Abstract
Background
Bolus timing is critical to optimal magnetic resonance angiography (MRA) acquisitions but can be challenging in some patients. Our purpose was to evaluate whether contrast-enhanced time-resolved magnetic resonance angiography (TR-MRA), a dynamic multiphase sequence that does not rely on bolus timing, is a viable alternative method to conventional 3D fast-long angle shot CE-MRA.
Methods
Coronal subtracted conventional CE-MRA images in 50 consecutive patients presenting for pre-atrial fibrillation ablation pulmonary venous (PV) mapping were compared with 50 TR-MRA images performed in 50 subsequent patients. The TR-MRA protocol was modified to optimize spatial resolution with slightly reduced temporal resolution (6.1s scan time). Three experienced readers evaluated each scan’s image quality and relative left atrial (LA) opacification based on a 4-point scale and diagnostic PV visualization in a binary fashion. Additionally, LA signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and PV dimensions were measured for both techniques.
Results
TR-MRA had significantly higher overall image quality (3.10 ±0.69 vs. 2.42 ±0.69, p<0.0001), and LA opacification scores (3.33 ±0.70 vs. 2.15 ±1.13, p<0.0001) compared to CE-MRA. The proportion of diagnostically visualized pulmonary veins was 137/150 (91%) in the CE-MRA group vs. 147/150 (98%) with TR-MRA (p=0.010). Both SNR and CNR were higher with TR-MRA vs. CE-MRA (277.9 ±48.9 vs 106.8 ±41, p=0.002 and 100.3 ±41.7 vs. 70.7 ±48.0, p=0.002, respectively). Inter-reader variance of individual PV measurements for each of the MR techniques ranged between 0.62 and 1.47mm and the ICC for vein measurements was higher with TR-MRA (range: 0.62–0.81) compared to CE-MRA (range: 0.47–0.64).
Conclusion
TR-MRA, modified to maximize spatial resolution, offers an alternative method for performing high quality MRA examinations in patients with AF. TR-MRA offers greater overall image quality, PV visualization, and similarly reproducible PV measurements compared to traditional CE-MRA, without the challenges of proper bolus timing.
Keywords: Atrial fibrillation, catheter ablation, contrast-enhanced magnetic resonance angiography, time-resolved magnetic resonance angiography
INTRODUCTION
Atrial fibrillation (AF), with a reported incidence of 33.5 million worldwide, is the most common cardiac arrhythmia and is thought to be primarily triggered by spontaneous ectopic beats originating from the pulmonary veins (PV) [1, 2]. Pulmonary vein isolation by radiofrequency ablation has emerged as an effective treatment of AF by interrupting signal conduction arising from ectopic triggers in the PV ostia to the left atrial (LA) myocardium [3]. High resolution contrast-enhanced images of the LA and PVs by non-invasive specific imaging methods, such as cardiac CT and magnetic resonance angiography (MRA), are widely used for procedure planning. Both techniques allow the detection of anatomical variants of the PV system, assist in electro-anatomic mapping and catheter positioning and avoid treatment-related complications, such as PV stenosis and use of incorrect catheter size [4–7]. Contrast-enhanced three-dimensional fast-long angle shot MRA (CE-MRA) does not expose patients to ionizing radiation and can be combined with a comprehensive cardiac MRI imaging of the heart in the same session, making it an appealing alternative to computed tomography [8, 9].
Bolus timing is critical to optimal CE-MRA acquisitions and can be challenging in some patients. Patient factors, such as vascular anatomy and cardiac output, and technologists’ comfort and expertise in bolus timing can affect quality of image acquisition and may result in suboptimal LA characterization. Dynamic, contrast-enhanced time-resolved interleaved stochastic trajectories (TR-MRA) is an appealing alternative to the standard CE-MRA. Indeed, the former does not rely on bolus timing, but rather uses rapidly acquired serial imaging obtained in a dynamic fashion after the injection of contrast. In a small study of 26 patients presenting for an AF ablation, Schonberger et al. reported comparable pulmonary venous conspicuity and dimensions between TR-MRA and traditional CE-MRA [10]. Based on these findings, we hypothesized that TR-MRA is a viable alternative method to CE-MRA and that TR-MRA may result in more optimally timed MRA images by removing the need for bolus timing. In this study, we sought to perform quantitative and qualitative comparisons of pulmonary vein maps acquired using TR-MRA and CE-MRA.
MATERIALS AND METHODS
PATIENT POPULATION
This retrospective cohort study included one hundred consecutive patients with symptomatic, drug-refractory paroxysmal or persistent AF undergoing cardiac MRI prior to an initial catheter ablation procedure at the Johns Hopkins Hospital. Patients were equally distributed in 2 groups depending upon the technique used for MRA sequence. In the first 50 consecutive cases, traditional 3D CE-MRA was performed; whereas the next 50 cases were acquired using 4D TR-MRA after it was implemented in the clinical routine. The Johns Hopkins Institutional Review Board (JH-IRB) approved the study and retrospective study data was obtained under a HIPPA compliant waiver of consent.
MRI ACQUISITION
All subjects underwent pre-ablation MRI up to one week prior to ablation. Images were acquired using a 1.5 T MAGNETOM Avanto MR scanner (Siemens, Erlangen, Germany) with a phased array cardiac coil.
Conventional CE-MRA of the pulmonary vasculature was obtained in a coronal orientation using a 3D gradient echo FLASH pulse sequence with the following scanning parameters: TR/TE 2.8/1.4 msec; flip angle, 25º; in-plane resolution 1.0 × 1.0 mm; mean slice thickness: 1.3 mm, range [1.2–1.4], parallel imaging acceleration factor × 2. A centric phase-encoding acquisition was used. A bolus of 0.2 mmol/kg of gadopentetate dimeglumine (Magnevist, Bayer) was injected at a rate of 2 mL/sec via IV cannula in the antecubital fossa. Image acquisition was trigerred using fluoro-prep. A sagittal image of the heart and thoracic aorta was acquired every second during contrast injection. Image acquisition is then manually triggered by the operator upon visualization of the contrast bolus in the ascending aorta. CE-MRA acquisition time was approximately 20 seconds.
TR-MRA was obtained in a coronal orientation centered on the left atrium. The scanning parameters were as follows: TR/TE 2.2/0.9 msec; flip angle 24º; in-plane resolution 1.0×1.0 mm; slab thickness 70–105 mm; slice thickness 1.5mm, range[1.5–1.8]; parallel imaging with acceleration factor ×2; partial Fourier 6/8 in x-y and z directions; acquisition time 6.1 sec/phase and 13 phase acquisitions. A bolus of 0.2 mmol/kg of gadopentetate dimeglumine (Magnevist, Bayer) was injected at a rate of 5 mL/s via IV cannula placed in the antecubital fossa. A 20 mL saline chaser was injected immediatedly following gadolinium contrast at the same rate. Breathhold was initiated by the technologist immediately following the start of contrast injection for TR-MRA scans.
Patients were instructed to perform an inspiratory breathold followed by a slow expiration. In-line digital subtraction of pre- and post-contrast 3D datasets was utilized for both techniques.
QUALITATIVE ANALYSIS
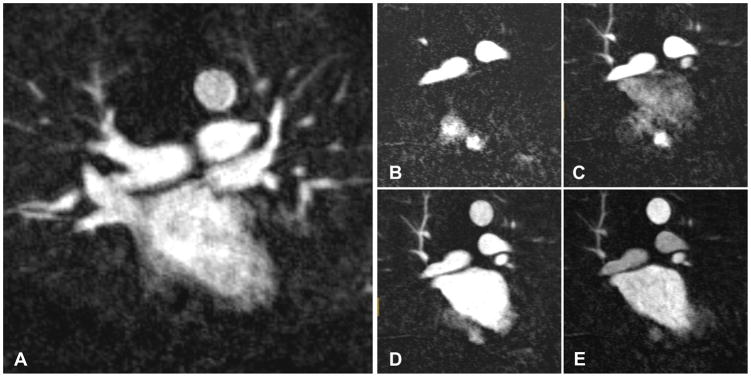
MRA scans were transferred to a dedicated imaging workstation (Leonardo; Siemens Medical Solutions, Erlangen, Germany) for analysis. All examinations were anonymized and randomized to avoid any recall by date. Scans were evaluated by three readers independently (CP, radiology resident with 3 years’ imaging experience; KP, radiology fellow with 4 years’ experience; and LC, radiology attending with 6 years’ experience), blinded to the imaging protocol and to each others’ scores. In the TR-MRA group, a separate (fourth) reader was used to first identify the phase demonstrating highest atrial enhancement (Figure 1). This phase was isolated from the remainder of the TR-MRA images and saved as a separate series for review, such that the three readers were unable to determine from the image data whether it was a TR-MRA or CE-MRA study. Overall image quality was subjectively evaluated on a four-point scale: (1) Non-diagnostic image quality, (2) poor-moderate image quality (marginal-moderate sharpness of vessels, diagnostic but with significant artifacts), (3) Good image quality (good contrast, minimal artifacts, if present), (4) Excellent image quality, (high contrast between vessels and background, high sharpness of vascular edges, no artifacts). Relative LA opacification was evaluated on a four-point scale as follows: (1) LA is less bright than right cardiac chambers, (2) LA is equal in brightness to right chambers, (3) LA is brighter than right chambers but less bright than aorta, (4) LA is the brightest structure. Scoring examples are displayed in Figure 2. Whether all PVs are visualized diagnostically was assessed in a binary fashion.
Figure 1.
Representative coronal subtracted magnetic resonance angiography (MRA) images displaying the left atrium and the neighboring structures. A: Conventional contrast-enhanced MRA; B–E: Time-resolved TR-MRA. Analysis of TR-MRA was performed on the phase demonstrating highest left atrial opacification relative to the neighboring structures. In this case, panel E was selected for the readers’ analysis.
Figure 2.
Example of scoring methodology used to assess overall image quality and relative left atrial opacification. Observers evaluated sequences in random order, while blinded to the imaging protocol. Overall image quality was evaluated on a 4-point scale: (1) Non-diagnostic image quality, (2) poor-moderate image quality, (3) Good image quality, (4) Excellent image quality. Relative LA opacification was evaluated on a 4-point scale: (1) LA is less bright than right cardiac chambers; (2) LA is equal in brightness to right chambers; (3) LA is brighter than right chambers but less bright than aorta; (4) LA is the brightest structure.
QUANTITATIVE ANALYSIS
Quantitative comparison included signal-to-noise ratio (SNR) and contrast-to-noise (CNR) ratio in addition to PV ostia measurements. A 3.0 × 3.0 cm region of interest (ROI) was placed in the mid-LA on coronal-subtracted images to quantify the signal intensity (SI) and an additional ROI of the same size was placed outside the body in air for background noise measurement. SNR for each sequence was defined as the ratio of mean SILA to the standard deviation of background noise (SIAIR). CNR was defined as difference in mean signal intensity between SILA and SIAIR divided by the standard deviation of SIAIR. PV ostia size was measured, by the three readers, on a perpendicular plane to the long axis of the vessel on a coronal view, as close to the LA cavity as possible. In the presence of a common ostium, the vascular trunk size was recorded instead and reported separately.
STATISTICAL ANALYSIS
Continuous variables were expressed as mean ±SD. Categorical variables were expressed as number (percentage). Differences in mean overall image quality and LA opacification scores as well as CNR and SNR between the protocols was assessed using Student’s t-test while differences in the proportion of diagnostically visualized PVs was assessed using z-test. Inter-reader variance for the two different protocols was measured using an ANOVA model of the difference in mean PV diameter among the readers. In order to assess inter-reader agreement on PV ostial diameter, intra-class correlation coefficient (ICC) in a two-way mixed-effects model was obtained, the readers being the fixed-effect in the model.
RESULTS
IMAGE ACQUISITION
One hundred patients were included in this study (74 males and 26 females; mean age of 60.7 ±8.8 years, range 40–81 years). All scans were completed successfully and no technical or data reconstruction problems occurred. Total mean contrast administered per scan was 34 ±7.3cc (32.9 ±8.3cc in CE-MRA vs. 35.1 ±6.1cc in TR-MRA, p=0.169). No adverse reactions were reported.
QUALITATIVE ANALYSIS
Each reader evaluated all 100 scans for a total of 300 observations. The results of the qualitative analysis are detailed in Table 1. In brief, 3(2%), 71(47%), 62(41%) and 14(9%) scans in the CE-MRA group vs. 41(27%), 83(55%), 24(16%) and 2(2%) scans in the TR-MRA group received an overall image quality score of 4 (best), 3, 2 and 1, respectively (p<0.0001). On pooled analysis, mean score was 2.42 ±0.69 in the CE-MRA vs. 3.10 ±0.69 in TR-MRA group (p<0.0001) and TR-MRA received a statistically significant higher image quality score by all three readers (Table 1). With regards to relative LA opacification, 30(20%), 17(11%), 48(32%) and 55(37%) scans in the CE-MRA group vs. 67(45%), 69(46%), 11(7%) and 3(2%) scans in the TR-MRA group received a score of 4 (best), 3, 2 and 1, respectively (p<0.0001). On pooled analysis, mean score was 2.15 ±1.13 in the CE-MRA vs. 3.33 ±0.70 in TR-MRA group (p<0.0001) and TR-MRA received a significantly higher LA opacification score by all three readers (Table 1). The proportion of diagnostically visualized pulmonary veins was 137/150 (91%) in the CE-MRA group vs. 147/150 (98%) with TR-MRA (p=0.010) (Table 1). Figure 3 displays a coronal slice as well as 3-dimensional reconstruction of the LA and neighboring structures in two patients using TR-MRA (Panels A & B) and CE-MRA (Panels C & D).
Table 1.
Qualitative comparison of image quality on TR-MRA and CE-MRA.
| Reader | Sequence | Mean Image Quality Score | Relative LA Opacification Score | Diagnostic PV Visualization, n (%) |
|---|---|---|---|---|
| Reader 1 | CE-MRA | 2.56 ±0.61 | 2.44 ±1.36 | 48 (96) |
| TR-MRA | 3.18 ±0.63 | 3.52 ±0.61 | 50 (100) | |
| p-value | <0.0001 | <0.0001 | 0.153 | |
|
| ||||
| Reader 2 | CE-MRA | 2.14 ±0.70 | 2.10 ±1.05 | 43 (86) |
| TR-MRA | 2.66 ±0.66 | 3.04 ±0.60 | 48 (96) | |
| p-value | 0.0002 | <0.0001 | 0.081 | |
|
| ||||
| Reader 3 | CE-MRA | 2.56 ±0.67 | 1.9 ±0.86 | 46 (92) |
| TR-MRA | 3.42 ±0.57 | 3.44 ±0.79 | 49 (98) | |
| p-value | <0.0001 | <0.0001 | 0.169 | |
|
| ||||
| Total | CE-MRA | 2.42 ±0.69 | 2.15 ±1.13 | 137 (91) |
| TR-MRA | 3.10 ±0.69 | 3.33 ±0.70 | 147 (98) | |
| p-value | <0.0001 | <0.0001 | 0.0102 | |
CE-MRA: contrast-enhanced (FLASH) MRA; LA: left atrium; PV: pulmonary vein.
Figure 3.
Examples of coronal slice as well as 3-dimensional reconstruction of the LA in two patients using TR-MRA (Panels A & B) and CE-MRA (Panels C & D).
QUANTITATIVE ANALYSIS
Mean SNR was significantly higher in the TR-MRA group compared to CE-MRA (106.8 ±41. vs. 277.9 ±48.9, p=0.002). Similarly, mean CNR was significantly higher in the TR-MRA group compared to CE-MRA (100.3 ±41.7 vs. 70.7 ±48.0, p=0.002). Seventeen left common PVs were reported and excluded from comparative PV diameter analysis while 28 right PV variants (middle veins) were reported but were not measured. Mean diameter of right superior PV, right inferior PV, left superior PV, and left inferior PV was 17.89 ±3.22mm, 17.56 ±3.27mm, 16.94 ±3.56mm, 16.55 ±2.81mm, respectively. PV measurements are summarized in Table 2. Comparison of mean PV diameter using ANOVA showed a statistically significant difference between readers for right superior PV (RSPV), right inferior PV (RIPV) and left superior PV (LSPV). However, inter-reader variance of individual PV measurements for both MR techniques ranged between 0.62 and 1.47mm, unlikely to be of clinical significance (Table 2). ICCs for PV measurements with CE-MRA ranged between 0.47 and 0.64, indicating a fair-good correlation (correlation being highest with LSPV, followed by RIPV, RSPV and lowest for LIPV). In comparison, ICC with TR-MRA were higher for each vein, ranging from 0.62–0.81, indicating a good-excellent correlation between readers (correlation was highest for RIPV, followed by LSPV, RSPV and lowest for LIPV).
Table 2.
Inter- and intra-reader comparison of pulmonary vein measurements on TR-MRA and CE-MRA.
| Vein | Reader | CE-MRA | TR-MRA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Diameter | P | IRV | ICC | Mean Diameter | p | IRV | ICC | ||
| RSPV | Reader 1 | 17.06 ±2.69 | <0.0001 | 1.33 | 0.58 | 18.15 ±3.58 | 0.0003 | 1.20 | 0.67 |
| Reader 2 | 16.11 ±3.04 | 17.33 ±2.74 | |||||||
| Reader 3 | 18.85 ±3.15 | 19.83 ±2.73 | |||||||
|
| |||||||||
| RIPV | Reader 1 | 17.42 ±2.98 | 0.059 | 0.62 | 0.62 | 18.49 ±3.25 | 0.002 | 1.07 | 0.81 |
| Reader 2 | 16.55 ±3.36 | 16.39 ±3.30 | |||||||
| Reader 3 | 18.09 ±3.19 | 18.33 ±3.04 | |||||||
|
| |||||||||
| LSPV | Reader 1 | 15.95 ±2.99 | 0.0004 | 1.50 | 0.64 | 17.27 ±2.96 | 0.0004 | 1.47 | 0.68 |
| Reader 2 | 14.14 ±2.98 | 16.09 ±2.99 | |||||||
| Reader 3 | 17.55 ±3.70 | 19.23 ±3.67 | |||||||
|
| |||||||||
| LIPV | Reader 1 | 15.94 ±2.44 | 0.498 | 0.69 | 0.47 | 17.40 ±2.91 | 0.118 | 0.49 | 0.62 |
| Reader 2 | 15.32 ±2.88 | 16.33 ±3.16 | |||||||
| Reader 3 | 16.10 ±2.67 | 17.68 ±2.38 | |||||||
CE-MRA: contrast-enhanced (FLASH) MRA; IRV: Inter-reader variance; ICC: intra-class coefficient; RSPV: right superior pulmonary vein; RIPV: right ingerior pulmonary vein; LSPV: left superior pulmonary vein; LIPV: left inferior pulmonary vein.;
DISCUSSION
In this study, we examined the hypothesis that TR-MRA, a dynamic multiphase sequence that does not rely on bolus timing, is a viable alternative method to the traditional CE-MRA for pre-ablation imaging in 100 patients with AF. The main finding of this study is that pulmonary venous maps displayed higher quality, better LA relative opacification, and a higher proportion of diagnostically visualized veins on TR-MRA compared to CE-MRA. Both SNR and CNR were significantly higher on TR-MRA images. While both techniques displayed minimal inter-reader variance of PV measurements, the inter-reader correlation was higher on TR-MRA for all PVs.
TECHNICAL FEATURES OF TR-MRA
Conventional 3D CE-MRA techniques rely on timing central k-space acquisition with the peak contrast concentration in the target vessel. Indeed, incorrect bolus timing may lead to sub-optimal contrast and undesired vascular overlay. CE-MRA only provides static structural information given long acquisition times. TR-MRA, in comparison, combines parallel imaging with a 3D view-sharing implementation. By acquiring multiple 3D-datasets with excellent temporal and spatial resolution, it can thus evaluate contrast flow hemodynamics [8]. TR-MRA differs in the trajectory of k-space sampling compared with other temporal under-sampling techniques described in the literature, such as TRICKS [11] and time-resolved echo-shared angiographic technique (TREAT) [12]. While all of these techniques use a keyhole imaging approach to sample the center of k-space more frequently compared with the periphery, TR-MRA uses a spiral and pseudo-stochastic rather than a linear trajectory to traverse a full range of k-space with every iteration, from center to periphery. The peripheral region of the k-space is sampled with a longer temporal footprint depending on the radial distance from the center of the k-space. Oversampling of the central portion of the k-space leads to high contrast resolution- a highly desirable property in pre-ablation PV imaging where relative LA opacification and contrast difference with the surrounding vessels are needed for accurate 3D reconstructions of the LA-PV complex during image-guided ablation procedures. TR-MRA, with its high temporal resolution, allows increased contrast flow-rate (5 ml/s for TR-MRA vs. 2ml/s for CE-MRA), leading to a more compact bolus with higher peak enhancement, rapid contrast clearance from the pulmonary arteries and a sharp arterio-venous separation. Finally, short acquisition time with TR-MRA reduces motion artifact, which typically hinders the interpretation of conventional MRA sequences. For the current study, we modified the “out-of-the-box” default vendor parameters to allow increased spatial resolution to more closely match that of CE-MRA (both 1.0 × 1.0 mm in-plane resolution; CE-MRA 1.3 mm slice thickness, TR-MRA 1.5 mm slice thickness). This required decreasing temporal resolution from 2 sec by default to 6.1 seconds. However, our results would suggest that this temporal resolution was adequate for diagnostic PV evaluation.
COMPARISON OF TR-MRA vs. CE-MRA
The above-mentioned features of TR-MRA make it an attractive choice for dynamic or static imaging of various vasculatures. In a study by of 26 patients with congenital cardiac and vascular anomalies, Vogt et al. compared arterial maps with 4D-TR-MRA vs. 3D-CE MRA [9]. While TR-MRA displayed slightly lower overall image quality compared to 3D CE-MRA, the diagnostic capacity, vessel borders sharpness, and SNR/CNR were identical between the two techniques. Moreover, the temporal resolution and dynamic component of TR-MRA provided information on the flow direction (for cardiac shunts and retrograde blood flow evaluation), vessel patency (for stenosis and stents evaluation) and vessel lumen (for dissection detection). TR-MRA has also been used in the evaluation of extra-cranial internal carotid arteries and compared with traditional CE-MRA [13]. TR-MRA demonstrated high accuracy only slightly inferior to CE-MRA in the diagnosis of carotid stenosis, and the correlation between the two techniques was excellent (r=0.82, p<0.001). Hemodynamic flow analysis on TR-MRA permitted the detection of differential rates of filling between normal and severely diseased vessels as well as collateral/retrograde flow in the setting of occlusion, providing valuable diagnostic information. Furthermore, in a small study of 26 patients presenting for AF ablation, Schonberger et al. evaluated the pulmonary venous circulation with time-resolved TR-MRA and compared it to conventional CE-MRA [10]. PV measurements, visualization of variant PV anatomy, PV conspicuity score, and overall image quality were all similar for both techniques and displayed high inter-reader agreement. Our study, which was performed in a larger cohort of 100 patients, extends and validates the findings of Schonberger et al, adding further information regarding differences in SNR, CNR, as well as more detailed measurements of image quality. On qualitative analysis, TR-MRA was attributed a significantly higher overall quality score by all 3 readers. LA-PVs opacification relative to neighboring vasculature and the proportion of diagnostically visualized veins were found to be significantly higher with TR-MRA compared to CE-MRA. On quantitative analysis, both SNR and CNR were higher on TR-MRA, likely a reflection of greater peak enhancement from faster injection rates, supported by higher image quality score from all 3 readers. As to the PV measurements, since we could not perform a direct comparison of the diameter of each PV using both techniques, we performed a per-vein ANOVA of the difference in mean diameter between the readers in order to assess inter-reader variance. This test allows us to assess whether the there is a similar level of vein diameter agreement between readers for each technique. The inter-reader variance was similar between both techniques and likely too small to constitute a clinically significant difference (0.62–1.47 mm). ICC of PV measurements between readers was found to be between 0.47 and 0.64 on CE-MRA (indicating a fair-good correlation), and between 0.62 and 0.81 on TR-MRA (indicating a good-excellent correlation).
LIMITATIONS
MRA was performed using one technique in each patient, precluding a direct, per-patient comparison of the two sequences. However, our study design avoids sequential contrast-based imaging which can skew the results in favor of one protocol over the other since some contrast can persist in the pulmonary veins, thereby having an additive effect on vessel enhancement. Differences in rate of injection likely at least partially account for differences in LA opacification between the techniques, with a tighter contrast bolus expected for the TR-MRA procedure given its higher rate of contrast injection, leading to better differentiation of LA from adjacent structures. A faster injection rate, however, is not widely used for CE-MRA given the longer acquisition times and risk of missing the bolus. Higher CNR and SNR values for the TR-MRA sequence could be partially accounted for by the slightly higher slice thickness (1.5 mm vs. 1.3 mm for CE-MRA). We did not analyze intrareader variability, however, given the high levels of interreader agreement, we expect intrareader variability to be low. We have only reviewed 3D reconstructions on image viewing workstations and have not compared 3D shells generated by both techniques on clinical workstations used in electrophysiological procedures. Although we would expect that better quality imaging on the viewing workstations would translate into better 3D shells on workstations in the electrophysiological lab, this was beyond the scope of the present study.
CONCLUSION
TR-MRA, modified to maximize spatial resolution, offers a superior alternative method to traditional contrast-enhanced CE-MRA technique for performing high quality MRA examinations in patients with AF. The dynamic multiphase acquisition obviates the need for reliance on accurate bolus timing to capture optimal pulmonary venous anatomy and is thus a useful alternative to traditional techniques.
Acknowledgments
Funding - The study was funded by the National Institutes of Health (grant nos. K23HL089333 and R01HL116280) as well as by a Biosense Webster grant to Dr Nazarian; the Roz and Marvin H. Weiner and Family Foundation; the Dr. Francis P. Chiaramonte Foundation; Marilyn and Christian Poindexter; and the Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund. Funding bodies had no role in the design of the study; collection, analysis, or interpretation of data; or in writing the manuscript.
Not applicable
LIST OF ABBREVIATIONS
- PV
pulmonary vein
- MRA
magnetic resonance angiography
- CE-MRA
contrast-enhanced magnetic resonance angiography
- TR-MRA
time-resolved magnetic resonance angiography
- LA
left atrium
- SI
signal intensity
- AF
atrial fibrillation
- TR
repetition time
- TE
echo time
- CNR
contrast to noise ratio
- SNR
signal to noise ratio
- ROI
regions of interest
- ICC
intra-class coefficient
- RSPV
right superior pulmonary vein
- RIPV
right inferior pulmonary vein
- LSPV
left superior pulmonary vein
- LIPV
left inferior pulmonary vein
Footnotes
Ethics approval and consent to participate – The Johns Hopkins Institutional Review Board (JH-IRB) approved the study and retrospective study data was obtained under a HIPPA compliant waiver of consent.
Competing interests - Dr. Nazarian has received research grant funding from Biosense Webster during the conduct of the study. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Tarek Zghaib, Division of Cardiology, Johns Hopkins Medicine, Baltimore, MD.
Adeel Shahid, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
Chiara Pozzessere, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
Kristin K. Porter, Department of Radiology, University of Alabama at Birmingham.
Linda C. Chu, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
John Eng, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
Hugh Calkins, Division of Cardiology, Johns Hopkins Medicine, Baltimore, MD.
Ihab R. Kamel, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
Saman Nazarian, Division of Cardiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Stefan L. Zimmerman, Russell A. Morgan Department of Radiology and Radiological Sciences, Johns Hopkins Medicine, Baltimore, MD.
References
- 1.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S-A, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 4.Dewire J, Calkins H. Update on atrial fibrillation catheter ablation technologies and techniques. Nat Rev Cardiol. 2013;10:599–612. doi: 10.1038/nrcardio.2013.121. [DOI] [PubMed] [Google Scholar]
- 5.Maksimović R, Dill T, Ristić AD, Seferović PM. Imaging in percutaneous ablation for atrial fibrillation. Eur Radiol. 2006;16:2491–504. doi: 10.1007/s00330-006-0235-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghaye B, Szapiro D, Dacher J-N, Rodriguez L-M, Timmermans C, Devillers D, et al. Percutaneous ablation for atrial fibrillation: the role of cross-sectional imaging. Radiographics. 2003;23(Spec No suppl_1):S19-33–50. doi: 10.1148/rg.23si035513. [DOI] [PubMed] [Google Scholar]
- 7.Hauser TH, Peters DC, Wylie JV, Manning WJ. Evaluating the left atrium by magnetic resonance imaging. Europace. 2008;10(Supplement 3):iii22–iii27. doi: 10.1093/europace/eun223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song T, Laine AF, Chen Q, Rusinek H, Bokacheva L, Lim RP, et al. Optimal k-space sampling for dynamic contrast-enhanced MRI with an application to MR renography. Magn Reson Med. 2009;61:1242–8. doi: 10.1002/mrm.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt FM, Theysohn JM, Michna D, Hunold P, Neudorf U, Kinner S, et al. Contrast-enhanced time-resolved 4D MRA of congenital heart and vessel anomalies: image quality and diagnostic value compared with 3D MRA. Eur Radiol. 2013;23:2392–404. doi: 10.1007/s00330-013-2845-7. [DOI] [PubMed] [Google Scholar]
- 10.Schonberger M, Usman A, Galizia M, Popescu A, Collins J, Carr JC. Time-Resolved MR Venography of the Pulmonary Veins Precatheter-Based Ablation for Atrial Fibrillation. J Magn Reson Imaging. 2013;137:127–37. doi: 10.1002/jmri.23808. [DOI] [PubMed] [Google Scholar]
- 11.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36:345–51. doi: 10.1002/mrm.1910360304. [DOI] [PubMed] [Google Scholar]
- 12.Pinto C, Hickey R, Carroll TJ, Sato K, Dill K, Omary RA, et al. Time-resolved MR Angiography with Generalized Autocalibrating Partially Parallel Acquisition and Time-resolved Echo-sharing Angiographic Technique for Hemodialysis Arteriovenous Fistulas and Grafts. J Vasc Interv Radiol. 2006;17:1003–9. doi: 10.1097/01.RVI.0000220395.05050.77. [DOI] [PubMed] [Google Scholar]
- 13.Lim RP, Shapiro M, Wang EY, Law M, Babb JS, Rueff LE, et al. 3D time-resolved MR angiography (MRA) of the carotid arteries with time-resolved imaging with stochastic trajectories: comparison with 3D contrast-enhanced Bolus-Chase MRA and 3D time-of-flight MRA. AJNR Am J Neuroradiol. 2008;29:1847–54. doi: 10.3174/ajnr.A1252. [DOI] [PMC free article] [PubMed] [Google Scholar]