Abstract
Background
The symptoms of major depressive disorder (MDD) are rapidly alleviated by administration of a single dose of the glutamatergic modulator ketamine. However, few studies have investigated the potential sustained neural effects of this agent beyond immediate infusion. This study used functional magnetic resonance imaging (fMRI) to examine the effect of a single ketamine infusion on the resting state default mode network (DMN) at two and 10 days after a single ketamine infusion in unmedicated MDD subjects as well as healthy controls (HCs).
Methods
Data were drawn from a double-blind, placebo-controlled, crossover study of 58 participants (33 with MDD and 25 HCs) who received an intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo on two separate test days spaced two weeks apart. Eight minutes of fMRI resting state data were acquired at baseline and at about two and 10 days after both infusions. The DMN was defined using seed-based correlation and compared across groups and scans.
Results
In MDD subjects, connectivity between the insula and the DMN was normalized compared to HCs two days post-ketamine infusion. This change was reversed after 10 days and did not appear in either of the placebo scans. Group-specific connectivity differences in drug response were observed, most notably in the insula in MDD subjects and the thalamus in HCs.
Conclusions
Connectivity changes in the insula in MDD subjects suggest that ketamine may normalize the interaction between the DMN and salience networks, supporting the triple network dysfunction model of MDD.
Keywords: major depressive disorder, ketamine, functional magnetic resonance imaging (fMRI), resting state, default mode network, glutamatergic modulator
Introduction
Major depressive disorder (MDD) is a common mental disorder (1, 2) predicted to be the leading cause of disease burden worldwide by 2030 (3). Despite the severity and prevalence of this disorder, currently available therapeutics may take weeks to exert an antidepressant effect, and only slightly more than one-third of patients will remit after a first treatment (4). Furthermore, up to one-third of patients with MDD will not enter remission even after treatment with four different antidepressants (4).
In the search for more effective treatments, numerous studies have found that a single, sub-anesthetic dose of the glutamatergic modulator ketamine has rapid, robust, and relatively sustained antidepressant effects (5–9), even in patients with treatment-resistant MDD or bipolar depression (7–10). Neural correlates of the acute effects of ketamine administration have been investigated in several functional magnetic resonance imaging (fMRI) studies. These identified immediate, robust, and reliable changes in blood oxygen level dependent (BOLD) resting state signal in healthy volunteers (11–15). However, few studies have investigated the potential sustained neural effects of ketamine beyond immediate infusion, nor correlated these changes with symptom improvement in MDD (16).
Intrinsic brain connectivity differences between MDD and healthy control (HC) samples have been well studied with fMRI (17, 18). In particular, the triple network model of dysfunction between the default mode (DMN), salience (SAL), and central executive (CEN) networks has been proposed to explain depressive symptomology as well as other neuropsychiatric disorders (19). With regard to MDD, this hypothesis posits that depressive symptoms result from increased activity in the DMN, a network responsible for introspection and rumination (20), in contrast with reduced activity in the SAL and CEN; the former mediates the processing of salient information from the external world, and the latter is responsible for working memory and attention (19).
Only two studies have investigated fMRI changes 24 hours post-ketamine infusion in MDD subjects. Murrough and colleagues (2015) found that reduced activation of the right caudate in response to negatively valenced emotional stimuli in treatment-resistant MDD patients was normalized after ketamine administration (21). They also found that resting state connectivity in the right caudate predicted treatment response, suggesting that the caudate was specifically affected by ketamine. In a resting state analysis, Abdallah and colleagues (2016) found that the decreased global connectivity observed in their MDD subjects at baseline was normalized to HC levels in ketamine responders (22).
In addition, only two fMRI studies have examined ketamine-induced changes in HCs the day after ketamine administration. One resting state study found that ketamine reduced connectivity of the dorsal nexus with the DMN and cognitive control network one day after blinded infusion (23). The other found reduced neural reactivity in the bilateral amygdalohippocampal complex during emotional stimulation with negative emotional faces (24). Taken together, these studies suggest that ketamine decreases brain response in regions typically identified as hyperactive in depression (25).
The current study sought to investigate the neural correlates of longer-term, sustained mood improvements within the first 10 days after ketamine infusion in medication-free, treatment-resistant MDD patients compared to a group of HCs. The DMN was used to investigate differences in resting state fMRI after ketamine infusion. Based on previous findings that ketamine normalizes BOLD activity in regions altered in depression (22, 26), we hypothesized that the DMN differences between the MDD and HC subjects would be reduced after ketamine administration, particularly in regions associated with the SAL and CEN.
Materials and Methods
Subjects
Thirty-three MDD subjects and 25 HCs who had a resting state fMRI scan as part of a larger study (NCT#00088699, NIH Protocol #04-M-0222, substudy 4) were included in this analysis. All subjects were between 18 and 65 years old and were recruited between 2011 and 2016. Subject demographics are listed in Supplemental Table S1. Each subject provided written informed consent as approved by the National Institutes of Health Combined Central Nervous System (CNS) Institutional Review Board.
MDD subjects were diagnosed with recurrent MDD without psychotic features and were experiencing a current depressive episode of at least moderate severity lasting at least four weeks; severity was defined as a Montgomery-Asberg Depression Rating Scale (MADRS) (27) total score ≥ 20 at screening and prior to each infusion. Patient diagnoses were confirmed using the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV Disorders with psychiatric screen, patient version (SCID-I/P) (28). MDD subjects were also required to be treatment-resistant, defined as not having responded to at least one adequate antidepressant dose/duration trial as assessed using the Antidepressant Treatment History Form (29). In addition, they were required to be free of comorbid substance abuse or dependence for at least three months (except for nicotine or caffeine) prior to inpatient admission, have a negative drug and alcohol urine toxicology screen and pregnancy test within 24 hours prior to each MRI session, have no unstable medical problems, and be in good physical health as assessed by medical history, physical examination, blood labs, urinalysis, and toxicology. Other exclusion criteria included concomitant treatment with psychotropic medications in the two weeks before randomization (five weeks for fluoxetine, three weeks for aripiprazole), and the presence of metallic (ferromagnetic) implants.
HCs were screened using the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV Disorders, non-patient version (SCID-NP) (30) and had no personal or family history (first-degree relative) of mood or Axis I disorder. All subjects were medically healthy as determined by medical history, physical examination, blood labs, urinalysis, and toxicology.
Study design
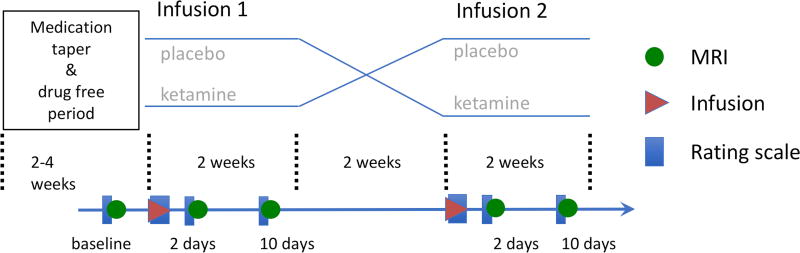
The double-blind, placebo-controlled, crossover study design is illustrated in Figure 1, which also lists the scans and rating scales obtained. All subjects received an intravenous infusion of either saline solution or 0.5 mg/kg of ketamine hydrochloride; two weeks later (to avoid carryover effects), subjects were blindly crossed over to the other arm of the study. Medical staff administering the infusion, investigators, raters, and subjects were all blind to randomization, which was performed by the National Institutes of Health Clinical Center pharmacy department. All subjects participated in both arms of the study because depressive symptoms returned for all MDD subjects before the second infusion. Ketamine infusions were administered intravenously over 40 minutes via an infusion pump on an inpatient unit by medical staff with advanced cardiac life support training.
Figure 1.
Summary of the study’s double-blind, placebo-controlled, crossover design showing the medication taper and drug-free period in relation to the imaging scans (green circles), infusions (red triangles), and rating scales (blue rectangles) in both the placebo and ketamine study arms.
All MDD subjects were medication-free for two weeks before randomization and throughout the entire study. Similarly, HCs were not permitted to take any medications with CNS effects throughout the study.
Rating scales
The MADRS was used throughout the study to obtain mood ratings. Ratings were obtained at -60 (baseline), 40, 80, 120, and 230 minutes post-infusion, as well as on Days 1, 2, 3, 7, 10, and 11. Average MADRS scores were estimated using a linear mixed effects model with baseline as a covariate using all time points. A separate model was used for each group.
fMRI scanning
Resting state fMRI scans (duration of 8 minutes, resolution of 3.75x3.75x3.5 mm) were acquired on a 3T scanner (HDx, General Electric Healthcare, Milwaukee, WI) along with an anatomical scan (1 mm isotropic resolution) using an eight-channel coil. The scans were conducted at baseline (b; in this case, two or three days before the first infusion) and at Days 2 or 3 and 10 or 11 after placebo (p2, p10) and ketamine (k2, k10) infusions, yielding an intended total of five scans per subject (b, p2, p10, k2, k10). Subjects were instructed to close their eyes, relax, and not fall asleep. Cardiac and respiration traces were also recorded using the manufacturer’s photo-plethysmograph and respiratory belt, respectively. Imaging acquisition parameters and details of the preprocessing methods can be found in the Supplemental Materials.
Data Analysis
Across all analyses, data were processed using AFNI version 17.3.05 (Nov. 2016) (31). The DMN was defined using a seed-based correlation method (3dTcorr) where the average time course from a 6mm radius sphere placed at the posterior cingulate cortex (3dROIstats) at the MNI template coordinates of (0,-52,27) (33) was correlated with all other brain voxels. The correlation values were converted to Z-scores using Fisher transform.
Group analyses were performed with 3dLME (34) using a linear mixed effects model including both the MDD and HC groups. The model had a fixed effect of scan type across all scan days (b, k2, k10, p2, p10) and a random effect of subject (to account for the repeated scans). Between-group differences were included for each scan time point (b, k2, k10, p2, p10). Post-hoc contrasts were also calculated in order to examine differences between each post-infusion scan and baseline (k2-b, k10-b, p2-b, p10-b) as well as the difference between post-ketamine and post-placebo scans at Days 2 and 10 (k2-p2, k10-p10). Family-wise error (FWE) multiple comparison correction for the group maps was performed by first estimating the smoothness of the data after pre-processing (3dFWHMx). Corrected cluster size was calculated using 3dClustSim with this value. Group maps were FWE-corrected to p<0.05 with an initial threshold of p<0.05 using a cluster size of >120.
In order to specifically investigate the triple network model, a region of interest (ROI) analysis was performed using the FIND ROI (35) set for the SAL (anterior and posterior) and CEN (left and right). Further details regarding the ROIs for these networks can be found in the Supplemental Materials and are also shown in Supplemental Figure S1. The average correlation values with the DMN within these ROIs was calculated per subject and averaged over the group. Statistics for these values were calculated using a linear mixed effect model in R (36) separately for each group, with scan type as a fixed factor (five levels) and subject as a random factor. Significance was established at p<0.05.
Results
Subject and scan characteristics
The MDD and HC groups did not differ significantly with regard to age (MDD: 36±10 years, HC: 33±10 years; t=0.96, p=0.34) or gender (MDD: 61% female, HC: 60%; chi2=0.05, p=0.82). Supplemental Table S2 lists the number of scans completed at each time point as well as the final number included in the analyses. Thirty-six of the 236 total scans obtained were excluded from the analysis; 10 scans (six MDD, four HC) were excluded due to incomplete physiological data, 20 scans (nine MDD, 11 HC) were excluded for excessive motion, and six scans (three MDD, three HC) were excluded due to high correlation between the respiration volume trace and the average global signal, which increased correlations across the brain. No significant differences in motion between groups or scan pairs were observed except for the p2 scan (average motion per TR=0.03 mm for MDD and 0.04 mm for HC (t=-3.17, p=0.003)). No significant differences in respiration or heart rate were found between groups or scans (details appear in Supplemental Figure S2) .
MADRS changes
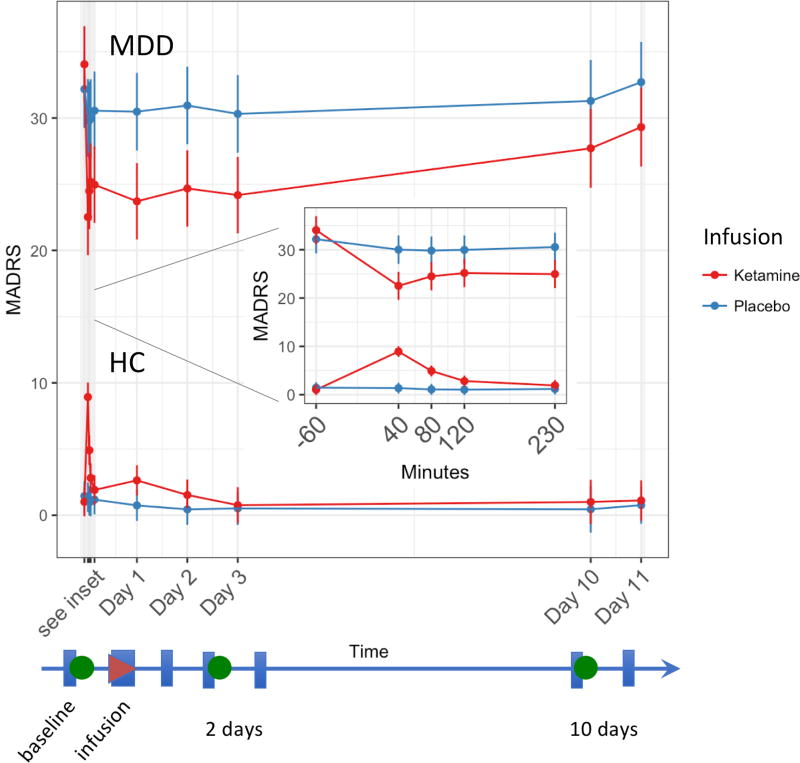
Within hours of ketamine infusion, MDD subjects had significantly improved MADRS scores, a change that was maintained at the Day 2 time point (mean difference of 9.4, p<0.001; see Figure 2). This change was significantly different (p<0.001) from the placebo response for this group at Day 2 and until the scan at Day 10 (p<0.02).
Figure 2.
Change in Montgomery-Asberg Depression Rating Scale (MADRS) total score for the healthy control (HC) and major depressive disorder (MDD) groups for both ketamine (red) and placebo (blue) infusions over the course of the study. The group mean is shown as a circle, and the error bars are the standard error. The linear timescale indicates the timing of the imaging scans (green circles), infusions (red triangle), and rating scale administrations (blue rectangles).
No significant differences in MADRS score were observed for the HCs from baseline or placebo at either Day 2 or Day 10. These results are consistent with findings from a recently published study of a larger cohort; details are available in (37).
DMN between-group differences for each scan day
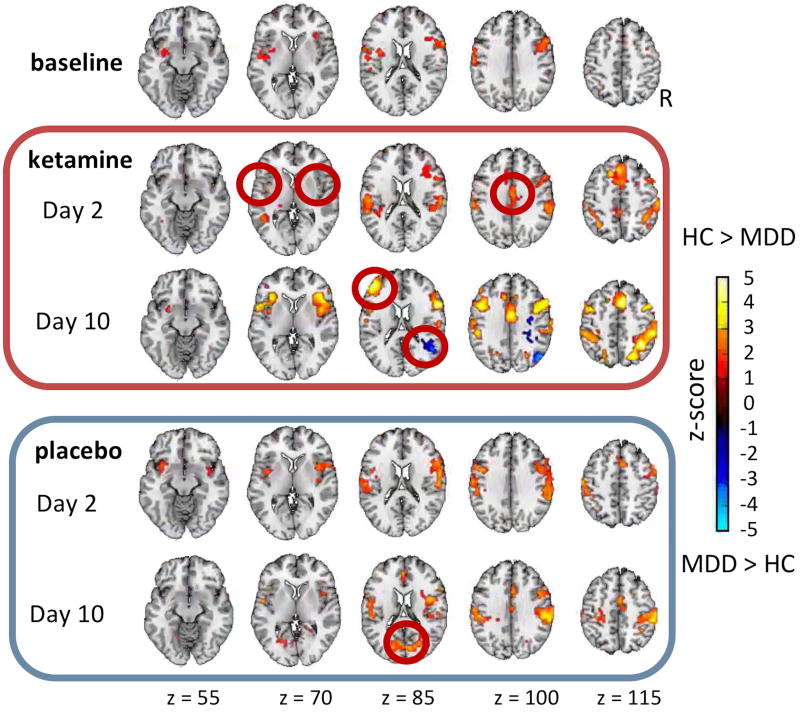
At baseline, the HCs had greater connectivity with the DMN than the MDD group in the right dorsolateral prefrontal cortex (BA6,9) and left post-central gyrus (insula to BA43) (see Figure 3 and Supplemental Table S3 for cluster coordinate locations). Across all the scans (b, k2, p2, k10, p10), the HCs had greater connectivity with the DMN than the MDD group in the right pre-central gyrus (BA44), as well as the left and right post-central gyrus (BA40).
Figure 3.
Group (healthy control (HC), major depressive disorder (MDD)) differences in connectivity with the posterior cingulate cortex (PCC) seed of the default mode network (DMN) across scans at each scan day. The mean Z-score maps are shown at a threshold of p< 0.05, familywise error (FWE) corrected. The red circles highlight regions of significant difference (second row: bilateral insula (salience network (SAL)) and anterior cingulate cortex (ACC, central executive network (CEN)); third row: right BA22 and left BA46 (SAL); fifth row: BA18).
A smaller difference unique to the k2 scan was noted with regard to connectivity of the insula with the DMN between the MDD and HC groups. This normalization between the groups returned to baseline by Day 10. The anterior cingulate cortex (ACC; BA24) showed increased connectivity in the HCs compared to MDD subjects at k2 that was still apparent at k10 but was not apparent at b or in the p2 scan. In the k10 scan, the right supra-marginal gyrus (BA22,39) showed increased connectivity in MDD subjects that was greater than in HCs; increased connectivity was also noted in the HCs compared to the MDD group in BA46 in this scan only. In the p10 scan, the occipital cortex (bilateral BA18) showed an increased difference in the HCs vs MDD subjects.
Many of the regions described above overlap with the CEN, which comprises the dorsolateral prefrontal cortex (DLPFC, BA8,9,10,46) as well as the SAL, which includes the ACC, insula, and ventrolateral prefrontal cortex (38). The DMN is composed of the ventromedial prefrontal cortex, the posterior cingulate cortex, bilateral inferior parietal cortex and the middle temporal lobe (39). These networks are illustrated in Supplemental Figure S1.
Group-specific differences in response to ketamine at Days 2 and 10
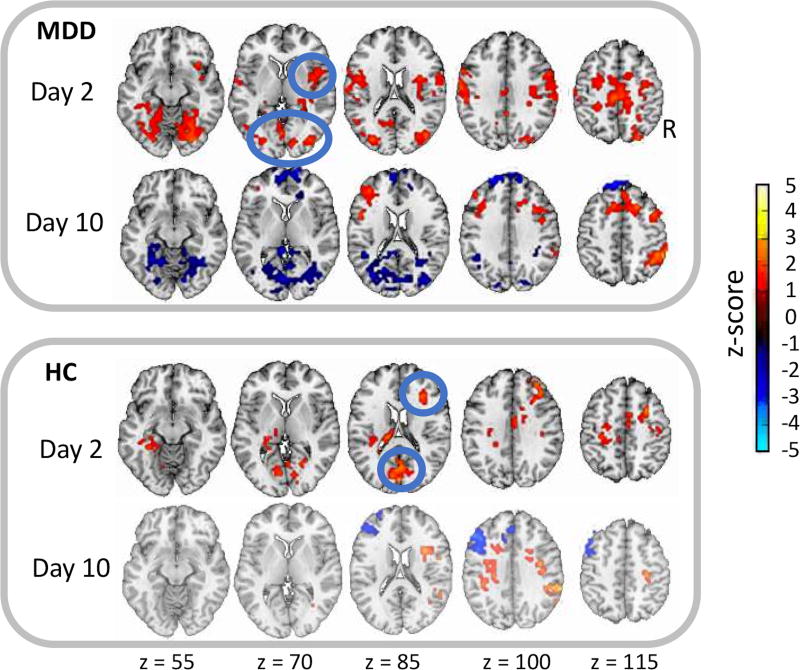
To further understand differences between the MDD and HC groups, group-specific maps were made to contrast the drug and placebo scans for each scan day (Day 2: k2-p2; Day 10: k10-p10) and each group (see Figure 4 for illustration and Supplemental Table S4 for cluster coordinates). Both groups had increased DMN connectivity at k2 compared to p2. MDD subjects had increases in the right and left insula, middle frontal gyrus (BA31), post-central gyrus (BA5), and occipital gyrus (BA18,19). HCs showed increases in the left thalamus, cingulate cortex (BA24), cuneus (BA18), and right middle frontal gyrus (BA6,8,9).
Figure 4.
Group-specific differences for connectivity with the posterior cingulate cortex (PCC) seed of the default mode network (DMN) across scans for the major depressive disorder (MDD) and healthy control (HC) groups. The mean Z-score maps illustrate the contrast between ketamine and placebo scans at two and 10 days post-infusion, respectively, and are shown at a threshold of p<0.05, familywise error (FWE) corrected. The blue circles highlight regions of significant difference (first row: insula and BA18, third row: BA6 and BA18).
At k10 (relative to p10), the MDD group showed reduced DMN connectivity to the occipital gyrus, a measure that had been elevated at k2. Other regions elevated at k2 for the MDD group (right and left insula, BA5 and BA31) were no longer increased at k10. However, the right post-central gyrus (BA40) showed an increase, and the left DLPFC (BA9) showed a decrease in DMN connectivity at k10.
Region-specific correlation changes between the CEN, SAL, and DMN
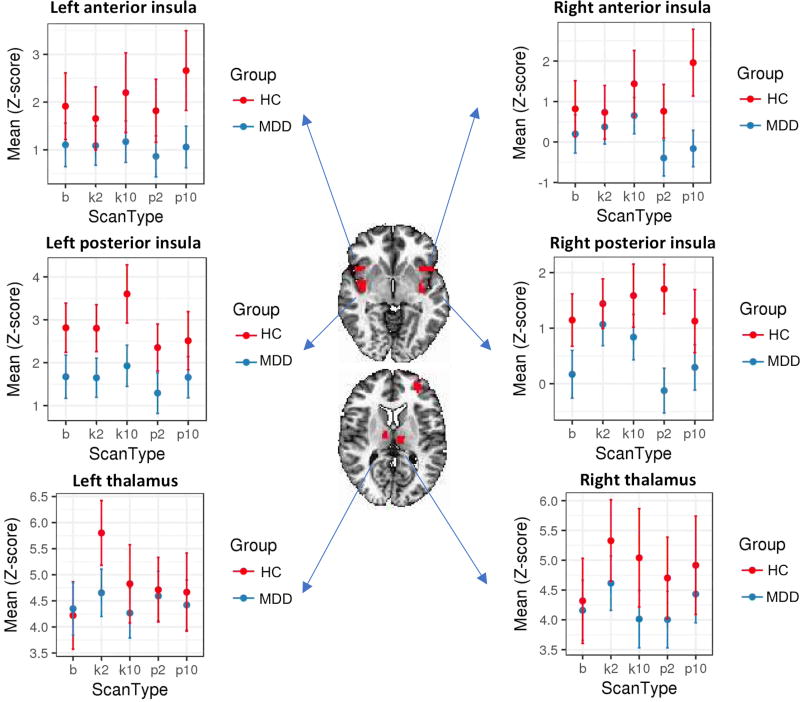
To compare the magnitude of the regional connectivity changes across scan days, group mean correlation values were calculated between ROIs for the CEN, SAL, and DMN. ROIs with significant within-group changes between the b and k2 scans—suggesting a ketamine effect—as well as the b and p2 scans were the right posterior insula for MDD and the left thalamus for HCs. For both the MDD and HC groups, Figure 5 displays the group mean correlations for these regions with the posterior cingulate cortex across each of the scan days, along with the opposite side ROI and the anterior insula for comparison.
Figure 5.
Mean connectivity for regions of interest (ROIs) with significant differences across scans for both healthy control (HC; red) and major depressive disorder (MDD; blue) groups for the bilateral anterior (first row), posterior insula (second row), and thalamus (third row). Error bars represent standard deviation.
Reduced connectivity was observed for all the ROIs in the MDD group compared to the HCs. Consistent with the whole-brain results, a significant increase in connectivity (Z score change=0.95, p<0.05) was observed in the right posterior insula for the MDD group. However, connectivity in the left posterior insula remained unchanged at all time points. In the left thalamus, a significant increase in connectivity was observed at k2 for the HCs (change=1.26, p=0.05) that was not found at other scan time points or in the MDD group.
Discussion
This double-blind, placebo-controlled, crossover fMRI study examined the effects of a single ketamine infusion on DMN connectivity in both MDD and HC subjects. We found that, compared with HCs, insular connectivity with the DMN was normalized in MDD subjects two days after a ketamine infusion, particularly in the right hemisphere. This change was reversed after 10 days and did not appear in either placebo scan. Furthermore, there were group-specific differences in regional connectivity with regard to drug response, notably for the insula in MDD subjects and for the thalamus in HCs.
Interestingly, connectivity regions were consistently different between the MDD and HC groups across the baseline and placebo scans, suggesting a reliable baseline difference between the groups. This is particularly important given the considerable overlap between previously described drug and placebo responses (40–42), and enabled the accurate determination of regions affected by ketamine. It should be noted, however, that several regions previously identified as having increased connectivity with the DMN in MDD subjects (compared to HCs) did not achieve significance in the present study, including the limbic regions (18, 43). One possible explanation is that DMN connectivity in our treatment-resistant population differed from that of other depressed populations, possibly due to the existence of depression subtypes(44). One study that focused specifically on differences between treatment-resistant (refractory) and non-refractory depression similarly found overall decreases in connectivity between MDD subjects and HCs (45). That study further identified the prefrontal areas (middle temporal and frontal gyri) regions as hypoactive in the treatment-resistant group compared to HCs, consistent with our study. It should be noted that the definition of treatment-resistance may vary between research groups, which may also contribute to variance in reported results. However, the use of subtypes that can be defined from resting state data (44) may help improve reproducibility.
In the present study, normalization of the connectivity between the insula and the DMN in MDD subjects two days post-ketamine infusion was consistent with the improvement in global brain connectivity previously observed in MDD patients one day post-ketamine infusion in this region (22). However, we also found that this region experienced a change in connectivity that corresponded to the response-relapse seen in the MADRS scores. This finding is particularly important because the insula shares substantial anatomical and functional connections with regions that have been implicated in the neurological differences observed in individuals with MDD (46). The insula is also implicated as a key node in the integration of external emotional stimuli and has been shown to play a role in interpreting emotional information and switching between the CEN and DMN (38). Thus, the post-ketamine increased connectivity between the insula and the DMN observed in the present study suggests an improved ability to process external stimuli which, in turn, may be linked to symptom improvement. Interestingly, the posterior insula, where we found the strongest pattern of normalization in MDD subjects, is linked to pain, sensorimotor processes, and language (47). This normalization may indicate relief of somatic depressive symptoms linked to the abnormal interoception associated with the insula (48). Indeed, a post-hoc correlation of connectivity between the right posterior insula and the DMN with the MADRS values for the MDD subjects supports the existence of this positive association (see Supplemental Figure S4).
A notable degree of change was also observed in the occipital cortex in the MDD group. Connectivity between the DMN and the occipital cortex was increased at Day 2 post-ketamine compared to baseline, but decreased at Day 10, potentially indicating a rebound effect. Changes in the occipital cortex have previously been associated with antidepressant use (49), and middle occipital activity has been shown to correlate with subsequent antidepressant response (50).
Our analysis also found regions of increased connectivity in the pre- and post-central gyri of the MDD group. Other studies have noted that gray matter is reduced in these regions in MDD subjects compared to HCs (51); our results may thus reflect a ketamine-modulated increase in neural plasticity. Overall, many of the regions showing increased connectivity with the DMN posterior cingulate cortex seed in MDD subjects post-ketamine overlapped with nodes in the CEN and SAL. This is consistent with the triple-network model of dysfunction, which posits that DMN connectivity with the CEN and SAL is disturbed in MDD and further suggests that ketamine may normalize the interaction of these networks with the DMN following symptom improvement. Although our discussion is presently limited to the regions used in our ROI analysis, future work using complex functional network analysis techniques (52) may provide more insight into the interplay between these networks.
In the HCs, the general increase of connectivity observed two days post-infusion is potentially inconsistent with the only study (23) that explored response to ketamine at one day post-infusion in HCs. That study found reduced functional connectivity of the pregenual ACC and medial prefrontal cortex with the DMN. Nevertheless, it is possible that the increase observed two days post-infusion may reflect a re-normalization effect occurring after the decrease seen at Day 1 by Scheidegger and colleagues.
Finally, the present study also found increased connectivity in the thalamus, occipital cortex, and prefrontal cortex; changes in these areas are consistent with other studies that examined changes during and immediately after ketamine infusion (53, 54). The increases we observed in the ACC and visual cortex may be attributable to changes in the balance of the SAL and CEN networks. It is interesting, however, that any connectivity differences were found at two days post-infusion in HCs, given that ketamine is quickly metabolized and that HCs showed no lasting behavioral effects beyond a few hours.
The study is associated with several limitations. First, we used an initial threshold of p<0.05 in order to enable investigation of regional differences between the groups and conditions in this study; however, increasing the initial threshold would have considerably decreased the number of significant regions identified (see Supplemental Figure S3). In addition, using a strict initial threshold of p<0.001, as recommended by some in the literature (55), would leave very few regions of significance. Thus, we chose a more lenient threshold in order to give a balanced report. This sensitivity to initial threshold is partly due to the relatively modest MDD and HC sample sizes compared to the heterogeneity of the population and drug response. Second, because this was a longitudinal study with repeated scans, we inevitably had a reduced number of subjects completing all scans, leading to missing data in the analysis; we controlled for this problem by using a linear mixed effects model that is specifically designed to handle this issue (34). Third, the baseline for a resting state study is not well defined, nor are the potential neural effects of expectancy, both of which are confounding factors. To address this, we included both a baseline and a placebo scan—each administered at two time points—to control for variations in the resting state data that would be unrelated to ketamine response, both in terms of drug anticipation or physiological/natural neural fluctuation. We further tried to mitigate confounding factors by measuring and directly regressing physiological noise, and the results presented here should be considered in this context. Lastly, we did not include rating scale as a covariate in this analysis because the depression rating scales used here did not have sufficient dynamic range to capture mood changes in the HC cohort at the scan time point of Day 2. Thus, our MDD results may be strengthened by using a behavioral response measure that captures inter-subject variability at that time or by investigating scans at earlier times, which is an area of future interest.
Taken together, the results of this study demonstrate that it is possible to characterize the neural correlates associated with the onset and offset of ketamine’s antidepressant effects. While MDD and HC subjects responded differently to ketamine, response was generally characterized by increased connectivity with the DMN at two days post-infusion, an increase that had dissipated by Day 10. Furthermore, the connectivity changes observed in the insula in MDD imply a normalization of the interaction between the DMN and SAL networks, supporting the triple network dysfunction theory in MDD. In the context of real world ketamine use and the increased interest in using repeat doses of this agent to maintain antidepressant response, these findings could help identify the window of plasticity and plan the optimal time for subsequent doses. The results also suggest an avenue whereby neural response to pharmaceutical drug interventions can be monitored and individual dose regimens optimized.
Supplementary Material
Acknowledgments
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA-MH002857), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). The authors thank the 7SE research unit and staff for their support and Elizabeth Ballard for helpful comments on the manuscript. Ioline Henter (NIMH) provided invaluable editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NCT#00088699; https://clinicaltrials.gov/ct2/show/NCT00088699
Financial Disclosures
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a coinventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The Epidemiology of Major Depressive Disorder. JAMA. 2003;289:3095. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global burden of mental disorders and the need for a comprehensive, coordinated response from health and social sectors at the country level Report by the Secretariat. 2012 Retrieved January 16, 2017, from http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_10-en.pdf.
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci. 2016;10:612. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SCR, Mehta MA. Quantifying the Attenuation of the Ketamine Pharmacological Magnetic Resonance Imaging Response in Humans: A Validation Using Antipsychotic and Glutamatergic Agents. J Pharmacol Exp Ther. 2013;345:151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- 12.De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, et al. Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage. 2013;64:75–90. doi: 10.1016/j.neuroimage.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Shcherbinin S, Doyle O, Zelaya FO, de Simoni S, Mehta MA, Schwarz AJ. Modulatory effects of ketamine, risperidone and lamotrigine on resting brain perfusion in healthy human subjects. Psychopharmacology (Berl) 2015;232:4191–4204. doi: 10.1007/s00213-015-4021-z. [DOI] [PubMed] [Google Scholar]
- 14.Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM, et al. Glutamate and the Neural Basis of the Subjective Effects of Ketamine. Arch Gen Psychiatry. 2008;65:154. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 15.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Walter M. Neuropathol Drug Addict Subst Misuse. Elsevier; 2016. The Acute and Chronic Effects of Ketamine as Revealed by Noninvasive Brain Imaging; pp. 689–702. [Google Scholar]
- 17.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509. doi: 10.1038/tp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine Decreases Resting State Functional Network Connectivity in Healthy Subjects: Implications for Antidepressant Drug Action. (SLSensi, editor) PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheidegger M, Henning A, Walter M, Lehmann M, Kraehenmann R, Boeker H, et al. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Hum Brain Mapp. 2016;37:1941–1952. doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murrough JW, Wan L Ben, Iacoviello B, Collins KA, Solon C, Glicksberg B, et al. Neurocognitive effects of ketamine in treatment-resistant major depression: Association with antidepressant response. Psychopharmacology (Berl) 2014;231:481–488. doi: 10.1007/s00213-013-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery SA, Asberg M, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon MWJ. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York: 2002. [Google Scholar]
- 29.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 1):10–17. [PubMed] [Google Scholar]
- 30.First Michael B, Spitzer Robert L, Gibbon Miriam, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York; 2002. [Google Scholar]
- 31.Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 32.Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–41. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–90. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: A Language and Environment for Statistical Computing. 2013 Retrieved from http://www.r-project.org/
- 37.Nugent A, Ballard E, Gould TD, Park LT, Moaddel R, Brutsche N, et al. Ketamine has distinct electrophysiological and behavioural effects in depressed and healthy subjects. Mol Psychiatry. doi: 10.1038/s41380-018-0028-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008:1124. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological Mechanisms of the Placebo Effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh BT, Seidman SN, Sysko R, Gould M, KJ R, M E, et al. Placebo Response in Studies of Major Depression. JAMA. 2002;287:1840. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 42.Mayberg HS, Arturo Silva FJ, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Paul Jerabek BA. The Functional Neuroanatomy of the Placebo Effect. Am J Psychiatry. 2002:1595. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Restingstate connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RCK, et al. Resting-State Functional Connectivity in Treatment-Resistant Depression. Am J Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 46.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased Occipital Cortex GABA Concentrations in Depressed Patients After Therapy With Selective Serotonin Reuptake Inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 50.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70:280–90. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. NeuroImage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Ganger S, et al. Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct. 2017;222:1533–1542. doi: 10.1007/s00429-016-1291-0. [DOI] [PubMed] [Google Scholar]
- 54.Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Windischberger C, et al. Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. Int J Neuropsychopharmacol. 2015;18:1–11. doi: 10.1093/ijnp/pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.