Abstract
In this case study, we report the longitudinal and multimodal follow‐up of a catastrophic initial presentation of cerebral fat embolism syndrome. We show that despite the initial severity, the cognitive outcome was ultimately very good but with a highly nonlinear time‐course and prolonged loss of consciousness (more than 2 months). Repeated clinical assessments and brain‐imaging techniques (electroencephalography, event‐related potential, 18‐Fluoro‐Deoxy‐Glucose‐PET and magnetic resonance imaging) allowed us to monitor and anticipate this dynamic, providing relevant information to guide decision making in front of withdrawal of life‐sustaining therapy discussions. This case illustrates the value of multimodal functional imaging in devastating brain injuries.
Introduction
Fat embolism syndrome (FES) is the collection of symptoms caused by embolization of bone marrow fat in the systemic circulation, typically after multiple fractures of long bones and pelvis. While systemic fat embolism events are frequently encountered in this context and during orthopedic surgery,1 neurological manifestations, cerebral FES (CFES), are rare. CFES is perceived as having a better prognosis than other causes of cerebral emboli, but description of neurological outcomes from severe clinical presentation of CFES remain sparse and poorly documented in the literature.2
Here we report the first detailed longitudinal multimodal description of a CFES case with, ultimately, an excellent neurological outcome despite a catastrophic CFES initial presentation and a late recovery of consciousness. This original multimodal approach yielded relevant information to monitor and anticipate the dynamics of this recovery.
Clinical presentation and diagnosis
A 26‐year‐old man was admitted in the Intensive Care Unit (ICU) in March 2017 for multiple fractures of the inferior limbs after a 20 m‐high fall from a roof. The patient was initially conscious with a Glasgow Coma Scale (GCS) of 15/15 without any focal deficit. Upon arrival at the hospital, he presented hemodynamic shock requiring intubation, mechanical ventilation support, fluid resuscitation, intravenous norepinephrine, and massive transfusion. Once stabilized, that is, 3 h following the trauma, a whole‐body computed tomography (CT) scan imaging showed complex open fractures of left tibia and fibula, luxation of left hip, fracture of right femur, and normal brain imaging. A 10‐h surgery resulted in fracture reduction and osteosynthesis with external fixation (left leg) and intramedullary nail (right femur). During the surgery, gas exchange dramatically deteriorated leading to refractory hypoxemia. Postoperative cerebral and thoracic CT‐scans, performed 16 h following the trauma, showed no cerebral abnormalities but diffuse bilateral pulmonary infiltrates comprising consolidations and ground‐glass opacities and bilateral pulmonary embolism. FES was highly suspected. Fundoscopy showed no ocular fat embolism at day 3. However, a bronchoalveolar lavage, performed at day 8 to document a suspected ventilator‐associated pneumonia, showed active alveolar hemorrhage and fat‐containing macrophages confirming the diagnosis. Cardiac echocardiography revealed no evidence of patent foramen ovale. After resolution of hypoxemia, sedations were stopped at day 7, but the patient remained comatose with a GCS of 5 (E1V1M3) and preserved brainstem reflexes. The diagnosis of CFES was proposed and confirmed by a typical MRI at day 10 showing “starfield” pattern on diffusion‐weighted imaging (DWI)3 in high‐signal associated with large high‐signal FLAIR lesions of the sus‐tentorial and brainstem deep white matter (DWM) and diffuse microbleeds on susceptibility‐weighted imaging (SWI) sequence (Fig. S1). Diffusion tensor imaging (DTI) furthermore revealed a marked reduction of fractional anisotropy (up to 40%) mainly involving the sus‐tentorial white matter.
Neurological recovery
At day 10, electroencephalograhy (EEG) showed a slow (3 Hz) monotonous and nonreactive background activity, associated with anterior bursts of slow complexes. Patient emerged from coma at day 14 (GCS = 8/15; and FOUR‐score = 10/16). Although he showed some signs of vigilance recovery with eyes opening, he was still unconscious according to the Coma Recovery Scale‐revised (CRS‐R)4: score = 5/23 1‐1‐2‐0‐0‐1, corresponding to a vegetative state (also coined unresponsive wakefulness syndrome ‐UWS). He presented severe neurovegetative crises. At day 16, the multivariate classification of EEG markers was also in favor of UWS.5, 6 However, cognitive event‐related potentials (ERP) using the “local‐global” paradigm7 showed preserved early cortical responses to sound stimuli, as well as a mismatch negativity (MMN), and a faint but significant late P3b component suggesting preserved conscious access to auditory stimuli.8 He was weaned from mechanical ventilation at day 20, but still required a tracheostomy tube for aspiration and airways protection.
At day 30, a 18‐Fluoro‐Deoxy‐Glucose‐PET (18FDG‐PET) scan showed on visual assessment a diffuse cortical hypometabolism, mainly of fronto‐parieto‐temporal associative cortices, preserving the primary sensory and subcortical areas. However, the quantitative metabolic index of the highest hemisphere (MIHH) was 3.34, compatible with a minimally conscious state (MCS) metabolic activity.9 Indeed, at day 33, he transitioned toward MCS with a CRS‐R of 10/23 (2‐3‐2‐1‐0‐2), meaning he showed consistent visual pursuit but no response to command or verbal response. MRI at day 36 showed a major reduction of the edematous DWM lesions, persisting microbleeds and the onset of a global cerebral atrophy. The tracheostomy tube was removed at day 45 and he was transferred to rehabilitation center at day 50, where he started exhibiting command following (day 60) and gradually recovered speech (day 66) and functional communication (day 72), exiting the MCS condition to a conscious state.
At 3 months, he was fully conscious (CRS‐R of 23/23), although he still suffered from cognitive and behavioral frontal syndrome with apathy, impulsivity, grasping and an anterograde amnesia due to encoding deficit. Mini‐Mental State Examination (MMSE) was 21/30 and Frontal Assessment Battery (FAB) 5/18. Retrograde memory, speech, praxis and visuospatial functions were quite preserved. EEG background activity was reactive but still slowed, in the theta range (6–7 Hz).
At 6 months, he improved further with an MMSE of 23/30 and a clear improvement of the frontal syndrome as shown by a FAB score of 13/18. EEG was normal, with a symmetric posterior alpha rhythm at 9 Hz, reactive to eyes opening and rapid microvolted anterior beta rhythm (Fig. 1). MRI was similar to the previous one and still showed some periventricular FLAIR hypersignal as well as a global reduction in fractional anisotropy. Atrophy was considered stable (Fig. S2). A second 18FDG‐PET showed a great improvement with near normal metabolism of the entire brain except for the internal temporal structures and the cerebellum. MIHH was 5.23, clearly in the range of conscious and healthy subjects (Fig. 2).
Figure 1.
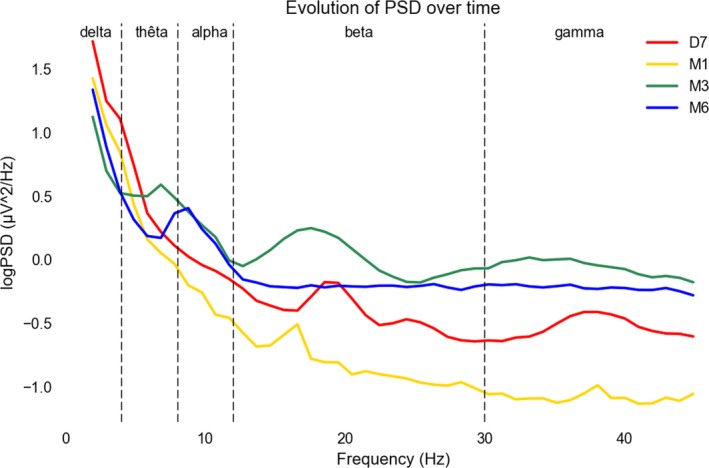
EEG power spectral density evolution. Legend. Evolution of the power spectral density of the EEG (log transform, unit log(μV2/Hz)) overt time showed the predominance of slow rhythm mainly in the delta (1–3‐Hz) frequency band at D7 and until M1. At M3, the background rhythm enriched with the diminution of the slow frequency for the benefit of theta (4–8 Hz) frequencies. At M6, theta frequencies left room to a normal alpha (9–12 Hz) background rhythm. Together with this rapid beta and gamma rhythm power spectra also increased over time probably due to more muscle artifacts with the patient regaining some mobility. See Data S1. D7, Day 7; Hz: Hertz; M1, Month 1; M3, Month 3; M6, Month 6; PSD, Power Spectral Density: V, Volt.
Figure 2.
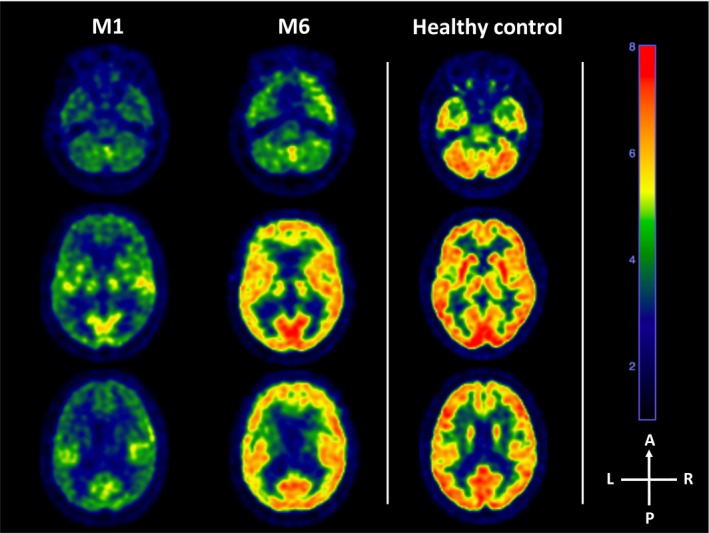
18‐FDG PET evolution. Legend.18‐Fluoro‐Deoxy‐Glucose Positron‐Emission‐Tomography (18‐FDG PET) evolution between M1 and M6 (left panel). At M1, the PET showed a diffuse cortical hypometabolism, mainly of the frontal, parietal and temporal associative cortices, preserving the primary sensory and internal temporal cortices as well as subcortical and cerebellar areas. However, the metabolic index of the highest hemisphere was 3.34, compatible with a minimally conscious state metabolic activity.9 At month 6, the metabolic activity of the entire brain was near normal except from the internal temporal lobe and the cerebellum. Metabolic index of the highest hemisphere was 5.23, clearly in the range of conscious and healthy subjects (example of an healthy control on the right panel). A, Anterior; L, Left; M1, Month 1; M6, Month 6; P, Posterior; R, Right.
At 9 months, MMSE was 26/30 and FAB 16/18. A full psychometric evaluation showed a mild dysexecutive syndrome with attentional deficits and fatigability. Nonetheless the patient was fit to be discharged home and could resume his social life (meeting‐up with friends, having dinner at the restaurant and going to the movies). At 1 year, MMSE was normal (29/30) and the FAB stable.
Discussion
The main findings of our report can be summarized as follows: (1) the time‐course of recovery from CFES is highly nonlinear and can be delayed up to several months; (2) neurological recovery can be good even in case of a catastrophic initial presentation; (3) a multimodal and repeated approach based on imaging and electrophysiology techniques helps monitoring and anticipating changes in CFES‐induced brain damage to guide decision making (Fig. 3 and Table 1).
Figure 3.
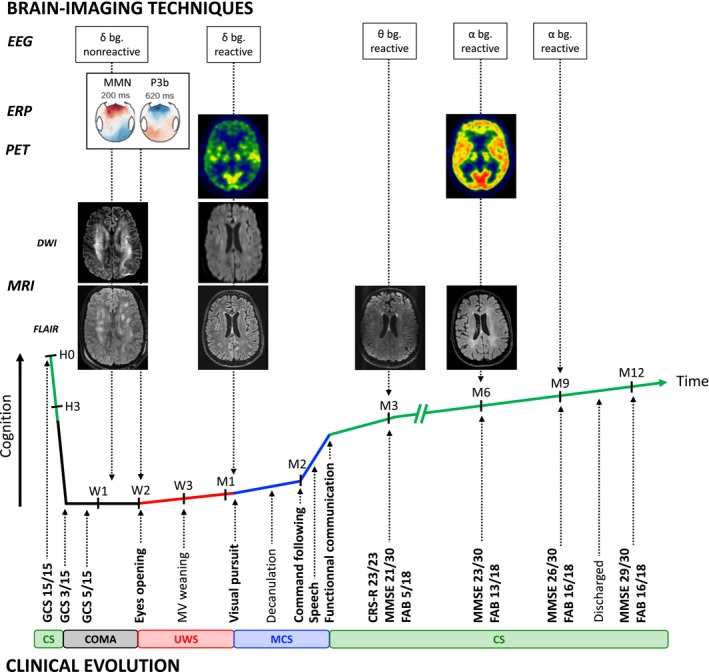
Multimodal clinical and brain‐imaging dynamic of recovery. Legend. (Bottom) Longitudinal clinical follow‐up of the patient's recovery according to different scores assessing vigilance (Glasgow Coma Scale), consciousness (Coma Recovery Scale‐ Revised) and cognitive function together with pivotal behavior. (Top) Concurrent brain function according to different functional and structural brain imaging techniques (electroencephalogram, auditory event‐related potential, 18‐FDG PET‐TDM and MRI). bg, background rhythm; CRS‐R, Coma recovery scale ‐revised; CS, Conscious state; D, Day; DWI, Diffusion‐weighted imaging; EEG, Electroencephalogram; ERP, Event‐related potential; FAB, Frontal assessment battery; FLAIR, Fluid‐attenuation inversion recovery; GCS, Glasgow coma scale; H, Hour; M, Month; MCS, Minimally conscious state; MMN, Mismatch negativity; MMSE, Mini‐mental state examination; MRI, Magnetic resonance imaging; MV, Mechanical ventilation; PET, Positron‐emission tomography; UWS, Unresponsive wakefulness syndrome; W, Week.
Table 1.
Patient's clinical evolution over a year together with brain imaging and electrophysiology and potential confounders of consciousness and cognition
| Time | Clinical | Neuroimaging | Electrophysiology | Confounders |
|---|---|---|---|---|
| D1 | Conscious with GCS 15 | |||
| W1 | Deep coma with GCS 3 (E1V1M1) (D1‐D7) | Normal brain CT scan normal (D1) | None |
Hemorrhagic shock and ARDS (D1‐D2) Midazolam and sufentanyl sedation (D1‐D7) Prophylactic amoxicilline‐clavulanate (D1‐D4) Piperacillin‐clavulanate for VAP (D4‐D6) |
| W2 | Coma with GCS 5 (E1V1M3) (D9) |
Periventricular DWM hypodensities on brain CT (D9) DWI starfield pattern, vasogenic and cytotoxic DWM edema, diffuse microbleeds and global FA decrease on MRI (D10) |
Nonreactive EEG with delta (3 Hz) background and anterior slow waves (1 Hz) (D7) |
Imipenem (D6‐D10) and meropenem (D11‐D19) for VAP Clonidine (D11‐D12) and levomepromazin (D16‐22) for neurovegetative crisis Hypernatremia (150 mmol/L) (D26‐27) |
| W3 |
Eyes opening with GCS 8 (E4V1M3) and CRS‐R 5 [1‐1‐2‐0‐0‐1] (D14) Neurovegetative crisis (D16‐D22) MV weaning (D20) Central fever (D19‐D22) Visual fixation with CRS‐R 9 [2‐2‐2‐1‐0‐2] (D23) |
None | Nonreactive EEG with slow delta background but with MMN and P3b on cognitive auditory evoked potentials (D16) | |
| M1 |
Visual pursuit with CRS‐R 10 [2‐3‐2‐1‐0‐2] (D33) Decannulation (D45) |
Diffuse PET hypometabolism with MMIH of 3.34 (D30) Regression of DWI and FLAIR abnormalities, diffuse microbleeds, global cerebral atrophy and FA reduction on MRI (D36) |
Reactive EEG with delta background (D30) | None |
| M2 |
Command following (D60) Speech production (D66) Functional communication with CRS‐R 21 [4‐5‐5‐3‐2‐2] (D72) |
None | None | Hyponatremia (132 mmol/L) (D64‐85) |
| M3 |
Fully conscious with CRS‐R 23/23 Posttraumatic amnesia and dysexecutive syndrome with MMSE 21/30, FAB 13/30 and GOAT 73/100 |
Normal DWI, diminution of DWM periventricular FLAIR hyperintensities, persistent diffuse microbleeds and global cerebral atrophy | Reactive but slowed EEG background in the theta range (6–7 Hz) | None |
| M6 | Moderate dysexecutive syndrome with MMSE 23/30 and FAB 13/30 |
Normal PET brain metabolism with MIHH of 5.23 Mild brain atrophy, periventricular FLAIR hypersignal and global FA reduction on MRI |
Normal reactive posterior alpha (9 Hz) background rhythm with rapid anterior beta rhythm on EEG | None |
| M9 | Mild dysexecutive syndrome with MMSE 26/30 and FAB 16/18 | None | Normal reactive posterior alpha (9 Hz) rhythm | None |
| Y1 | Autonomous at home with MMSE 29/30 and FAB 16/18 | None | None | None |
ARDS, acute respiratory distress syndrome; CRS‐R, coma recovery scale‐revised; CT, computed tomography; FA, fractional anisotropy; FAB, frontal assessment battery; GCS, Glasgow coma scale; GOAT, Galveston orientation and amnesia test; M, month; MMSE, mini mental state examination; MRI, magnetic resonance imaging; PET, 18‐fluoro‐deoxyglucose positron emission tomography; VAP, ventilator acquired pneumonia.
Although the prognosis is usually considered better than other causes of emboli, serious complications are described (refractory status epilepticus,10 raised intracranial pressure requiring monitoring,11 decompressive hemicraniectomy,2 or sometimes resulting in brain death12). Few publications have studied the neurological and functional outcome of patients suffering from CFES. The largest series reported overall 7.4% mortality and 72.2% good outcome2 but with differences according to the neurological presentation. Focal deficits and/or epilepsy with mild mental status change was associated with 90.5% probability of good outcome, whereas deep alteration of vigilance and consciousness up to coma with abnormal postures, as seen in our case, was associated with only 57.6% probability of good outcome. Moreover, the definition of “good” and “bad” outcome is often unclear in the aforementioned cases. To date, there are only two case reports in the literature assessing cognitive outcome of CFES using neuropsychological testing13, 14 showing some mild difficulties in frontal function as seen in our patient. However, they lack detailed description of recovery. We show that the dynamic of recovery can be delayed,15 and most importantly extremely nonlinear. Indeed, after 2 weeks of coma, it took another 2 months for the patient to fully recover consciousness with functional communication that subsequently led to fast improvement of the cognitive function. This highlights the importance of the distinction between alteration of vigilance and loss of consciousness and prompts to carefully assess the latter, using dedicated scale, such as the CRS‐R. This scale remains rarely used in the literature of severe brain injury, while it yields more relevant information about the patient's condition after the acute phase. Taken together, the prolonged loss of consciousness and catastrophic initial presentation (rapid‐onset and deep coma, extended MRI lesions16 and nonreactive EEG) led to multidisciplinary discussions in the medical team regarding withdrawal of care, which was finally not retained considering the result of functional brain imaging. Indeed, auditory ERP revealed a MMN suggestive of unconscious processing of auditory novelty which has been shown to predict awakening,17, 18 but more importantly a P3b evocative of the preservation of high‐order cortical structures and conscious processing.7, 8 18FDG‐PET also showed a metabolic activity higher than the behavioral examination suggested. This outlines the interest of repeated assessment with combined multimodal techniques based on neurophysiology and neuroimaging in noncommunicative patients to probe the covert cognitive function19 and overall brain activity. These techniques also showed different time‐courses that yielded complementary information to the behavioral assessment in the dynamic of recovery.
The two mains etiologies of devastating brain injuries are anoxic brain injury (ABI) following cardiac arrest and severe traumatic brain injury (TBI). Neuroprognostication differs in these two conditions due to very different dynamic of recovery and overall better outcome of TBI.20, 21 This results in an earlier and more standardized approach in ABI than in TBI where lesions and prognosis are more heterogenous and late recoveries are possible. Recent guidelines on prognostication after ABI provided a hierarchical and multimodal algorithm22 in which a GCS motor response of 1 or 2, the absence of corneal and pupillary reflexes and/or the bilateral absence of the N20 wave on short‐latency somatosensory‐evoked potential from day 3 to 5 are predictive of a poor outcome with very low false positive rate. If this is not the case, a second step relies on less robust markers, that need to be combined and eventually repeated, such as high serum neuron‐specific enolase, unreactive malignant EEG patterns (status epilecticus or burst‐suppression, the prognostic value of isolated discontinuous or unreactive EEG being less clear) and/or brain imagery showing diffuse anoxic injury (CT or MRI). Recently, the global FA proved to be very accurate in prognosing outcome at 1 year of patients still comatose 7 days after cardiac arrest.23 In severe TBI, prognosis is more heterogenous and is often delayed by the initial management of high intracranial pressure (sedation, hypothermia, etc). Classic early predictive factors are age, initial GCS and pupillary response, hypoxia and hypotension on admission, mass lesion, subarachnoid hemorrhage or signs of raised intracranial pressure on brain CT (Marshall CT class III–VI) and to a lesser extent, blood glucose, and hemoglobin at admission. These predictors have been included in a score which predicts the 6 month Glasgow outcome scale with acceptable accuracy (area under the curve 0.8).24 Brainstem lesion or diffuse axonal injury on MRI are also associated with bad functional outcome25 and quantitative EEG may help but is still an open area of research.26 In the end, prognostication after severe TBI is more subjective and heavily relies on the clinical examination and the dynamic of recovery in the first few days to weeks.
Neuroprognostication thus depends on the underlying cause of brain injury and some authors have suggested that withdrawal of care decision should not be made before 1 month in CFES27 but our case demonstrates that this delay can be too short to properly assess the outcome of this complex etiology. In any case, potential confounders of consciousness and cognitive status (septic or metabolic encephalopathy, drugs, etc.) must be taken into account and in face of discrepancies or uncertainty, withdrawal of care decision should also be withhold.
Ethical Considerations
The patient signed an informed consent for this publication. The study conformed the Declaration of Helsinki, the French regulations, and was approved by the ethics committee (Comité de Protection des Personnes; CPP n° 2013‐A01385‐40) Ile de France 1 (Paris, France).
Conflict of Interest
None declared.
Supporting information
Figure S1. Initial MRI (day 10).
Figure S2. MRI evolution.
Data S1. EEG power spectral density evolution.
Acknowledgments
We respectfully thank the patient and his family for their trust and agreement on the publication of this case. We also thank all the caregivers that participated in the patient's recovery, from the ICU to the rehabilitation center.
Funding Information
None.
References
- 1. Christie J, Robinson CM, Pell AC, et al. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995;77:450–455. [PubMed] [Google Scholar]
- 2. Kellogg RG, Fontes RB, Lopes DK. Massive cerebral involvement in fat embolism syndrome and intracranial pressure management: case report. J Neurosurg 2013;119:1263–1270. [DOI] [PubMed] [Google Scholar]
- 3. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion‐weighted MRI (Starfield Pattern). Stroke 2001;32:2942–2944. [PubMed] [Google Scholar]
- 4. Kalmar K, Giacino JT. The JFK coma recovery scale‐revised. Neuropsychol Rehabil 2005;15:454–460. [DOI] [PubMed] [Google Scholar]
- 5. King J‐R, Sitt JD, Faugeras F, et al. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol 2013;23:1914–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sitt JD, King J‐R, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bekinschtein TA, Dehaene S, Rohaut B, et al. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci 2009;106:1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faugeras F, Rohaut B, Weiss N, et al. Probing consciousness with event‐related potentials in the vegetative state. Neurology 2011;77:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stender J, Mortensen KN, Thibaut A, et al. The minimal energetic requirement of sustained awareness after brain injury. Curr Biol 2016;26:1494–1499. [DOI] [PubMed] [Google Scholar]
- 10. Fernández‐Torre JL, Burgueño P, Ballesteros MA, et al. Super‐refractory nonconvulsive status epilepticus secondary to fat embolism: a clinical, electrophysiological, and pathological study. Epilepsy Behav 2015;49:184–188. [DOI] [PubMed] [Google Scholar]
- 11. Beretta L, Calvi MR, Frascoli C, Anzalone N. Cerebral fat embolism, brain swelling, and severe intracranial hypertension. J Trauma Inj Infect Crit Care 2008;65:E46–E48. [DOI] [PubMed] [Google Scholar]
- 12. Aggarwal R, Pal S, Soni K, Gamangatti S. Massive cerebral fat embolism leading to brain death: a rare presentation. Indian J Crit Care Med 2015;19:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manousakis G, Han DY, Backonja M. Cognitive outcome of cerebral fat embolism. J Stroke Cerebrovasc Dis. 2012;21:906.e1–906.e3. [DOI] [PubMed] [Google Scholar]
- 14. Gray AC, Torrens L, White TO, et al. The cognitive effects of fat embolus syndrome following an isolated femoral shaft fracture: a case report. J Bone Joint Surg Am 2007;89:1092. [DOI] [PubMed] [Google Scholar]
- 15. Rajasekaran S, Srikanth K, Sundararajan S. Late recovery in cerebral fat embolism. Indian J Orthop 2014;48:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma Acute Care Surg 1999;46:324. [DOI] [PubMed] [Google Scholar]
- 17. Kane NM, Curry SH, Butler SR, Cummins BH. Electrophysiological indicator of awakening from coma. The Lancet 1993;341:688. [DOI] [PubMed] [Google Scholar]
- 18. Fischer C, Morlet D, Bouchet P, et al. Mismatch negativity and late auditory evoked potentials in comatose patients. Clin Neurophysiol 1999;110:1601–1610. [DOI] [PubMed] [Google Scholar]
- 19. Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140:2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Multi‐Society Task Force on PVS . Medical aspects of the persistent vegetative state (1). N Engl J Med 1994;330:1499–1508. [DOI] [PubMed] [Google Scholar]
- 21. Luauté J, Maucort‐Boulch D, Tell L, et al. Long‐term outcomes of chronic minimally conscious and vegetative states. Neurology 2010;75:246–252. [DOI] [PubMed] [Google Scholar]
- 22. Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post‐resuscitation care. Intensive Care Med 2015;41:2039–2056. [DOI] [PubMed] [Google Scholar]
- 23. Velly L, Perlbarg V, Boulier T, et al. Use of brain diffusion tensor imaging for the prediction of long‐term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol 2018;17:317–326. [DOI] [PubMed] [Google Scholar]
- 24. Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5:e165; discussion e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haghbayan H, Boutin A, Laflamme M, et al. The prognostic value of MRI in moderate and severe traumatic brain injury: a systematic review and meta‐analysis. Crit Care Med 2017;45:e1280–e1288. [DOI] [PubMed] [Google Scholar]
- 26. Tolonen A, Särkelä MOK, Takala RSK, et al. Quantitative EEG parameters for prediction of outcome in severe traumatic brain injury: development study. Clin EEG Neurosci. 2017;1550059417742232. [DOI] [PubMed] [Google Scholar]
- 27. Meyer N, Pennington WT, Dewitt D, Schmeling GJ. Isolated cerebral fat emboli syndrome in multiply injured patients: a review of three cases and the literature. J Trauma Acute Care Surg 2007;63:1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Initial MRI (day 10).
Figure S2. MRI evolution.
Data S1. EEG power spectral density evolution.


