Abstract
The hemodynamic response function (HRF), a model of brain blood-flow changes in response to neural activity, reflects communication between neurons and the vasculature that supplies these neurons in part by means of glial cell intermediaries (e.g., astrocytes). Intact neural-vascular communication might play a central role in optimal cognitive performance. This hypothesis can be tested by comparing healthy individuals to those with known white-matter damage and impaired performance, as seen in Multiple Sclerosis (MS). Glial cell intermediaries facilitate the ability of neurons to adequately convey metabolic needs to cerebral vasculature for sufficient oxygen and nutrient perfusion. In this study, we isolated measurements of the HRF that could quantify the extent to which white-matter affects neural-vascular coupling and cognitive performance. HRFs were modeled from multiple brain regions during multiple cognitive tasks using piecewise cubic spline functions, an approach that minimized assumptions regarding HRF shape that may not be valid for diseased populations, and were characterized using two shape metrics (peak amplitude and time-to-peak). Peak amplitude was reduced, and time-to-peak was longer, in MS patients relative to healthy controls. Faster time-to-peak was predicted by faster reaction time, suggesting an important role for vasodilatory speed in the physiology underlying processing speed. These results support the hypothesis that intact neural-glial-vascular communication underlies optimal neural and cognitive functioning.
1. Introduction
The hemodynamic response function (HRF) models the time course of the change in blood flow in response to a brief change in neural activity that is measured with functional magnetic resonance imaging (fMRI; Kwong et al., 1992; Ogawa et al., 1990, 1992). As such, in the healthy system, it is generally considered to be a proxy for underlying neural activity originating in gray-matter. It is known to result from a complex interplay of neurons, glial cell intermediaries (e.g., astrocytes), and blood vessels that constitute the mechanism by which neurons communicate their metabolic needs to blood vessels, and by which blood vessels return oxygen and metabolites to neurons (e.g., Attwell et al., 2010; Cauli and Hamel, 2010; Iadecola, 2017; Lundgaard et al., 2014; Metea & Newman, 2006; Rossi, 2006; Takano et al., 2006). Thus, in healthy young adults, the HRF actually indexes regional displacement of metabolically generated deoxyhemoglobin by the flow of oxyhemoglobin following neural activity in the form of the blood-oxygen-level-dependent (BOLD) signal.
Due to the reliability of the HRF, it assumes a canonical shape in young healthy individuals (see Fig 1). Thus, most fMRI studies utilize a canonical HRF in analysis (see Lindquist et al., 2009). However, it is known to vary considerably in aging and disease (e.g., Bonakdarpour, Parrish, & Thompson, 2007; D'Esposito et al., 1999; D'Esposito, Deouell, & Gazzaley, 2003; Hubbard et al., 2016a; Rypma & D'Esposito, 2001; Zou et al., 2011). Because of the reliance upon neural-vascular communication to produce this canonical shape (e.g., Buxton et al., 2004; Martin et al., 2006), comparisons between healthy individuals and those with known neural-vascular coupling compromise would permit testing of hypotheses regarding the importance of an intact neural-vascular coupling system to optimal neural and cognitive performance.
Figure 1.
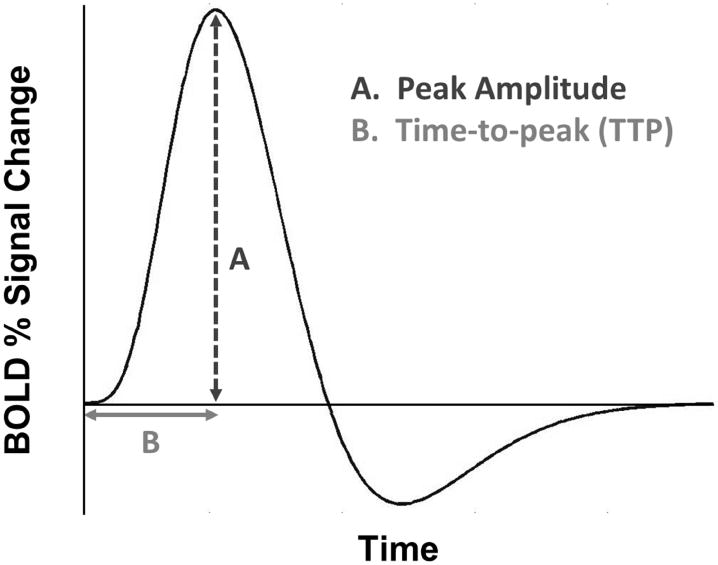
Canonical hemodynamic response function (HRF), a model of the change in blood-oxygen-level-dependent signal through time.
In Multiple Sclerosis (MS), the integrity of the glial cell intermediaries, known to facilitate neural-vascular communication, is damaged (De Keyser et al., 2008; D'Haeseleer et al., 2011; Gareau et al., 1999; Jukkola et al., 2013; Lassmann, 2003, 2014; Lundgaard et al., 2014; Mulligan & MacVicar, 2004; Petzold & Murthy, 2011; Trapp & Nave, 2008; Trapp & Stys, 2009). One potential consequence of this damage is a compromised ability of neurons to adequately convey their metabolic needs to vasculature, resulting in insufficient oxygen and nutrient perfusion (see De Keyser et al., 2008; Debernard et al., 2013; D'Haeseleer et al., 2011). Because reduced white-matter integrity in MS probably disrupts neural-vascular communicating structures, comparisons to healthy individuals can elucidate the roles of these structures in the healthy brain, including in neural-vascular coupling.
Ascertaining the best model of the HRF for group comparisons has been debated in fMRI literature. Several studies have provided evidence for the appropriateness of gamma functions (e.g., Friston et al., 1998; Maus et al., 2012; cf Lindquist & Wager, 2007; Lindquist et al., 2009). However, these models rely on the validity of group-equivalence assumptions regarding HRF shape (e.g., that maximum BOLD signal occurs at a fixed time point after stimulus onset; but see Henson et al., 2002). Neurocognitive aging research, for instance, has demonstrated the consequences of HRF between-groups equivalence assumptions, yielding both false positives and false negatives (Ances et al., 2009; Hutchison et al., 2013a,b; Lindquist et al., 2009; Mohtasib et al., 2012; Pasley et al., 2007; Restom et al., 2007; cf. Aizenstein et al., 2004; Buckner et al., 2006; D'Esposito et al., 1999, Huettel, Singerman, & McCarthy, 2001). Canonical HRF modeling may not be any more appropriate in MS-healthy comparisons than in young-old comparisons. Greater variation in underlying hemodynamic systems seen in groups such as older adults (Hutchison et al., 2013a,b; Tsvetanov et al., 2015) and MS patients (DeLuca et al., 2008; Pantano et al., 2005; Rocca et al., 2002; Wegner et al., 2008; cf. Genova et al., 2009; Hubbard et al., 2016a; Lee et al., 2000; White et al., 2009) challenges the assumptions necessary for use of canonical HRFs in group comparison studies. In this way, HRFs derived from the use of a canonical function could be biased in their characterization of the BOLD signal response (cf. Calhoun et al., 2004; Handwerker, Ollinger, & D'Esposito, 2004).
Such bias could underlie the diversity of results that have been observed in BOLD-fMRI of MS. Some studies have observed MS-related increases in BOLD signal of motor cortex during finger tapping (e.g., Pantano et al., 2005; Rocca et al., 2002; Wegner et al., 2008) and prefrontal cortex during a processing speed task (e.g., DeLuca et al., 2008), while others have observed MS-related decreases in motor cortex BOLD signal during finger tapping (e.g., Hubbard et al., 2016a; Lee et al., 2000; White et al., 2009) and prefrontal cortex during a processing speed task (e.g., Genova et al., 2009). Increases in BOLD signal have been suggested to reflect adaptive reorganization (e.g., Bonnet et al., 2010; Kern et al., 2012), but the underlying mechanisms remain poorly understood.
One solution to this problem involves HRF modeling techniques that minimize shape assumptions, such as spline interpolation (Carew et al., 2003; Gibbons et al., 2004; see also Glover, 1999; Gouette, Nielsen, and Hansen, 2000; Wink, Hoogduin, & Roerdink, 2008). Instead of fitting a single pre-conceived function to measured data points by minimizing squared error, the spline interpolation method used in this study modulates the shape of a set of (polynomial) basis functions to smoothly connect the measured data points (called knots) in piecewise fashion with the overall curvature of the entire set of functions minimized (Ramsay, 2006).
MS-related damage to white-matter structures might cause neural dysconnectivity and vascular change (Bonzano et al., 2009; Dineen et al., 2009). Law and colleagues (2004), for instance, showed reduced cerebral blood flow and prolonged transit time in MS patients' white-matter. Inability of astrocytes to mediate vasodilation in MS leads to neural-vascular communication deficits in and around active transient lesions (Carmignoto & Gómez-Gonzalo, 2010; De Keyser et al., 2008; Metea & Newman, 2006). Blood flow changes at transient-lesion sites persist after exacerbation resolution (Ge et al., 2005; Haselhorst et al., 2000). Thus, transient lesions leave in their wake white-matter dysfunction, resulting in disruption of cortical transmission necessary for efficient cognition, vascular-dependent cell metabolism, and the magnetic signature vital to fMRI known as the BOLD signal.
Cerebral vascular dynamics are known to be altered in MS (Brooks et al., 1984; Lycke et al., 1993; Mulholland et al., 2017; Rashid et al., 2004; Sun et al., 1998; Swank, Roth, & Woody, 1983). However, based on what is known about MS neuropathology, these dynamics probably reflect microstructural damage to glial cell intermediaries in white (i.e., fibrous astrocytes) and gray matter (i.e., protoplasmic astrocytes). Global reductions in perfusion of oxygen and metabolites (De Keyser et al., 2008; Debernard et al., 2013) probably result from astrocyte dysfunction (e.g., Blanco, Stern, & Filosa, 2008; Brosnan & Raine, 2013; De Keyser et al., 1999; D'Haeseleer et al., 2011; Takano et al., 2006). One study (Marshall et al., 2014) has shown MS-related cerebrovascular reactivity reductions, but this phenomenon probably results from chronic vasodilation secondary to elevated nitric oxide concentration in cerebral tissue (e.g., Brown 2007, 2010; Brown & Bal-Price, 2003; Brown & Borutaite 2002; Su et al., 2009).
Optimal cognitive performance could depend on the integrity of the neural-glial-vascular system. Studies employing increased oxygen availability (i.e., hyperoxia) have demonstrated that increases in perfusion (mediated by intact neural-glial-vascular function) are associated with decreases in neural activity (Xu et al., 2012) and improvements in cognitive performance (Chung et al., 2006). Such relationships implicate neural efficiency as a mechanism underlying processing speed, the speed with which an individual can execute elementary cognitive operations (Rypma et al., 2006; Salthouse, 1992). Cognitive slowing is the most commonly observed neuropsychological deficit in MS patients, and is primarily indexed by processing speed measures such as the Digit Symbol Substitution Task (DSST; Strober et al., 2014). Variation in this basic ability is thought to mediate higher-order cognitive functions (e.g., working memory and reasoning; Ackerman et al., 2002; Rypma et al., 2006; Rypma & D'Esposito, 1999; Rypma & Prabhakaran, 2009; Salthouse, 1996; Vernon, 1983). Thus, comparisons between MS patients and healthy controls could elucidate the role of neural-glial-vascular function in processing speed.
In this study, we utilized a modeling approach not dependent on the validity of shape assumptions to quantify differences between a healthy group and one with known neural-vascular compromise that probably affects the canonical shape of their HRF (i.e., MS patients; Hubbard et al., 2016a). Thus, we tested two hypotheses. The first was that HRF shape metrics (as measured by peak amplitude and time-to-peak) will differ between MS patients and controls. The second was that these HRF shape metrics will be more associated with processing speed in MS patients than in controls. On one hand, the finding that multiple HRF metrics account for variance in task performance would indicate widespread disruption of the neural-glial-vascular system. The finding of a single HRF metric accounting for this variance, on the other hand, would isolate a relationship between a specific component of the neural-glial-vascular system and cognition. Our findings suggest that the canonicality of the HRF indexes the health of the neural-glial-vascular system necessary for optimal cognitive performance. Thus, a canonical HRF reflects a healthy neural-vascular coupling system, critical to supporting neural function. Deviations from canonicality, and their relationships to performance, may index the extent to which the integrity of this system is compromised.
2. Methods
2.1. Participants
A total of fifty-five participants were enrolled in this study. Twenty-five healthy controls were recruited from the greater Dallas-Fort Worth Metroplex, and thirty relapsing-remitting MS patients were recruited from the University of Texas Southwestern Medical Center (UTSW) Clinical Center for Multiple Sclerosis. All participants provided informed written consent prior to scanning, and all were compensated financially for their participation. Procedures were jointly approved by Institutional Review Boards of both UTD and UTSW.
Participant recruitment was designed to age- and sex-match controls to patients, and neither attribute was significantly different between groups (see Table 1). All participants had normal or corrected-to-normal vision (one patient did report a previous history of optic neuritis, but vision was normal at scan time). Patients had neither experienced an exacerbation nor had been treated with corticosteroids for at least one month prior to scanning. Eighty-two percent of patients indicated a history of immunomodulatory therapy (i.e., interferon beta, glatiramer acetate, and/or natalizumab). Average time from initial diagnosis for MS patients was 153.19 months (SEM = 14.72; n = 27). Several participants were excluded for the following reasons: use of a psychostimulant prior to the fMRI scan (one MS patient), history of taking medication for seizures (one healthy control), and a failure to align functional MRI data with a standardized brain template (one healthy control, one MS patient; see section 2.4). Data analysis was then possible for the remaining fifty-one participants (nPatient = 28; nControl = 23).
Table 1.
Participant demographics, neuropsychometric performance, and MS disease measures.
| Characteristic | Healthy controls (n = 23) | MS patients (n = 28) | p-value |
|---|---|---|---|
| Age, mean (SEM) | 42.13 (2.56) | 47.36 (2.04) | n.s. |
| Sex, n (%) | |||
| Male | 6 (26.09%) | 5 (17.86%) | - |
| Female | 17 (73.91%) | 23 (82.14%) | n.s. |
| Handedness | |||
| Right | 23 (100%) | 28 (100%) | - |
| SDMT, mean correct (SEM) | 58.26 (1.44) | 51.88 (2.52) | 0.046 |
| PASAT, mean correct (SEM) | 49.96 (1.69) | 45.84 (2.28) | 0.176 |
| Box completion, mean correct (SEM) | 55.65 (2.04) | 46.60 (2.41) | 0.009 |
| Trails A, mean RT (SEM) | 21.07 (1.09) | 27.51 (2.04) | 0.013 |
| Trails B, mean RT (SEM) | 40.98 (1.95) | 64.99 (9.43) | 0.028 |
| EDSS, mean score (SEM) | - | 2.78 (0.35) | - |
| MFIS, mean score (SEM) | - | 28.65 (2.49) | - |
| Lesion burden, mean volume in mm³ (SEM) | - | 15264.60 (2521.91) |
2.2. Experimental Paradigm
Participants underwent fMRI scanning during performance of two tasks. The first was a simple and commonly-employed sensorimotor button-press task (BPT). An event-related, fixed-paced experimental design was used to minimize the effects of differences in RT between groups on the HRF. Stimuli presentations were broken up by jittered rest periods of durations of 14±1 seconds, in which a white fixation cross was displayed on-screen. In this event-related paradigm, participants were instructed to press thumb-buttons bilaterally and simultaneously as rapidly as possible after onset of a radial black-and-white checkerboard flickering at 6 Hz for 500 ms. There were 20 trials in total.
The second task involved a version of the Digit Symbol Substitution Task (DSST), modified for use in the fMRI environment (Rypma et al., 2006). In each trial, participants viewed a key of nine digit-symbol pairs and one probe digit-symbol pair for 4000 ms (see Fig 2). Participants were asked to indicate as quickly and accurately as possible via left- or right-thumb button-press whether the probe digit-symbol pair matched one of the digit-symbol pairs in the key. Inter-trial intervals were jittered at 0, 2, 4, and 6 second intervals. Accuracy and reaction time (RT) were recorded. RT was calculated for both groups only for correct responses. Further, as a measure of external validity, we examined processing speed performance outside of the fMRI environment. There were 225 total trials across three runs (75 trials per run).
Figure 2.
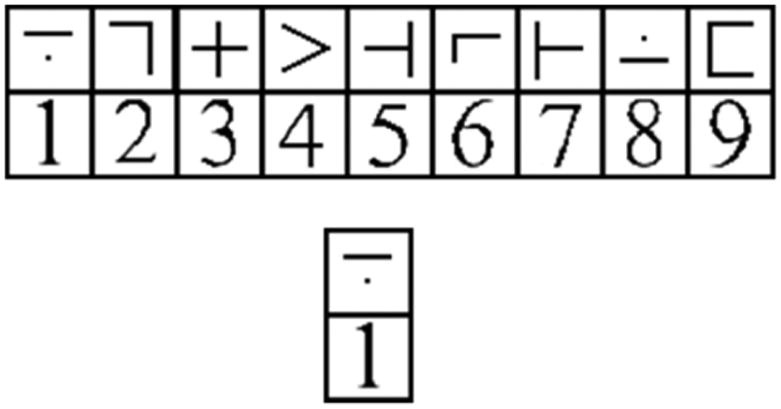
Sample stimuli from a single trial of the DSST.
After scanning, participants completed a battery of neuropsychometric tests outside the scanner environment to characterize the samples. This battery included the Symbol Digit Modalities Test (SDMT) from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 2008), the Paced Auditory Serial Addition Task (PASAT; Gronwall, 1977), Trail Making Tests A & B (Tombaugh et al., 1998), and a box completion task (Salthouse, 1996). MS patients also completed several assessments commonly administered in MS research, including the Modified Fatigue Impact Scale (MFIS; Fisk et al., 1994) and the Expanded Disability Status Scale (EDSS; Bowen et al., 2001).
2.3. Scanning Parameters
Neuroimaging data were collected at the UTSW Advanced Imaging Research Center using a Philips 3 Tesla MRI scanner (Philips Medical Systems, Best, The Netherlands) with an 8-channel SENSE head coil. Structural data were acquired using a T1-weighted MPRAGE pulse sequence, using the following parameters: 160 slices/volume, sagittal slice orientation, 12° flip angle, 256 × 204 matrix. The scan lasted a total of 237 seconds. Functional data were collected using gradient-echo echo planar imaging with the following parameters: echo time (TE) = 30 ms, repetition time (TR) = 2000 ms, 39 transverse slices with no slice gap acquired in interleaved fashion, voxel dimensions of 3.43 mm × 3.43 mm × 4.00 mm, 70° flip angle, 64 × 64 matrix. Functional scanning for both tasks lasted 18 minutes and 12 seconds in total. Additionally, a T2-fluid attenuated inversion recovery (FLAIR) image was acquired for each participant (except for one MS patient and one control) with the following parameters: TE = 125 ms, TR = 11000 ms, 33 5-mm transverse slices with no gap, 1 × 1 mm³ in-plane resolution, 120° refocusing angle, 352 × 212 matrix. The T2-FLAIR image allowed for quantification of lesion burden in MS patients. For a detailed description of T2-FLAIR image processing and results, see the Supplemental Materials, section S.1.
2.4. Data Processing Pipeline
Functional data were converted from the Philips PAR/REC proprietary format into the HEAD/BRIK format used by AFNI (Analysis of Functional NeuroImages; Cox, 1996). The functional volumes were then preprocessed to correct for slice timing and realigned to the initial functional volume using a rigid-body transformation to minimize effects of participant motion in the scanner. Motion parameter files for each scanning run were reviewed to ensure motion did not exceed half the length of one voxel on its shortest side (1.71 mm). The MPRAGE structural image was skull-stripped, and functional volumes were aligned to the MPRAGE image. Functional data were then high-pass filtered (0.015625 Hz), eliminating a significant portion of the noise spectrum (< .008 Hz), and spatially smoothed using a Gaussian kernel (FWHM = 6 mm) to increase the signal-to-noise ratio of the data. Extra-cranial noise was removed by masking out voxels that were either located outside of the anatomical brain region or exhibited a high degree of functional signal loss. Participants' structural scans were warped to the Colin TTN27 template, and participants' functional data were then warped to their structural scans within Talairach space using the @auto_tlrc program in AFNI. Spatial normalization allowed for demarcations of regions of interest (ROIs) using standard stereotaxic coordinates. All functional and anatomical data were visually inspected before and after preprocessing for artifacts and data processing issues. Preprocessed functional data were then analyzed for each participant using a general linear model (AFNI's 3dDeconvolve command; Ward, 2000).
ROIs were delineated in Talairach space using the AFNI Talairach Daemon. This method yielded a cortical map of three bilateral ROIs: Brodmann's area 4 (BA 4; Brodmann, 1909/2006), composed of precentral gyrus/primary motor cortex, BA 17, composed of striate cortex/primary visual cortex, and BA 9, composed of dorsolateral prefrontal cortex. ROI selection was determined a priori and motivated by each region's involvement in the tasks participants completed in the scanner, based on comparable tasks used in previous studies that measured BOLD in primary motor cortex (e.g., Aguirre, Zarahn, & D'Esposito, 1998; D'Esposito et al., 1999; Handwerker, Ollinger, & D'Esposito, 2004), primary visual cortex (e.g., Boynton et al., 1996; Dale & Buckner, 1997; Handwerker, Ollinger, & D'Esposito, 2004), and prefrontal cortex (e.g., DeLuca et al., 2008; Genova et al., 2009; Hubbard et al., 2016b; Leavitt et al., 2011; Rypma et al., 1999, 2006, 2007; Turner et al., 2016).
2.5. HRF Spline Fitting
The spline-fitting method used a finite number of basis functions to permit modeling of participant-specific HRFs, without requiring assumptions that the contour of individual participants' HRFs conform to a canonical shape. The HRF was modeled from baseline during a window of time beginning at stimulus onset using cubic Hermite spline interpolation, fitting piecewise functions for each participant HRF and overall group HRF using BOLD signal calculated at eight time-points, spaced equally at intervals of two seconds (1 TR). These parameter estimates represented percent signal change from baseline, beginning at stimulus onset (t0) and extending 14 seconds (t7) past the initial event (e.g., Dale and Buckner, 1997). This resulted in a maximal fit of the function to the data (R2 =1 in all cases) and resulted in smooth curves approximating each HRF, within unilateral and bilateral ROIs for each task.
2.6. HRF Shape Differences
Canonicality
A canonical HRF is one that follows the general contour of a standard impulse response function. One caveat to using an approach that minimizes shape assumptions is that non-canonical HRFs are possible, depending on the time course of the BOLD signal. HRFs were categorized as either canonical or non-canonical using the following criteria. A function was deemed canonical unless it met any one of the following criteria to be deemed non-canonical: (1) the spline-fit function contained more than three critical points (i.e., the points at which the slope of the fit function changes direction), (2) the spline-fit function contained only a single critical point, or (3) the maximum of the function occurred beyond the second critical point.
Metrics
We used two metrics to isolate quantifiable differences in overall HRF shape (see Fig 1). The first metric was peak amplitude, the critical point of the HRF with the maximum value. The second metric was time-to-peak (TTP), the point in time at which each HRF reached peak amplitude. Metrics were calculated using MATLAB code written by one of the authors (MT). Because spline functions can exhibit increased curvature nearer to boundary knots (t0 and t7), HRF metrics for each group and individual participant were visually inspected to ensure each metric was appropriately measured.
3. Results
3.1. Performance
RT data for the BPT were analyzed for 45 of the 51 participants, as equipment failure caused loss of data for six participants (nPatient = 3; nControl = 3). Patients and healthy controls showed similar performance on the BPT as measured by RT (MPatient = 386.26 [SEM = 9.54] vs. MControl = 374.37 [SEM = 16.67]), t(43) = .65, p = 0.520. DSST performance accuracy was not significantly different between MS patients and healthy controls (MPatient = 93.24% [SEM = .010] vs. MControl = 94.97% [SEM = .004]), t(36.90) = -1.51, p = 0.141. MS patients were significantly slower on the DSST compared to healthy controls (MPatient = 1804.00 [SEM = 61.64] vs. MControl = 1595.11 [SEM = 57.64]), t(49.86) = 2.42, p < 0.019. Results of tests from the neuropsychometric battery are listed in Table 1.
3.2. HRF Shape and Performance
Individual and group HRFs modeled in each ROI during both BPT and DSST performance are illustrated in Fig 3.
Figure 3.
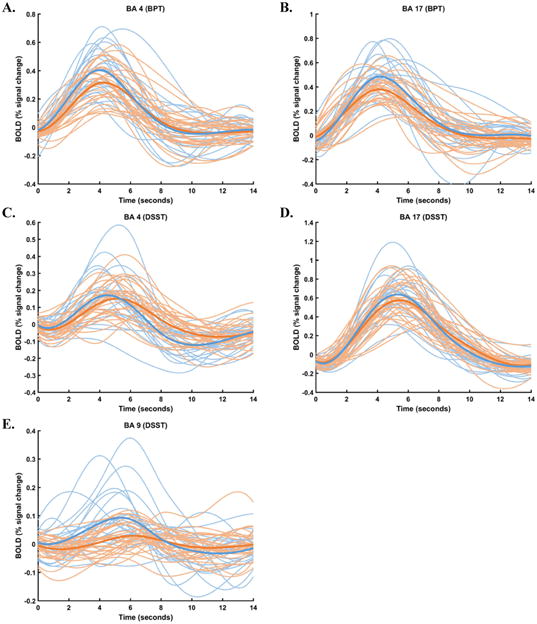
Individual and group HRFs modeled in bilateral (A) BA 4 during BPT performance, (B) BA 17 during BPT performance, (C) BA 4 during DSST performance, (D) BA 9 during DSST performance, and (E) BA 17 during DSST performance. Individual HRFs are in lighter blue (healthy controls) and orange (MS patients), and group HRFs are darker, bolder curves.
Canonicality
A two-proportion Z-test revealed a significantly greater rate of non-canonical HRFs across all regions (visual, motor, and prefrontal cortex) in MS patients (PPatient=32.14%, 95% CI [28.21%, 36.34%]) and controls (PControl=25.12%, [21.18%, 29.51%]), Z = -2.358, p < 0.018. These results support our hypothesis that MS-related neural-vascular coupling changes are reflected in the shape of the HRF. In subsequent analyses, we quantitatively assessed HRF shape differences to ascertain precisely which metrics differed between groups.
Metrics
To test each of our hypotheses, that (1) HRF canonicality (as measured by peak amplitude and TTP) will differ between MS patients and controls, and (2) HRF metrics will be more associated with processing speed in MS patients than in controls, we utilized multiple regression to predict each HRF Metric from Group and RT separately for BA 4 and BA 17 during BPT performance, and for BA 9 during DSST performance.
Peak Amplitude
In MS patients, peak amplitude was reduced compared to healthy controls in BA 17 during BPT performance, and in BA 9 during DSST performance. During BPT performance, there was a significant main effect of Group in BA 17, F(1,41) = 7.71, p < 0.008. During DSST performance there was a significant main effect of Group in BA 9, F(1,47) = 10.77, p < 0.002 (see Fig 4). No other effects were significant (all ps > 0.05).
Figure 4.
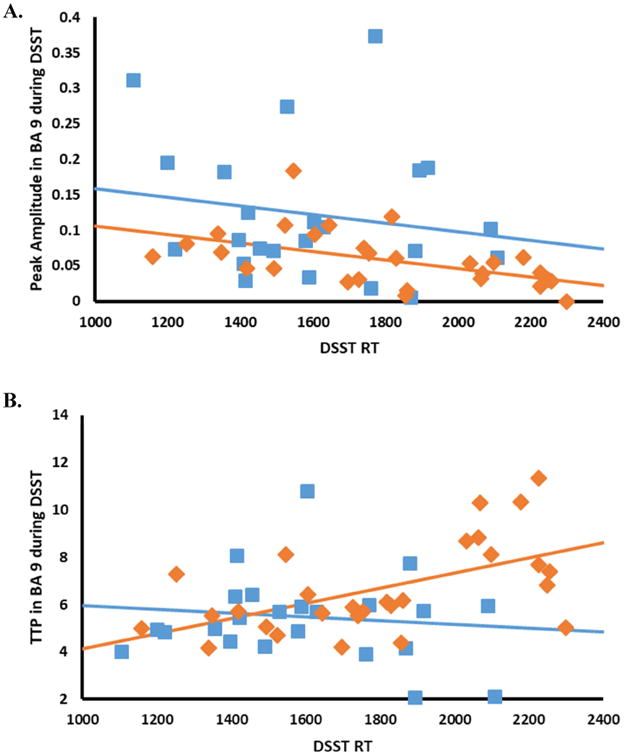
Relationships between performance (as measured by RT on the DSST) and (A) peak amplitude and (B) TTP in bilateral BA 9. Lines represent the best fit to the data using least-squares linear regression. Healthy controls are in blue, and MS patients are in orange.
TTP
In MS patients, TTP was longer compared to healthy controls only in BA 9 during DSST performance. There was a significant main effect of Group, F(1,47) = 6.27, p < 0.016. TTP was also longer for slower participants than faster participants. There was a significant main effect of RT, F = 4.77, p < 0.034. This effect was greater in the MS group than in the control group. There was a significant Group × RT interaction, F = 5.78, p < 0.020 (see Fig 4).
Medication effects
Multivariate analysis of variance was used to determine whether MS immunomodulatory medications significantly affected any HRF shape metrics. Across all tasks, regions, and metrics, no significant effects of medication were observed (all ps > 0.05, uncorrected).
4. Discussion
In this study, we compared BOLD-HRFs between MS patients and controls without existing parametric canonical models. This approach allowed us to assess differences between characteristics of HRFs while minimizing shape assumptions that could bias the ability to estimate BOLD signal. Results suggested a greater likelihood for the HRFs of MS patients to exhibit a departure from canonicality compared to healthy controls. Additionally, analysis of HRF metrics revealed attenuated peak amplitude and greater TTP in task-related ROIs in MS patients relative to controls. Prefrontal TTP was the sole measure predicted by individual differences in processing speed. Specifically, faster TTP was predicted by faster RT, suggesting an important role for vasodilatory speed in processing speed. Based on the fact that BOLD signal measures relative venous deoxyhemoglobin concentration, and that deoxyhemoglobin concentration changes in response to functional hyperemia, the different HRF shape metrics could reflect the critical role that glial cells play in neural-vascular coupling as moderators of nutrient perfusion in response to neurometabolic demand in healthy brains.
The HRF shape characteristics that we compared between MS patients and healthy controls are known to be uniform and reliable in the healthy brain (e.g., Aguirre, Zarahn, & D'Esposito, 1998; Boynton et al., 1996; Buckner, 1998; Friston et al., 1998; Lindquist et al., 2009). Such reliability reflects the integrity of glial (e.g., astrocytes; see Haydon & Carmignoto, 2006; Petzold & Murthy, 2011; Rossi et al., 2006; Takano et al., 2007; and passive diffusion mechanisms; see Cauli & Hamel, 2010) and vascular structures (e.g., endothelium, smooth muscle cells; e.g., Chen et al., 2014; Davis et al., 1998; Hoge et al., 1999; Hutchison et al., 2013a,b; Stefanovic et al., 2004) that facilitate changes in neurometabolic demand. Reductions in this integrity, as we observed in MS, underscore the importance of mechanisms that (1) allow neurons to feed forward their metabolic needs to vasculature via communicating structures (e.g., Attwell et al., 2010; Hillman, 2014), and (2) allow vasculature to feed back oxygen and nutrients to neurons via glial cells (e.g., Lee et al., 2012; Rinholm & Bergerson, 2012), ultimately facilitating neural performance (and, the current results suggest, cognitive performance).
In this study, we tested the hypothesis that dysfunction in underlying physiology in MS could be indexed by departures of HRF shape from canonicality. HRF canonicality was preserved at a greater rate in healthy controls than in MS patients (a group for which glial disruption is known; see Brosnan & Raine, 2013; De Keyser et al., 2008; D'Haeseleer et al., 2011; Gareau et al., 1999; Jukkola et al., 2013; Lassmann, 2003, 2014; Lundgaard et al., 2014; Mulligan & MacVicar, 2004; Trapp & Stys, 2009). Interestingly, HRF variability in all regions tested was greater in healthy controls relative to MS patients (see Fig 3). This finding might reflect a floor effect in MS patients, where smaller task-related excursions from baseline might result in a narrower envelope through which HRFs may vary across time.
Peak amplitude was lower in MS patients compared to healthy controls, possibly reflecting reduced vascular dilation (e.g., Devor et al., 2005; Metea & Newman, 2006), or a reduced proportion of oxygen extracted from capillary blood (e.g., Griffeth & Buxton, 2011; Hyder et al., 2001; Lu & Van Zijl, 2005; Trapp & Stys, 2009). TTP was higher in MS patients compared to healthy controls, possibly reflecting a delay of the system to reach maximal oxygen perfusion. This delay could be due to either disrupted glial vasodilatory signaling (e.g., Bonakdarpour, Parrish, & Thompson, 2007; Devor et al., 2005; Metea & Newman, 2006), or to differences in the efficiency of oxygen extraction from capillaries (e.g., Griffeth & Buxton, 2011; Hyder et al., 2001; Lu & Van Zijl, 2005; Trapp & Stys, 2009). Immunomodulatory medications did not exert significant effects on any of these metrics. These results suggest that canonical HRFs reflect intact neural-glial-vascular communication, oxygen extraction, and vascular compliance, fundamental to optimal functional hyperemia, neural efficiency, and cognitive performance.
It is worth noting that significant complexity accompanies interpretation of BOLD-HRFs in the context of underlying neural-vascular activity (e.g., Lindquist & Wager, 2007; Lindquist et al., 2009). Measurement of the HRF occurs on a time scale three orders of magnitude greater than that of underlying neuronal activity (Logothetis, 2002). Heterogeneity in the evolution of the HRF through time exists across cortical regions (Aguirre, Zarahn, & D'Esposito, 1998; Handwerker, Ollinger, & D'Esposito, 2004), wherein the relationship between BOLD signal and underlying neural activity is sometimes linear (e.g., Boynton et al., 1996), but sometimes nonlinear (e.g., Birn, Saad, & Bandettini, 2001; Martindale et al., 2005). More advanced techniques, such as calibrated fMRI (that measures both BOLD and cerebral blood flow and permits calculation of cerebral oxygen metabolism; e.g., Hutchison et al., 2013) or combined EEG-fMRI, will be needed to assess the validity of these hypotheses.
The hypothesis that intact communication between neurons, glia, and vasculature is essential to optimal neural performance is supported by relationships we observed between HRF shape metrics and behavioral performance. MS-related physiologic dysfunction reflected in non-canonical HRFs impairs neural performance and, the present results suggest, efficient cognitive performance. While group differences were found in both HRF metrics, processing speed performance exclusively predicted TTP in prefrontal cortex. Further, the effect of processing speed performance was stronger in MS patients than in healthy controls as evidenced by a significant Group × RT interaction effect. This result suggests the hypothesis that mechanisms underlying HRF latency (e.g., delay in functional hyperemia) play a larger role in speed of processing (an ability known to be central to general cognitive performance; Salthouse, 1996; Vernon, 1983) than those underlying peak amplitude (e.g., oxygen extraction).
Earlier work from our lab (Rypma et al., 1999, 2006, 2007; Turner et al., 2016), and from others (e.g., Bachman et al., 2010; Boone et al., 1998; DeLuca et al., 2008; Genova et al., 2009), has localized processing-speed ability to prefrontal cortex, a region not known for frank MS lesions. This result is consistent with others showing that MS pathology exerts pervasive deleterious effects well beyond white-matter lesion sites. Previous research has demonstrated that global attenuation in fractional anisotropy predicted HRF peak amplitude in visual and motor cortex, whereas lesion location in MS patients did not (Hubbard et al., 2016a). This result, combined with our finding that lesion burden was not related to behavioral performance (see Supplemental Material), suggests that MS-related damage is not limited to lesioned regions, and instead represents dysfunction occurring at a system-wide level. Further work using more advanced white-matter imaging techniques (such as diffusion kurtosis imaging; see e.g., Lu et al., 2006) will be needed to better characterize white-matter-HRF relationships.
There are several caveats to interpretation of our results. First, much remains to be elucidated about MS pathophysiology, which limits the confidence with which we can provide a straightforward interpretation of altered HRF shape. However, strong relationships between HRF shape measures and performance provide compelling evidence that altered hemodynamic processes in instances of white-matter compromise have consequences for cognition. Second, the standard-space analyses that we employed to facilitate like-to-like spatial between-groups comparisons may not account for cortical gray-matter atrophy often seen in neurodegenerative diseases such as MS (Azevedo & Pelletier, 2016; Calabrese et al., 2007, 2009; Fisher et al., 2008; Fisniku et al., 2008; Geurts & Barkhof, 2008; Geurts et al., 2012; Pirko et al., 2007; Vercellino et al., 2009). Calibrated imaging work shows that altered gray-matter metabolism in MS is related to white-matter compromise (Hubbard et al., 2017; see also Varga et al., 2009). More work is certainly needed to disentangle relative influences of white- and gray-matter on HRF shape and cognitive performance. Third, the MS patients scanned in our study were permitted to take their regular courses of medications (e.g., glatiramer acetate, interferon-beta, natalizumab) on the day of their scans. Although we did not observe significant effects of these medications on the HRF, effects of these medications on the BOLD signal remain unknown. Additional work is certainly needed to pursue answers to these unresolved questions.
Imaging studies of MS commonly focus on the involvement of white-matter. Other populations feature differences in white-matter structure relative to healthy young-adult brains. White-matter volume is known to decline, for instance, even as a consequence of healthy aging (e.g., Bartzokis et al., 2003; Bennett et al., 2010; Gunning-Dixon et al., 2009; Salat, Kaye, & Janowsky, 1999). Degradation of white-matter integrity is also observed in cases of neurodegeneration other than MS, including Alzheimer's disease (Acosta-Cabronero et al., 2010; Bozzali et al., 2002), Parkinson's disease (Hattori et al., 2012; Rae et al., 2012), and amyotrophic lateral sclerosis (Abrahams, Leigh, & Goldstein, 2005; Zhang et al., 2007). Further, a prominent hallmark of healthy brain development in children is maturation of white-matter (e.g., Barnea-Goraly et al., 2005; Klingberg et al., 1999; Mabbott et al., 2006; Nagy, Westerberg, & Klingberg, 2004). These groups also exhibit cognitive performance differences relative to healthy young adult controls. Such cognitive performance differences provide further evidence for the importance of intact neural-vascular coupling to intact neural performance, and in turn, cognitive performance.
Conclusions
We isolated differences in HRF metrics between MS patients and healthy controls to assess how HRF shape differed between groups, and to assess the extent to which departures from a canonical HRF shape were related to performance differences. The use of an approach that (1) compared healthy individuals to a group with known white-matter damage, and (2) minimized assumptions regarding HRF shape, made this assessment sensitive enough to isolate group differences that might not have been apparent if a standard impulse response function had been used. HRF shape was significantly altered between groups, and was related to processing speed differences. Together, these results provide support for the hypothesis that alterations to glial cell intermediaries are associated with neural-vascular coupling deficits, that are in turn related to reductions in neural function and processing speed. Neural-glial-vascular communication might form the basis for optimal neural performance, and provide a plausible physiological mechanism for processing speed differences between healthy younger, older, and diseased groups.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Dianne Cash Predoctoral Fellowship (to MT), the Friends of Brain Health and the Linda and Joel Roebuck Distinguished New Scientist endowments (to NAH), the National Multiple Sclerosis Society (RG4453A1/2 to BR and EF), and the National Institutes of Health (1R01AG047972 and 1R01AG029523 to BR). The content does not necessarily reflect the position or the policy of the Federal government or the sponsoring agencies, and no official endorsement should be inferred.
Footnotes
Conflicts of Interest: MT, NAH, DKS, LMH, JLH, JH, JS, and BR are not aware of any potential financial conflicts of interest related to the current study. EF, TF, and DO have received honoraria from speaking engagements with pharmaceutical companies related to multiple sclerosis, but unrelated to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Leigh PN, Goldstein LH. Cognitive change in ALS A prospective study. Neurology. 2005;64(7):1222–1226. doi: 10.1212/01.WNL.0000156519.41681.27. [DOI] [PubMed] [Google Scholar]
- Ackerman PL, Beier ME, Boyle MD. Individual differences in working memory within a nomological network of cognitive and perceptual speed abilities. Journal of Experimental Psychology: General. 2002;131(4):567. [PubMed] [Google Scholar]
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133(2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Adhya S, Johnson G, Herbert J, Jaggi H, Babb JS, Grossman RI, Inglese M. Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. NeuroImage. 2006;33(4):1029–1035. doi: 10.1016/j.neuroimage.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8(4):360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, et al. Carter CS. The BOLD hemodynamic response in healthy aging. Journal of Cognitive Neuroscience. 2004;16(5):786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Al-Omari MH, Rousan LA. Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. International Angiology. 2010;29(2):115. [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2011;1812(2):252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Human brain mapping. 2009;30(4):1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald C, Fisk J. Information processing efficiency in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 2000:686–701. doi: 10.1076/1380-3395(200010)22:5;1-9;FT686. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. (2010) Glial and neuronal control of brain blood flow. Nature. 2010 Nov 11;468(7321):232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo CJ, Pelletier D. Whole-brain atrophy: ready for implementation into clinical decision-making in multiple sclerosis? Current opinion in neurology. 2016;29(3):237–242. doi: 10.1097/WCO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- Bachman P, Reichenberg A, Rice P, Woolsey M, Chaves O, Martinez D, et al. Glahn DC. Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophrenia research. 2010;118(1):6–11. doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of neurology. 2003;60(3):393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr 2010. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging. Human Brain Mapping. 2010 Mar;31(3):378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. NeuroImage. 2001;14(4):817–826. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(6):H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Parrish TB, Thompson CK. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. NeuroImage. 2007;36(2):322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MC, Allard M, Dilharreguy B, Deloire M, Petry KG, Brochet B. Cognitive compensation failure in multiple sclerosis. Neurology. 2010;75(14):1241–1248. doi: 10.1212/WNL.0b013e3181f612e3. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L. Structural connectivity influences brain activation during PVSAT in multiple sclerosis. Neuroimage. 2009;44(1):9–15. doi: 10.1016/j.neuroimage.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Boone KB, Pontón MO, Gorsuch RL, González JJ, Miller BL. Factor analysis of four measures of prefrontal lobe functioning. Archives of Clinical Neuropsychology. 1998;13(7):585–595. [PubMed] [Google Scholar]
- Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Multiple Sclerosis Journal. 2001;7(3):201–206. doi: 10.1177/135245850100700311. [DOI] [PubMed] [Google Scholar]
- Boynton G, Engel S, Glover G, Heeger D. Linear Systems Analysis of Functional Magnetic Resonance Imaging in Human V1. Journal of Neuroscience. 1996:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, et al. Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(6):742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Brodmann's Localisation in the Cerebral Cortex 2005. Springer; US: 1909/2006. [DOI] [Google Scholar]
- Brooks DJ, Leenders KL, Head G, Marshall J, Legg NJ, Jones T. Studies on regional cerebral oxygen utilisation and cognitive function in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 1984;47(11):1182–1191. doi: 10.1136/jnnp.47.11.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61(4):453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12(6):1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and neuronal death. Nitric oxide. 2010;23(3):153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Molecular neurobiology. 2003;27(3):325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radical Biology and Medicine. 2002;33(11):1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. MIT Press; 2006. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Atzori M, Bernardi V, Morra A, Romualdi C, Rinaldi L, et al. Battistin L. Cortical atrophy is relevant in multiple sclerosis at clinical onset. Journal of neurology. 2007;254(9):1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, et al. Perini P. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Archives of neurology. 2009;66(9):1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Liddle PF, Pearlson GD. Aberrant localization of synchronous hemodynamic activity in auditory cortex reliably characterizes schizophrenia. Biological psychiatry. 2004;55(8):842–849. doi: 10.1016/j.biopsych.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JD, Wahba G, Xie X, Nordheim EV, Meyerand ME. Optimal spline smoothing of fMRI time series by generalized cross-validation. NeuroImage. 2003;18(4):950–961. doi: 10.1016/s1053-8119(03)00013-2. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Gómez-Gonzalo M. The contribution of astrocyte signalling to neurovascular coupling. Brain research reviews. 2010;63(1):138–148. doi: 10.1016/j.brainresrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Frontiers in Neuroenergetics. 2010;2(9):1–8. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. Journal of the American Heart Association. 2014;3(3):e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SC, Iwaki S, Tack GR, Yi JH, You JH, Kwon JH. Effect of 30% oxygen administration on verbal cognitive performance, blood oxygen saturation and heart rate. Applied psychophysiology and biofeedback. 2006;31(4):281–293. doi: 10.1007/s10484-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Cox RW. (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human brain mapping. 1997;5(5):329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. (1998) Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1834–9. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser J, Steen C, Mostert JP, Koch MW. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow & Metab. 2008;28:1645–1651. doi: 10.1038/jcbfm.2008.72. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Wilczak N, Leta R, Streetland C. Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology. 1999;53(8):1628–1628. doi: 10.1212/wnl.53.8.1628. [DOI] [PubMed] [Google Scholar]
- Debernard L, Melzer TR, Van Stockum S, Graham C, Wheeler-Kingshott CA, Dalrymple-Alford JC, et al. Mason DF. Reduced grey matter perfusion without volume loss in early relapsing-remitting multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2013 doi: 10.1136/jnnp-2013-305612. jnnp-2013. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Genova HM, Hillary FG, Wylie G. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. Journal of the neurological sciences. 2008;270(1):28–39. doi: 10.1016/j.jns.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, et al. Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(10):3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS, Auer DP. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132(1):239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. NeuroImage. 1999;10(1):6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D'haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. (2011) Vascular aspects of multiple sclerosis. Lancet Neurol. 2011 Jul;10(7):657–66. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Annals of neurology. 2008;64(3):255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases. 1994;18(Supplement_1):S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, et al. Miller DH. Gray matter atrophy is related to long-term disability in multiple sclerosis. Annals of neurology. 2008;64(3):247–254. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes AW, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gareau PJ, Gati JS, Menon RS, Lee D, Rice G, Mitchell JR, et al. Karlik SJ. Reduced visual evoked responses in multiple sclerosis patients with optic neuritis: comparison of functional magnetic resonance imaging and visual evoked potentials. Multiple Sclerosis Journal. 1999;5(3):161–164. doi: 10.1177/135245859900500304. [DOI] [PubMed] [Google Scholar]
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, Grossman RI. Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. American Journal of Neuroradiology. 2005;26(6):1539–1547. [PMC free article] [PubMed] [Google Scholar]
- Genova H, Hillary F, Wylie G, Rypma B, Deluca J. Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. Journal of the International Neuropsychological Society. 2009:383–93. doi: 10.1017/S1355617709090535. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. The Lancet Neurology. 2008;7(9):841–851. doi: 10.1016/S1474-4422(08)70191-1. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Calabrese M, Fisher E, Rudick RA. Measurement and clinical effect of grey matter pathology in multiple sclerosis. The Lancet Neurology. 2012;11(12):1082–1092. doi: 10.1016/S1474-4422(12)70230-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R, Lazar N, Bhaumik D, Sclove S, Chen H, Thulborn K, et al. Patterson D. Estimation and classification of fMRI hemodynamic response patterns. NeuroImage. 2004:808–14. doi: 10.1016/j.neuroimage.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related bold fmri 1. NeuroImage. 1999;9(4):416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Goutte C, Nielsen FA, Hansen KH. Modeling the hemodynamic response in fMRI using smooth FIR filters. IEEE transactions on medical imaging. 2000;19(12):1188–1201. doi: 10.1109/42.897811. [DOI] [PubMed] [Google Scholar]
- Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011;58(1):198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall DMA. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and motor skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. International journal of geriatric psychiatry. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21(4):1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Haselhorst R, Kappos L, Bilecen D, Scheffler K, Möri D, Radü EW, Seelig J. Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: Application of an extended blood-brain barrier leakage correction. Journal of magnetic resonance imaging. 2000;11(5):495–505. doi: 10.1002/(sici)1522-2586(200005)11:5<495::aid-jmri5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, et al. Mizusawa H. Cognitive status correlates with white matter alteration in Parkinson's disease. Human brain mapping. 2012;33(3):727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological reviews. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15(1):83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annual review of neuroscience. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. (1999) Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magnetic Resonance in Medicine. 1999 Nov;42(5):849–63. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hubbard NA, Turner M, Hutchison JL, Ouyang A, Strain J, Oasay L, Sundaram S, Davis S, Remington G, Brigante R, Huang H, Hart J, Jr, Frohman T, Frohman E, Biswal BB, Rypma B. Multiple sclerosis-related white matter microstructural change alters the BOLD hemodynamic response. Journal of Cerebral Blood Flow & Metabolism. 2016a;36(11):1872–1884. doi: 10.1177/0271678X15615133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Turner MP, Sundaram S, Oasay L, Robinson D, Strain J, Weaver T, Davis SL, Remington GM, Huang H, Biswal BB, Hart J, Jr, Frohman TC, Frohman EM, Rypma B. Asynchrony in executive networks predicts cognitive slowing in multiple sclerosis. Neuropsychology. 2016b;30(1):75. doi: 10.1037/neu0000202. [DOI] [PubMed] [Google Scholar]
- Hubbard NA, Turner MP, Ouyang M, Himes L, Thomas BP, Hutchison JL, Faghihahmadabadi S, Davis SL, Strain JF, Spence J, Krawczyk DC, Huang H, Lu H, Hart J, Jr, Frohman TC, Frohman EM, Okuda DT, Rypma B. Calibrated imaging reveals altered grey matter metabolism related to white matter microstructure and symptom severity in multiple sclerosis. Human brain mapping. 2017;38(11):5375–5390. doi: 10.1002/hbm.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001;13(1):161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Hutchison JL, Lu H, Rypma B. Neural mechanisms of age-related slowing: the ΔCBF/ΔCMRO2 ratio mediates age-differences in BOLD signal and human performance. Cerebral Cortex. 2013a doi: 10.1093/cercor/bhs233. bhs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JL, Shokri-Kojori E, Lu H, Rypma B. A BOLD perspective on age-related neurometabolic-flow coupling and neural efficiency changes in human visual cortex. Frontiers in psychology. 2013b;4 doi: 10.3389/fpsyg.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR in biomedicine. 2001;14(7-8):413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukkola P, Guerrero T, Gray V, Gu C. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta neuropathologica communications. 2013;1(1):70. doi: 10.1186/2051-5960-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern KC, Ekstrom AD, Suthana NA, Giesser BS, Montag M, Arshanapalli A, et al. Sicotte NL. Fornix damage limits verbal memory functional compensation in multiple sclerosis. Neuroimage. 2012;59(3):2932–2940. doi: 10.1016/j.neuroimage.2011.09.071. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. Journal of the neurological sciences. 2003;206(2):187–191. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia. 2014;62(11):1816–1830. doi: 10.1002/glia.22597. [DOI] [PubMed] [Google Scholar]
- Law M, Saindane AM, Ge Y, Babb JS, Johnson G, Mannon LJ, et al. Grossman RI. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231(3):645–652. doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- Leavitt VM, Lengenfelder J, Moore NB, Chiaravalloti ND, DeLuca J. The relative contributions of processing speed and cognitive load to working memory accuracy in multiple sclerosis. Journal of clinical and experimental neuropsychology. 2011;33(5):580–586. doi: 10.1080/13803395.2010.541427. [DOI] [PubMed] [Google Scholar]
- Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S, et al. Matthews PM. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Annals of neurology. 2000;47(5):606–613. [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Pellerin L. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Wager TD. Validity and power in hemodynamic response modeling: a comparison study and a new approach. Human brain mapping. 2007;28(8):764–784. doi: 10.1002/hbm.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Loh JM, Atlas LY, Wager TD. Modeling the hemodynamic response function in fMRI: efficiency, bias and mis-modeling. Neuroimage. 2009;45(1):S187–S198. doi: 10.1016/j.neuroimage.2008.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. Philosophical Transactions of the Royal Society of London. 2002. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal; pp. 1003–37. Series B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, van Zijl P. Experimental measurement of extravascular parenchymal BOLD effects and tissue oxygen extraction fractions using multi-echo VASO fMRI at 1.5 and 3.0 T. Magnetic resonance in medicine. 2005;53(4):808–816. doi: 10.1002/mrm.20379. [DOI] [PubMed] [Google Scholar]
- Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR in Biomedicine. 2006;19(2):236–247. doi: 10.1002/nbm.1020. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Osório MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke J, Wikkelsö C, Bergh AC, Jacobsson L, Andersen O. Regional cerebral blood flow in multiple sclerosis measured by single photon emission tomography with technetium-99m hexamethyl-propyleneamine oxime. European neurology. 1993;33(2):163–167. doi: 10.1159/000116926. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. NeuroImage. 2006;33(3):936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural–hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32(1):33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Martindale J, Berwick J, Martin C, Kong Y, Zheng Y, Mayhew J. Long duration stimuli and nonlinearities in the neural–haemodynamic coupling. Journal of Cerebral Blood Flow & Metabolism. 2005;25(5):651–661. doi: 10.1038/sj.jcbfm.9600060. [DOI] [PubMed] [Google Scholar]
- Marshall O, Lu H, Brisset JC, Xu F, Liu P, Herbert J, et al. Ge Y. Impaired cerebrovascular reactivity in multiple sclerosis. JAMA neurology. 2014;71(10):1275–1281. doi: 10.1001/jamaneurol.2014.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus B, van Breukelen GJ, Goebel R, Berger MP. Optimal design for nonlinear estimation of the hemodynamic response function. Human brain mapping. 2012;33(6):1253–1267. doi: 10.1002/hbm.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. Journal of Neuroscience. 2006;26(11):2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtasib RS, Lumley G, Goodwin JA, Emsley HC, Sluming V, Parkes LM. Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. Neuroimage. 2012;59(2):1143–1151. doi: 10.1016/j.neuroimage.2011.07.092. [DOI] [PubMed] [Google Scholar]
- Mulholland AD, Vitorino R, Hojjat SP, Ma AY, Zhang L, Lee L, Carroll TJ, Cantrell CG, Figley CR, Aviv RI. Spatial Correlation of Pathology and Perfusion Changes within the Cortex and White Matter in Multiple Sclerosis. American Journal of Neuroradiology. 2017 doi: 10.3174/ajnr.A5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–200. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of cognitive neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee T, Kay A, Tank D. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, et al. Pozzilli C. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128(9):2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36(2):269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68(9):634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- Prakash R, Snook E, Lewis J, Motl R, Kramer A. Cognitive impairments in relapsing-remitting multiple sclerosis: a meta-analysis. Multiple Sclerosis. 2008:1250–1261. doi: 10.1177/1352458508095004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB. White matter pathology in Parkinson's disease: the effect of imaging protocol differences and relevance to executive function. NeuroImage. 2012;62(3):1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JO. Functional data analysis. John Wiley & Sons, Inc; 2006. [Google Scholar]
- Rashid W, Parkes LM, Ingle GT, Chard DT, Toosy AT, Altmann DR, et al. Miller DH. Abnormalities of cerebral perfusion in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(9):1288–1293. doi: 10.1136/jnnp.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. NeuroImage. 2007;37(2):430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Bergersen LH. The wrap that feeds neurons. Nature. 2012;487(7408):435. doi: 10.1038/487435a. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Falini A, Colombo B, Scotti G, Comi G, Filippi M. Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Annals of neurology. 2002;51(3):330–339. doi: 10.1002/ana.10120. [DOI] [PubMed] [Google Scholar]
- Rossi DJ. Another BOLD role for astrocytes: Coupling blood flow to neural activity. Nature Neuroscience. 2006;9:159–60. doi: 10.1038/nn0206-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proceedings of the National Academy of Sciences. 1999;96(11):6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Age-related changes in brain–behaviour relationships: Evidence from event-related functional MRI studies. European Journal of Cognitive Psychology. 2001;13(1-2):235–256. [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37(2):207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D'Esposito M. (2006) Neural correlates of cognitive efficiency. NeuroImage. 2006 Nov 15;33(3):969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43(1):65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9(2):216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of neurology. 1999;56(3):338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta psychologica. 1992;79(2):155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Salthouse T. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22(2):771–778. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Strober LB, Rao SM, Lee JC, Fischer E, Rudick R. Cognitive impairment in multiple sclerosis: An 18 year follow-up study. Multiple sclerosis and related disorders. 2014;3(4):473–481. doi: 10.1016/j.msard.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Current neurology and neuroscience reports. 2009;9(5):411–417. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tanaka M, KoNDo S, Okamoto K, Hirai S. Clinical significance of reduced cerebral metabolism in multiple sclerosis: a combined PET and MRI study. Annals of nuclear medicine. 1998;12(2):89–94. doi: 10.1007/BF03164835. [DOI] [PubMed] [Google Scholar]
- Swank RL, Roth JG, Woody DC., Jr Cerebral blood flow and red cell delivery in normal subjects and in multiple sclerosis. Neurological research. 1983;5(1):37–59. doi: 10.1080/01616412.1983.11739631. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nature neuroscience. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Rees L, McIntyre N. A compendium of neuropsychological tests: administration. Oxford University Press; New York: 1998. Normative data for the trail making test. [Google Scholar]
- Trapp BD, Nave KA. Multiple Sclerosis: an immune or neurodegenerative disorder? Annual Reviews Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. The Lancet Neurology. 2009;8(3):280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RN, Tyler LK, Davis SW, Shafto MA, Taylor JR, et al. Rowe JB. The effect of ageing on fMRI: correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human brain mapping. 2015;36(6):2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MP, Hubbard NA, Himes LM, Faghihahmadabadi S, Hutchison JL, Bennett IJ, Motes MA, Haley RW, Rypma B. Cognitive Slowing in Gulf War Illness Predicts Executive Network Hyperconnectivity: Study in a Population-Representative Sample. NeuroImage: Clinical. 2016;12:535–541. doi: 10.1016/j.nicl.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Johnson G, Babb JS, Herbert J, Grossman RI, Inglese M. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. Journal of the neurological sciences. 2009;282(1):28–33. doi: 10.1016/j.jns.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellino M, Masera S, Lorenzatti M, Condello C, Merola A, Mattioda A, et al. Giordana MT. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. Journal of Neuropathology & Experimental Neurology. 2009;68(5):489–502. doi: 10.1097/NEN.0b013e3181a19a5a. [DOI] [PubMed] [Google Scholar]
- Vernon PA. Speed of information processing and general intelligence. Intelligence. 1983;7(1):53–70. [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data [software manual] 1998/2006 Retrieved from https://afni.nimh.nih.gov/pub/dist/doc/manual/Deconvolvem.pdf.
- Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wegner C, Filippi M, Korteweg T, Beckmann C, Ciccarelli O, De Stefano N, et al. Hirsch J. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. European journal of neurology. 2008;15(2):113–122. doi: 10.1111/j.1468-1331.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- White AT, Lee JN, Light AR, Light KC. Brain activation in multiple sclerosis: a BOLD fMRI study of the effects of fatiguing hand exercise. Multiple Sclerosis Journal. 2009;15(5):580–586. doi: 10.1177/1352458508100034. [DOI] [PubMed] [Google Scholar]
- Wink AM, Hoogduin H, Roerdink JB. Data-driven haemodynamic response function extraction using Fourier-wavelet regularised deconvolution. BMC Medical Imaging. 2008;8(1):7. doi: 10.1186/1471-2342-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F LiuP, Pascual JM XiaoG, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. Journal of Cerebral Blood Flow & Metabolism. 2012;32:1909–18. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Avants BB, Yushkevich PA, Woo JH, Wang S, McCluskey LF, et al. Gee JC. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE transactions on medical imaging. 2007;26(11):1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- Zou P, Helton KJ, Smeltzer M, Li CS, Conklin HM, Gajjar A, et al. Ogg RJ. Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain imaging and behavior. 2011;5(4):295–306. doi: 10.1007/s11682-011-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


