Abstract
This review provides a historical account of the discovery of secondary visual pathways (from retina to the superior colliculus to the dorsal thalamus and extrastriate cortex), and Vivien Casagrande’s pioneering studies of this system using the tree shrew as a model. Subsequent studies of visual pathways in the tree shrew are also reviewed, beginning with a description of the organization and central projections of the tree shrew retina. The organization and connectivity of 2nd visual system components, that include the retino-recipient superior colliculus, tecto-recipient pulvinar nucleus and its projections, and the tecto-recipient dorsal lateral geniculate nucleus and its projections are detailed. Potential functions of the 2nd visual system are discussed in the context of this work and in the context of the behavioral studies that initially inspired the secondary visual system concept.
Indexing Terms: Tupaia, pulvinar, superior colliculus, thalamus, striate cortex, temporal cortex
Graphical abstract
This review provides a historical account of the discovery of secondary visual pathways (from retina to the superior colliculus to the dorsal thalamus and extrastriate cortex), and Vivien Casagrande’s pioneering studies of this system using the tree shrew as a model. The organization and connectivity of 2nd visual system components are detailed. Potential functions of the 2nd visual system also are discussed in the context of this work and in the context of the behavioral studies that initially inspired the 2nd visual system concept.
“Until recently study of the central mechanisms of vision in mammals has been concerned chiefly with the visual cortex and its thalamic relay nucleus, the lateral geniculate body. Only in the last decade has there been any concerted effort to gain an understanding of the anatomy, physiology, and function of the superior colliculus which, from the phylogenetic point of view, is the oldest visual center.”
These first two sentences of Vivien Casagrande’s doctoral dissertation accurately convey the state of the field before she, and many other students and collaborators of Irving Diamond at Duke University, contributed significantly to the knowledge of extra-geniculate visual processing in mammals over the past 50 years. In this review, we will differentiate these two visual systems, and focus primarily on the 2nd visual system of the tree shrew (Tupaia), the model system chosen by Vivien Casagrande for many of her anatomical, physiological and behavioral studies of visual processing.
1. The 1st and 2nd visual systems in vertebrates
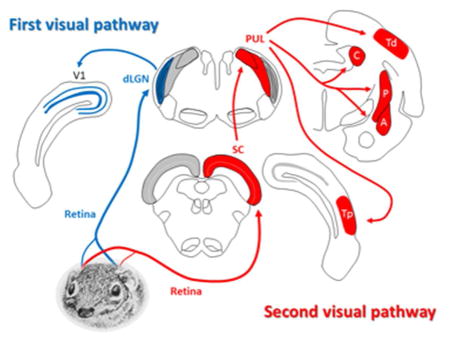
The observation that retinal ganglion cells project to two major targets, the tectum and the thalamus, kindled a number of papers in the late 1960’s to propose that there are two major visual systems. One system was delineated by retinal projections to the lateral geniculate nucleus of the thalamus, which projects to the striate visual cortex (also known as primary visual cortex, V1 or Brodman’s Area 17). The other lesser-studied pathway consisted of retinal projections to the tectum (or superior colliculus), which transfers visual signals to the extrastriate cortex by way of the pulvinar nucleus of the thalamus. As they were initially proposed, in addition to consisting of different pathways, these two systems differed primarily in that activation of the 1st visual system resulted in conscious visual perception (the other being unconscious), and that whereas the 1st system was more concerned with the identification of objects, the 2nd system was concerned with detection, localization and creating orienting responses.
In his 1967 paper “Two visual mechanisms underlying the behavior of fish”, Ingle differentiated one system involving “processes by which the fish “evaluates” the identity or activity of the object” and a second system providing visual processes subserving “orientation” of the fish to a moving object. The following year, building on reports of preserved visual function following striate cortex lesions in monkeys (e.g., Cowey and Weiskrantz, 1967; Humphrey and Weiskrantz, 1967), Trevarthen (1968) proposed that in macaques “vision involves two parallel processes…one system contributing to a focal level which examined detail in small areas of space, and a second being ambient, involved in determining the space at large around the body”. One year later, Schneider (1969) proposed the phrase “two visual systems” to characterize his findings in hamsters that brain mechanisms for the identification of (or discrimination between) objects and for the localization of objects could be dissociated by tectal or cortical lesions. These papers, all published within two years, proposed two distinguishable visual systems in fish, in macaques, and in hamsters. In each case, the basic difference between these visual systems was apparent: identification of objects vs. locating them and orienting to them. These and other studies showed that an intact retino-geniculo-striate system was NOT necessary for the ability to respond to visual stimuli. Within the next decade, Weiskrantz et al (1974) described preserved vision in human patients with damage to striate cortex, and conversely, Zihl and von Cramon (1979) showed deficits in visual detection and directed visual attention in a patient with specific damage to part of the extra-geniculate visual pathway (specifically the pulvinar nucleus). These findings had implications for the definition of “blindness”, and for understanding the ability of patients with primary visual cortex damage to respond to visual targets in the blind visual field without being consciously aware of them, a phenomenon known as “blindsight” (see Stoerig and Cowey, 1997).
An important part of this re-assessment of the visual system was an early report by Snyder, Hall and Diamond (1966) in which they described the behavior of three tree shrews following complete ablation of striate cortex and corresponding complete degeneration of the dorsal lateral geniculate nucleus (dLGN). Subsequent testing showed the animals’ ability to retain (or quickly re-learn) visual pattern discriminations in a test box. In their home cage they displayed normal ability to avoid obstacles, and to recognize and react to moving or stationary food that was offered. The de-striate animals could not be distinguished from unoperated animals. Snyder et al. concluded that the geniculo-striate pathway is not necessary for pattern vision and object vision in the tree shrew, as these functions were apparently mediated by the tecto-pulvino-temporal cortex pathway. In later studies, Casagrande (1973; Casagrande and Diamond, 1974) found that ablation of the retinal target of that pathway, the superior colliculus, produced significant visual deficits, later characterized as “midbrain blindness” by Kaas (1995). A fuller description of these and related experiments from this lab, and the 2nd visual system’s involvement in visual perception and visually-guided motion, will be presented in Section 12 after a review of the tree shrew visual system.
2. The tree shrew as a model system for study of vision and visual pathways
Tree shrews are small, fast moving animals with excellent motion vision and visually-guided behavior. They are extremely facile at navigating an arboreal environment, which requires well-developed mechanisms of attention and coding of visual signal movement. Tree shrews have approximately 45 degrees of binocular overlap (Conway and Schiller, 1983), and very likely have stereopsis, but that has not been measured empirically.
Tree shrews can be trained in visual detection and discrimination tasks and their visual capacities for color vision, form vision and motion vision have been studied behaviorally (e.g., Polson, 1968; Petry et al, 1984, Petry and Kelly, 1991; Callahan and Petry, 2000; Petry et al., 2012). Visual structures of the tree shrew brain are well-developed and display many features in common with primates, including a six-layered dLGN (Figure 2b,c), and a high degree of functional specialization within the striate cortex (e.g., Conway and Schiller, 1983; Muly and Fitzpatrick, 1992; Holdefer and Norton, 1995; Fitzpatrick, 1996; Mooser et al., 2004; Johnson et al., 2010; van Hooser et al, 2013; Balaram and Kaas, 2014). The tree shrew cortex is not folded into gyri and sulci, thus the smooth surface of both the striate and extrastriate cortical areas provides an advantageous model for studies using intrinsic optical imaging techniques to visualize global cortical activity patterns during the presentation of visual stimuli (e.g., Bosking et al., 2000; Johnson et al., 2010; Van Hooser et al., 2013; Vanni et al., 2016). The fact that the tree shrew brain possesses many primate characteristics (Kaas and Preuss, 1993; Kaas, 2002), led at one time to their classification in the Order Primates (LeGros Clark, 1934; Campbell, 1966). Although now classified in the Order Scandentia, tree shrews still are considered to represent a prototype of early prosimian primates. Species of Tupaia commonly used in neuroscience studies include the “common tree shrew” (T. glis), the “northern tree shrew (T. belangeri) and the “Chinese tree shrew” (T. belangeri chinensis), but no differences in brain structure or function between species has been noted.
Figure 2.
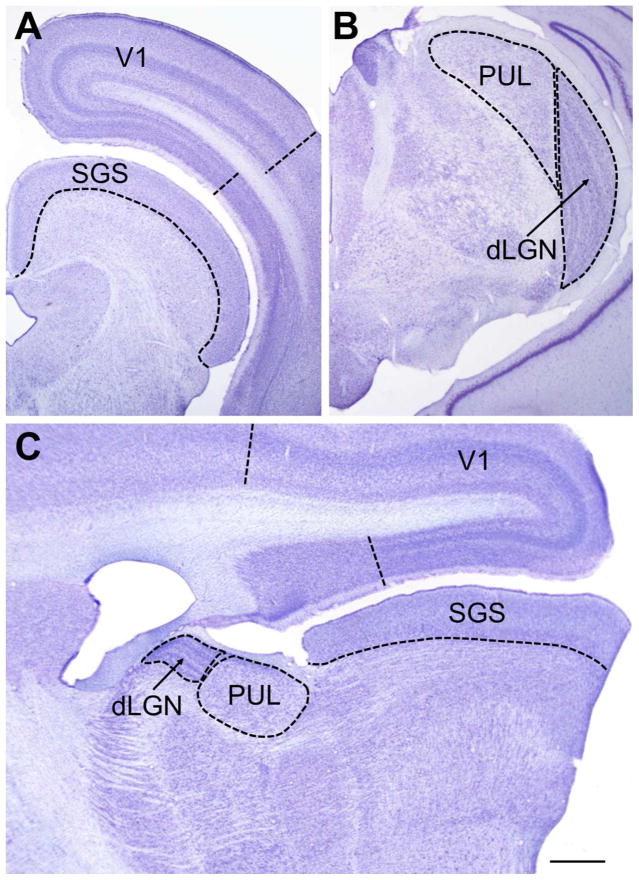
a) and b) Nissl-stained coronal sections of the tree shrew brain. (a) shows the superior colliculus with the retino-recipient stratum griseum superficiale (SGS) indicated, and the primary cortical visual area (V1, Area 17, striate cortex) defined by the “stripe of Gennari” shown here ending in the 17/18 border (dashed line). (b) shows the posterior region of the dorsal lateral geniculate nucleus (dLGN) and the pulvinar nucleus (PUL) of the thalamus. (c) Nissl-stained sagittal section of the tree shrew brain showing the large relative sizes of the tree shrew pulvinar nucleus and the upper layer of the superior colliculus (the SGS). Scale bar = 1 mm and applies to all panels.
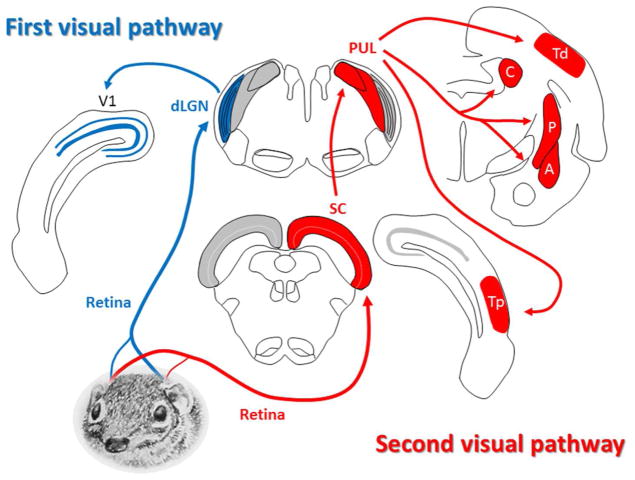
An overview of the components of the 1st and 2nd visual systems of the tree shrew is shown in Figure 3. These structures will be considered more fully in Sections 5–11. Particularly striking features of the tree shrew brain are the two greatly enlarged components of the 2nd visual system: the superior colliculus (Figure 2a, c) and pulvinar nucleus of the thalamus (Figure 2b, c). These structures, and their connectivity, are described in detail in Sections 6 and 7.
Figure 3.
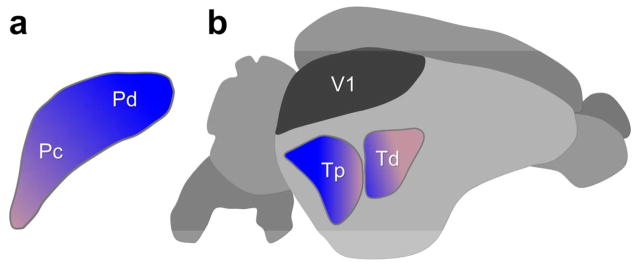
Overview of the 1st and 2nd visual pathways in the tree shrew brain. (For clarity, the “1st visual pathway” is shown on the left and the “2nd visual pathway” is shown on the right.) In both cases the retinal projection is primarily contralateral. In the 1st visual system (the “geniculo-striate pathway”), retinal ganglion cells terminate in the dorsal lateral geniculate nucleus (dLGN), which projects to the striate cortex (V1). In the 2nd visual pathway (the “extrastriate pathway”), retinal fibers project to the superior colliculus (SC), which in turn projects to the pulvinar (PUL). The pulvinar displays reciprocal connections with two areas of temporal cortex (Td and Tp), and also projects to nuclei in the basal ganglia (C: caudate, and P: putamen) and to the limbic system (A: amygdala). See description in text for more details. (Tree shrew drawing by Mary K. L. Baldwin.)
3. Retinal organization and retinal ganglion cell properties
The tree shrew retina is cone-dominant and contains two types of cones: long-wave-sensitive (LWS) cones and short-wave-sensitive (SWS) cones that are arranged in a single layer (Müller & Peichl, 1989; Petry & Harosi, 1990; Petry and Murphy, 1995). The small population of rods (.ca 5–8% of photoreceptors) are displaced slightly deeper (closer to the vitreous) in the retina. Both LWS and SWS cones show peak densities in the central, slightly ventral, retina, and rods show peak density in the extreme ventral retina (Petry et al., 1993). Since the ventral retina corresponds to the upper visual field (from where most of the tree shrew’s predators are likely to appear), this arrangement provides the animal with the best detection system at dusk and dawn.
The inner nuclear layer (INL) and inner plexiform layer (IPL) are relatively thick, and dendrites of retinal ganglion cells (RGCs) with ON responses terminate in the superficial (more scleral) portion of the IPL, whereas those of cells with OFF responses terminate in the deep (more vitreal) sublamina of the IPL (DeBruyn, 1983). The retinal ganglion cell layer varies in thickness from one to three cells deep. Although there is no fovea in the tree shrew retina, RGC density peaks in the temporal retina, which corresponds to the area of central vision or “area centralis”. DeBruyn (1983) reported a peak density of 16,000 cells/mm2 in the “area centralis”. From there, the area of maximum RGC density is elongated along the horizontal meridian, much like the “visual streak” that characterizes RGC distribution in other species (Hughes, 1977). Density falls off in a concentric elliptical manner that tapers off to approximately 9,000 cells/mm2 in the most peripheral regions (DeBruyn, 1983).
As in other species, morphological and physiological characteristics have been used to group RGCs into classes that may be related to their function in visual processing (for review, see, Rodieck, 1973; Rowe and Stone, 1977). Using morphological characteristics, DeBruyn (1983) distinguished three major types of RGCs in the tree shrew retina, each composing roughly 30%–35% of the total. Noticing bimodal distributions of some characteristics for two of the types, he used factor analysis to subdivide the 3 types in to 5 clusters. Type I cells (clusters 1 and 2) had large somas, large primary dendritic fields and thick axons. DeBruyn compared these to alpha-type RGCs in the cat (Boycott & Wassle, 1974). Type II cells had small somas, small primary dendrites and medium size axons. DeBruyn compared this type (which showed a unimodal distribution) to beta-type RGCs in the cat (Boycott and Wassle, 1974). Type III cells (clusters 4 an 5) were very heterogeneous, but showed similarities in their small somas, very large primary dendritic fields, and thin axons. DeBruyn compared these to gamma-type cat RGCs (Boycott and Wassle, 1974). Drenhaus et al. (1997) measured axon diameters in the tree shrew optic nerve and found three distinct groupings of axon diameter corresponding to the RGC types reported by DeBruyn. However these measurements suggested different ratios of RGC sub-types, and Drenhaus et al. estimated that large cells made up only 10% of the total, medium cells 20% and small cells 70%. Overall, 90% of the axons in the tree shrew optic nerve are myelinated (Drenhaus et al., 1997).
A recent study by Johnson et al. (2017) used immunohistochemical methods to identify the presence of intrinsically photosensitive RGCs (ipRGCs) in the tree shrew retina. Two classes of neurons were described that shared some properties with M1 and M2 type ipRGCs found in rodent and primate retinas, and a novel third class was identified that appears to be dopaminergic ipRGCs. These three classes show differences in their morphology, stratification patterns in the IPL, and density gradients across the retina, suggesting that they are involved in different functions.
Physiological attributes have been measured in extracellular recordings from tree shrew retinal ganglion cells or from their axons in the optic nerve or optic tract in a number of studies (Dongen et al., 1976; Thijssen, et al., 1976; ter Laak and Thijssen, 1979; ter Laak et al., 1980; Lu and Petry, 2003). Common among these studies is the reporting of at least two clear sub-types of RGCs based on their responses to changes in luminance: transient cells (that displayed a brief, phasic response to the onset or offset of light), and sustained cells (that responded continuously to the presence of the light stimulus). These classes show similarities to X-cells and Y-cells in other vertebrate retinas (see Sherman and Spear, 1982). Other cells with receptive field characteristics that are similar to W-cells in other species have also been reported in the tree shrew retina (Dongen et al. 1976; Lu and Petry, 2003).
Electrophysiological recordings made in the optic tract (Dongen et al., 1976) revealed that cells with sustained and transient response properties each comprise about one-third of the RGCs. The other third exhibited response properties ascribed to W-like cells. Recording from RGC axons, Lu and Petry (2003) used a transient-sustained index that revealed a bimodal distribution, with a 1:1.7 ratio of transient to sustained cells, and reported only a few W-like cell responses. The ratios of transient to sustained types recorded in electrophysiological studies (Dongen et al, 1976; Lu and Petry, 2003) is in rough agreement to the morphologically-determined ratio of large and medium sized cells (i.e., Type I and Type II) measured by DeBruyn (1983) and Drenhaus et al. (1997). Keeping in mind that microelectrode recordings are more sensitive to sampling error (larger cells or axons are more likely to be measured), differences in the physiologically-based vs. morphologically-based estimates of the number of small-sized W-like cells is not pertinent.
4. Central projections of tree shrew retina
Tigges (1966) reported that roughly 80% of RGCs project to the midbrain component of the 2nd visual system, the SC. The retinal projection to the SC and thalamus is predominantly contralateral, but ipsilateral projections are also present in smaller numbers (Campbell et al., 1967; Glickstein, 1967; Laemle, 1968). Retinal projections to individual layers of the dLGN and superior colliculus were reported in a number of studies using unilateral enucleation (Campbell et al., 1967; Laemle, 1968), autoradiographic methods (Casagrande and Harting, 1975; Hubel 1975), and retrograde tracer studies (DeBruyn and Casagrande, 1978, 1983; DeBruyn, 1983). More details on the local termination of retinal fibers in the dLGN and SC will be described in Sections 5 and 6.
Several degeneration studies also reported a direct, but minor, retinal projection to the pulvinar (Campbell et al., 1967; Laemle, 1968; Ohno et al., 1975; Somogyi et al., 1981), although DeBruyn (1983) was not able to demonstrate this projection using retrograde labeling of HRP. Label in the pulvinar was also noted by Casagrande and Harting (1975), following retinal tracer injections, but attributed to transneuronal transport from the SC. Other retinal projections include a bilateral input to the suprachiasmatic nucleus (SCN; Casagrande and Harting, 1975; DeBruyn, 1983; Murakami and Fuller, 1990), and contralateral input to the ventral LGN (vLGN), pretectum (PT) and accessory optic nuclei (Campbell et al., 1967; Casagrande and Harting, 1975; DeBruyn, 1983).
Central projections related to RGC type were reported by DeBruyn (1983). Type I RGCs (large somas, large primary dendritic fields and thick axons) were found to project to the dLGN, vLGN, SC, accessory optic nuclei, and possibly the PT. Type II RGCs (small somas, small primary dendrites and medium size axons) were found to project to the dLGN, SC, PT and possible to the vLGN. Type III RGCs (small somas, very large primary dendritic fields, and thin axons) were found to project to the dLGN, vLGN, SC, and SCN.
5. Geniculo-striate pathway: the 1st visual system of the tree shrew
Since the primary purpose of this review is to highlight the 2nd visual system, the geniculo-striate pathway will only be described in brief. The dLGN consists of 6 layers and associated interlaminar zones. Ocular input is segregated with ipsilateral RGCs terminating in layers 1 & 5 and contralateral RGCs terminating in layers 2, 3, 4 & 6 (Glickstein, 1967; Harting et al., 1973). (Layers are numbered medial to lateral.) The projection of different RGC morphological types is also segregated (DeBruyn, 1983; Conley et al., 1984). This results in an organization of the dLGN that is most easily thought of as three pairs of layers (Holdefer and Norton, 1995). Layers 1 and 2 form a matched pair of layers that receive ipsilateral and contralateral projections (respectively) from medium and large sized RGCs whose dendrites originate in the superficial (“ON”) IPL of the retina. These dLGN cells display ON-center receptive fields. Layers 4 and 5 form a matched pair that receive contralateral and ipsilateral projections (respectively) from medium and large sized RGCs whose dendrites originate in the deep (“OFF”) IPL of the retina. These dLGN cells display OFF-center receptive fields. Layers 3 and 6 form a pair of layers that receive contralateral projections from small-sized RGCs and display W-like ON and ON-OFF center receptive field organization, respectively (Conway and Schiller, 1983; Holdefer and Norton, 1995). Layers 3 and 6 are also the only dLGN layers to receive a projection from the SC (Diamond et al., 1991), that is described in more detail in Section 8. An early physiological report (Sherman et al., 1975) suggested the presence of the X-cell and Y-cell dichotomy in the tree shrew dLGN, but with a larger sample and more extensive tests and analysis, Holdefer and Norton (1995) were not able to find evidence for a clear Y-cell class of neurons. Histochemical studies have shown that layers 1, 2, 4, and 5 stain more heavily for cytochrome oxidase (Wong-Riley and Norton, 1988). Calcium binding protein distributions are also differentiated by layer with layers 1, 2, 4, and 5 showing parvalbumin reactivity and layers 3 and 6 showing calbindin reactivity (Diamond et al., 1993).
The dominant cortical target of the dLGN is the striate area, and the dLGN afferents maintain segregation of the ON- and OFF-center organization in that projection (Conley et al., 1984; Raczkowski and Fitzpatrick, 1990; Usrey et al, 1992). Layers with ON-center responses (layers 1 and 2) project to the upper part of striate layer IV (IVa) whereas afferents with OFF-center responses (from layers 4 and 5) project to the lower part of layer IV (IVb). Ocular input is not organized into columns, but rather horizontally within each tier, although there is some overlapping of ipsilateral and contralateral eye input (Humphrey et al, 1977). Labeling geniculocortical axons originating from dLGN layers 3 or 6 (Usrey et al., 1992) showed that both axon types were found to extend to layer I of the striate cortex, and in addition layer 6 was found to project heavily to lower layer IIIc while axons originating from layer 3 of the dLGN terminated densely in layer IIIb and IVm. The vertical organization of striate cortex reveals a precise visuotopic map (Sesma et al., 1984) and orientation columns (Skeen et al., 1978; Humphrey and Norton, 1980; Humphrey et al., 1980; Bosking et al., 1997; Mooser et al., 2004). Injection of retrograde tracers in area V2 or area TD of temporal cortex have revealed a small population of neurons in dLGN layers 3 and 6 that project outside the striate cortex (Lyon et al., 2003b). This projection is reminiscent of similar minor projections of koniocellular neurons to extrastriate cortex in primates (Stepniewska et al., 1999; Sincich and Horton, 2003). In addition to involving fewer neurons, this projection in the tree shrew reflected less precise retinotopy than the projections from the other dLGN layers.
6. Organization of retino-recipient superior colliculus
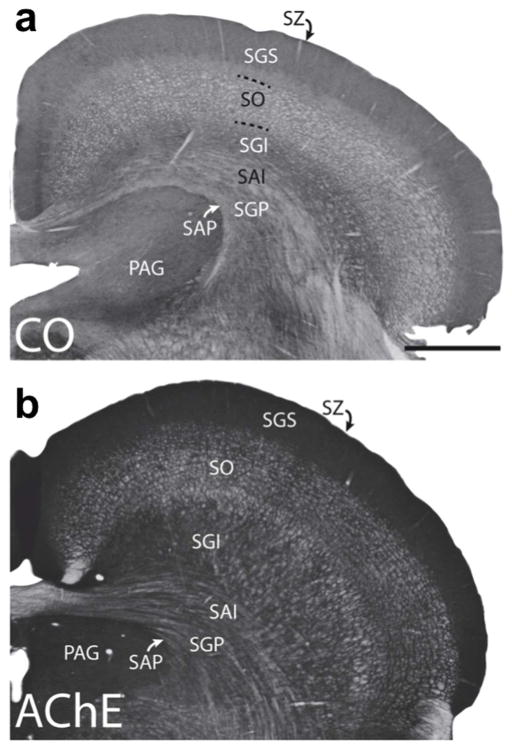
The SC is a structure generally known to be involved in initiating eye and head movements toward novel stimuli. It’s laminar organization can be seen in the coronal sections shown in Figure 4, stained for cytochrome oxidase (CO; Figure 4a) and for acetylcholinesterase (AChE; Figure 4b). The importance of the tree shrew SC can be inferred from its size relative to other visual structures, as is evident in Figure 2(a,c). It has been estimated that the tree shrew stratum griseum superficiale (SGS) has a volume that is approximately one-half the size of its striate cortex and six times greater than its dLGN (Norton, 1982). In comparison, the monkey SGS is about one-fifth the volume of the dLGN. Only the upper layers of the tree shrew SC (i.e., the SGS and the underlying stratum opticum or SO) receive direct retinal input. Cells in these layers also receive sensory input from the visual cortex and are organized in a retinotopic manner (Lane et al., 1971; Casagrande et al, 1972; Kaas et al., 1974). Degeneration studies have shown that the majority of retinal inputs terminate in the upper SGS layers, whereas the majority of visual cortex terminals terminate in the lower SGS (Graham and Casagrande, 1980). This is consistent with evidence from a variety of studies (i.e., studies employing anatomical, tract-tracing, ablation and electrophysiological methods) that have suggested that the SGS is functionally subdivided into an upper two-thirds region and a lower one-third region (Albano et al., 1979; Casagrande et al., 1972; Graham & Casagrande, 1980; Norton, 1982). Further distinction of the upper SGS is seen in the distribution of vesicular glutamate transporters (VGLUTs). The dorsal division of the upper SGS reacts weakly for VGLUT1 and strongly for VGLUT2, and the ventral division of the shows the opposite pattern (Balaram et al., 2015).
Figure 4.
Laminar organization of the superior colliculus (SC) is shown in coronal sections stained for cytochrome oxidase (a) and acetylcholinesterase (b). The seven main layers of the superior colliculus are labeled. Stratum zonale (SZ); stratum grisium superficiale (SGS); stratum opticum (SO); stratum griseum intermediale (SGI); stratum album intermediale (SAI); stratum griseum profundum (SGP); and stratum album profundum (SAP). Also shown is the periaqueductal gray (PAG). Scale bar is 1mm. (from Baldwin et al., 2013)
In support of this functional subdivision, most neurons in the upper SGS layers (i.e., the stationary-responsive (S-R) cells described by Albano et al. (1979) and Norton, (1982)) possess visual receptive field characteristics that are very similar to the retinal ganglion cells (RGCs) that innervate them (e.g., relatively small receptive field centers; good response to standing contrast; no orientation selectivity or direction selectivity). These receptive field properties are also similar in many ways to those of projection neurons in the dLGN. In contrast, Albano et al. (1979) and Norton (1982) also described properties of neurons in the lower SGS layer that are unlike those of tree shrew RGCs (e.g., brisk response to movement; direction selectivity; larger receptive fields). Furthermore, retrograde labeling studies have revealed that 20% of upper SGS cells project to the dLGN and none were found to project to the pulvinar, whereas 50% of lower SGS neurons were found to project to the pulvinar and none to the dLGN (Graham and Casagrande, 1980). Cell morphology in these SGS sub-layers also differs. Upper SGS neurons have spindle-shaped somas and vertically-oriented dendritic fields, whereas lower SGS neurons have large triangular-shaped somas, and broad, obliquely-oriented dendrites (Graham and Casagrande, 1980). These differences in receptive field properties and morphology of the lower SGS neurons likely correlate with the properties of pulvinar neurons described in the following section.
The organization and neuron characteristics of the superficial visual layers of the SC that receive retinal input, and input from striate and extrastriate cortex (Baldwin et al., 2013), contrast sharply with the deeper motor layers of this structure. Connections between the SGS and deep layers are sparse at best, and the SO appears to serve as an intermediary between visual and motor layers (Hall and Lee, 1993; Lee and Hall, 1995; Hall and Lee; 1997; Lee et al., 1997; Pettit et al., 1999). The role of the deep layers of the SC in the 2nd visual system is further considered in more detail in Section 12.
7 Organization of pulvinar
Like the SC, the tree shrew pulvinar nucleus is also relatively large (Figure 2b, c). Kaas and colleagues delineated its boundaries using histochemical and tract tracing techniques (Lyon et al., 2003a, b). These studies indicate that the tree shrew pulvinar nucleus, similar to the primate pulvinar nucleus, is the largest thalamic territory. Volume estimates indicate that the tree shrew pulvinar nucleus occupies about 4.4 mm3 while the dLGN occupies 2.7 mm3 (Lyon et al., 2003a). As discussed in detail in Section 8, and shown in Figure 5, the tree shrew pulvinar nucleus can be subdivided into two zones that receive distinct tectal connections (Luppino et al., 1988; Chomsung et al., 2008). We have proposed that these two tectopulvinar projections are involved in coding different aspects of visual movement (Chomsung et al., 2008; Day-Brown et al., 2010; Wei, Masterson et al., 2011).
Figure 5.
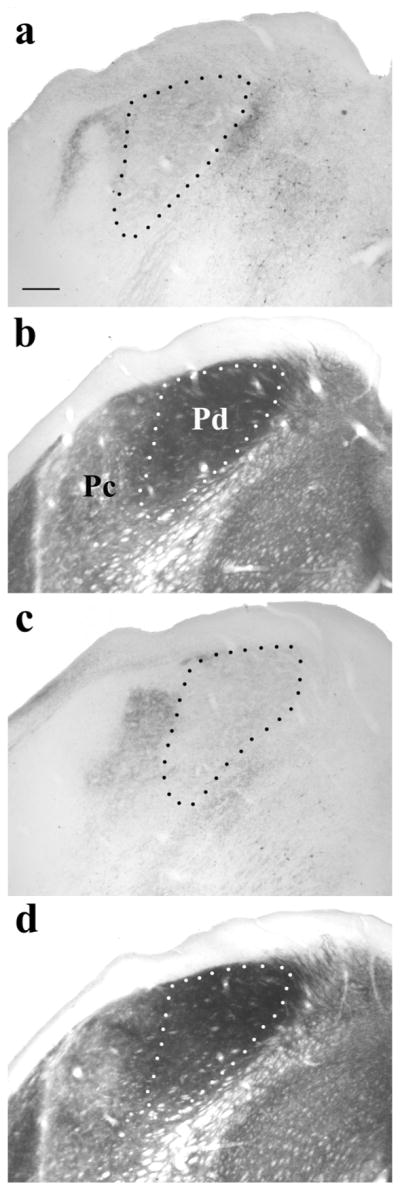
Photomicrographs of coronal sections stained to reveal the distribution of tectopulvinar terminals labeled via a pressure injection of the anterograde tracer biotinylated dextran amine (BDA) in the SC (a, c) and adjacent sections stained for acetylcholinesterase (AChE; b, d). In the dorsomedial regions of the pulvinar nucleus, terminals are diffusely distributed (outlined by black dotted lines in a,c). The region of diffuse terminals (indicated by white dotted lines in b,d) corresponds to the Pd subdivision of the pulvinar nucleus, which stains darkly with the AChE reaction (Lyon et al., 2003). Dense clusters of terminals are located in the Pc subdivision of the pulvinar nucleus, which stains lightly with the AChE reaction. Scale bar is 250um. (from Chomsung et al., 2008).
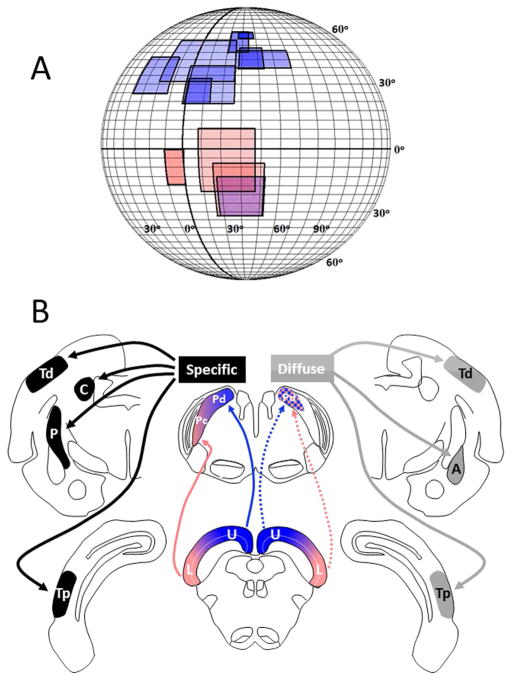
As shown in Figure 6, neurons in both the dorsal and central pulvinar (Pd and Pc) of the tree shrew have very large receptive fields, although on average those of Pd neurons are larger than Pc (Dang et al., 2012). Large receptive fields have also been reported for neurons in the tecto-recipient zones of the pulvinar in other species (e.g., cat: Chalupa and Abramson, 1989; mouse: Roth et al., 2016; macaque: Benevento and Miller, 1981, Felsten et al., 1983; squirrel monkey: Mathers and Rapisardi, 1973). Neurons in the Pd and Pc of the tree shrew also represent different parts of the visual field. Receptive fields of neurons in the Pd are located primarily in the upper visual field of the contralateral eye, while those of the Pc are located primarily in the lower visual field of the contralateral eye (Dang et al., 2012). Neurons in both regions display direction selectivity, but prefer different types of movement. The most preferred stimuli for Pd neurons are fields of drifting dots, which do not excite Pc neurons. In contrast, Pc neurons respond best to large, rapidly moving bars. These movement responses are likely dependent, at least in part, on input from the SC.
Figure 6.
a) Receptive field (RF) sizes and locations of 12 pulvinar neurons recorded in the Pd and Pc. The color of the RF plots correspond to the schematic drawing below. As expected from the results of Chomsung et al. (2008), the RFs of Pd neurons were found to be located in the upper and lower visual fields and those of Pc neurons in the lower visual field. Darker color indicates overlapping RFs. The sizes of pulvinar RFs overall are considerably larger compared to those of SC neurons (Lane et al. 1971) most likely due to convergence in the tectopulvinar projection (from Dang et al., 2012). b) Schematic drawing of the specific and diffuse pulvinar systems diagramed on coronal sections of the tree shrew brain. (For clarity, the “specific” system is shown on the left and the “diffuse” system shown on the right although both exist bilaterally in the tree shrew brain.) The distribution of tecto-pulvinar projections is shown relative to the retinotopic representation of the visual field in the superior colliculus (SC). Pulvinar projections to other brain regions are indicated for each sub-system. (U: upper visual field; L: lower visual field; Tp: posterior temporal cortex; Td: dorsal temporal cortex; C: caudate nucleus; P: putamen; A: amygdala.
Wei, Bonjean et al. (2011) reported whole-cell recordings made from tree shrew pulvinar neurons and dLGN neurons in a brain slice preparation that revealed two basic firing patterns: tonic and burst. Pulvinar neurons had a much greater propensity to fire in bursts than is seen in other thalamic nuclei, such as the dLGN. With the application of histochemical methods Wei, Bonjean et al. (2011) discovered that the tree shrew pulvinar nucleus contains more T-type calcium channels and more small conductance potassium channels (SK2) than dLGN neurons. The pulvinar was also found to be characterized by a higher glia-to-neuron ratio than the dLGN. These factors likely contribute to the propensity for burst firing in the pulvinar nucleus, a phenomenon also reported in the monkey (Ramcharan et al., 2005).
8. Tectothalamic pathways
Lesions of the superficial and deep layers of the tree shrew superior colliculus (SC), carried out by Vivien Casagrande and colleagues in the early 1970s, demonstrated that the superficial SC layers project to the dLGN and pulvinar nucleus (Casagrande et al., 1972; Harting, Hall et al., 1973). Specifically, the tree shrew SC innervates layers 3 and 6 of the dLGN, and the Pd and Pc zones of the pulvinar nucleus (Luppino et al, 1988; Lyon et al., 2003a; Chomsung et al, 2008). Subsequent retrograde tracing experiments determined that these projections originate from distinct SC cell types. Tectogeniculate cells were found to be distributed in the upper regions of the SGS, whereas tectopulvinar cells were found to be located in the ventral SGS and underlying SO (Albano et al., 1979; Graham and Casagrande, 1980).
The dendritic morphology of each tectothalamic cell type has also been found to be distinct. Tectal projections to layer 3 of the dLGN originate from cells in the upper regions of the SGS and exhibit vertically oriented narrow dendritic fields (around 100 μm in the mediolateral dimension); these cells also project to the vLGN. Tectal projections to the dLGN layer 6 also originate from cells in the upper regions of the SGS that exhibit dendritic arbors restricted to around 100 μm in the mediolateral dimension, but these cells also project to the pretectum (Diamond et al., 1991). In contrast to the limited dendritic fields of the tectogeniculate cells, tectopulvinar cells exhibit very widespread dendritic arbors that extend laterally and vertically from the somata in the SO and deep SGS to the dorsal surface of the SC; the dendritic arbors of these widefield vertical cells can extend over a millimeter in the mediolateral dimension (Chomsung et al., 2008). In the mouse, widefield vertical cells have been shown to have very large receptive fields (Gale and Murphy, 2014), which suggests that widefield vertical cells in the tree shrew SC likely contributes to the large receptive fields of tree shrew pulvinar neurons (Dang et., 2012).
Anterograde tract tracing experiments revealed further specializations of tectothalamic projections in the tree shrew. Tectogeniculate projections that terminate in layer 6 are elongated and organized parallel to the layer, whereas those that terminate in layer 3 form discrete clusters that are organized orthogonal to the layer. Likewise, two types of tectopulvinar projections were identified. After restricted tracer injections in the SC, most of the Pd was filled with terminals irrespective of the size or location of the injection site. In contrast, the Pc exhibited dense, spatially restricted patches of labeled terminals following the same injections in the colliculus, and their location varied with the location of the injection site. Consequently, the tectal projections to the Pd were described as “diffuse”, while those to the Pc were described as “specific” (Luppino et al, 1988). Further anterograde and retrograde tracing studies suggested that the Pd may also contain “specific” projections from regions of the SC that represent the upper visual field (Chomsung et al., 2008).
Electron microscopic and in vitro physiological investigations of tectopulvinar terminals in the tree shrew revealed that the nontopographic “diffuse” tectal projections are highly convergent. These tectopulvinar terminals form dense clusters that surround and synapse on single pulvinar dendrites. In contrast, the topographic “specific” projections are less convergent and form smaller, more discrete, synaptic clusters (Chomsung et al., 2008; Wei et al., 2011). Thus, the superficial layers of the SC provide at least 4 distinct patterns of projections to the dorsal thalamus. As discussed below, these tectothalamic channels are further characterized by the projections of thalamic neurons innervated by the SC.
9. Cortical connections of the pulvinar nucleus
The cortical projections of the tree shrew dorsal thalamus were first studied using retrograde degeneration techniques (Diamond et al. 1970). Small lesions of the striate cortex produced severe degeneration of cells in restricted regions of the dLGN suggesting a precise topographic organization of geniculocortical axons. In contrast, the severity of degeneration in the pulvinar nucleus increased as a function of lesion size in the temporal cortex. This result suggested that pulvinocortical axons have widespread collateral branches. Subsequent anterograde degeneration studies confirmed the precision of axon projections to the striate cortex following lesions confined to the dLGN, but found gaps in the distribution of degenerating axons in the temporal cortex following single lesions of the pulvinar nucleus (Harting, Diamond et al., 1973).
It was later confirmed that each region of the pulvinar nucleus projects topographically to 2 separate regions of the temporal cortex (Chomsung et al., 2010). Small injections of anterograde tracers placed in either the Pd or Pc labeled 2 foci of terminals in the temporal cortex. These pulvinocortical projections were found to densely innervate layers I–IV in the temporal cortex, where they primarily synapsed on dendritic spines. The connections of the temporal cortex and pulvinar nucleus were also found to be reciprocal. Projections from the temporal cortex to the Pd and Pc originate from layer VI cells, and form small terminals that synapse on small caliber dendrites within the pulvinar nucleus (Chomsung et al., 2010). These reciprocal connections are summarized in Figure 7.
Figure 7.
Reciprocal pulvino-cortical connections. Areas Pc and Pd of the tree shrew (depicted in a schematic view of a coronal section through the pulvinar nucleus, (a) project to layers I–IV of two areas of temporal cortex (depicted in a schematic lateral view of the whole tree shrew brain (b)), one more posterior (Tp) and one more dorsal (Td). In turn, cells in layer VI of those areas of temporal cortex project in a topographic fashion to pulvinar areas Pc and Pd. Layer V of these temporal areas also projects to the superior colliculus Baldwin et al., 2013). This provides a mechanism for motion signals relayed from the superior colliculus to initiate action to be constantly modified by cortical circuits underlying visual perception. Striate cortex (V1) is shown for reference, but does not receive a projection from the Pd or Pc (modified from Chomsung et al., 2010).
The two pulvinocortical projection areas Td and Tp may be homologous to primate areas MT and V3. The Td in particular has been suggested to be a homologue of area MT based on its extensive reciprocal connections with V1 (Sesma et al., 1984; Lyon et al., 1998). Similar dual projections have been revealed in primates. Following small lesions in the tectorecipient pulvinar of the galago, Glendenning et al. (1975) showed two projections to the middle temporal area and a more ventral area of the temporal cortex using degeneration techniques. In addition, Lyon et al. (2010) revealed that tectopulvinar neurons were labeled by transynaptic retrograde labeled following injections in either area MT or area V3 in macaque. However, some regions of the primate pulvinar that project to area MT have been found to receive direct retinal input rather than tectal input (Kwan et al., 2018). For a detailed comparative review of the subcortical projections to the extrastiate cortex across species, see Baldwin and Bourne (2017).
10. Cortical projections of the tectorecipient dLGN
As described in Section 5, the projections to striate cortex from dLGN layers that receive tectal input (layers 3 and 6) are distinct from those dLGN layers that do not receive tectal input. Anterograde and retrograde tracer studies have shown that dLGN layers 3 and 6 terminate in the supragranular layers of striate cortex, whereas projections from the remaining dLGN layers (1, 2, 4 and 5) terminate primarily within layer IV of the striate cortex (Carey et al., 1979a, b; Raczkowski and Fitzpatrick, 1990; Usrey et al., 1992; Familtsev et al., 2015). Also, as described in Section 5, these two layers can be distinguished from the other dLGN layers by their RGC inputs (DeBruyn, 1983), by the receptive field characteristics of their neurons (Holdefer and Norton, 1995), by cytochrome oxidase histochemistry (Wong-Riley and Norton, 1988) and by calcium binding protein labeling (Diamond et al., 1993). Because of these differences, the neurons in dLGN layers 3 and 6 and their striate targets cannot be clearly designated as a component of the 1st or 2nd visual pathway. Perhaps they may be a component of a 3rd visual pathway not yet characterized.
11. Pulvinar projections to striatum and amygdala
In addition to the projections to the temporal cortex, tract tracing and electron microscopy revealed that projections from the tectorecipient pulvinar innervate spiny output cells of the dorsal striatum (caudate and putamen) and lateral amygdala (as depicted schematically in Figures 3 and 6). However, while both the Pd and Pc project to the caudate and putamen, only the Pd was found to innervate the lateral amygdala (Day-Brown et al., 2010). Since the Pd receives “diffuse” projections from the SC, this pathway could potentially relay nontopographic visual information to the amygdala to alert the animal to potentially dangerous visual images. In contrast, the “specific” tectopulvinar pathway to dorsal striatum is likely involved in coordinating topographic visual information to aid in guiding precise movements. (see Day-Brown et al. (2010) for more extensive consideration of these pathways.)
12. Behavioral and lesion studies of tree shrews implicating the 2nd visual system in visual perception and action
Snyder et al.’s (1966) report (described in Section 1) that the geniculo-striate system was not necessary for pattern vision and object vision in tree shrews led to a multitude of subsequent studies from that lab, and others, designed to determine what aspects of visual perception might be subserved by the 2nd visual system in the tree shrew. (For a detailed review of the consequences of V1 lesions in other species, see Baldwin and Bourne (2017).)
Diamond and Hall (1969) compared tree shrews with complete ablation of striate cortex (and corresponding complete dLGN degeneration) to tree shrews that had undergone ablation of striate cortex plus ablation of adjacent temporal cortex areas, and also to other animals that had received only temporal cortex ablations (i.e., with the striate cortex left intact). In the animals with temporal cortex ablations, pattern discrimination was never relearned, deficits were seen in object vision and reversal learning was abolished. These studies caused Diamond and Hall to classify temporal cortex as a sensory area, rather than an association area. As histological verification revealed that the temporal cortex ablations resulted in severe degeneration of the pulvinar (as well as the lateral and ventral nuclei), the 2nd visual system was thus implicated in pattern vision, object vision and reversal learning. Interestingly, behavioral measurement of visual acuity showed no differences between normal tree shrews and those with striate cortex ablated (Ware et al., 1972; 1974). Follow-up studies from this lab suggested that the striate cortex’s contribution to form and pattern discrimination centered on the ability to attend to the pertinent portion of the stimulus and extract relevant cues from distractors (Killackey and Diamond, 1971; Killackey et al., 1971; Killackey et al, 1972). Interestingly, removal of striate cortex produced an inability to discriminate form when hue is an irrelevant and distracting cue, and removal of temporal cortex alone showed the opposite effect (Killackey et al, 1971). A basic difficulty with all of these studies was the issue of separating learning deficits from perceptual loss.
The locus of hue discrimination and color vision in the tree shrew was also addressed by lesion studies. Snyder et al (1969) found that color vision was preserved in tree shrews that had complete ablations of striate cortex and temporal cortex. Since these lesions resulted in complete degeneration of the dLGN and severe degeneration of the pulvinar, these authors concluded that a component of the 2nd visual system, the superior colliculus, was the most likely location for color vision function. As will be described below, the presence of deficits in color vision following ablation of the upper layers of the SC (with cerebral cortex intact) support this conclusion.
The effects of lesions of the superior colliculus on visual perception and behavior were reported in several studies by Casagrande and colleagues (Casagrande et al., 1972; Casagrande, 1973; Casagrande and Diamond, 1974). Ablations of either the superficial layers of the SC alone, or of the superficial and deep layers, were performed in adult and infant tree shrews (tested as adults). Those with ablations of the superficial layers were later able to make discriminations based on pattern, but shape discrimination and aspects of hue discrimination were impaired although not eliminated (i.e., deficits were seen in discriminations of a chromatic stimulus from an equally bright achromatic stimulus, but the animals were able to distinguish a red stimulus from a blue one.) On the other hand, tree shrews with lesions of the superficial plus deep layers sat motionless in their cage and appeared blind. They were unable to track objects despite their ability to move their neck normally for grooming. This result was supported by other work showing that ablation of striate cortex in tree shrews resulted in only a subtle visual impairment, whereas unilateral ablation of the superior colliculus produced a profound vision loss (Jane et al. 1969; 1972).
Based on these results, Casagrande and her colleagues (Casagrande et al., 1972; Casagrande, 1973; Casagrande and Diamond, 1974) proposed that there are two functional divisions of the SC. The first division being the upper layers that are involved in visual perception, that receive input from retina and visual cortex, and that project to the pulvinar, dLGN, pretectum and vLGN. The second division being the deep layers that are involved in the control of head and eye movements, that receive multimodal input and that project to the motor areas of brainstem and thalamus, posterior nucleus, interlaminar complex, and subthalamus. In an attempt to parcel out the function of the deep layers, Raczkowski et al. (1976) performed electrolytic lesions of the pre-dorsal bundle to disrupt the output of the deep layers while leaving the superficial layers intact. The lesioned tree shrews did show an attention deficit in that they remained still and did not visually orient towards food unless it was accompanied by noise. However other visual functions remained intact. They were able to run and avoid obstacles; displayed normal head, neck and eye movements and mastered visual tasks involving pattern discrimination and shape discrimination.
Other species also show rapid recovery of some functions after striate cortex ablations (e.g., Sprague and Meikle, 1965; Sprague, 1966), however tree shrews appear to be particularly apt at recovery. Norton (1982) suggested that the relatively large sizes of the pulvinar and SC (particularly the SGS) may provide the basis for this capability. The upper SGS projection to the vLGN also may be relevant, but the vLGN doesn’t project to cortex. This raises the question of whether the neural information being used by tree shrews to perform visual tasks may be mediated by subcortical structures.
13. Potential functions of the tree shrew 2nd visual system
As is clear from the work described in this review, the 2nd visual system of the tree shrew plays a significant role in the animal’s vision. It cannot simply be relegated as a system that regulates directed eye movements and head movements to visual stimuli. The 2nd visual system clearly plays a role in visual perception as well.
Visual motion
The tree shrew’s 2nd visual pathway is likely involved in the coordination of visually guided movement with the perception of moving visual stimuli. This hypothesis is supported by efferent pathways from the pulvinar to the striatum. Furthermore, pulvinocortical projections may modify cortical activity such that moving visual stimuli are represented in the context of the animal’s own movement (or impending movements). A variety of studies suggest that projections from MT to V1 are critical for motion awareness (Pascual-Leone and Walsh, 2001; Silvanto et al., 2005; Laycock et al., 2007; Koivisto et al., 2010), and recently, Wilke et al. (2009) found that pulvinar activity correlates as robustly to the perceptual suppression of a visual stimulus as it does to its physical removal. If pulvinocortical projections activate corticocortical cells, such as those projecting from MT to V1, this could explain why lesions of the pulvinar nucleus can produce visual neglect (Karnath et al., 2002; Saalmann and Kastner, 2011).
Color vision
As discussed in Section 12, the results of several lesion studies (Snyder et al. 1969; Casagrande, 1973; Casagrande and Diamond, 1974) showed that an intact superior colliculus is necessary for color vision, although single-neuron recordings from that structure have not revealed any color-opponent cells (Albano et al., 1978). Interestingly, neurons in the supragrannular layers of tree shrew striate cortex have been shown to display SWS-cone modulated responses that are not characteristic of a typical color-opponent channel (Johnson et al., 2010). There, SWS-cone stimulation modulated a population of neurons with receptive field properties tuned for orientation, luminance and various spatiotemporal properties. These results suggest that color and form are not processed separately in the tree shrew striate cortex. It should also be noted that the striate cortex layers studied by Johnson et al. (layers II/III) are those that receive input from tecto-recipient dLGN layers 3 and 6 (as described in Section 10). The extent to which the processing of color information in the tree shrew represents a species difference, or whether the involvement of the phylogenitically older SC represents a basic color vision function maintained by tree shrews, but which was further evolved in the geniculo-striate system of primates, is unknown.
Form vision
The striking result obtained by Ware et al. (1972; 1974) that removal of striate cortex (and subsequent degeneration of dLGN) in tree shrews had no effect on post-operative visual acuity clearly implicates a role in form vision for the 2nd visual pathway. Norton (Albano et al., 1978; Norton, 1982) has suggested that the S-R cells in upper SGS of the SC have the characteristics to provide that function. The S-R cells provide an unusually large population of cells with small RF size at all eccentricities, which Norton compares to dLGN neurons that likely provide the basis for form vision in tree shrews and other species. Furthermore, ablation of striate cortex has little impact on SC function. Recording in the SGS of de-striate tree shrews, Kuyk and Norton (1981) found no differences from normal in the responses of upper SGS neurons and only a small decrease in lower SGS neurons. The only difference being that a portion of the lower SGS neurons had RFs with weaker suppressive surrounds.
Escape behavior
As described in Section 11, the Pc and Pd zones of the pulvinar project to motor nuclei (e.g., the caudate and putamen nuclei of the basal ganglia), with the Pd also projecting to the amygdala, a component of the limbic system (Day-Brown et al., 2010). The activation of the amygdala by the Pd, which receives nontopographic visual information (i.e., “diffuse” projections) from the SC, may be involved in initiating behaviors to permit the tree shrew to escape from dangerous situations and predators. This output system of the pulvinar is in contrast to the “specific” tectopulvinar pathway described above that may provide guidance of precise movements.
The studies reviewed here show that the 2nd visual system is capable of mediating a variety of visual functions in the absence of the geniculo-striate visual pathway. It is possible that this is due to a duplication of function between the 1st and 2nd visual systems. As has been pointed out by Norton (1982) and others, some sort of duplication is likely, given how rapidly the tree shrews with striate cortex lesions can perform visual tasks that were presumed to be mediated solely by the geniculo-striate pathway. Interestingly, Casagrande (1973) observed that the retinas of tree shrews that had undergone ablation of the superior colliculus showed no noticeable difference from normal retinas. The lack of retrograde degeneration of tectal-projecting RGCs implies that due to sustaining collaterals, most or all of the retinal projection to the SC must arise from bifurcation of retinal axons that are also projecting elsewhere (presumably mostly to the dLGN). Thus, common RGC input may provide the basis for some overlap in visual processing by the 1st and 2nd visual systems in the tree shrew. This also suggests that both systems are normally used in visual perception.
Figure 1.
The northern tree shrew (Tupaia belangeri). (drawing by Mary K. L. Baldwin)
TABLE 1.
ABBREVIATIONS
| AChE | acetylcholinesterase |
| dLGN | dorsal lateral geniculate nucleus |
| CO | cytochrome oxidase |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| LWS | long-wavelength-sensitive |
| MT | middle temporal area |
| Pc | central pulvinar |
| Pd | dorsal pulvinar |
| PT | pretectum |
| RGC | retinal ganglion cell |
| SC | superior colliculus |
| SCN | suprachiasmatic nucleus |
| SGS | stratum griseum superficiale |
| SO | stratum opticum |
| S-R | stationary-responsive |
| SWS | short-wavelength-sensitive |
| vLGN | ventral lateral geniculate nucleus |
Acknowledgments
We are very fortunate to have trained with and collaborated with Vivien Casagrande. Her excitement and curiosity about neuroscience was contagious. Her insights and generosity were much appreciated, and her overall enthusiasm made working with her a pleasure.
Grant support: NIH R01EY016155 and R01EY024173
LITERATURE CITED
- Albano JE, Humphrey AL, Norton TT. Laminar organization of receptive-field properties in tree shrew superior colliculus. Journal of Neurophysiology. 1978;41(5):1140–64. doi: 10.1152/jn.1978.41.5.1140. [DOI] [PubMed] [Google Scholar]
- Albano JE, Norton TT, Hall WC. Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Research. 1979;173(1):1–11. doi: 10.1016/0006-8993(79)91090-4. [DOI] [PubMed] [Google Scholar]
- Balaram P, Isaamullah M, Petry HM, Bickford ME, Kaas JH. Distributions of vesicular glutamate transporters 1 and 2 in the visual system of tree shrews (Tupaia belangeri) The Journal of Comparative Neurology. 2015;523(12):1792–808. doi: 10.1002/cne.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaram P, Kaas J. Towards a unified scheme of cortical lamination for primary visual cortex across primates: insights from NeuN and VGLUT2 immunoreactivity. Frontiers in Neuroanatomy. 2014;8(81):1–14. doi: 10.3389/fnana.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Bourne JA. The evolution of subcortical pathways to the extrastriate cortex. In: Kaas J, editor. Evolution of Nervous systems 2e. Vol. 3. Oxford: Elsevier; pp. 165–185. 2107. [Google Scholar]
- Baldwin MKL, Wei H, Reed JL, Bickford ME, Petry HM, Kaas JH. Cortical projections to the superior colliculus in tree shrews (Tupaia belangeri) The Journal of Comparative Neurology. 2013;521(7):1614–32. doi: 10.1002/cne.23249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Miller J. Visual responses of single neurons in the caudal lateral pulvinar of the macaque monkey. The Journal of Neuroscience. 1981;1(11):1268–78. doi: 10.1523/JNEUROSCI.01-11-01268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosking WH, Kretz R, Pucak ML, Fitzpatrick D. Functional specificity of callosal connections in tree shrew striate cortex. The Journal of Neuroscience. 2000;20(6):2346–59. doi: 10.1523/JNEUROSCI.20-06-02346.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. The Journal of Neuroscience. 1997;17(6):2112–27. doi: 10.1523/JNEUROSCI.17-06-02112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat’s retina. The Journal of Physiology. 1974;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan TL, Petry HM. Psychophysical measurement of temporal modulation sensitivity in the tree shrew (Tupaia belangeri) Vision Research. 2000;40(4):455–8. doi: 10.1016/s0042-6989(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Campbell CB. Taxonomic status of tree shrews. Science (New York, N Y) 1966;153(3734):436. doi: 10.1126/science.153.3734.436. [DOI] [PubMed] [Google Scholar]
- Campbell CB, Jane JA, Yashon D. The retinal projections of the tree shrew and hedgehog. Brain Research. 1967;5(3):406–18. doi: 10.1016/0006-8993(67)90047-9. [DOI] [PubMed] [Google Scholar]
- Carey RG, Fitzpatrick D, Diamond IT. Layer I of striate cortex of Tupaia glis and Galago senegalensis: Projections from thalamus and claustrum revealed by retrograde transport of horseradish peroxidase. The Journal of Comparative Neurology. 1979;186(3):393–437. doi: 10.1002/cne.901860306. [DOI] [PubMed] [Google Scholar]
- Carey RG, Fitzpatrick D, Diamond IT. Thalamic projections to layer I of striate cortex shown by retrograde transport of horseradish peroxidase. Science (New York, N Y ) 1979;203(4380):556–9. doi: 10.1126/science.760205. [DOI] [PubMed] [Google Scholar]
- Casagrande VA. Doctoral dissertation. Duke University; Durham NC: 1973. Behavioral changes following ablation of the superior colliculus in the tree shrew (Tupaia glis) [Google Scholar]
- Casagrande VA, Diamond IT. Ablation study of the superior colliculus in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1974;156(2):207–237. doi: 10.1002/cne.901560206. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK. Transneuronal transport of tritiated fucose and proline in the visual pathways of tree shrew Tupaia glis. Brain Research. 1975;96(2):367–72. doi: 10.1016/0006-8993(75)90749-0. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science (New York, N Y ) 1972;177(4047):444–7. doi: 10.1126/science.177.4047.444. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Abramson BP. Visual receptive fields in the striate-recipient zone of the lateral posterior-pulvinar complex. The Journal of Neuroscience. 1989;9(1):347–57. doi: 10.1523/JNEUROSCI.09-01-00347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsung RD, Petry HM, Bickford ME. Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. The Journal of Comparative Neurology. 2008;510(1):24–46. doi: 10.1002/cne.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsung RD, Wei H, Day-Brown JD, Petry HM, Bickford ME. Synaptic organization of connections between the temporal cortex and pulvinar nucleus of the tree shrew. Cerebral Cortex (New York, N Y 5: 1991) 2010;20(4):997–1011. doi: 10.1093/cercor/bhp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JL, Schiller PH. Laminar organization of tree shrew dorsal lateral geniculate nucleus. Journal of Neurophysiology. 1983;50(6):1330–42. doi: 10.1152/jn.1983.50.6.1330. [DOI] [PubMed] [Google Scholar]
- Cowey A, Weiskrantz L. A comparison of the effects of inferotemporal and striate cortex lesions on the visual behaviour of rhesus monkeys. Quarterly Journal of Experimental Psychology. 1967;19(3):246–253. doi: 10.1080/14640746708400099. [DOI] [PubMed] [Google Scholar]
- Dang W, Masterson SP, Day-Brown JD, Maire PS, Bickford ME, Petry HM. Neuroscience Meeting Planner. New Orleans LA: Society for Neuroscience; 2012. Receptive field characteristics of visually-responsive neurons in the tree shrew pulvinar nucleus. Program No. 467.13. Online. [Google Scholar]
- Day-Brown JD, Wei H, Chomsung RD, Petry HM, Bickford ME. Pulvinar projections to the striatum and amygdala in the tree shrew. Frontiers in Neuroanatomy. 2010 doi: 10.3389/fnana.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyn EJ. Doctoral dissertation. Vanderbilt University; Nashville TN: 1983. The organization and central terminations of retinal ganglion cells in the tree shrew (Tupaia glis) [Google Scholar]
- DeBruyn EJ, Casagrande VA. Density and central projections of retinal ganglion cells in the tree shrew. Neuroscience Abstracts. 1978;4:624. [Google Scholar]
- DeBruyn EJ, Casagrande VA. Tree shrew retinal ganglion cells: differences in nasal and temporal retina. Neuroscience Abstracts. 1980;6:348. [Google Scholar]
- DeBruyn EJ, Casagrande VA. Morphological differences in retinal ganglion cells projecting to different layers of the dorsal lateral geniculate nucleus in the tree shrew. Neuroscience Abstracts. 1983;9:809. [Google Scholar]
- Diamond IT, Conley M, Fitzpatrick D, Raczkowski D. Evidence for separate pathways within the tecto-geniculate projection in the tree shrew. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(4):1315–9. doi: 10.1073/pnas.88.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT, Fitzpatrick D, Schmechel D. Calcium binding proteins distinguish large and small cells of the ventral posterior and lateral geniculate nuclei of the prosimian galago and the tree shrew (Tupaia belangeri) Proceedings of the National Academy of Sciences of the United States of America. 1993;90(4):1425–9. doi: 10.1073/pnas.90.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT, Hall WC. Evolution of neocortex. Science (New York, N Y ) 1969;164(3877):251–62. doi: 10.1126/science.164.3877.251. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Snyder M, Killackey H, Jane J, Hall WC. Thalamo-cortical projections in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1970;139(3):273–306. doi: 10.1002/cne.901390303. [DOI] [PubMed] [Google Scholar]
- Drenhaus U, von Gunten A, Rager G. Classes of axons and their distribution in the optic nerve of the tree shrew (Tupaia belangeri) The Anatomical Record. 1997;249(1):103–16. doi: 10.1002/(SICI)1097-0185(199709)249:1<103::AID-AR13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Familtsev D, Quiggins R, Masterson SP, Dang W, Slusarczyk AS, Petry HM, Bickford ME. Ultrastructure of geniculocortical synaptic connections in the tree shrew striate cortex. The Journal of Comparative Neurology. 2016;524(6):1292–306. doi: 10.1002/cne.23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsten G, Benevento LA, Burman D. Opponent-color responses in macaque extrageniculate visual pathways: the lateral pulvinar. Brain Research. 1983;288(1–2):363–7. doi: 10.1016/0006-8993(83)90119-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cerebral Cortex (New York, N Y 5: 1991) 1996;6(3):329–41. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- Gale SD, Murphy GJ. Distinct Representation and Distribution of Visual Information by Specific Cell Types in Mouse Superficial Superior Colliculus. Journal of Neuroscience. 2014;34(40):13458–13471. doi: 10.1523/JNEUROSCI.2768-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning KK, Hall JA, Diamond IT, Hall WC. The pulvinar nucleus of Galago senegalensis. The Journal of Comparative Neurology. 1975;161(3):419–457. doi: 10.1002/cne.901610309. [DOI] [PubMed] [Google Scholar]
- Glickstein M. Laminar structure of the dorsal lateral geniculate nucleus in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1967;131(2):93–102. doi: 10.1002/cne.901310203. [DOI] [PubMed] [Google Scholar]
- Graham J, Casagrande VA. A light microscopic and electron microscopic study of the superficial layers of the superior colliculus of the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1980;191(1):133–151. doi: 10.1002/cne.901910108. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. I. The superficial gray layer. The Journal of Comparative Neurology. 1993;332(2):213–223. doi: 10.1002/cne.903320206. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. III: The optic layer. Visual Neuroscience. 1997;14(4):647–61. doi: 10.1017/s095252380001261x. [DOI] [PubMed] [Google Scholar]
- Harting JK, Diamond IT, Hall WC. Anterograde degeneration study of the cortical projections of the lateral geniculate and pulvinar nuclei in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1973;150(4):393–439. doi: 10.1002/cne.901500403. [DOI] [PubMed] [Google Scholar]
- Harting JK, Glendenning KK, Diamond IT, Hall WC. Evolution of the primate visual system: Anterograde degeneration studies of the tecto-pulvinar system. American Journal of Physical Anthropology. 1973;38(2):383–392. doi: 10.1002/ajpa.1330380237. [DOI] [PubMed] [Google Scholar]
- Harting JK, Hall WC, Diamond IT, Martin GF. Anterograde degeneration study of the superior colliculus in Tupaia glis: Evidence for a subdivision between superficial and deep layers. The Journal of Comparative Neurology. 1973;148(3):361–386. doi: 10.1002/cne.901480305. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Norton TT, Mize RR. Laminar organization and ultrastructure of GABA-immunoreactive neurons and processes in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia belangeri) Visual Neuroscience. 1988;1(2):189–204. doi: 10.1017/s0952523800001462. [DOI] [PubMed] [Google Scholar]
- Hubel DH. An autoradiographic study of the retino-cortical projections in the tree shrew (Tupaia glis) Brain Research. 1975;96(1):41–50. doi: 10.1016/0006-8993(75)90568-5. [DOI] [PubMed] [Google Scholar]
- Hughes A. The topography of vision in mammals of contrasting life style: Comparative optics and retinal organization. In: Crescitelli F, editor. Handbook of Sensory Physiology. VII/5. Berlin: Springer-Verlag; 1977. pp. 613–756. [Google Scholar]
- Humphrey AL, Albano JE, Norton TT. Organization of ocular dominance in tree shrew striate cortex. Brain Research. 1977;134(2):225–36. doi: 10.1016/0006-8993(77)91069-1. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Norton TT. Topographic organization of the orientation column system in the striate cortex of the tree shrew (Tupaia glis). I. Microelectrode recording. The Journal of Comparative Neurology. 1980;192(3):531–547. doi: 10.1002/cne.901920311. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Skeen LC, Norton TT. Topographic organization of the orientation column system in the striate cortex of the tree shrew (Tupaia glis). II. Deoxyglucose mapping. The Journal of Comparative Neurology. 1980;192(3):549–566. doi: 10.1002/cne.901920312. [DOI] [PubMed] [Google Scholar]
- Humphrey NK, Weiskrantz L. Vision in Monkeys after Removal of the Striate Cortex. Nature. 1967;215:595. doi: 10.1038/215595a0. [DOI] [PubMed] [Google Scholar]
- Jane JA, Carlson NJ, Levey N. A comparison of the effects of lesions of striate cortex and superior colliculus on vision in the Malayan tree shrew (Tupaia glis) Anatomical Record. 1969;163:306–307. [Google Scholar]
- Jane JA, Levey N, Carlson NJ. Tectal and cortical function in vision. Experimental Neurology. 1972;35(1):61–77. doi: 10.1016/0014-4886(72)90060-x. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Van Hooser SD, Fitzpatrick D. The representation of S-cone signals in primary visual cortex. Journal of Neuroscience. 2010;30(31):10337–10350. doi: 10.1523/JNEUROSCI.1428-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Westbrook T, Shayesteh R, Chen EL, Schumacher JW, Fitzpatrick D, Field GD. Distribution and diversity of intrinsically photosensitive retinal ganglion cells in tree shrew. Journal of Comparative Neurology. 2017;00:1–17. doi: 10.1002/one.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Vision without awareness. Nature. 1995;373(6511):195–195. doi: 10.1038/373195a0. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behavior and Evolution. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Harting JK, Guillery RW. Representation of the complete retina in the contralateral superior colliculus of some mammals. Brain Research. 1974;65(2):343–6. doi: 10.1016/0006-8993(74)90047-x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Archonton affinities as reflected in the visual system. In: Szalay R, Novacek M, Mckenna M, editors. Mammal phyologeny: placentals. New York: Springer-Verlag; 1993. pp. 115–128. [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain5: A Journal of Neurology. 2002;125(Pt 2):350–60. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Killackey H, Diamond IT. Visual attention in the tree shrew: an ablation study of the striate and extrastriate visual cortex. Science (New York, N Y ) 1971;171(3972):696–9. doi: 10.1126/science.171.3972.696. [DOI] [PubMed] [Google Scholar]
- Killackey H, Snyder M, Diamond IT. Function of striate and temporal cortex in the tree shrew. Journal of Comparative and Physiological Psychology. 1971;74(1) Suppl 2:1–29. doi: 10.1037/h0020531. [DOI] [PubMed] [Google Scholar]
- Killackey H, Wilson M, Diamond IT. Further studies of the striate and extrastriate visual cortex in the tree shrew. Journal of Comparative and Physiological Psychology. 1972;81(1):45–63. doi: 10.1037/h0033320. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Mäntylä T, Silvanto J. The role of early visual cortex (V1/V2) in conscious and unconscious visual perception. NeuroImage. 2010;51(2):828–834. doi: 10.1016/j.neuroimage.2010.02.042. [DOI] [PubMed] [Google Scholar]
- Kuyk TK, Norton TT. Effects of striate cortex ablation on single-unit responses in tree shrew superior colliculus. Invest Ophthalmol Vision Sci Suppl. 1981;20:15. [Google Scholar]
- Kwan WC, Mundinano IC, de Souza MJ, et al. Unraveling the subcortical and retinal circuitry of the primate inferior pulvinar. Journal of Comparative Neurology. 2018;00:1–19. doi: 10.1002/cne.24387. [DOI] [PubMed] [Google Scholar]
- Lane RH, Allman JM, Kaas JH. Representation of the visual field in the superior colliculus of the grey squirrel (Sciurus carolinensis) and the tree shrew (Tupaia glis) Brain Research. 1971;26(2):277–92. [PubMed] [Google Scholar]
- Laycock R, Crewther DP, Fitzgerald PB, Crewther SG. Evidence for Fast Signals and Later Processing in Human V1/V2 and V5/MT+: A TMS Study of Motion Perception. Journal of Neurophysiology. 2007;98(3):1253–1262. doi: 10.1152/jn.00416.2007. [DOI] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13299–304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Hall WC. Interlaminar connections of the superior colliculus in the tree shrew. II: Projections from the superficial gray to the optic layer. Visual Neuroscience. 1995;12(3):573–88. doi: 10.1017/s0952523800008464. [DOI] [PubMed] [Google Scholar]
- LeGros Clark WE. Early Forerunners of Man. London: Baillere, Tyndall and Cox; 1934. [Google Scholar]
- Lu HD, Petry HM. Temporal modulation sensitivity of tree shrew retinal ganglion cells. Visual Neuroscience. 2003;20(4):363–72. doi: 10.1017/s0952523803204028. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Carey RG, Fitzpatrick D, Diamond IT. New view of the organization of the pulvinar nucleus in Tupaia as revealed by tectopulvinar and pulvinar-cortical projections. The Journal of Comparative Neurology. 1988;273(1):67–86. doi: 10.1002/cne.902730107. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. Cortical connections of striate and extrastriate visual areas in tree shrews. Journal of Comparative Neurology. 1998;401:109–128. doi: 10.1002/(sici)1096-9861(19981109)401:1<109::aid-cne7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. The visual pulvinar in tree shrews I. Multiple subdivisions revealed through acetylcholinesterase and Cat-301 chemoarchitecture. The Journal of Comparative Neurology. 2003a;467(4):593–606. doi: 10.1002/cne.10939. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. The Journal of Comparative Neurology. 2003b;467(4):607–627. doi: 10.1002/cne.10940. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. A Disynaptic Relay from Superior Colliculus to Dorsal Stream Visual Cortex in Macaque Monkey. Neuron. 2010;65(2):270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers LH, Rapisardi SC. Visual and somatosensory receptive fields of neurons in the squirrel monkey pulvinar. Brain Research. 1973;64:65–83. doi: 10.1016/0006-8993(73)90171-6. [DOI] [PubMed] [Google Scholar]
- Mooser F, Bosking WH, Fitzpatrick D. A morphological basis for orientation tuning in primary visual cortex. Nature Neuroscience. 2004;7(8):872–879. doi: 10.1038/nn1287. [DOI] [PubMed] [Google Scholar]
- Müller B, Peichl L. Topography of cones and rods in the tree shrew retina. Journal of Comparative Neurology. 1989;282(4):581–594. doi: 10.1002/cne.902820409. [DOI] [PubMed] [Google Scholar]
- Muly EC, Fitzpatrick D. The morphological basis for binocular and ON/OFF convergence in tree shrew striate cortex. The Journal of Neuroscience. 1992;12(4):1319–34. doi: 10.1523/JNEUROSCI.12-04-01319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami DM, Fuller CA. The retinohypothalamic projection and oxidative metabolism in the suprachiasmatic nucleus of primates and tree shrews. Brain, Behavior and Evolution. 1990;35(5):302–12. doi: 10.1159/000115876. [DOI] [PubMed] [Google Scholar]
- Norton TT. Geniculate and extrageniculate visual systems in the tree shrew. In: Morrison AR, Strick PL, editors. Changing Concepts of the Nervous System. New York: Academic Press; 1982. pp. 377–409. [Google Scholar]
- Ohno T, Misgeld U, Kitai ST, Wagner A. Organization of the visual afferents into the LGd and the pulvinar of the tree shrew Tupaia glis. Brain Research. 1975;90(1):153–8. doi: 10.1016/0006-8993(75)90691-5. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast Backprojections from the Motion to the Primary Visual Area Necessary for Visual Awareness. Science. 2001;292(5516):510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Petry HM, Clark CT, Day-Brown JD, Bolin RT, Bickford ME. Behavioral measurement of RDK velocity discrimination thresholds in the tree shrew [Abstract] Journal of Vision. 2012;12(9):1223. [Google Scholar]
- Petry HM, Erichsen JT, Szél A. Immunocytochemical identification of photoreceptor populations in the tree shrew retina. Brain Research. 1993;616(1–2):344–50. doi: 10.1016/0006-8993(93)90230-k. [DOI] [PubMed] [Google Scholar]
- Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Research. 1984;24(9):1037–42. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- Petry HM, Hárosi FI. Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus): a microspectrophotometric investigation. Vision Research. 1990;30(6):839–51. doi: 10.1016/0042-6989(90)90053-n. [DOI] [PubMed] [Google Scholar]
- Petry HM, Kelly JP. Psychophysical measurement of spectral sensitivity and color vision in red-light-reared tree shrews (Tupaia belangeri) Vision Research. 1991;31(10):1749–57. doi: 10.1016/0042-6989(91)90024-y. [DOI] [PubMed] [Google Scholar]
- Petry HM, Murphy HA. Differentiation of short-wavelength-sensitive cones by NADPH diaphorase histochemistry. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):5121–3. doi: 10.1073/pnas.92.11.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Helms MC, Lee P, Augustine GJ, Hall WC. Local excitatory circuits in the intermediate gray layer of the superior colliculus. Journal of Neurophysiology. 1999;81(3):1424–7. doi: 10.1152/jn.1999.81.3.1424. [DOI] [PubMed] [Google Scholar]
- Polson MC. Doctoral dissertation. Indiana University; Bloomington. IN: 1968. Spectral sensitivity and color vision in Tupaia glis. [Google Scholar]
- Raczkowski D, Casagrande VA, Diamond IT. Visual neglect in the tree shrew after interruption of the descending projections of the deep superior colliculus. Experimental Neurology. 1976;50(1):14–29. doi: 10.1016/0014-4886(76)90231-4. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Fitzpatrick D. Terminal arbors of individual, physiologically identified geniculocortical axons in the tree shrew’s striate cortex. The Journal of Comparative Neurology. 1990;302(3):500–514. doi: 10.1002/cne.903020307. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proceedings of the National Academy of Sciences. 2005;102(34):12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. The Vertebrate Retina: Principles of Structure and Function. New York: W.H. Freeman; 1973. [Google Scholar]
- Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature Neuroscience. 2015;19(2):299–307. doi: 10.1038/nn.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MH, Stone J. Naming of neurons. Classification and naming of cat retinal ganglion cells. Brain, Behavior and Evolution. 1977;14(3):185–216. doi: 10.1159/000125660. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71(2):209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma MA, Casagrande VA, Kaas JH. Cortical connections of area 17 in tree shrews. Journal of Comparative Neurology. 1984;230:337–351. doi: 10.1002/cne.902300303. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. X- and Y-cells in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia glis) Brain Research. 1975;93(1):152–7. doi: 10.1016/0006-8993(75)90294-2. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiological Reviews. 1982;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double Dissociation of V1 and V5/MT activity in Visual Awareness. Cerebral Cortex. 2005;15(11):1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. Journal of Neuroscience. 2003;23:5684–5692. doi: 10.1523/JNEUROSCI.23-13-05684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen LC, Humphrey AL, Norton TT, Hall WC. Deoxyglucose mapping of the orientation column system in the striate cortex of the tree shrew, Tupaia glis. Brain Research. 1978;142(3):538–45. doi: 10.1016/0006-8993(78)90915-0. [DOI] [PubMed] [Google Scholar]
- Snyder M, Hall WC, Diamond IT. Vision in tree shrews (Tupaia glis) after removal of striate cortex. Psychonomic Science. 1966;6(5):243–244. [Google Scholar]
- Somogyi G, Hajdu F, Hassler R, Wagner A. An experimental electron microscopical study of a direct retino-pulvinar pathway in the tree shrew. Experimental Brain Research. 1981;43(3–4):447–50. doi: 10.1007/BF00238389. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science (New York, N Y ) 1966;153(3743):1544–7. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprague JM, Meikle TH. The role of the superior colliculus in visually guided behavior. Experimental Neurology. 1965;11:115–46. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? European Journal of Neuroscience. 1999;11:469–480. doi: 10.1046/j.1460-9568.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Cowey A. Blindsight in man and monkey. Brain5: A Journal of Neurology. 1997;120(3):535–59. doi: 10.1093/brain/120.3.535. [DOI] [PubMed] [Google Scholar]
- ter Laak HJ, Thijssen JM. Definition of neural response and incremental sensitivity of retinal ganglion cells in a tree shrew (Tupaia chinensis) Vision Research. 1979;19(8):885–9. doi: 10.1016/0042-6989(79)90022-1. [DOI] [PubMed] [Google Scholar]
- ter Laak HJ, Thijssen JM, Vendrik AJ. Intensity discrimination capacity of retinal ganglion cells of a tree shrew (Tupaia chinensis) Vision Research. 1980;20(2):149–57. doi: 10.1016/0042-6989(80)90157-1. [DOI] [PubMed] [Google Scholar]
- Thijssen JM, van Dongen PA, ter Laak HJ. Maintained activity of cells in the three shrew’s optic tract. Experimental Brain Research. 1976;25(3):279–90. doi: 10.1007/BF00234019. [DOI] [PubMed] [Google Scholar]
- Trevarthen CB. Two mechanisms of vision in primates. Psychologische Forschung. 1968;31(4):299–348. doi: 10.1007/BF00422717. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Muly EC, Fitzpatrick D. Lateral geniculate projections to the superficial layers of visual cortex in the tree shrew. The Journal of Comparative Neurology. 1992;319(1):159–171. doi: 10.1002/cne.903190113. [DOI] [PubMed] [Google Scholar]
- van Dongen PA, ter Laak HJ, Thijssen JM, Vendrik AJ. Functional classification of cells in the optic tract of a tree shrew (Tupaia chinensis) Experimental Brain Research. 1976;24(4):441–6. doi: 10.1007/BF00235010. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Roy A, Rhodes HJ, Culp JH, Fitzpatrick D. Transformation of receptive field properties from lateral geniculate nucleus to superficial V1 in the tree shrew. Journal of Neuroscience. 2013;33(28):11494–11505. doi: 10.1523/JNEUROSCI.1464-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni MP, Thomas S, Petry HM, Bickford ME, Casanova C. Spatiotemporal profile of voltage-sensitive dye responses in the visual cortex of tree Shrews evoked by electric microstimulation of the dorsal lateral geniculate and pulvinar nuclei. The Journal of Neuroscience. 2015;35(34):11891–6. doi: 10.1523/JNEUROSCI.0717-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CB, Casagrande VA, Diamond IT. Does the acuity of the tree shrew suffer from removal of striate cortex? A commentary on the paper by ward and Masterton. Brain, Behavior and Evolution. 1972;5(1):18–29. doi: 10.1159/000123768. [DOI] [PubMed] [Google Scholar]
- Ware CB, Diamond IT, Casagrande VA. Effects of ablating the striate cortex on a successive pattern discrimination: further study of the visual system in the tree shrew (Tupaia glis) Brain, Behavior and Evolution. 1974;9(4):264–79. doi: 10.1159/000123670. [DOI] [PubMed] [Google Scholar]
- Wei H, Bonjean M, Petry HM, Sejnowski TJ, Bickford ME. Thalamic burst firing propensity: a comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. The Journal of Neuroscience. 2011;31(47):17287–99. doi: 10.1523/JNEUROSCI.6431-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Masterson SP, Petry HM, Bickford ME. Diffuse and specific tectopulvinar terminals in the tree shrew: synapses, synapsins, and synaptic potentials. PloS One. 2011;6(8):e23781. doi: 10.1371/journal.pone.0023781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain5: A Journal of Neurology. 1974;97(4):709–28. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proceedings of the National Academy of Sciences. 2009;106(23):9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT, Norton TT. Histochemical localization of cytochrome oxidase activity in the visual system of the tree shrew: Normal patterns and the effect of retinal impulse blockage. The Journal of Comparative Neurology. 1988;272(4):562–578. doi: 10.1002/cne.902720409. [DOI] [PubMed] [Google Scholar]
- Zihl J, von Cramon D. The contribution of the “second” visual system to directed visual attention in man. Brain5: A Journal of Neurology. 1979;102(4):835–56. doi: 10.1093/brain/102.4.835. [DOI] [PubMed] [Google Scholar]