Abstract
The period of in utero development is one of the most critical windows during which adverse conditions and exposures may influence the growth and development of the fetus as well as its future postnatal health and behavior. Maternal substance use during pregnancy remains a relatively common but nonetheless hazardous in utero exposure. For example, previous epidemiological studies have associated prenatal substance exposure with reduced birth weight, poor developmental and psychological outcomes, and increased risk for diseases and behavioral disorders (e.g., externalizing behaviors like ADHD, conduct disorder, and substance use) later in life. Researchers are now learning that many of the mechanisms whereby adverse in utero exposures may affect key pathways crucial for proper fetal growth and development are epigenetic in nature, with the majority of work in humans considering DNA methylation specifically. This review will explore the research to date on epigenetic alterations tied to maternal substance use during pregnancy and will also discuss the possible role of DNA methylation in the robust relationship between maternal substance use and later behavioral and developmental sequelae in offspring.
1. Introduction
The period of in utero development is one of the most critical windows during which adverse conditions and exposures may influence the growth and development of the fetus as well as its future postnatal health and behavior. Yet, a considerable proportion of women in the United States continue to use substances while pregnant. The most frequently used substance is tobacco, followed by alcohol, cannabis and other illicit substances (Forray and Foster, 2015). According to the National Survey on Drug Use and Health, the Pregnancy Risk Assessment and Monitoring System (PRAMS), and the Centers for Disease Control (CDC), 12.3–15.1% of women in the United States report smoking during pregnancy (SDP; Tong et al., 2013; United States Department of Health and Human Services et al., 2015), with the rates of smoking among pregnant teenagers ranging from 19.5% to as high as 50% (Cornelius et al., 2002; Ventura et al., 2001). These rates persist despite a large literature suggesting undesirable outcomes in children exposed to SDP (e.g., Knopik et al., 2016a; Knopik et al., 2016b; Kuja-Halkola et al., 2014) as well as warnings encouraging women to stop smoking while pregnant. Findings also suggest that there are a variety of placental complications linked to prenatal exposure to cigarette smoke (Einarson and Riordan, 2009), which could effectively translate into a number of sequelae (e.g., intrauterine growth retardation and later behavioral problems; Joya et al., 2014; Knopik, 2009).
A number of women also report drinking alcohol during pregnancy. In fact, an estimated 16–23% of US women report alcohol use during pregnancy (Marceau et al., 2016a). According to the CDC Behavioral Risk Factor Surveillance System, about 1 in 10 pregnant women (10.2%) report any alcohol use, and about 1 in 33 pregnant women (3.1%) report binge drinking (4+ drinks on one occasion) in the past 30 days (Tan et al., 2015). In a recent review of the effects of prenatal alcohol exposure on fetal alcohol spectrum disorders, the American Academy of Pediatrics concluded, “There is no known absolutely safe quantity, frequency, type, or timing of alcohol consumption during pregnancy, but having no [prenatal alcohol exposure] translates into no [fetal alcohol spectrum disorders]” (Williams and Smith, 2015).
With regard to illicit drugs, 5.9% of pregnant women report illicit drug use, with approximately 2.5% of pregnant women admitting to consistent use of cannabis; although, actual use is probably higher (Hayatbakhsh et al., 2011). Importantly, cannabinoids (constituents of the cannabis plant that act on cannabinoid receptors in the human body and brain) have been shown to cross the placenta as well as the blood brain barrier and can also be concentrated in breast milk (e.g., Jaques et al., 2014). Cannabis has been associated with preterm labor, low birth weight, intrauterine growth retardation, reduced attention and executive function, as well as increased behavioral problems in exposed children (Warner et al., 2014). Cocaine and methamphetamine use during pregnancy are also related to several adverse pregnancy and birth-related outcomes, such as low birthweight, later developmental issues, and behavioral problems (Dyk et al., 2014; Strathearn and Mayes, 2010). Finally, consistent with the current epidemic of opioid use in the United States, between 2000 and 2009, there has been a fivefold increase in opiate use during pregnancy (Desai et al., 2014; Forray, 2016; Hayes and Brown, 2012). Opiate use during pregnancy has been linked to postnatal growth deficiency, neurobehavioral problems, and increased use of healthcare modalities (Minozzi et al., 2013). Given the widespread use of substances during pregnancy, it is important to understand the mechanisms by which substance use exposure can affect child development. One highly plausible mechanism is epigenetic changes, as detailed below.
Broadly, the field of epigenetics is focused on understanding a type of slow-motion, developmentally stable change in certain mechanisms of gene expression that (i) do not alter DNA sequence, and (ii) can be passed on from one cell to its daughter cells (Bird, 2007). It may be through epigenetic modifications that environmental factors like diet, stress, prenatal nutrition, and for the purposes of this review, prenatal substance exposure, can change gene expression from one cell to its daughter cells and, in some cases, from one generation to the next. Theoretically, epigenetic changes are an important part of any biological pathway that includes both genetic influences and environmental exposures, as epigenetic changes are one mechanism by which environmental exposures can ‘get under the skin’ to affect the underlying biology of a system.
Taken together, since substance use during pregnancy continues to be a common occurrence and significant public health concern, understanding the potential pathways underlying the associations between early life exposures and later health and behavior, therefore, has important public health implications. Thus, the purpose of this review is to (i) highlight the prenatal period as a critical window of development of adverse offspring health and behavioral outcomes, and (ii) discuss the potential role that epigenetic modifications, particularly DNA methylation, might play in this developmental relationship. We will briefly summarize epidemiological data to establish the critical nature of the prenatal period. We then outline results from genetically-informed studies that suggest that the relationships between maternal substance use during pregnancy and later outcomes may be, in part, confounded by genetic and environmental factors that families share (e.g., genetic transmission from mother to child and aspects of the postnatal family environment). Finally, we end with current theory and evidence in humans for epigenetic changes linked to exposure to in utero substance use and how these epigenetic changes might play a potential role in child and adolescent developmental and behavioral outcomes.
2. The critical window of prenatal development
A number of epidemiological studies have explored the links between adverse prenatal conditions and increased risk for diseases, health problems, and psychological outcomes later in development. Perhaps most notable are examinations of the Dutch Famine Birth Cohort, which consists of men and women born as term singletons (single births) in The Netherlands during or immediately following the Dutch Famine of 1944–45 (de Rooij et al., 2010; Stein et al., 1972). Work on this cohort suggests that an adverse intrauterine environment influenced by famine is associated with multiple negative outcomes, including, but not limited to, increased risk for diabetes mellitus, cardiovascular disease, other metabolic disorders, decreased cognitive function later in life (Argente et al., 2010; Barker and Clark, 1997; de Rooij et al., 2010), and (in males) increased risk of affective disorders (Brown et al., 1995). There is also some evidence that adverse health outcomes related to prenatal famine exposure are not just limited to the children directly exposed to prenatal famine, but are also evident in the grandchildren of the Dutch Famine Cohort individuals (Painter et al., 2008). While these findings focus on famine as an adverse prenatal environment rather than substance use, these studies underscore the importance of considering timing of prenatal exposure to adverse conditions, as well as potential confounding elements (Brown et al., 1995), when looking at outcomes across development and generations.
Observations from the Dutch Famine Birth Cohort, in part, influenced the theory of fetal programming, which was initially proposed in the 1980s and highlights the prenatal period as a critical developmental window. More specifically, fetal programming (commonly called Barker's hypothesis) explains the influence of the in utero environment on the development of body structure, function, and metabolism and how these factors contribute to later disease (Barker and Clark, 1997; Hales and Barker, 1992). While there is ample research pointing broadly to associations between the prenatal environment and fetal programming for later childhood outcomes, causal pathways and specific mechanisms have been hard to pinpoint. Research aimed at better understanding the underlying mechanisms of fetal programming is ongoing. The focal points of this research include areas devoted to potential genetic and epigenetic mechanisms as well as adaptive responses of the fetus to a broad range of environmental cues, including exposure to viruses, such as influenza, increased levels of stress during pregnancy, and of importance to this review, maternal substance use during pregnancy.
3. Substance use during pregnancy: evidence from genetically informed studies
Prenatal substance exposure, or to use a more specific example for descriptive purposes, maternal smoking during pregnancy (SDP), certainly contributes to an adverse intrauterine environment. In brief, epidemiological studies (i.e., non-genetically informed studies) suggest that SDP is associated with multiple adverse birth related outcomes, such as preterm delivery (Castles et al., 1999; Kaddar et al., 2009; Shah and Bracken, 2000), increased risk for spontaneous abortion (Castles et al., 1999), and lower birth weight (e.g., Knopik et al., 2016b; Marceau et al., 2016b; Smith et al., 2016). SDP has also been associated with prenatal ischemia-hypoxia (Smith et al., 2016), respiratory disease (Cook and Strachan, 1999), cancer later in life (Doherty et al., 2009), and a host of later neurodevelopmental and behavioral outcomes, including externalizing behaviors, such as conduct disorder, ADHD, and later substance use (see Knopik, 2009 for a review). However, evidence that maternal SDP is correlated with other potential contributors to an adverse in utero environment can make causal attribution difficult (see Knopik, 2009; Knopik et al., 2012 for reviews). Indeed, recent work from our own group and others suggests that maternal SDP is correlated with many risk factors (e.g., obstetric complications, anxiety and depression during pregnancy, prenatal infections) and exposures (e.g., exposure to toxins like chemicals, pesticides) experienced during pregnancy (Marceau et al., 2013). SDP is also associated with lower levels of maternal education (D'Onofrio et al., 2010), spousal/significant other substance dependence (Knopik et al., 2006; Knopik et al., 2005), nicotine dependence (Agrawal et al., 2008), as well as maternal ADHD and other psychopathology (D'Onofrio et al., 2010; Huizink and Mulder, 2006; Knopik, 2009; Knopik et al., 2009), all of which may also influence the intrauterine environment and thus, predict later offspring behavior. Of additional note is evidence that SDP is genetically-influenced (or heritable; Agrawal et al., 2008) and most of the outcomes that have been studied in children exposed to SDP are also heritable. In fact, one of the main limitations of studying familial influence on child development is that the parents are providing both the environment and the genes to their offspring (D'Onofrio et al., 2003).
These findings as a whole have led to increased interest in understanding the nature of the reported SDP-child outcome associations, particularly in light of more recent genetically-informed studies that suggest that the SDP-externalizing link is less clear. Genetically-informed approaches to the effects of substance use during pregnancy, sometimes called quasi-experimental designs, consist of adoption designs, twin designs and their extensions, children-of-twin designs, sibling-comparison designs, and to a lesser degree, molecular genetic studies (see Knopik, 2009 for detailed review of these designs). In general, the intent of using these genetically-informed approaches is to attempt to control for genetic and environmental variables that family members share as a means to disentangle a more robust effect of prenatal exposure. Some genetically-informed approaches suggest that, even after controlling for shared genetic and familial effects, SDP is potentially causally linked to disruptive behavior (e.g., Gaysina et al., 2013; Knopik et al., 2016a; Marceau et al., 2017). However, others suggest that certain SDP-externalizing associations seen in epidemiological studies may be due to an inability to adequately control for shared familial influences, including genetic and shared family environmental factors. In other words, after control for confounding factors, they do not see a robust effect of SDP on certain outcomes, including offspring conduct problems (D'Onofrio et al., 2008; Jaffee et al., 2012), criminality (D'Onofrio et al., 2010; Kuja-Halkola et al., 2014), ADHD (Skoglund et al., 2014; Thapar et al., 2009) and substance use initiation (Bidwell et al., 2017). While much less has been done in terms of genetically-informed approaches and maternal use of alcohol during pregnancy, there is a similar picture of inconsistent results (D'Onofrio, 2009; D'Onofrio et al., 2007). To the best of our knowledge, at the time of this report, there are no genetically-informed studies on prenatal cannabis or other illicit drugs in humans. Given the different conclusions across studies on the nature of the effects of exposure to substances during pregnancy on later offspring behavioral outcomes, there is growing interest in epigenetics as one potential mechanism by which some of these inconsistencies might be explained.
4. Substance use during pregnancy: the role of epigenetics
Various subfields have emerged to explore epigenetic effects in a variety of settings. For example, the field of “environmental epigenetics” studies how environmental exposures affect epigenetic mechanisms (Reamon-Buettner et al., 2008). Many researchers are interested in uncovering how environmental exposures at sensitive periods of development, such as maternal substance use during pregnancy, might influence epigenetics and thus, affect the developing fetus. There is ongoing research using animals (primarily mice and rat models) to characterize the influence of environmental exposures, specifically prenatal drug use, on epigenetic changes. While not the focus of this review, we summarize selected animal work in Table 1 in order to provide a context within which the human data can be considered. In brief, animal models of the epigenetics of prenatal substance exposure show, in general, altered epigenetic profiles (as measured by DNA methylation, histone modification, and microRNA) in a variety of tissues (most often brain tissue) of exposed animals. In the animal models that then consider later behavioral outcomes, studies suggest these differential epigenetic profiles have an effect on offspring locomotor activity, hyperactivity, inattention, impulsivity, anxiety, increased drug sensitivity, and impaired spatial learning and memory (see Table 1). The importance of animal work to human problems cannot be overstated (England et al., 2017). For example, animal models have the ability to design studies that incorporate a specific controlled dose of a specific drug thus providing valuable information on the effects of specific doses of prenatal substance use on tissues (e.g., brain) that are not generally available in human research. Animal work also provides support of a possible epigenetic mechanism by which prenatal substance exposure leads to later offspring outcomes. However, the human condition is considerably more complex. In humans, fetal drug exposure is typically correlated with polysubstance use, a variety of doses, and environmental variables as well as genetic predisposition. In addition, the human brain is very different from the rodent brain. The effects of prenatal substance exposure in humans often show up as higher level cognitive functions, which are controlled by the prefrontal cortex (Knopik, 2009), a structure which, according to functional and structural studies, might not exist in the rodent brain (Preuss, 1995). Importantly, while we can use the evidence of negative effects of prenatal substance exposure that we garner from animal work as a guide to narrow our focus on potential effects in humans, we cannot directly extrapolate from animal findings to the complex human condition (Knopik, 2009).
Table 1.
Selected animal models of maternal substance use during pregnancy and epigenetic effects.
| Authors and publication date | Sample size (N) and animal studied | Measure of prenatal exposure | Epigenetic alteration studied | Tissue of interest | Outcome measures | Brief results (exposed offspring) |
|---|---|---|---|---|---|---|
| Li et al. (2013) | Male Sprague-Dawley rat pups | Nicotine was administered to pregnant rats from day 4 of gestation to day 10 after birth (through osmotic minipumps (4 μg/kg/min) implanted subcutaneously) | DNA Methylation | Brain | Vulnerability to brain hypoxic-ischemic injury in prenatally exposed male pups | Perinatal nicotine exposure increases methylation of a single CpG−52 locus near the TATA element at AT2R gene promoter, resulting in a decrease in the binding of TATA-binding protein (TBP) to AT2R promoter and a repression of AT2R gene expression in the developing brain. |
| Jabbar et al. (2016) | N = 60 adult female rats of Charles River, | 24 females received a liquid diet containing ethanol (6.7%) for 30 days | DNA Methylation | Brain | Stop Watch behavioral monitoring software; weight | Longer gestation time; smaller litter size; lower birth weight; reduced body growth throughout nursing period; stress hyperresponse; increased anxiety behavior |
| Zhao et al. (2015) | Pregnant C57BL/6 J mice | On gestational days 8 through 18, an injection of either saline (0.9% NaCl) or 20mg/k cocaine subcutaneously (s.c.) twice per day. | DNA Methylation | Brain (hippocampus) | Morris Water Maze; open field test; weight | No significant effect on birth weight; impaired spatial learning and memory; increased anxiety behavior |
| Itzhak., Ergui, & Young (2015) | N = 22, C57BI/6 J mice Jackson Laboratory; N = 62 saline pups and N = 60 Meth pups | 4 mg/kg of METH every other day subcutaneously | DNA methylation | Brain (hippocampus) | Cocaine conditioned place preference paradigm (CPP); fear conditioning; spontaneous locomotor activity; preference for black and white compartments | No effect on dam or pup body weight; increased spontaneous locomotor activity and anxiety-like behavior; male pups showed significantly higher responses than females; dampened freezing response |
| Marjonen et al. (2015) | 18 control (123 offspring, 74 male offspring), 19 ethanol-exposed (137 offspring, 75 male offspring) and 19 cross-fostering control dam; inbred; C57BL/6 J Rcc (Harlan, Netherlands) | 10% v/v ethanol; free drinking | DNA Methylation | Brain (hippocampus) | Weight | Reduced fetal body weight |
| Ngai et al. (2015) | 35.5% ethanol-derived calories; liquid diet | DNA Methylation | Brain (hippocampus) | Weight | Reduced fetal body weight | |
| Wang et al. (2009) (Wang et al., 2009) | N = 706 | Ethanol 2.0, 4.0 or 6.0gAg/day | microRNA | Brain | Weight; open field test; Morris water maze | Reduced fetus weight; lower locomotor activity in ethanol group; impairment of task acquisition in ethanol group |
| Haycock and Ramsay, 2009 | N = 337 | 1.5 and 2.5 dpc, 0.015 ml/g of 25% ethanol (2.9 gAg) | DNA Methylation | Embryo and placenta | Weight | Reduced embryonic weights in ethanol versus control groups |
| Kaminen-Ahola et al. (2010) | N = 242; C57BL/6 J mouse | Free access to 10% (v/v) ethanol for 8 days after fertilization | DNA Methylation | Tails and Brain | Weight | Lower birth weight |
| Kim et al. (2013) | N ranged from 4 to 13 for mice and 6-29 for rat depending on experiment | 0.5, 1, 2, 4 and 6gAg/day; 25v/v% of ethanol from gestational day 6 to 15 via intragastric intubation | Histone modification | Brain | Open field test; Y-Maze Test; The Electro-Foot Shock Aversive Water Drinking Test | No difference in birth weight; Hyperactivity; inattention; impulsive behaviors |
| Asimes et al., 2017 | N = 132 male and female Wistar rats | repeated binge-pattern alcohol paradigm; 3 g/Kg body weight (20% v/v solution) | DNA Methylation | Brain (hypothalamus) | Enhanced Reduced Representation Bisulfite Sequencing (ERRBS); High Capacity Reverse Transcription Kit | No difference in litter size, pup weight, or sex ratio; altered genome-wide DNA methylation patterns |
| Novikova et al., 2008 | N = 20 CD1 dams (10 exposed, 10 saline-control); 10 male pups (1 per litter) for offspring outcomes | Cocaine-treatment group: subcutaneously injected (at the dorsum of the neck) with 20 mg/Kg cocaine hydrochloride dissolved in 200 μl of 0.9% saline, twice a day (at 8:00 AM and 8:00 PM) from the 8th through the 19th day of gestation (E8–E19). Saline-control group: same schedule of injections but with 200 μl of 0.9% saline only. | DNA Methylation | Brain (hippocampus) | Global DNA methylation, methylated DNA immunoprecipitation followed by CGI2 microarray profiling and bisulfite sequencing, as well as quantitative real-time RT-PCR gene expression analysis, were evaluated in hippocampal pyramidal neurons excised by laser capture microdissection. | Global DNA methylation was significantly decreased at P3 and increased at P30. Endogenous expression of selected genes linked to the abnormally methylated CGIs was correspondingly decreased or increased by as much as 4–19-fold. By P30, some of the cocaine-associated effects at P3 endured, reversed to opposite directions, or disappeared. |
| DiNieri et al. (2011) | N=5–6 prenatally exposed male rats per THC and Control groups | Females were treated with daily i.v. injections of either THC (0.15 mg/kg) or vehicle (VEH, 0.3% Tween 80-sterile saline solution) from GD5-PND2. This treatment period corresponds to the neurodevelopmental period examined in our human fetal population (midgestation, ∼20 weeks) (16). The dose of THC used in this paradigm is comparable to current estimates of low dose cannabis cigarettes (∼16 mg of THC) | Histone modification | Morphine place conditioning task | Chromatin immunoprecipitation (ChIP) on extracts isolated from the NAc of adult male rats with prenatal THC exposure and immunopreciptated with antibodies specific for dimethylated lysine 9 (2meH3K9) and trimethylated lysine 4 (3meH3K4) on histone H3. | Increased opiate reward sensitivity in adult male rat. Data suggest that the association between prenatal cannabis exposure and addiction vulnerability can be explained, at least in part, by cannabis-induced alterations in the epigenetic regulation of the DRD2 gene in the NAc. |
Importantly, research in both humans (the focus of this review) and animal models (see Table 1) continues to characterize the influence of environmental exposures on epigenetic changes. Despite the complexity in humans, this work is significant because epigenetic mechanisms, such as DNA methylation profiles, may have utility not only as diagnostic biomarkers capable of predicting increased risk for exposure, behavioral deficits, and disease, but also as therapeutic targets (Ladd-Acosta, 2015; Maccani et al., 2013; Pajer et al., 2012).
5. Epigenetics: DNA methylation
While there are multiple modes of epigenetic gene regulation (see Allis et al., 2015; Januar et al., 2015; Maccani and Marsit, 2009; Nelissen et al., 2011), DNA methylation is the most heavily studied (Bird, 2007). In human research, there are very few studies that look at other modes of epigenetic regulation as they relate to prenatal substance exposure. There is only limited work considering the effects of prenatal substance exposure on differential microRNA expression (see Vrijens et al., 2015) or histone modifications, thus we focus this review on DNA methylation. DNA methylation is performed by one of a number of DNA methyltransferases which add a methyl group to a specific cytosine residue. These cytosine residues often reside in cytosine- and guanine-rich stretches of DNA called “CpG islands.” Research has determined that the blocking of transcription in a methylated gene is not due to the methylation of DNA alone, but rather due to the irregular binding of a variety of proteins. In the presence of DNA methylation, proteins which normally bind DNA and enable transcription to proceed are unable to bind as well, or at all, which effectively limits or stops transcription. Exactly how DNA methylation in a gene's promoter region controls the complex regulatory environment necessary for transcription and thus gene expression remains to be completely understood. There is some evidence to suggest that DNA methylation in different regions of the gene can have different impact. For example, methylation in the gene promotor region can reduce gene transcription due to altered binding of transcriptional factors, while methylation in the body of the gene can increase gene expression (Szulwach and Jin, 2014).
During the period of embryonic development, methylation patterns of the germline and somatic cell lineages are beginning to be established (Maccani and Marsit, 2009; Reik, 2007). During the cleavage phase, which includes the early cell divisions that occur as a fertilized egg begins to develop into an embryo, methylation in the zygote's genome is almost completely removed. After implantation, as the cells produced during the cleavage phase begin to organize themselves, a process called gastrulation, the organism's methylation patterns are reestablished by de novo methylation (Jaenisch, 1997; Ariel et al., 1992; Monk et al., 1987; Razin and Shemer, 1995). Such patterning and re-patterning of methylation marks also occurs in trophoblast lineages, the various specialized cells comprising the placenta (Jaenisch, 1997; Wossidlo et al., 2011). These epigenetic marks are involved in modulating functional pathways. Thus, proper setting and resetting of methylation marks throughout development is key for the proper health and development of the embryo, setting the stage for future protections and vulnerabilities.
There is growing evidence to suggest that direct exposure to toxins, such as drug use or pollution, is associated with changes in offspring methylation patterns (Yang and Schwartz, 2012; Zhou et al., 2014). Of particular interest to the current review, a number of studies have characterized associations between prenatal substance exposure and aberrant DNA methylation patterns in a variety of tissues. The following sections, as well as Table 2, briefly summarize these findings for the two most prevalent and common exposures: (i) prenatal cigarette smoke exposure, and (ii) prenatal alcohol exposure. There is growing interest in the epigenetic effects associated with prenatal exposure to other substances (cannabis, opioids, and other illicit drugs), particularly given the rise in opioid use in the United States; however, to date and to the best of our knowledge, there is a paucity of research in humans.
Table 2.
Human models of maternal substance use during pregnancy and DNA methylation.
| Authors and publication date | Sample size (iV) | Measure of prenatal exposure | Epigenetic alteration studied | Tissue of interest | Outcome measures | Brief results (exposed offspring) | |
|---|---|---|---|---|---|---|---|
| DNA methylation in placental tissue | |||||||
| Appleton et al. (2013) | N= 444 | Interviewer-administered structured questionnaire of smoking during pregnancy | DNA Methylation | Placenta | Bisulfite pyrosequencing of the HSD11B2 region. Methylation across each of the four HSD11B2 CpG sites was averaged to obtain an overall measure of methylation. | Prenatal tobacco use significantly associated with 8.8% less HSD11B2 methylation compared to those not exposed to smoking during pregnancy | |
| Chhabra et al. (2014) | N= 80 | Nicotine exposure was determined by measuring placental cotinine concentration in nanograms per gram of placental tissue. | DNA Methylation | Placenta | llumina HumanMethylation450 BeadChip array | Percent methylation in exposed and unexposed placental tissue for all CpG sites in the GTF2H2C and GTF2H2D gene suggest a region of relative hypomethylation associated with nicotine exposure | |
| Maccani et al. (2013) | N= 206 | Smoking status at any time during pregnancy recorded from patient charts | DNA Methylation | Placenta | Lumina HumanMethylation27 BeadChip array; bisulfite pyrosequencing | Seven loci within the intronic and promoter regions of the RUNX3 gene displayed differential methylation patterns that were significantly associated with maternal smoking during pregnancy. | |
| Paquette et al. (2013) | N= 444 | Medical chart review of smoking during pregnancy | DNA Methylation | Placenta | Bisulfite pyrosequencing; Infant neurobehavior (NICU Network Neurobehavioral Scales [NNNS]) | Marginally significant relationship between smoking and HTR2A promoter methylation; smokers had a 4.3% higher mean methylation (41.3% vs. 36.9%). Significant positive correlation between HRT2A methylation and infant attention score (P = 0.0008) and a significant negative correlation between methylation and quality of movement (P = 0.02). | |
| Suter et al. (2011) | N= 36 | Maternal-reported tobacco exposure during pregnancy | DNA Methylation | Placenta | Puregene Kit; EZ DNA methylation kit; Infinium Human Methylation 27 BeadChip; Human HG-12 BeadChip, birth weight | 1024 CpG sites were differentially methylated; 622 genes were differentially expressed; 438 of genes differentially expressed were correlated with methylation. STX5, FUT11, TUSC3, FAN1, and ZNF671 associated with both smoking and birth weight; PURA, GTF2H2, GCA, GPR135, and HKR1 associated with smoking | |
| Suter et al. (2010) | N= 34 | Maternal-reported tobacco exposure during pregnancy | DNA Methylation | Placenta | Machery Nagel NucleoSpin kit; Applied Biosystems High Capacity cDNA Reverse Transcription Kit; EZ DNA methylation kit | Increases in placental CYP1A1 expression is associated with differential methylation at xenobiotic response elements | |
| Stroud et al. (2014) | N= 45 | Maternal smoking during pregnancy was assessed by timeline followback interview, verified by saliva and meconium cotinine | DNA Methylation | Placenta | Degree of methylation at the NR3C1 promoter ex on IF - examined via quantitative pyrosequencing using PyroMark MD Pyrosequencing System; infant attention, self-regulation and lethargy | Increased placental NR3C1 in exposed infants. NR3C1 methylation associated with infant basal and reactive Cortisol over the first postnatal month | |
| Stroud et al. (2016) | N = 45 mother/infant pairs | Timeline Follow Back interview of maternal smoking during pregnancy | DNA Methylation | Placenta | Degree of methylation at the NR3C1 promoter region - examined via quantitative pyrosequencing using PyroMark MD Pyrosequencing System; infant attention, self-regulation and lethargy | MSDP associated with decreased methylation of NR3C1, decreased infant attention and self-regulation, and increased lethargy and need for examiner soothing over the first postnatal month. No evidence for mediation by NR3C1. | |
| Wilhelm-Benartzi et al. (2012) | N= 479 | Medical record review of alcohol use during pregnancy | DNA Methylation | Placenta | Bisulfite pyrosequencing of the LINE-1 and the AluYb8 repetitive elements; fetal growth | Alcohol exposure was related to methylation; methylation markers were associated with fetal growth | |
| DNA methylation in cord blood | |||||||
| Ivorra et al. (2015) | N = 20; newborns | Maternal self-report of tobacco exposure; blood cotinine levels | DNA Methylation | Cord blood; maternal peripheral venous blood | Infinium Human Methylation 450 BeadChip Infinium Human Methylation 450 BeadChip | In utero tobacco exposure contributes to hypomethylation | |
| Joubert et al. (2012) | N = 1062; newborns | Maternal plasma cotinine for tobacco exposure | DNA Methylation | Cord blood | Differential DNA methylation at epigenome-wide statistical significance (P-value < 1.06 × 10−7) for 26 CpGs mapped to 10 genes. 8 of the 26 were within the coding region of growth factor independent 1 transcription repressor (GFI1) on chromosome 1; 4 were within the coding region of arylhydrocarbon receptor repressor (AHRR) on chromosome 5; 4 were in a region upstream of cytochrome P450 isoform CYP1A1 on chromosome 15; and 4 were within the coding region of myosin 1G (MYOIG). | ||
| Ladd-Acosta et al., (2016) | N= 572, 3–5yr./old | Maternal self-report of tobacco exposure (SEED caregiver interview) | DNA Methylation | Cord blood | Infinium 450 K array | In utero tobacco exposure contributes to differential DNAm signatures | |
| Novakovic et al. (2014) | N = 46 twin pregnancies from The Peri/postnatal Epigenetics Twin Study (PETS) | Maternal self-report of tobacco exposure | DNA Methylation | Cord blood mononuclear cells, buccal epithelium, and placenta tissue | Illumina Human Methylation 450 BeadChip | Association between maternal smoking and AHRR methylation in neonatal blood and plasma cotinine levels; DNA methylation changes are limited to the CpG island shore, and are tissue-specific; intrauterine exposure can induce persistent DNA methylation change for up to 18 mo of age | |
| Breton et al. (2014) | N = 527 children (5–12 yo) from the Childhood Asthma Management Program | Caregiver report of tobacco exposure during pregnancy | DNA Methylation | Cord blood | IIHM 450 K BeadChip | Increased methylation in FRMD4A and Cllorf52 | |
| Guerrero-Preston et al. (2010) | N= 30 | Cord serum cotinine levels indicated tobacco exposure during pregnancy | DNA Methylation | Cord blood | ELISA based commercial kit; Quantifiler Y Human Male DNA quantification Kit | Global DNA methylation were lowest in newborns exposed to tobacco; global DNA methylation is inversely correlation with serum PFOA | |
| Wang et al. (2013) | N= 14 | Cord blood cotinine levels indicated tobacco exposure during pregnancy | DNA Methylation | Cord blood | Illumina Infinium 27 K methylation arrays | Thymic stromal lymphopoietin (TSLP) genes displayed differential methylation, in a separate cohort of children TSLP is association with atopic dermitis | |
| Murphy et al. (2012) | N= 418 | Maternal self-reported tobacco exposure during pregnancy | DNA Methylation | Cord blood | Gentra Puregen Reagents; SQ Assay Design Software; Pyromark Q96 MD Pyrosequencer (Qiagen); 2 U Platinum Taq DNA polymerase; 1 U Platinum Taq DNA polymerase | High methylation at the IGF2 DMR; methylation was most pronounced in male offspring; tobacco related low-birth weight was mediated by aberrant IGF2 methylation differentially for male and female infants | |
| Kupers et al. (2015) | N = 129 Dutch children exposed to maternal smoking vs 126 unexposed; replication with 175 exposed and 1248 unexposed newborns | Maternal self-report of tobacco exposure during pregnancy | DNA Methylation | Cord blood | EZ-96 DNA methylation kit; Infinium HumanMethylation450 BeadChip | Differences in cord blood methylation; reduced birth weight; methylation partially mediated the association between smoking during pregnancy and birthweight | |
| Richmond et al. (2015) | N = 800 mother-offspring pairs | Maternal self-report of tobacco use during pregnancy administered at 18 and 32 weeks of gestation | DNA Methylation | Cord blood | Zymo EZ DNA MethylationTM kit; Infinium HM450 BeadChip; Infinium HM450 BeadChip | Methylation at 15 CpG sites in seven gene regions; samples at 7 and 17 years showed reversibility at some CpG sites of methylation, whereas others showed persistently perturbed patterns; in comparisons between paternal and maternal smoking, maternal associations were consistently stronger | |
| Hoyo et al. (2012) | N = 300 pregnant women | Maternal self-report of tobacco use during pregnancy, verified through medical records | DNA Methylation | Cord blood | Bisulfite pyrosequencing; birth weight | Increase in IGF2 protein concentrations; decrease in methylation levels at the IGF2 DMR; increased IGF2 protein concentrations are related to higher birth weight | |
| Lee et al. (2015) | N = 164 pairs of pregnant women and their spouse | Maternal self-report of alcohol exposure | DNA Methylation | Cord blood | Methylation-specific endonuclease digestion; quantitative real-time polymerase chain reaction | Alcohol exposure during the periconceptional period decreases SERT promoter region methylation and increases MeCP2 promoter region methylation | |
| Sharp et al. (2018) | N = 1147 mothers who consumed alcohol both before and throughout pregnancy and N = 1928 mothers who consumed alcohol before pregnancy/during the first trimester but not during the second and/or third trimester | Maternal self-report during pregnancy | DNA methylation | Cord blood | Bisulfite conversion using the EZ-96 DNA methylation kit. DNA methylation was measured using the Illumina Infinium HumanMethylation450k BeadChip assay at Illumina or in cohort-specific laboratories. | No consistent or strong evidence of differential methylation after correcting for multiple testing. | |
| DNA methylation in other tissues | |||||||
| Terry et al. (2008) | N= 262 | Maternal self-report of tobacco exposure during pregnancy | DNA Methylation | White blood cells | Genomic hypomethylation; blood collection | Tobacco exposure is significantly related to overall levels of DNA methylation | |
| Flom et al. (2011) | N = 90 women born between 1959 and 1963 in New York City | Maternal self-report of tobacco exposure | DNA Methylation | Blood | EZ DNA Methylation Kit (Zymo Research) | Tobacco exposure is related to DNA methylation in adulthood | |
| Toledo-Rodriguez et al. (2010) | N = 156 adolescents from the Saguenay Youth Study | Maternal retrospective report of tobacco use during pregnancy | DNA Methylation | Blood | Bisulfite sequencing of DNA | Tobacco exposure is associated with methylation in the BDNF-6 exon | |
| Breton et al. (2009) | N = 348 children participating in the Children's Health Study | Parent/guardian report of tobacco exposure during pregnancy | DNA Methylation | Buccal Cells | EZ-96 DNA Methylation-Gold Kit; PURGENE DNA isolation Kit; MGB probes on an ABI PRISM 7700 Sequence Detector | Lower methylation of AluYb8; increased methylation of AXL and PTPRO; Differential tobacco-related effects on LINE1 methylation in children with the common GSTM1 null genotype. | |
| Markunas et al. (2014) | N = 889 infants shortly after delivery | Maternal self-report of tobacco use 3–4 months after delivery | DNA Methylation | Whole Blood | Illumina HumanMethylation450 BeadChip | 185 CpGs with altered methylation | |
| Laufer et al. (2015) | N = 6 children (3–6 years old) with FASD | Clinically diagnosed with FASD by J Kapalanga in Ontario, Canada | DNA Methylation | Cheek Swabs | QIAamp DNA Mini Kit; Illiumina HumanMethylation450 BeadChip | Increased methylation in genes: expressed in nervous system, mediate cell-to-cell interactions, related to transmission of glutamate, and that are associated with the growth of some stem cell precursors that control organ size | |
| Portales- Casamar. et al. (2016) | N = 110 children with confirmed fetal alcohol syndrome and 96 controls | Confirmed fetal alcohol syndrome disorder (FASD) diagnosis | DNA Methylation | Saliva samples; Buccal epithelial cells | Illumina HumansMethylation450 array; Infinium HumanOmni2.5-Quad vl.0 BeadChip | 658 differentially methylated sites between FASD cases and controls; 41% displaying differences in percent methylation change > 5% | |
5.1. DNA methylation and the placenta
The placenta is one of the most important functional organs that supports the development of the fetus. In fact, it has been considered an important and accessible record or history of prenatal events (Maccani and Marsit, 2009). It not only protects the fetus, but it also provides nutrients, assists in waste transfer, and secretes hormones. These hormones help to regulate the stages of pregnancy and protect the fetus from harmful exposures, such as substance use during pregnancy (Sood et al., 2006). Finally, placental gene expression, which is regulated by epigenetic markers, can also be affected by environmental insults, such as drugs, pollution and maternal stress (Guo et al., 2008; Sood et al., 2006). Placental DNA methylation patterns may serve as mechanistic links between prenatal exposures, altered gene expression, and adverse outcomes across development (Maccani and Maccani, 2015).
5.1.1. Maternal smoking during pregnancy
Both epigenome-wide association studies (EWAS) and gene-specific methylation studies have yielded significant associations between maternal smoking during pregnancy (SDP) and placental methylation patterns (see Table 2 and Maccani and Maccani (2015) for a comprehensive review of genes in which one or more CpG sites show differential methylation associated with SDP). In short, epigenome-studies of placental tissue have suggested association between SDP and methylation of genes involved in (i) cellular differentiation and development in neuronal cells, and (ii) normal brain development, synapse formation, and proliferation of various cell types in the central nervous system (Maccani and Maccani, 2015). Candidate gene studies yielded associations between SDP and methylation of genes involved (i) in the metabolism of the potentially carcinogenic compounds found in cigarette smoke (Suter et al., 2010), (ii) the serotonergic and glucocorticoid systems (e.g., Paquette et al., 2013; Stroud et al., 2016).
5.1.2. Maternal alcohol use during pregnancy
While numerous animal models have suggested that alcohol exposure is linked to methylation differences in placental tissue (e.g., see Bekdash et al., 2014; Haycock, 2009; Mead and Sarkar, 2014; Varadinova and Boyadjieva, 2015 for reviews), studies examining these effects in humans are exceedingly rare perhaps because of (i) the difficulty of recruiting large samples of mothers who do and do not drink during pregnancy prospectively, (ii) the reluctance to admit alcohol use during pregnancy, and (iii) obtaining placental tissue samples at or after birth. Only one study was identified that examines alcohol use during pregnancy and methylation differences in repetitive elements (Wilhelm-Benartzi et al., 2012). However, in this study alcohol use during pregnancy was very rare (3 cases compared with 377 controls reporting no alcohol use during pregnancy). Thus, future work is needed before the reliability of this finding can be ascertained.
5.2. DNA methylation in cord blood
5.2.1. Maternal smoking during pregnancy
Epigenome-wide association studies using cord blood as the tissue of interest have also been conducted and suggest that prenatal smoke exposure may alter the epigenome resulting in global DNA hypo-methylation (when considering all CpG sites across the genome; Ivorra et al., 2015). In one of the largest EWAS studies to date, Joubert et al. (2012) screened 1062 newborn cord blood samples and found significant methylation changes at four genes (see Table 1). Similar patterns of methylation changes associated with prenatal smoke exposure were also recently found in an independent sample of 3–5 year old children, suggesting that prenatal-exposure driven methylation changes persist and are still detectable in later childhood (Ladd-Acosta et al., 2016). Specific interest has been paid to a gene (AHRR) that is involved in the detoxification of chemicals found in tobacco smoke (i.e., the xenobiotic response) and which also acts as a feedback inhibition modulator of the aryl hydrocarbon receptor (AHR). AHRR exerts its effects by competing with AHR for binding with its related nuclear dimer partner, AHR nuclear transporter (Haarmann-Stemmann et al., 2007). Both roles play a pivotal role in AHR regulation and may be involved in altered immune function (Opitz et al., 2011). Joubert et al. (2012) found the methylation levels of AHRR cg05575921decreased with cotinine in a dose-dependent manner. All other statistically significant AHRR CpGs had lower methylation with increasing cotinine levels except for cg23067299, which is upstream of the other significant AHRR CpGs and had higher methylation with increasing cotinine (Joubert et al., 2012). Lower methylation may be a cellular response to the presence of the chemicals found in cigarette smoke, resulting in higher expression of this gene (Novakovic et al., 2014). Four additional studies of AHRR in adult smokers have linked a decrease in methylation to smoking exposure in multiple tissues: lymphoblasts and alveolar macrophages (Monick et al., 2012), whole blood (Shenker et al., 2013; Zeilinger et al., 2013), and lymphocytes (Philibert et al., 2012). Recent efforts replicated the finding of decreased AHRR methylation in cord blood, but did not find similar results in placental tissue or saliva cells (Novakovic et al., 2014). This is not surprising given that research suggests that AHHR is expressed at low levels in the placenta while cord blood cells show high interindividual variation (Yamamoto et al., 2004). One particular challenge with AHRR is that this gene contains at least 3 large CpG islands that are interspersed throughout the gene and at least 11 AHRR transcripts, each of which codes for a differently sized protein that may have unique competitive properties with respect to AHR (Monick et al., 2012). Given this epigenetic complexity, considerably more work examining specific splice variants altered by smoking is warranted. Taken together, these findings highlight the importance of looking across tissue types and understanding the level of gene expression in various tissues.
5.2.2. Maternal alcohol use during pregnancy
To date, there has been limited and inconsistent work in humans examining alcohol use during pregnancy and epigenetic changes in offspring cord blood. Studies have investigated cord blood in infants for methylation changes in genes involved in the dopaminergic pathway, the serotonergic pathway, and genes which play key roles in controlling fetal growth and metabolism after exposure to drinking during pregnancy (Lee et al., 2015). Depending on which gene was considered, there was evidence of both increased and decreased methylation in infants whose mothers reported drinking up to 14 drinks/week while pregnant. Interestingly, there was also evidence of decreased methylation in the dopaminergic pathway in infants whose fathers reported heavy binge drinking (5+ drinks per occasion), suggesting that alcohol exposure pre-conception may also exert influence on epigenetic changes in offspring that can affect fetal and postnatal development (Lee et al., 2015).
However, a more recent meta-analysis, including data from six independent cohorts within the Pregnancy and Childhood Epigenetics consortium, examined the effects of sustained exposure during pregnancy on DNA methylation in cord blood (Sharp et al., 2018). The analyses incorporated 3075 mother-child pairs (N = 1147 mothers who consumed alcohol both before pregnancy and during the second and third trimesters versus N =1928 mothers who consumed alcohol before pregnancy or during the first trimester but not during the second and third trimester). Despite the larger overall sample size, a higher prevalence of alcohol use during pregnancy, and various ways of defining alcohol use during pregnancy (i.e., including dose and timing of exposure), there was no consistent or strong evidence for an association between sustained alcohol use and methylation changes in cord blood at either individual level CpG sites or larger genomic regions (Sharp et al., 2018).
5.3. DNA methylation in other tissues
5.3.1. Maternal smoking during pregnancy
There are a handful of studies that have examined the effects of SDP on DNA methylation in tissues other than placental cells and umbilical cord blood (see Table 2). For example, Terry et al. (2008) analyzed DNA methylation profiles in leukocyte, or white blood cell, DNA in a multiethnic birth cohort from New York City. Multivariable models indicated that overall levels of DNA methylation were significantly associated with maternal smoking during pregnancy and a number of other covariates. Flom et al. (2011) also used this same birth cohort to show that SDP is associated with decreased methylation of certain repetitive elements in adult offspring (mean age 43) who had been exposed prenatally. These data suggest that exposures experienced throughout the course of life – from fertilization onward – may be associated with DNA methylation in adulthood, although replication is needed. Longitudinal studies capable of measuring within-individual changes in DNA methylation in a variety of tissues over time will yield important data informative of the intragenerational plasticity of DNA methylation (Knopik et al., 2012).
5.3.2. Maternal alcohol use during pregnancy
Examining methylation differences from buccal swab (i.e., cheek cell) samples, it has been shown that youth (aged 3–6 years; Laufer et al., 2015) with fetal alcohol spectrum disorder (FASD) had increased methylation in genes that (i) are expressed in the developing nervous system, (ii) mediate cell-to-cell interactions, (iii) are related to the synaptic transmission of glutamate (the most abundant excitatory neurotransmitter in the nervous system), and (iv) play roles in the regulation of the growth of some stem cell precursors and for controlling organ size, when compared to matched controls who were not exposed to alcohol during pregnancy. These findings were replicated in two independent samples of FASD cases and controls, and mapped onto results from the examination of mouse brains that were subjected to neurodevelopmental alcohol exposure (Laufer et al., 2015). More recently, Portales-Casamar et al. (2016) corroborated some of these findings when examining children (aged 5–18) with and without FSAD. Results of this more recent study also found altered methylation patterns within genes related to the immune response. Specifically, differentially methylated regions were found in the body of the HLA-DPB1 gene, an HLA class II histocompatibility antigen, and in the body of the ITGAL gene (integrin alpha L chain). Altered methylation patterns between FASD cases and controls were also found in genes involved in the stress response (e.g., in the 1st exon and 5′ UTR region of UCN3, an antagonist of the CRF type 2 receptor). Further, results held up to sensitivity analyses suggesting that DNA methylation is altered in patients with FASD, but other factors (age, sex, medication history) should also be considered (Laufer et al., 2015).
6. Implications and future directions
There is a great degree of interest in better understanding the biological pathways and mechanisms underlying the reported associations between maternal substance use during pregnancy and later developmental and behavioral outcomes, including child and adolescent externalizing behavior and substance use. However, investigations considering epigenetic pathways as a causal mechanism are only beginning to emerge. In general, research to date suggests effects of prenatal substance use across specific biologically relevant epigenetic systems; however, the literature is still in its infancy. All reviewed findings are in need of further replication. Further, as observed by the review above and in Table 2, very few human studies have examined behavioral outcomes in the offspring, particularly during childhood and adolescence. Thus, while there is emerging evidence to suggest that prenatal smoke and alcohol exposure result in epigenetic alterations, the role of these epigenetic changes in manifesting later behavioral problems during childhood and adolescence remains unclear (see Fig. 1). Taking the work of animal researchers into account, certain developmental and behavioral deficits consistent with externalizing behaviors, including ADHD, conduct disorder, and substance use are possible within the context of the human condition. However, as also noted, humans are increasingly complex and the ability to disentangle genetic, epigenetic, prenatal and postnatal/familial environmental effects on child and adolescent behavior is in many ways, a daunting task.
Fig. 1.
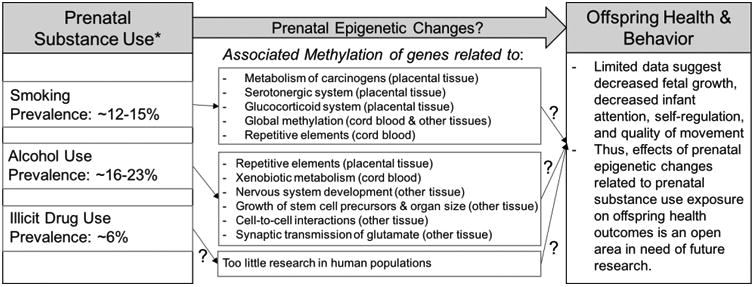
Prenatal substance use exposure, DNA methylation, and offspring health and behavior outcomes – summary of human studies. *Prevalence of prenatal exposure from Tong et al. (2013) and United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center forBehavioral Health Statistics and Quality (2015).
Nonetheless, an important goal of this epigenetic work is to delineate mechanistic pathways by which prenatal exposures can increase risk for adverse health and behavioral outcomes. However, there is variability across studies and debate in the field in terms of the nature of the effects of prenatal exposures (Slotkin, 2013). Carefully designed genetic and epigenetic studies, such as those focused on methylation changes that also account for appropriate confounding variables, can help clarify whether there is a causal role for prenatal exposure on child and adolescent developmental and behavioral outcomes. Thus, this kind of mechanistic research is imperative in order to provide meaningful data on the timing and level of exposures that predict poor outcomes and how those early exposures interact with other risk factors in order to glean what information is best to provide to clinicians and social workers on the frontline.
Based on current data, we know enough to say that asking pregnant women to stop using substances during pregnancy is critically important; however, this is unlikely to be enough to wholly improve outcomes for children who continue to be exposed to the myriad of confounding genetic and environmental factors linked with maternal substance use during pregnancy. Thus, expanding findings in order to understand which intervention points can improve offspring health outcomes is critical. It may be that if data suggest a causal effect of prenatal exposure on child and adolescent behavior via methylation changes, these findings can be the basis for developing interventions focused on altering particular epigenetic components in relevant biological pathways. For example, medications that focus on methylation changes and epigenetic processes as treatment targets are already emerging for cancer and psychiatric disease (e.g., Valproate for schizophrenia; Guidotti et al., 2009). Additional data could lead to these types of targeted medication approaches for altering epigenetic processes induced by prenatal exposures and linked with later child and adolescent outcomes. In addition, findings that support a role for familial and other environmental factors in contributing to the associations among prenatal substance exposure and adolescent behavioral problems can be the basis for building interventions that treat the entire maternal and family system. Such comprehensive approaches are key to affect better child outcomes. For example, a mother may struggle in smoking cessation efforts during pregnancy in part due to a co-occurring mental illness, which also requires treatment and attention. However, given the nascency of the field and mixed and complicated findings, the clinical implications of epigenetic work are speculative. Below we outline some critical steps for future work in this area.
6.1. Quality data on the effects of exposure specific substances, timing, and quantity
Given that the use of particular substances is correlated (e.g., tobacco use is correlated with alcohol use, cannabis use, and other illicit drug use), it can be hard to design human research protocols that effectively test for effects of specific drugs, resulting in large gaps in the scientific literature. Research efforts in this domain have primarily focused on maternal smoking during pregnancy, while epigenetic research in the field of prenatal alcohol exposure is more limited. In addition, although it is widely reported that in utero cannabinoid exposure is associated with a myriad of neurobehavioral consequences for the individual as both a child and adolescent, including an increased tendency for drug abuse (Jaques et al., 2014), no human studies have directly examined the effects of maternal marijuana use during pregnancy on methylation changes (or any other epigenetic change) in offspring tissue. Similarly, no human studies have examined the effects of maternal illicit drug use during pregnancy on epigenetic changes. Quality data on the effects of specific substances related to timing and quantity of exposure is needed in order to develop better therapeutic targets (i.e., delivering certain interventions during specific points during pregnancy).
6.2. Larger longitudinal studies with a wider range of epigenetic data
Much of the data on epigenetics as a mechanism for linking prenatal exposure to substance use with later developmental outcomes is based on small cross-sectional studies that could be subject to sampling biases. Epigenome wide studies with larger samples and longitudinal tracking of mothers and offspring with carefully defined outcomes will help to clarify findings and relevant biological systems over time.
6.3. Creative designs that appropriately control for confounders
Overall, conducting well-controlled human studies in this area is quite challenging. Because maternal use of a particular substance during pregnancy is often correlated with other risk behaviors and environmental risk factors, pinpointing specific effects, whether genetic, epigenetic or environmental, is often difficult. In addition, effects can be magnified when comparing groups of offspring whose mothers did or did not use substances during their pregnancies without properly dealing with these correlated and confounding risk factors (see Knopik, 2009). Some studies of offspring outcomes have dealt with this issue, for example, by selecting comparison groups consisting of offspring of mothers who have a history of smoking, but who did not smoke during pregnancy, providing a relative index of the risk of smoking during pregnancy for mothers with a history of smoking (Palmer et al., 2016). While this choice is expected to result in a smaller effect size than that of a comparison to offspring of “non-smoking” mothers, it is a more clinically meaningful comparison group (since it would be the rare case that a lifetime non-smoker would start smoking during pregnancy). This approach also provides data on the impact of successfully avoiding and/ or limiting offspring's smoke exposure during pregnancy (i.e., none, early part of pregnancy, versus throughout; Palmer et al., 2016). A similar approach, noted above, is the sibling-comparison design which compares siblings where mothers smoked in one pregnancy but not another in order to control for familial confounds including maternal characteristics. In this type of analysis, a significant within-family association of smoking during pregnancy with child outcomes suggests a potentially causal effect of exposure to smoking during pregnancy (Bidwell et al., 2017; Knopik et al., 2015; Marceau et al., 2017; Micalizzi et al., in press) This design, and others, can also account for the role of maternal and child genetic background (e.g. at the DNA level) and how an individual's genetic variation impacts the degree of possible or probable methylation at particular sites. Other studies have used statistical approaches to control for confounders, such as propensity score matching, that match families on relevant risk factors and attempt to isolate and quantify any specific effects of a prenatal exposure to a particular substance (e.g., Boutwell et al., 2011).
Summary
Despite the difficulty of disentangling risk factors, there are studies suggesting that prenatal substance use exposure may exert a unique environmental influence on later developmental and behavioral outcomes (e.g., O'Brien and Hill, 2014; Richardson et al., 2013; Sonon et al., 2015). Given evidence of lasting effects of epigenetic changes on gene expression in biological systems related to externalizing behavior and substance use (e.g., related to various aspects of brain development, as well as serotonin, dopamine, and glucocorticoid function), epigenetic changes are likely to be an important mechanisms of the influence on prenatal substance use exposure on later behavioral outcomes. Although the literature on epigenetic effects of prenatal substance use exposure is still in its infancy, data suggest the importance of these marks as a potential mechanistic link between maternal substance use and negative offspring outcomes. Sophisticated research designs will hone these findings and could help define specific biological pathways by which substance use during pregnancy can impact risk for adverse neurobehavioral and medical outcomes later in life. Eventually, identifying specific epigenetic changes that lead to developmental outcomes and/or impact disease risk is likely to advance prevention and intervention efforts at the individual and family level.
Footnotes
Transparency document: The http://dx.doi.org/10.1016/j.ntt.2018.01.009 associated with this article can be found, in the online version.
References
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, Heath AC, Madden PA. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine Tob Res. 2008;10(4):567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- Allis CD, Caparros ML, Jenuwein T, Reinberg D, Lachner M. Epigenetics. 2. Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- Appleton AA, Armstrong DA, Lesseur C, Lee J, Padbury JF, Lester BM, Marsit CJ. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS One. 2013;8(9):e74691. doi: 10.1371/journal.pone.0074691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J, Mehls O, Barrios V. Growth and body composition in very young SGA children. Pediatr Nephrol. 2010;25(4):679–685. doi: 10.1007/s00467-009-1432-2. [DOI] [PubMed] [Google Scholar]
- Asimes A, Torcaso A, Pinceti E, Kim CK, Zeleznik-Le NJ, Pak TR. Adolescent binge-pattern alcohol exposure alters genome-wide DNA methylation patterns in the hypothalamus of alcohol-naive male offspring. Alcohol. 2017;60:179–189. doi: 10.1016/j.alcohol.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2(2):105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- Bekdash R, Zhang C, Sarkar D. Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress. Alcohol Clin Exp Res. 2014;38(9):2323–2330. doi: 10.1111/acer.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Marceau K, Brick LA, Karoly HC, Todorov AA, Palmer RH, Heath AC, Knopik VS. Prenatal exposure effects on early adolescent substance use: preliminary evidence from a genetically informed bayesian approach. J Stud Alcohol Drugs. 2017;78(5):789–794. doi: 10.15288/jsad.2017.78.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Boutwell BB, Beaver KM, Gibson CL, Ward JT. Prenatal exposure to cigarette smoke and childhood externalizing behavioral problems: a propensity score matching approach. Int J Environ Health Res. 2011;21(4):248–259. doi: 10.1080/09603123.2010.544032. [DOI] [PubMed] [Google Scholar]
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Siegmund KD, Joubert BR, Wang X, Qui W, Carey V, Nystad W, Haberg SE, Ober C, Nicolae D, Barnes KC, Martinez F, Liu A, Lemanske R, Strunk R, Weiss S, London S, Gilliland F, Raby B. Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One. 2014;9(6):e99716. doi: 10.1371/journal.pone.0099716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry J Ment Sci. 1995;166(5):601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16(3):208–215. doi: 10.1016/s0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- Chhabra D, Sharma S, Kho AT, Gaedigk R, Vyhlidal CA, Leeder JS, Morrow J, Carey VJ, Weiss ST, Tantisira KG, DeMeo DL. Fetal lung and placental methylation is associated with in utero nicotine exposure. Epigenetics. 2014;9(11):1473–1484. doi: 10.4161/15592294.2014.971593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DG, Strachan DP. Health effects of passive smoking-10: summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, Day NL, Larkby C. Alcohol, tobacco and marijuana use among pregnant teenagers: 6-year follow-up of offspring growth effects. Neurotoxicol Teratol. 2002;24(6):703–710. doi: 10.1016/s0892-0362(02)00271-4. 2002 Nov-Dec. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci U S A. 2010;107(39):16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70(8):763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty SP, Grabowski J, Hoffman C, Ng SP, Zelikoff JT. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 2009;14(Suppl. 1):97–101. doi: 10.1080/13547500902965898. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM. The need for more quasi-experimental studies of alcohol consumption during pregnancy. Addiction. 2009;104(8):1278–1279. doi: 10.1111/j.1360-0443.2009.02631.x. discussion 1279-1280. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, Emery RE. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J Child Psychol Psychiatry. 2003;44(8):1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Arch Gen Psychiatry. 2007;64(11):1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Singh AL, Iliadou A, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in sweden. Arch Gen Psychiatry. 2010;67(5):529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyk J, Ramanjam V, Church P, Koren G, Donald K. Maternal methamphetamine use in pregnancy and long-term neurodevelopmental and behavioral deficits in children. J Popul Ther Clin Pharmacol. 2014;21(2):e185–196. [PubMed] [Google Scholar]
- Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol. 2009;65(4):325–330. doi: 10.1007/s00228-008-0609-0. [DOI] [PubMed] [Google Scholar]
- England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, Gould TJ, Hatsukami D, Jensen F, Kandel D, Lanphear B, Leslie F, Pauly JR, Neiderhiser J, Rubinstein M, Slotkin TA, Spindel E, Stroud L, Wakschlag L. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev. 2017;72:176–189. doi: 10.1016/j.neubiorev.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom JD, Ferris JS, Liao Y, Tehranifar P, Richards CB, Cho YH, Gonzalez K, Santella RM, Terry MB. Prenatal smoke exposure and genomic DNA methylation in a multiethnic birth cohort. Cancer Epidemiol Biomark Prev. 2011;20(12):2518–2523. doi: 10.1158/1055-9965.EPI-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A. Substance use during pregnancy. F1000Res. 2016;5 doi: 10.12688/f1000research.7645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, Foster D. Substance use in the perinatal period. Curr Psychiatry Rep. 2015;17(11):91–102. doi: 10.1007/s11920-015-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiat. 2013;70(9):956–963. doi: 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, Apelberg BJ, Hernandez-Roystacher M, Jaffe A, Halden RU, Sidransky D. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5(6):539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci. 2009;30(2):55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Guo L, Choufani S, Ferreira J, Smith A, Chitayat D, Shuman C, Uxa R, Keating S, Kingdom J, Weksberg R. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev Biol. 2008;320(1):79–91. doi: 10.1016/j.ydbio.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Haarmann-Stemmann T, Bothe H, Kohli A, Sydlik U, Abel J, Fritsche E. Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab Dispos. 2007;35(12):2262–2269. doi: 10.1124/dmd.107.016253. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Kingsbury AM, Flenady V, Gilshenan KS, Hutchinson DM, Najman JM. Illicit drug use before and during pregnancy at a tertiary maternity hospital 2000–2006. Drug Alcohol Rev. 2011;30(2):181–187. doi: 10.1111/j.1465-3362.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod. 2009;81(4):607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Ramsay M. Exposure of mouse embryos to ethanol during pre-implantation development: effect on DNA methylation in the h19 imprinting control region. Biol Reprod. 2009;81(4):618–627. doi: 10.1095/biolreprod.108.074682. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Brown MS. Epidemic of prescription opiate abuse and neonatal abstinence. JAMA. 2012;307(18):1974–1975. doi: 10.1001/jama.2012.4526. [DOI] [PubMed] [Google Scholar]
- Hoyo C, Fortner K, Murtha AP, Schildkraut JM, Soubry A, Demark-Wahnefried W, Jirtle RL, Kurtzberg J, Forman MR, Overcash F, Huang Z, Murphy SK. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control. 2012;23(4):635–645. doi: 10.1007/s10552-012-9932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ergui I, Young JI. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol Psychiatry. 2015;20(2):232–239. doi: 10.1038/mp.2014.7. [DOI] [PubMed] [Google Scholar]
- Ivorra C, Fraga MF, Bayon GF, Fernandez AF, Garcia-Vicent C, Chaves FJ, Redon J, Lurbe E. DNA methylation patterns in newborns exposed to tobacco in utero. J Transl Med. 2015;13:25. doi: 10.1186/s12967-015-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar S, Chastain LG, Gangisetty O, Cabrera MA, Sochacki K, Sarkar DK. Preconception alcohol increases offspring vulnerability to stress. Neuropsychopharmacology. 2016;41(11):2782–2793. doi: 10.1038/npp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet. 1997;13(8):323–329. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Strait LB, Odgers CL. From correlates to causes: can quasi-experimental studies and statistical innovations bring us closer to identifying the causes of antisocial behavior? Psychol Bull. 2012;138(2):272–295. doi: 10.1037/a0026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januar V, Desoye G, Novakovic B, Cvitic S, Saffery R. Epigenetic regulation of human placental function and pregnancy outcome: considerations for causal inference. Am J Obstet Gynecol. 2015;213(4):S182–S196. doi: 10.1016/j.ajog.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Jaques S, Kingsbury A, Henshcke P, Chomchai C, Clews S, Falconer J, Abdel-Latif M, Feller J, Oei J. Cannabis, the pregnant woman and her child: weeding out the myths. J Perinatol. 2014;34(6):417–424. doi: 10.1038/jp.2013.180. [DOI] [PubMed] [Google Scholar]
- Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, Huang Z, Hoyo C, Midttun O, Cupul-Uicab LA, Ueland PM, Wu MC, Nystad W, Bell DA, Peddada SD, London SJ. 450 K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120(10):1425–1431. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya X, Manzano C, Alvarez AT, Mercadal M, Torres F, Salat-Batlle J, Garcia-Algar O. Transgenerational exposure to environmental tobacco smoke. Int J Environ Res Public Health. 2014;11(7):7261–7274. doi: 10.3390/ijerph110707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddar T, Rouault JP, Chien WW, Chebel A, Gadoux M, Salles G, Ffrench M, Magaud JP. Two new miR-16 targets: caprin-1 and HMGA1, proteins implicated in cell proliferation. Biol Cell. 2009;101(9):511–524. doi: 10.1042/BC20080213. [DOI] [PubMed] [Google Scholar]
- Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev. 1992;6(5):705–714. doi: 10.1101/gad.6.5.705. [DOI] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, Whitelaw E, Chong S. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6(1):e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Park JH, Choi CS, Choi I, Joo SH, Kim MK, Kim SY, Kim KC, Park SH, Kwon KJ, Lee J, Han SH, Ryu JH, Cheong JH, Han JY, Ko KN, Shin CY. Effects of ethanol exposure during early pregnancy in hyperactive, inattentive and impulsive behaviors and MeCP2 expression in rodent offspring. Neurochem Res. 2013;38(3):620–631. doi: 10.1007/s11064-012-0960-5. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34(1):462593. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, McLaughlin TL, Todorov A, Todd RD, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006;36(10):1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Jacob T, Haber JR, Swenson LP, Howell DN. Paternal alcoholism and offspring ADHD problems: a children of twins design. Twin Res Hum Genet. 2009;12(1):53–62. doi: 10.1375/twin.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 2012;24(4):1377–1390. doi: 10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Marceau K, Palmer RH, McGeary JE, Todorov A, Schettini Evans A. Missouri Mothers and Their Children: a family study of the effects of genetics and the prenatal environment. Twin Res Hum Genet. 2015;18(5):485–496. doi: 10.1017/thg.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Marceau K, Bidwell LC, Palmer RH, Smith TF, Todorov A, Evans A, Heath AC. Smoking during pregnancy and ADHD risk: a genetically informed, multiple rater approach. Am J Med Genet B Neuropsychiatr Genet. 2016a;107(7):971–981. doi: 10.1002/ajmg.b.32421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Marceau K, Palmer RH, Smith TF, Heath AC. Maternal smoking during pregnancy and offspring birth weight: a genetically-informed approach comparing multiple raters. Behav Genet. 2016b;46(3):353–364. doi: 10.1007/s10519-015-9750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Halkola R, D'Onofrio BM, Larsson H, Lichtenstein P. Maternal smoking during pregnancy and adverse outcomes in offspring: genetic and environmental sources of covariance. Behav Genet. 2014;44(5):456–467. doi: 10.1007/s10519-014-9668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers LK, Xu X, Jankipersadsing SA, Vaez A, la Bastide-van Gemert S, Scholtens S, Nolte IM, Richmond RC, Relton CL, Felix JF, Duijts L, van Meurs JB, Tiemeier H, Jaddoe VW, Wang X, Corpeleijn E, Snieder H. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol. 2015;44(4):1224–1237. doi: 10.1093/ije/dyv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C. Epigenetic signatures as biomarkers of exposure. Curr Environ Health Rep. 2015;2(2):117–125. doi: 10.1007/s40572-015-0051-2. [DOI] [PubMed] [Google Scholar]
- Ladd-Acosta C, Shu C, Lee BK, Gidaya N, Singer A, Schieve LA, Schendel DE, Jones N, Daniels JL, Windham GC, Newschaffer CJ, Croen LA, Feinberg AP, Daniele Fallin M. Presence of an epigenetic signature of prenatal cigarette smoke exposure in childhood. Environ Res. 2016;144(Pt A):139–148. doi: 10.1016/j.envres.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer BI, Kapalanga J, Castellani CA, Diehl EJ, Yan L, Singh SM. Associative DNA methylation changes in children with prenatal alcohol exposure. Epigenomics. 2015;7(8):1259–1274. doi: 10.2217/epi.15.60. [DOI] [PubMed] [Google Scholar]
- Lee BY, Park SY, Ryu HM, Shin CY, Ko KN, Han JY, Koren G, Cho YH. Changes in the methylation status of DAT, SERT, and MeCP2 gene promoters in the blood cell in families exposed to alcohol during the periconceptional period. Alcohol Clin Exp Res. 2015;39(2):239–250. doi: 10.1111/acer.12635. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiao D, Yang S, Zhang L. Promoter methylation represses AT2R gene and increases brain hypoxic-ischemic injury in neonatal rats. Neurobiol Dis. 2013;60:32–38. doi: 10.1016/j.nbd.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani JZ, Maccani MA. Altered placental DNA methylation patterns associated with maternal smoking: current perspectives. Adv Genomics Genet. 2015;2015(5):205–214. doi: 10.2147/AGG.S61518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol. 2009;62(2):78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani JZ, Koestler DC, Houseman EA, Marsit CJ, Kelsey KT. Placental DNA methylation alterations associated with maternal tobacco smoking at the RUNX3 gene are also associated with gestational age. Epigenomics. 2013;5(6):619–630. doi: 10.2217/epi.13.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Hajal N, Leve LD, Reiss D, Shaw DS, Ganiban JM, Mayes LC, Neiderhiser JM. Measurement and associations of pregnancy risk factors with genetic influences, postnatal environmental influences, and toddler behavior. Int J Behav Dev. 2013;37(4):366–375. doi: 10.1177/0165025413489378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, De Arujo-Greecher M, Miller ES, Massey SH, Mayes LC, Ganiban JM, Reiss D, Shaw DS, Leve LD, Neiderhiser JM. The Perinatal Risk Index: early risks experienced by domestic adoptees in the united states. PLoS One. 2016a;11(3):e0150486. doi: 10.1371/journal.pone.0150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Palmer RH, Neiderhiser JM, Smith TF, McGeary JE, Knopik VS. Passive rGE or developmental gene-environment cascade? An investigation of the role of xenobiotic metabolism genes in the association between smoke exposure during pregnancy and child birth weight. Behav Genet. 2016b;46(3):365–377. doi: 10.1007/s10519-016-9778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Bidwell L, Cinnamon, Karoly HC, Evans AS, Todorov AA, Palmer RH, Heath AC, Knopik VS. J Abnorm Child Psychol. 2017. Within-family effects of smoking during pregnancy on ADHD: the importance of phenotype; pp. 1–15. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjonen H, Sierra A, Nyman A, Rogojin V, Grohn O, Linden AM, Hautaniemi S, Kaminen-Ahola N. Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLoS One. 2015;10(5):e0124931. doi: 10.1371/journal.pone.0124931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markunas CA, Xu Z, Harlid S, Wade PA, Lie RT, Taylor JA, Wilcox AJ. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2014;122(10):1147–1153. doi: 10.1289/ehp.1307892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EA, Sarkar DK. Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms. Front Genet. 2014;5:154. doi: 10.3389/fgene.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi L, Marceau K, Brick LA, Palmer RH, Todorov AA, Heath AC, Evans A, Knopik VS. Inhibitory control in siblings discordant for exposure to maternal smoking during pregnancy. Dev Psychol. 2017 doi: 10.1037/dev0000423. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev. 2013;12:Cd006318. doi: 10.1002/14651858.CD006318.pub3. [DOI] [PubMed] [Google Scholar]
- Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, Philibert RA. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159b(2):141–151. doi: 10.1002/ajmg.b.32021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99(3):371–382. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494(1):36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum Reprod Update. 2011;17(3):397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- Ngai YF, Sulistyoningrum DC, O'Neill R, Innis SM, Weinberg J, Devlin AM. Prenatal alcohol exposure alters methyl metabolism and programs serotonin transporter and glucocorticoid receptor expression in brain. Am J Physiol Regul Integr Comp Physiol. 2015;309(5):R613–622. doi: 10.1152/ajpregu.00075.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic B, Ryan J, Pereira N, Boughton B, Craig JM, Saffery R. Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics. 2014;9(3):377–386. doi: 10.4161/epi.27248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JW, Hill SY. Effects of prenatal alcohol and cigarette exposure on offspring substance use in multiplex, alcohol-dependent families. Alcohol Clin Exp Res. 2014;38(12):2952–2961. doi: 10.1111/acer.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG Int J Obstet Gynaecol. 2008;115(10):1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, Campo J, Bridge J, Blizinsky K, Dennis K, Vedell P, Churchill GA, Redei EE. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry. 2012;2:e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Bidwell LC, Heath AC, Brick LA, Madden PA, Knopik VS. Effects of maternal smoking during pregnancy on offspring externalizing problems: contextual effects in a sample of female twins. Behav Genet. 2016;46(3):403–415. doi: 10.1007/s10519-016-9779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lesseur C, Armstrong DA, Koestler DC, Appleton AA, Lester BM, Marsit CJ. Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics. 2013;8(8):796–801. doi: 10.4161/epi.25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Beach SR, Brody GH. Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics. 2012;7(11):1331–1338. doi: 10.4161/epi.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portales-Casamar E, Lussier AA, Jones MJ, MacIsaac JL, Edgar RD, Mah SM, Barhdadi A, Provost S, Lemieux-Perreault LP, Cynader MS, Chudley AE, Dube MP, Reynolds JN, Pavlidis P, Kobor MS. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin. 2016;9:25. doi: 10.1186/s13072-016-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Razin A, Shemer R. DNA methylation in early development. Hum Mol Genet. 1995;4 Spec No:1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Reamon-Buettner SM, Mutschler V, Borlak J. The next innovation cycle in toxicogenomics: environmental epigenetics. Mutat Res. 2008;659(1–2):158–165. doi: 10.1016/j.mrrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J Am Acad Child Adolesc Psychiatry. 2013;52(1):37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Smith AD, Timpson NJ, Tilling K, Davey Smith G, Relton CL. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) Hum Mol Genet. 2015;24(8):2201–2217. doi: 10.1093/hmg/ddu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465–472. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp GC, Arathimos R, Reese SE, Page CM, Felix J, Kupers LK, Rifas-Shiman SL, Liu C, Burrows K, Zhao S, Magnus MC, Duijts L, Corpeleijn E, DeMeo DL, Litonjua A, Baccarelli A, Hivert MF, Oken E, Snieder H, Jaddoe V, Nystad W, London SJ, Relton CL, Zuccolo L. Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics. 2018;10(1):27–42. doi: 10.2217/epi-2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker NS, Polidoro S, van Veldhoven K, Sacerdote C, Ricceri F, Birrell MA, Belvisi MG, Brown R, Vineis P, Flanagan JM. Epigenome-wide association study in the European prospective investigation into cancer and nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum Mol Genet. 2013;22(5):843–851. doi: 10.1093/hmg/dds488. [DOI] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, D'Onofrio BM, Lichtenstein P, Larsson H. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry. 2014;55(1):61–68. doi: 10.1111/jcpp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. MAternal smoking and conduct disorder in the offspring. JAMA Psychiat. 2013;70(9):901–902. doi: 10.1001/jamapsychiatry.2013.1951. [DOI] [PubMed] [Google Scholar]
- Smith TF, Schmidt-Kastner R, McGeary JE, Kaczorowski JA, Knopik VS. Prenatal ischemia-hypoxia, the ischemia-hypoxia response pathway, and ADHD risk: a multi-omic perspective. Behav Genet. 2016;46(3):467. doi: 10.1007/s10519-016-9784-4. [DOI] [PubMed] [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol Teratol. 2015;47:10–15. doi: 10.1016/j.ntt.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103(14):5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178:708–713. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann N Y Acad Sci. 2010;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, Lester B, Huestis MA, Niaura R, Padbury JF, Marsit CJ. Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014;48:29–40. doi: 10.1016/j.psyneuen.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Salisbury AL, Phipps MG, Huestis MA, Niaura R, Padbury JF, Marsit CJ, Lester BM. Epigenetic regulation of placental NR3C1: mechanism underlying prenatal programming of infant neurobehavior by maternal smoking? Child Dev. 2016;87(1):49–60. doi: 10.1111/cdev.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metab Clin Exp. 2010;59(10):1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C, Showalter L, Abramovici A, Aagaard-Tillery KM. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6(11):1284–1294. doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Jin P. Integrating DNA methylation dynamics into a framework for understanding epigenetic codes. Bioessays. 2014;36(1):107–117. doi: 10.1002/bies.201300090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2015;64(37):1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, Gamble MV, Susser E. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomark Prev. 2008;17(9):2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Pausova Z, Paus T. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153b(7):1350–1354. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in smoking before, during, and after pregnancy–pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010. Morb Mortal Wkly Rep Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. ICPSR35509-v3. Inter-university Consortium for Political and Social Research [distributor]; Ann Arbor, MI (-11-23): 2015. National Survey on Drug Use and Health, 2013. [DOI] [Google Scholar]
- Varadinova M, Boyadjieva N. Epigenetic mechanisms: a possible link between autism spectrum disorders and fetal alcohol spectrum disorders. Pharmacol Res. 2015;102:71–80. doi: 10.1016/j.phrs.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: final data for 1999. Natl Vital Stat Rep. 2001;49(1):1–100. [PubMed] [Google Scholar]
- Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect. 2015;123(5):399–411. doi: 10.1289/ehp.1408459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24(3):562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Chen SL, Lu TP, Chuang EY, Chen PC. Prenatal smoke exposure, DNA methylation, and childhood atopic dermatitis. Clin Exp Allergy. 2013;43(5):535–543. doi: 10.1111/cea.12108. [DOI] [PubMed] [Google Scholar]
- Warner TD, Roussos-Ross D, Behnke M. It's not your mother's marijuana: effects on maternal-fetal health and the developing child. Clin Perinatol. 2014;41(4):877–894. doi: 10.1016/j.clp.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, Gagne LA, Banister CE, Padbury JF, Marsit CJ. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012;120(2):296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JF, Smith VC. Fetal alcohol spectrum disorders. Pediatrics. 2015;136(5):e1395–1406. doi: 10.1542/peds.2015-3113. [DOI] [PubMed] [Google Scholar]
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Ihara K, Nakayama H, Hikino S, Satoh K, Kubo N, Iida T, Fujii Y, Hara T. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 2004;74(8):1039–1049. doi: 10.1016/j.lfs.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Yang IV, Schwartz DA. Epigenetic mechanisms and the development of asthma. J Allergy Clin Immunol. 2012;130(6):1243–1255. doi: 10.1016/j.jaci.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilinger S, Kuhnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, Strauch K, Waldenberger M, Illig T. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8(5):e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Hou J, Chen B, Shao X, Zhu R, Bu Q, Gu H, Li Y, Zhang B, Du C, Fu D, Kong J, Luo L, Long H, Li H, Deng Y, Zhao Y, Cen X. Prenatal cocaine exposure impairs cognitive function of progeny via insulin growth factor II epigenetic regulation. Neurobiol Dis. 2015;82:54–65. doi: 10.1016/j.nbd.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Enoch MA, Goldman D. Gene expression in the addicted brain. Int Rev Neurobiol. 2014;116:251–273. doi: 10.1016/B978-0-12-801105-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


