0) |
| Model 2† | −0.37 (−0.99, 0.26) | 0.2516 | −0.5 (−1.6, 0.6) | −0.8 (−1.9, 0.4) | −0.9 (−2.2, 0.4) |
| HbA1c, % | |||||
| Model 1 | −0.04 (−0.06, −0.01) | 0.0094 | −0.08 (−0.13, −0.02) | −0.08 (−0.14, −0.02) | −0.06 (−0.12, 0.01) |
| Model 2† | −0.01 (−0.04, 0.02) | 0.3878 | −0.05 (−0.11, 0.00) | −0.05 (−0.10, 0.01) | −0.01 (−0.07, 0.05) |
| log(HOMA-IR) | |||||
| Model 1 | −0.27 (−0.33, −0.21) | <0.0001 | −0.30 (−0.44, −0.17) | −0.36 (−0.49, −0.22) | −0.54 (−0.67, −0.42) |
| Model 2† | −0.10 (−0.16, −0.05) | 0.0003 | −0.14 (−0.26, −0.03) | −0.10 (−0.21, 0.01) | −0.22 (−0.34, −0.10) |
|
| |||||
| Lipids | |||||
|
| |||||
| Cholesterol, mg/dL | |||||
| Model 1 | −4.94 (−7.43, −2.46) | 0.0001 | −3.1 (−8.2, 2.0) | −5.4 (−11.2, 0.5) | −10.1 (−15.2, −5.0) |
| Model 2† | −4.03 (−6.62, −1.43) | 0.0025 | −2.2 (−7.4, 3.0) | −3.9 (−9.9, 2.2) | −8.2 (−13.4, −2.9) |
| HDL, mg/dL | |||||
| Model 1 | 1.74 (0.79, 2.70) | 0.0004 | 2.4 (0.4, 4.3) | 2.4 (0.4, 4.4) | 3.8 (1.8, 5.8) |
| Model 2† | −0.37 (−1.29, 0.54) | 0.4217 | 0.4 (−1.3, 2.2) | −0.7 (−2.6, 1.2) | −0.2 (−2.1, 1.8) |
| LDL, mg/dL | |||||
| Model 1 | −3.71 (−5.85, −1.56) | 0.0008 | −2.9 (−7.4, 1.6) | −4.2 (−9.2, 0.9) | −8.0 (−12.5, −3.5) |
| Model 2† | −2.19 (−4.39, 0.01) | 0.0511 | −1.5 (−5.9, 2.9) | −1.9 (−7.1, 3.2) | −5.1 (−9.6, −0.5) |
| log(TG, mg/dL) | |||||
| Model 1 | −0.18 (−0.23, −0.13) | <0.0001 | −0.14 (−0.25, −0.03) | −0.22 (−0.33, −0.10) | −0.36 (−0.46, −0.25) |
| Model 2† | −0.09 (−0.15, −0.04) | 0.0007 | −0.06 (−0.16, 0.04) | −0.09 (−0.20, 0.02) | −0.19 (−0.30, −0.09) |
Lowest quartile of CRF is the reference category for quartile analysis; values are betas (95% confidence intervals) derived from survey linear regression models adjusted for age, sex, field center, Hispanic/Latino background, place of birth, annual household income, and parent education level, MVPA, and sedentary behavior.
Model 2 is further adjusted for waist circumference z-score.
BF, body fat; BMI, body mass index; CRF, cardiorespiratory fitness; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high density lipoprotein; HOMA-IR, homeostasis model assessment – insulin resistance; LDL, low density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
We also observed strong inverse associations between CRF and cardiometabolic risk factors (p-values <0.05 for all markers except fasting plasma glucose)(Table 2), even after the adjustment for MVPA and sedentary behaviors. These associations were attenuated when models were further adjusted for waist circumference, but remained statistically significant for systolic and diastolic blood pressure, HOMA-IR, total cholesterol, and triglycerides. As expected, there was a positive association between CRF and HDL-c, and an inverse association between CRF and LDL-c independent of MVPA and sedentary behaviors. However, after the adjustment for waist circumference, the association between CRF and HDL was no longer statistically significant. When examining the association of cardiometabolic risk factors across quartiles of CRF, we observed protective associations even at moderate levels of CRF (quartiles 2 and 3) but associations appeared to be stronger at highest levels of fitness (quartile 4). Furthermore, the number of cardiovascular disease risk factors, an index of cardiovascular health, was also inversely related to CRF (Figure 2). The odds of having one, or ≥2 cardiovascular risk factors decreased across higher quartiles of CRF. This trend was independent of MVPA, sedentary behavior and waist circumference.
Figure 2. Odds ratios (95% Confidence Interval) for the association of CRF (quartiles) with number of CVD risk factors. HCHS/SOL Youth (n=1380).
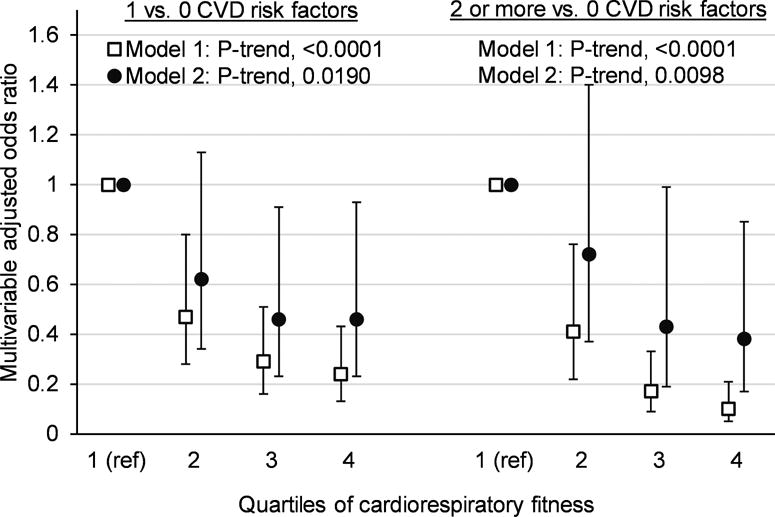
*CVD risk factors include obesity (BMI ≥95th percentile), high blood pressure, high fasting plasma glucose, high HbA1c, high total cholesterol, high LDL-cholesterol, high triglycerides, and low HDL-cholesterol
**Derived from proportional odds model for ordinal dependent variable
***Odds ratios for each level of the dependent variable relative to zero cardiovascular risk factors, derived from multivariable adjusted generalized logit models
****Tests for a trend for increasing odds of 1 and ≥2 CVD risk factors, relative to 0 CVD risk factors, across higher quartiles of cardiorespiratory fitness
Model 1 adjusted for age, sex, field center, Hispanic/Latino background, place of birth, annual household income, and parent education level, physical activity and sedentary behavior
Model 2 is further adjusted for waist circumference
We also explored potential effect modification by sex, age group, and obesity status. There was an interaction by sex in the association of CRF with percent body fat, which suggested a stronger effect among boys (supplemental eTable 1). However, no interactions by sex were observed for the association between CRF and cardiometabolic risk factors (supplemental Table e1). Age and obesity status did not modify the associations of CRF with adiposity or cardiometabolic risk factors (supplemental eTables 2 and 3).
Cardiorespiratory fitness, inflammatory makers, and endothelial function biomarkers
CRF was inversely related to C-reactive protein and PAI-1, in models adjusted for sociodemographic characteristics, sedentary behavior and MVPA (Table 3). Furthermore, these inverse associations were observed at moderate (quartile 3) and high (quartile 4) levels of CRF. Associations were attenuated but remained significant after adjusting for waist circumference. The association of CRF with PAI-1 was stronger in boys compared to girls (p-value for interaction=0.016; supplemental eTable 1). On average, each 10 mL/kg/min increase in CRF was associated with a 0.39 (95% CI −0.49, −0.28) decrease in log-transformed PAI-1 in boys and 0.24 (95% CI −0.34, −0.13) decrease in log-transformed PAI-1 in girls. These associations were consistent by age group or obesity status (supplemental eTables 2–3). Adiponectin levels were significantly higher at higher levels of fitness (p-value = 0.0019), but the association did not remain significant after adjustment for waist circumference (P=0.67).
Table 3.
Multiple linear regression (β; 95% Confidence interval) for the association of cardiorespiratory fitness (CRF) with adiponectin, inflammatory markers, and e-selectin. HCHS/SOL Youth (n=1380)*
| CRF | Quartiles of CRF | ||||
|---|---|---|---|---|---|
|
| |||||
| Each 10mL/kg/min | p-value | Quartile 2 | Quartile 3 | Quartile 4 | |
| log(CRP, mg/dL) | |||||
| Model 1 | −0.44 (−0.55, −0.33) | <0.0001 | −0.46 (−0.69, −0.24) | −0.70 (−0.94, −0.46) | −0.88 (−1.13, −0.63) |
| Model 2† | −0.14 (−0.24, −0.04) | 0.0088 | −0.18 (−0.36, 0.00) | −0.25 (−0.46, −0.04) | −0.31 (−0.54, −0.07) |
|
| |||||
| log(PAI-1, ng/mL) | |||||
| Model 1 | −0.29 (−0.37, −0.20) | <0.0001 | −0.28 (−0.43, −0.13) | −0.36 (−0.52, −0.20) | −0.59 (−0.78, −0.40) |
| Model 2† | −0.09 (−0.17, −0.01) | 0.0300 | −0.10 (−0.24, 0.04) | −0.07 (−0.22, 0.08) | −0.22 (−0.39, −0.04) |
|
| |||||
| Adiponectin, ug/mL | |||||
| Model 1 | 0.59 (0.22, 0.96) | 0.0019 | 0.72 (−0.08, 1.52) | 0.82 (0.02, 1.61) | 1.31 (0.54, 2.08) |
| Model 2† | −0.08 (−0.47, 0.31) | 0.6783 | 0.11 (−0.61, 0.84) | −0.16 (−0.98, 0.65) | 0.06 (−0.73, 0.85) |
|
| |||||
| log(e-selectin, ng/mL) | |||||
| Model 1 | −0.08 (−0.12, −0.04) | <0.0001 | −0.07 (−0.14, 0.01) | −0.08 (−0.15, −0.01) | −0.18 (−0.26, −0.10) |
| Model 2† | −0.03 (−0.07, 0.01) | 0.1206 | −0.02 (−0.09, 0.05) | 0.00 (−0.07, 0.07) | −0.08 (−0.16, 0.00) |
Lowest quartile of CRF is the reference category for quartile analysis; values are betas (95% confidence intervals) derived from survey linear regression models adjusted for age, sex, field center, national background, place of birth, annual household income, and parent education level, physical activity, and sedentary behavior.
Model 2 is additionally adjusted for waist circumference z-score.
CRF, cardiorespiratory fitness; CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor-1.
We also observed evidence for an association of CRF with endothelial dysfunction. (Table 3),. Each 10 mL/kg/min increase in CRF was associated with a 0.8 (95% CI −0.12, −0.04) reduction in log-transformed e-selectin, independent of physical activity and sedentary behavior (P<0.0001). Similar to other cardiometabolic risk factors, lower levels of e-selectin were observed at moderate levels of CRF, with effects stronger at the highest quartile of fitness. After adjusting for waist circumference the association of CRF with e-selectin was no longer significant. Lastly, the association of CRF with e-selectin was stronger in younger children (8–12 years old) compared to adolescents (13–16 years old) (Supplemental eTable2). No other interactions were observed (Supplemental eTables 1 and 3).
DISCUSSION
As we hypothesized, the study showed protective associations of CRF for cardiometabolic risk factors and biomarkers of endothelial dysfunction, independently of time spent in moderate-to-vigorous physical activity and sedentary behaviors. These results showed graded associations with all but one (fasting glucose) of studied biomarkers. Importantly, the inverse associations of CRF with measures of cardiovascular health were also observed at moderate levels of CRF, albeit associations were stronger among the fittest youth. Furthermore, the protective associations of CRF were observed in youth with and without obesity. Despite differences in CRF between boys and girls, associations were consistent across sex, except for PAI-1. The study results are consistent with other studies (33). The European Heart study, which included youths of similar age, showed inverse associations of CRF with cardiometabolic risk factors in boys and girls (34, 35), but the study did not include measures of endothelial function. A study of South Korean school children reported inverse associations of CRF with adiposity and lipids, but no associations with glycemic traits or blood pressure.(9) Similarly, other studies in youth have reported inverse associations of cardiorespiratory fitness and low-grade inflammation (7, 8, 36). Few studies examined the role of CRF on early markers of atherosclerosis. One such study, showed an association of CRF with left ventricular mass, but endothelial function was not assessed (37).
Taken together, these studies and ours, indicate that there is an independent association of CRF on cardiometabolic risk factors in youth. However, the mechanisms linking CRF to cardiovascular health remains to be elucidated. In our study, relationships were attenuated after adjusting for waist circumference, suggesting that central adiposity may mediate this association but the cross-sectional design prevented us from formally testing for mediation. In addition, as CRF is related to muscles’ ability to utilize oxygen and nutrients, improvements in muscle metabolism may be another explanation for the association of CRF with cardiovascular health. In fact, a small study of children showed that fat-free mass is associated with higher CRF (38). CRF is influenced by aerobic exercise levels, but it is also hypothesized to have a substantial genetic component.(39, 40) However, genetic studies offering insights about the biological mechanisms behind CRF are still limited. Available studies identified genes that regulate the aerobic response to exercise training (41, 42). Yet functional studies are needed to understand the common pathways that may regulate aerobic fitness and cardiometabolic traits (43, 44).
Some important study limitations have to be noted. First, the cross-sectional design limits any causal inference. Second, the study sample is not representative of the overall Hispanic/Latino youth population in the US, but only of the communities from which the study enrolled; although these four urban communities are among the 15 metropolitan areas with the largest concentration of Hispanic/Latinos (45). The sample size does not allow us to test differences by Hispanic/Latino national background. However, we previously reported similar prevalence of cardiometabolic risk factors in youth of Mexican background and youth of other backgrounds were similar (1). The potential for measurement error is present; for example, using bioelectrical impedance method may underestimate percentage body fat in children (46, 47). In addition, our index of overall cardiovascular health had modest reliability. Furthermore, the study was limited to a measure of aerobic fitness, and other dimensions of fitness that may be relevant to cardiovascular health, such as strength and endurance (48), were not included. Despite these limitations, the study contributes to the growing literature about the importance of CRF for cardiovascular health. Unique contributions of this research are an understudied population, young age group, and measures of endothelial function, a marker of early atherosclerosis. The study also has important implications related to youths’ future health once they reach adulthood given that high levels of fitness during adolescence predict lower risk of metabolic syndrome (10), CVD, and lower mortality in adulthood (11, 12, 49). Furthermore, low aerobic capacity in adolescence is associated with higher risk of hypertension and stroke later in life (50, 51).
CONCLUSION
The protective associations of fitness were observed in youth with and without obesity; suggesting that programs that aim to improve CRF could be useful to improve cardiovascular health among at-risk youth. Other than increases in vigorous activity, there is little information of other modifiable factors that may lead to improvements in CRF. Since our findings were independent of physical activity, studies exploring the biological pathways are needed to identify other strategies than increases in aerobic exercise that may lead to improvements of CRF.
Supplementary Material
Acknowledgments
The SOL Youth Study was supported by Grant Number R01HL102130 from the National Heart, Lung, and Blood Institute. The children in SOL Youth are drawn from the study of adults: The Hispanic Community Health Study/Study of Latinos, which was supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. Additional support was provided by the Life Course Methodology Core of the New York Regional Center for Diabetes Translation Research (DK111022- 8786). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. No financial disclosures were reported by the authors of this paper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: No financial disclosures were reported by the authors of this paper
Conflict of Interest: The authors were supported by Grant Number R01HL102130 from the National Heart, Lung, and Blood Institute. Additional support was provided by the Life Course Methodology Core of the New York Regional Center for Diabetes Translation Research (DK111022- 8786) The study sponsors did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Contributor Information
Carmen R Isasi, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY 1300 Morris Park Ave, Bronx, NY, 10461 Phone: 718-430-3345.
Garrett M Strizich, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY 1300 Morris Park Ave, Bronx, NY, 10461 Phone: 718-430-3345.
Robert C Kaplan, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY 1300 Morris Park Ave, Bronx, NY, 10461 Phone: 718-430-3345.
Martha L Daviglus, Institute for Minority Health Research, University of Illinois at Chicago, Chicago, IL.
Daniela Sotres-Alvarez, Collaborative Studies Coordinating Center, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, 137 East Franklyn Street, Suite 203. Chapel Hill, NC 27514.
Denise C Vidot, Department of Psychology, University of Miami Miller School of Medicine, 1120 NW 14th Street. Miami, FL 33136, Phone: 561-301-7904.
Maria M Llabre, Department of Psychology, University of Miami, 5665 Ponce de Leon Boulevard, Room 458. Miami, FL 33124, Phone: 305-284-6698.
Gregory Talavera, Institute for Behavioral and Community Health, Graduate School of Public Health, San Diego State University, San Diego, CA.
Mercedes Carnethon, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, 680 N. Lake Shore Drive, Suite 1400. Chicago, IL 60611, Phone: 312-503-4479.
References
- 1.Isasi CR, Parrinello CM, Ayala GX, Delamater AM, Perreira KM, Daviglus ML, et al. Sex Differences in Cardiometabolic Risk Factors among Hispanic/Latino Youth. The Journal of pediatrics. 2016;176:121–7 e1. doi: 10.1016/j.jpeds.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laukkanen JA, Kurl S, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. European heart journal. 2004;25(16):1428–37. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262(17):2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 4.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. American journal of epidemiology. 2007;165(12):1413–23. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada SS, Lee IM, Naito H, Kakigi R, Goto S, Kanazawa M, et al. Cardiorespiratory fitness, body mass index, and cancer mortality: a cohort study of Japanese men. BMC public health. 2014;14:1012. doi: 10.1186/1471-2458-14-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. Journal of psychopharmacology. 2010;24(4 Suppl):27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isasi CR, Starc TJ, Tracy RP, Deckelbaum R, Berglund L, Shea S. Inverse association of physical fitness with plasma fibrinogen level in children: the Columbia University BioMarkers Study. American journal of epidemiology. 2000;152(3):212–8. doi: 10.1093/aje/152.3.212. [DOI] [PubMed] [Google Scholar]
- 8.Isasi CR, Deckelbaum RJ, Tracy RP, Starc TJ, Berglund L, Shea S. Physical fitness and C-reactive protein level in children and young adults: the Columbia University BioMarkers Study. Pediatrics. 2003;111(2):332–8. doi: 10.1542/peds.111.2.332. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Lee KJ, Jeon YJ, Ahn MB, Jung IA, Kim SH, et al. Relationships of physical fitness and obesity with metabolic risk factors in children and adolescents: Chungju city cohort study. Annals of pediatric endocrinology & metabolism. 2016;21(1):31–8. doi: 10.6065/apem.2016.21.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MD, Magnussen CG, Rees E, Dwyer T, Venn AJ. Childhood fitness reduces the long-term cardiometabolic risks associated with childhood obesity. International journal of obesity. 2016;40(7):1134–40. doi: 10.1038/ijo.2016.61. [DOI] [PubMed] [Google Scholar]
- 11.Hogstrom G, Nordstrom A, Nordstrom P. Aerobic fitness in late adolescence and the risk of early death: a prospective cohort study of 1.3 million Swedish men. International journal of epidemiology. 2016;45(4):1159–68. doi: 10.1093/ije/dyv321. [DOI] [PubMed] [Google Scholar]
- 12.Hogstrom G, Nordstrom A, Nordstrom P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. European heart journal. 2014;35(44):3133–40. doi: 10.1093/eurheartj/eht527. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. The Journal of clinical endocrinology and metabolism. 2009;94(9):3171–82. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 14.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. The American journal of medicine. 1999;107(1):85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 15.Caballero AE, Bousquet-Santos K, Robles-Osorio L, Montagnani V, Soodini G, Porramatikul S, et al. Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes care. 2008;31(3):576–82. doi: 10.2337/dc07-1540. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Miyashita M, Kwon YC, Park HT, Kim EH, Park JK, et al. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children: a randomised controlled study. BMC pediatrics. 2012;12:111. doi: 10.1186/1471-2431-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruyndonckx L, Hoymans VY, De Guchtenaere A, Van Helvoirt M, Van Craenenbroeck EM, Frederix G, et al. Diet, exercise, and endothelial function in obese adolescents. Pediatrics. 2015;135(3):e653–61. doi: 10.1542/peds.2014-1577. [DOI] [PubMed] [Google Scholar]
- 18.Qi Q, Hua S, Perreira KM, Cai J, Van Horn L, Schneiderman N, et al. Sex Differences in Associations of Adiposity Measures and Insulin Resistance in US Hispanic/Latino Youth: The Hispanic Community Children–s Health Study/Study of Latino Youth (SOL Youth) The Journal of clinical endocrinology and metabolism. 2016 doi: 10.1210/jc.2016-2279. jc20162279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunet M, Chaput JP, Tremblay A. The association between low physical fitness and high body mass index or waist circumference is increasing with age in children: the ‘Quebec en Forme’ Project. International journal of obesity. 2007;31(4):637–43. doi: 10.1038/sj.ijo.0803448. [DOI] [PubMed] [Google Scholar]
- 20.Zaqout M, Vyncke K, Moreno LA, De Miguel-Etayo P, Lauria F, Molnar D, et al. Determinant factors of physical fitness in European children. International journal of public health. 2016;61(5):573–82. doi: 10.1007/s00038-016-0811-2. [DOI] [PubMed] [Google Scholar]
- 21.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and Implementation of the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology. 2010;20(8):629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isasi CR, Carnethon MR, Ayala GX, Arredondo E, Bangdiwala SI, Daviglus ML, et al. The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth): design, objectives, and procedures. Annals of epidemiology. 2014;24(1):29–35. doi: 10.1016/j.annepidem.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis K, Feinstein R. A simple height-specific and rate-specific step test for children. Southern medical journal. 1991;84(2):169–74. doi: 10.1097/00007611-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 24.McMurray RG, Guion WK, Ainsworth BE, Harrell JS. Predicting aerobic power in children. A comparison of two methods. The Journal of sports medicine and physical fitness. 1998;38(3):227–33. [PubMed] [Google Scholar]
- 25.McMurray RG, Bangdiwala SI, Harrell JS, Amorim LD. Adolescents with metabolic syndrome have a history of low aerobic fitness and physical activity levels. Dynamic medicine: DM. 2008;7:5. doi: 10.1186/1476-5918-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill CSCCaTUoNCaC. Hispanic Community Children’s Health/Study of Latino Youth (SOL Youth) Manual 1: Field Center Procedures Version 3.1. 2013 [Google Scholar]
- 27.NHCS. 2000 CDC Growth Charts: United States. 2000 [Available from: http://www.cdc.gov/growthcharts/
- 28.Prevention CfDCa. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [Google Scholar]
- 29.NHLBI/NHBPEP. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114(2 (Suppl 4th Report)):555–76. [PubMed] [Google Scholar]
- 30.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Medicine and science in sports and exercise. 2011;43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanzini M, Petroski EL, Ohara D, Dourado AC, Reichert FF. Calibration of ActiGraph GT3X, Actical and RT3 accelerometers in adolescents. European journal of sport science. 2014;14(1):91–9. doi: 10.1080/17461391.2012.732614. [DOI] [PubMed] [Google Scholar]
- 32.Cossio S, Messiah SE, Garibay-Nieto N, Lopez-Mitnik G, Flores P, Arheart KL, et al. How do different indices of obesity correlate with cardiometabolic disease risk factors in multiethnic youths? Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009;15(5):403–9. doi: 10.4158/EP08354.OR. [DOI] [PubMed] [Google Scholar]
- 33.Ortega FB, Ruiz JR, Castillo MJ, Sjostrom M. Physical fitness in childhood and adolescence: a powerful marker of health. International journal of obesity. 2008;32(1):1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz JR, Ortega FB, Rizzo NS, Villa I, Hurtig-Wennlof A, Oja L, et al. High cardiovascular fitness is associated with low metabolic risk score in children: the European Youth Heart Study. Pediatric research. 2007;61(3):350–5. doi: 10.1203/pdr.0b013e318030d1bd. [DOI] [PubMed] [Google Scholar]
- 35.Hurtig-Wennlof A, Ruiz JR, Harro M, Sjostrom M. Cardiorespiratory fitness relates more strongly than physical activity to cardiovascular disease risk factors in healthy children and adolescents: the European Youth Heart Study. Eur J Cardiovasc Prev Rehabil. 2007;14(4):575–81. doi: 10.1097/HJR.0b013e32808c67e3. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Gomez D, Eisenmann JC, Warnberg J, Gomez-Martinez S, Veses A, Veiga OL, et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: the AFINOS Study. International journal of obesity. 2010;34(10):1501–7. doi: 10.1038/ijo.2010.114. [DOI] [PubMed] [Google Scholar]
- 37.Janz KF, Dawson JD, Mahoney LT. Predicting heart growth during puberty: The Muscatine Study. Pediatrics. 2000;105(5):E63. doi: 10.1542/peds.105.5.e63. [DOI] [PubMed] [Google Scholar]
- 38.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24(7):841–8. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 39.Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, et al. The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Medicine and science in sports and exercise. 2009;41(1):35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 40.Bouchard C. Genomic predictors of trainability. Experimental physiology. 2012;97(3):347–52. doi: 10.1113/expphysiol.2011.058735. [DOI] [PubMed] [Google Scholar]
- 41.Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. Journal of applied physiology. 2011;110(1):46–59. doi: 10.1152/japplphysiol.00634.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, et al. Genomic predictors of the maximal O(2) uptake response to standardized exercise training programs. Journal of applied physiology. 2011;110(5):1160–70. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loos RJ, Hagberg JM, Perusse L, Roth SM, Sarzynski MA, Wolfarth B, et al. Advances in exercise, fitness, and performance genomics in 2014. Medicine and science in sports and exercise. 2015;47(6):1105–12. doi: 10.1249/MSS.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circulation research. 2015;116(5):909–22. doi: 10.1161/CIRCRESAHA.116.302888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pew Research center. Hispanic Trends. Hispanic Population in Select US Metropolitan Areas. 2014 [Google Scholar]
- 46.Lee LW, Liao YS, Lu HK, Hsiao PL, Chen YY, Chi CC, et al. Validation of two portable bioelectrical impedance analyses for the assessment of body composition in school age children. PloS one. 2017;12(2):e0171568. doi: 10.1371/journal.pone.0171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguirre CA, Salazar GD, Lopez de Romana DV, Kain JA, Corvalan CL, Uauy RE. Evaluation of simple body composition methods: assessment of validity in prepubertal Chilean children. European journal of clinical nutrition. 2015;69(2):269–73. doi: 10.1038/ejcn.2014.144. [DOI] [PubMed] [Google Scholar]
- 48.Fraser BJ, Huynh QL, Schmidt MD, Dwyer T, Venn AJ, Magnussen CG. Childhood Muscular Fitness Phenotypes and Adult Metabolic Syndrome. Medicine and science in sports and exercise. 2016;48(9):1715–22. doi: 10.1249/MSS.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 49.Farrell SW, Cortese GM, LaMonte MJ, Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity. 2007;15(12):3140–9. doi: 10.1038/oby.2007.374. [DOI] [PubMed] [Google Scholar]
- 50.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive Effects of Physical Fitness and Body Mass Index on the Risk of Hypertension. JAMA internal medicine. 2016;176(2):210–6. doi: 10.1001/jamainternmed.2015.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on risk of stroke: A national cohort study. International journal of stroke: official journal of the International Stroke Society. 2016;11(6):683–94. doi: 10.1177/1747493016641961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


