Abstract
We utilized the well‐validated University of Pennsylvania Smell Identification Test (UPSIT) to examine whether olfactory dysfunction occurs in ALS participants. After adjusting for relevant confounders in a multiple linear regression model, ALS participants scored significantly lower on the UPSIT compared with control participants, with an estimated mean difference of 2.31 points (P = 0.015). ALS participants also had twice the rate of olfactory dysfunction (microsmia or anosmia). This study suggests that olfactory dysfunction exists in ALS patients, which expands our understanding of the extramotor findings in ALS. Future investigations could determine whether there are correlations between olfactory dysfunction and specific ALS phenotypes.
Introduction
The concept of ALS as a purely motor neuron disease has been challenged by the discovery of cognitive impairment in up to half of patients with sporadic ALS.1 ALS patients with frontotemporal dementia and executive dysfunction tend to have more rapid disease progression.2 Therefore, the ability to detect other extramotor abnormalities in ALS patients could be helpful diagnostically and prognostically.
Olfactory dysfunction is a powerful biomarker in other neurodegenerative disorders such as Parkinson's disease (PD), Alzheimer's disease (AD), and frontotemporal dementia (FTD).3, 4 It has been widely studied in PD and is now recognized as one of its initial symptoms, preceding motor symptoms by several years.5 Olfactory deficits have also been identified in a small cohort of Guamanian patients with ALS, as well as those with parkinsonism, dementia, and the parkinsonism‐dementia complex.6 Interestingly, olfactory dysfunction has been shown to predict the degree of cognitive impairment associated with PD.7 This relationship between olfaction and cognitive impairment is also shared with AD, where olfactory dysfunction is associated with an increased risk of dementia.8 It is thought that olfaction is affected in these disorders due to the pathologic changes found in cortical areas associated with smell perception and identification.3, 4, 5 Olfactory structures have also been found to be involved in ALS, with postmortem reports of TDP‐43‐positive inclusions in the olfactory bulb, hippocampus, and primary olfactory cortex.9
Olfaction in ALS has been previously investigated in studies, with a small sample size, with mixed findings. A difference in smell identification has been reported in 17 patients with bulbar onset ALS compared with controls, as well as in 15 patients with motor neuron disease.10, 11 A more recent study found olfactory dysfunction in patients in the ALS‐FTD spectrum, but not in ALS patients with normal cognitive function.12 However, these studies included a small number of participants and did not account for additional confounders (such as smoking history or current nasal congestion) that may have affected the results. Moreover, they did not explore the effect of other disease characteristics on olfaction.
To expand our understanding of the extramotor involvement in ALS, we tested the prevalence of olfactory dysfunction in a cohort of ALS patients, analyzed potential confounders of olfactory dysfunction, and studied whether olfactory dysfunction was correlated with ALS disease characteristics.
Methods
Participants
Patients aged 18–80 with either sporadic or familial ALS and El Escorial criteria of possible, probable, laboratory‐supported probable, or definite ALS were included in the study. Age‐matched, unrelated controls without a history of neurological disease were obtained by enrolling spouses of ALS participants as well as by advertisement. The study protocol was approved by the Johns Hopkins Medicine Institutional Review Board. All participants gave written informed consent prior to inclusion in the study. ALS participants as well as healthy controls were administered the olfactory metrics and clinical history simultaneously and did not share results with one another to minimize bias.
Olfactory metrics
ALS and control participants were administered the University of Pennsylvania Smell Identification Test (UPSIT) as a measure of olfactory function. The UPSIT is a validated, easily administered test that has been widely used in other neurodegenerative disorders such as PD.4 It consists of a “scratch and sniff” test where participants are exposed to 40 odors and asked to identify them from a list of options. The scores range from 0 to 40, with 40 indicating correct identification of all odors. Participants were categorized as normosmic, microsmic, or anosmic based on the sex‐adjusted clinical impairment classifications used by the UPSIT scoring system. We also assessed basic history of common causes of olfactory impairment (e.g., smoking history, current nasal congestion, and head trauma) in ALS and control groups through a questionnaire.13
Clinical and cognitive evaluation
Disease duration, site of ALS onset (bulbar, spinal, respiratory), and a history of familial ALS were recorded as reported by the patients and family members. ALS participants were administered the ALS Functional Rating Scale‐Revised (ALSFRS‐R) and the ALS Cognitive Behavioral Screen (ALS‐CBS). Their pulmonary forced vital capacity (FVC) was also obtained. Patients were also characterized using King's criteria as a measure of clinical disease stage (Stage 1: Functional involvement of one CNS region, Stage 2: Functional involvement of two CNS regions, Stage 3: Functional involvement of three CNS regions, Stage 4: Need for gastrostomy (4A) or noninvasive ventilation (4B), and Stage 5: Death).14
Statistical analysis
Categorical variables were analyzed with chi‐square test or Fisher's exact test based on sample size. Normally distributed continuous variables were analyzed using Student's t‐test, and those that were not normally distributed were analyzed with Wilcoxon rank sum test. UPSIT score differences between controls and ALS participants were analyzed with a multiple linear regression model, with robust standard error estimates, adjusting for age, sex, smoking history, past head trauma, current nasal congestion, past nasal surgery, and a past exposure to potentially toxic chemicals. Linear regression models adjusting for age, with robust standard error estimates, were carried out to examine the relationship between UPSIT scores and ALS characteristics among the ALS participants. A P‐value < 0.05 was considered to be significant. The analyses were performed with STATA version 12.
Results
A total of 78 ALS and 69 control participants were enrolled over a period of 6 months. Demographic data are detailed in Table 1. There was no statistically significant difference in the age, race, and smoking history of ALS and control participants. There was a statistically significant difference in the gender, presence of nasal congestion, and exposure to potentially toxic chemicals among ALS and control participants. The gender differences are related to the fact that more men with ALS were enrolled in the study, with their female spouses enrolled as controls. Among the ALS participants, the most common site of onset was spinally followed by bulbar onset, with percentages of 71.80% and 25.64%, respectively. The majority of patients had sporadic ALS (87.18%) with the remaining carrying known mutations as outlined in Table 1. The average ALSFRS‐R and ALS‐CBS performances were 36.6 ± 8.2 and 16.8 ± 2.7, respectively.
Table 1.
Baseline characteristics of ALS and control participants
| Variables | ALS participants (n = 78) | Control participants (n = 69) | P‐value |
|---|---|---|---|
| Age (years), mean ± SD | 58.9 ± 10.1 | 56.2 ± 10.6 | 0.13 |
| Male, n (%) | 47 (60.3%) | 25 (36.2%) | 0.004 |
| Caucasian, n (%) | 69 (88.5%) | 63 (91.3%) | 0.60 |
| Smoking, n (%) | 0.86 | ||
| Current smoker | 5 (6.4%) | 4 (5.8%) | |
| Past smoker | 27 (34.6%) | 21 (30.4%) | |
| Never smoker | 46 (59.0%) | 44 (63.8%) | |
| Past head trauma, n (%) | 19 (25.3%) | 8 (11.6%) | 0.053 |
| Current nasal congestion, n (%) | 24 (32.0%) | 7 (10.3%) | 0.002 |
| Exposure to toxic chemicals, n (%) | 0.047 | ||
| Yes | 8 (10.3%) | 1 (1.5%) | |
| No | 65 (83.3%) | 66 (95.7%) | |
| Not sure | 5 (6.4%) | 2 (2.9%) | |
| Past nasal surgery, n (%) | 11 (14.1%) | 4 (5.8%) | 0.11 |
| UPSIT, mean ± SD | 31.8 ± 7.2 | 35.1 ± 3.0 | 0.004 |
| Time since onset (years) | 3.3 ± 6.8 | ||
| Time since diagnosis (years) | 1.4 ± 3.4 | ||
| Site of onset, n (%) | |||
| Bulbar | 20 (25.6%) | ||
| Arms | 23 (29.5%) | ||
| Legs | 33 (42.3%) | ||
| Respiratory | 1 (1.3%) | ||
| Other | 1 (1.3%) | ||
| Genetic diagnosis, n (%) | |||
| C9ORF72 | 5 (6.4%) | ||
| SOD1 | 3 (3.9%) | ||
| SETX | 1 (1.3%) | ||
| ALS2 | 1 (1.3%) | ||
| Sporadic | 68 (87.2%) | ||
| ALSFRS‐R | 36.6 ± 8.2 | ||
| ALS‐CBS | 16.8 ± 2.7 | ||
| Pulmonary FVC, (% of predicted value) | 80.5 ± 21.3 | ||
| King's staging, n (%) | |||
| Stage 1 | 20 (25.6%) | ||
| Stage 2 | 21 (26.9%) | ||
| Stage 3 | 17 (21.8%) | ||
| Stage 4a | 3 (3.9%) | ||
| Stage 4b | 17 (21.8%) | ||
| Individuals taking riluzole | 50 (64.1%) | ||
Values are mean ± SD unless stated otherwise.
The mean UPSIT scores of ALS participants and controls were 31.8 ± 7.2 and 35.1 ± 3.0, respectively (P = 0.004). According to the UPSIT's score interpretation, eight ALS participants were anosmic, 26 had microsmia, and 44 were normosmic. Comparatively, no control patients were anosmic, 15 had microsmia, and 54 were normosmic. Taken together, 43.59% of ALS participants had olfactory dysfunction, as opposed to 21.74% of control participants (P = 0.003) (Fig. 1).
Figure 1.
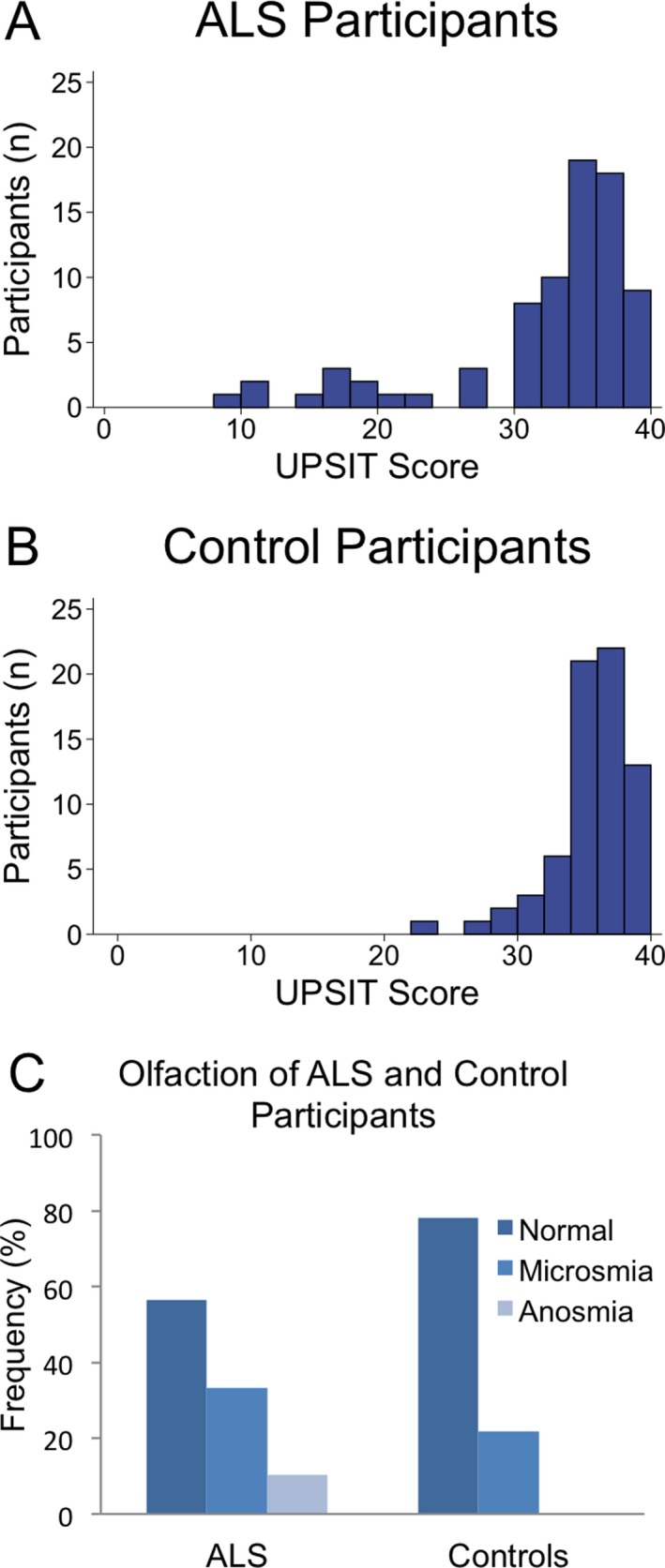
Olfactory dysfunction in ALS and control participants. (A) UPSIT scores in ALS participants. (B) UPSIT scores in control participants. (C) 43.59% of ALS participants have olfactory dysfunction—33.33% had microsmia, and 10.26% total smell loss (anosmia). Conversely, 21.74% of controls had olfactory dysfunction—21.74% had microsmia, and none had anosmia. P = 0.003.
The study also collected information for factors that could lower the UPSIT score, independent of ALS. While there were some differences in the summary statistics among the groups as indicated in Table 1, we explored whether any of these or other factors could account for the differences in UPSIT scores between ALS and control participants. After adjusting for age, sex, race, smoking history, past head trauma, current nasal congestion, past nasal surgery, and past exposure to potentially toxic chemicals in a multiple regression, ALS participants scored significantly lower on the UPSIT as compared to control participants, with an estimated lower score of 2.22 points (P = 0.02) (Table 2). Age was also found to be significantly associated with UPSIT—for every year increase in age, UPSIT score decreased by 0.12 points (P = 0.02). Gender and smoking history were not found to be significant in predicting UPSIT in our cohort.
Table 2.
Multiple regression model results for UPSIT scores adjusting for variables ALS versus Control, age, sex, race, smoking history, past head trauma, current nasal congestion, past nasal surgery, and past exposure to potentially toxic chemicals (n = 147)
| Predictors | Coefficient | 95% confidence interval | P‐value |
|---|---|---|---|
| Control vs. ALS | 2.22 | 0.36, 4.07 | 0.02 |
| Age | −0.12 | −0.21, −0.02 | 0.02 |
| Sex: F vs. M | 0.40 | −1.66, 2.47 | 0.70 |
| Race: Non‐Caucasian vs. Caucasian | −2.01 | −4.71, 0.68 | 0.14 |
| Smoking history | |||
| Past vs. Current smoker | −1.46 | −4.75, 1.83 | 0.38 |
| Never vs. Current smoker | −0.34 | −3.02, 2.33 | 0.80 |
| Past head trauma | −0.09 | −2.96, 2.77 | 0.95 |
| Current nasal congestion | −1.12 | −3.93, 1.69 | 0.43 |
| Exposure to toxic chemicals | |||
| No vs. Yes | 0.75 | −5.06, 6.57 | 0.80 |
| Not sure vs. Yes | −0.64 | −9.04, 7.75 | 0.88 |
| Past nasal surgery | −2.16 | −7.76, 3.44 | 0.45 |
We also explored whether UPSIT scores differed among a number of ALS subgroups. We performed a linear regression model that adjusted for age and excluded outliers in time since ALS symptom onset, time since ALS diagnosis, and ALS‐CBS. There were no statistically significant differences among the groups when comparing the USPIT scores of ALS participants by time since ALS symptom onset, time since ALS diagnosis, site of onset, genetic diagnosis, FVC, ALSFRS‐R (ALSFRS‐R bulbar, motor, respiratory, and total scores), ALS‐CBS, and Kings stage (Table 3).
Table 3.
Linear regression models adjusting for age between UPSIT scores and ALS characteristics among the ALS participants (n = 78)
| ALS characteristics | Coefficient | 95% confidence interval | P‐value |
|---|---|---|---|
| Years since ALS diagnosis | 0.35 | −0.68, 1.38 | 0.50 |
| Years since ALS onset | 0.30 | −0.22, 0.82 | 0.25 |
| ALS site of onset | |||
| Arms vs. Bulbar | −1.66 | −5.66, 2.34 | 0.41 |
| Legs vs. Bulbar | −0.03 | −3.78, 3.72 | 0.99 |
| Genetic diagnosis | |||
| SOD1 vs. C9ORF72 | 5.08 | −0.76, 10.92 | 0.09 |
| Sporadic vs. C9ORF72 | 1.39 | −4.39, 7.17 | 0.63 |
| Pulmonary FVC | 0.01 | −0.09, 0.11 | 0.82 |
| ALSFRS‐R bulbar | −0.06 | −0.60, 0.48 | 0.83 |
| ALSFRS‐R motor | −0.05 | −0.32, 0.21 | 0.68 |
| ALSFRS‐R respiratory | −0.10 | −0.85, 0.64 | 0.78 |
| ALSFRS‐R total | −0.04 | −0.28, 0.19 | 0.72 |
| ALS‐CBS | 0.40 | −0.21, 1.01 | 0.20 |
| Kings stage | |||
| Stage 2 vs. 1 | 0.07 | −4.51, 4.66 | 0.98 |
| Stage 3 vs. 1 | 1.11 | −3.73, 5.95 | 0.65 |
| Stage 4a vs. 1 | −0.04 | −3.93, 3.87 | 0.99 |
| Stage 4b vs. 1 | −0.43 | −5.34, 4.47 | 0.86 |
Discussion
The data from this pilot study demonstrate that ALS participants scored significantly lower on the UPSIT, a validated and widely used measure of olfactory dysfunction, and have twice the prevalence of olfactory dysfunction than controls. It is possible that the cortical changes found in ALS also involve olfactory regions and lead to olfactory dysfunction, as is speculated in AD, PD, and FTD.3, 4, 5 This theory is consistent with previous postmortem reports of pathological TDP‐43‐positive inclusions in the olfactory structures of patients with ALS, including the olfactory bulb, hippocampus, and primary olfactory cortex.9
Our findings support past studies that have shown a difference in olfaction baseline in ALS patients compared with controls.10, 11 Additionally, we have confirmed that olfactory dysfunction is present in a large cohort of ALS patients and is not limited to those with cognitive dysfunction. While age, gender, and smoking history are factors known to impact olfaction, our cohort only showed the effects of age.15 Although gender did not influence UPSIT performance in our ALS cohort, one limitation of our study is that the majority of patients were male, and we did not have a gender‐matched cohort. It is possible that smoking history was not found to be significant due to the small number of participants that are smoking currently. We did not examine the impact of olfactory dysfunction in this ALS population on taste, appetite, or other quality of life measures related to smell. While we investigated whether there was a correlation in ALS variables to account for differences in UPSIT score, we did not appreciate any significant differences within these ALS subgroups. Given that there are such data supporting olfactory dysfunction in patients with future cognitive impairment in other neurodegenerative disorders, and that up to half of ALS patients develop cognitive impairment, we had hypothesized that ALS‐CBS would correlate with UPSIT.1 However, because this is a pilot trial, we likely lacked the statistical power to observe true differences in these subgroups. Additional studies may allow us to investigate these correlations in ALS subgroups.
Taken together, this study extends previous studies that have shown extramotor involvement in ALS to include olfactory dysfunction. With validation and further investigation, measures of olfactory dysfunction could become useful in predicting ALS subtype, disease severity, or disease progression.
Author Contributions
Cristina Viguera involved in study concept and design, analysis and interpretation of data, study supervision, and critical revision of manuscript for intellectual content.
Jiangxia Wang performed analysis and interpretation of data.
Elizabeth Mosmiller carried out study concept and design, data collection and analysis, and critical revision of manuscript for intellectual content.
Aiana Cerezo involved in study concept and design and data collection and analysis.
Nicholas J. Maragakis performed study concept and design, analysis and interpretation of data, study supervision, and critical revision of manuscript for intellectual content.
Conflict of Interests
Cristina Viguera: Nothing to disclose.
Jiangxia Wang: Nothing to disclose.
Elizabeth Mosmiller: Nothing to disclose.
Aiana Cerezo: Nothing to disclose.
Nicholas J. Maragakis: Scientific Advisory Board, Above and Beyond, LLC, Consultant to Biogen/Idec, Biohaven Pharmaceuticals, Cytokinetics, Q Therapeutics, Inc., Navigant, Izumi.
Funding Information
This study was funded by the Johns Hopkins School of Medicine Dean's Funding program. Support for the statistical analysis comes from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Funding Statement
This work was funded by Johns Hopkins School of Medicine Dean's Funding Program grant ; National Center for Research Resources grant ; National Center for Advancing Translational Sciences (NCATS) grant ; National Institutes of Health grant 1UL1TR001079.
References
- 1. Ringholz GM, Appel SH, Bradshaw M, et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005;65:586–590. 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- 2. Elamin M, Phukan J, Bede P, et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 2011;76:1263–1269. 10.1212/WNL.0b013e318214359f. [DOI] [PubMed] [Google Scholar]
- 3. Orasji SS, Mulder JL, de Bruijn SF, Wirtz PW. Olfactory dysfunction in behavioral variant frontotemporal dementia. Clin Neurol Neurosurg 2016;141:106–110. 10.1016/j.clineuro.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4. Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta‐analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 1998;55:84–90. [DOI] [PubMed] [Google Scholar]
- 5. Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 2008;63:167–173. 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 6. Ahlskog JE, Waring SC, Petersen RC, et al. Olfactory dysfunction in Guamanian ALS, parkinsonism, and dementia. Neurology 1998;51:1672–1677. [DOI] [PubMed] [Google Scholar]
- 7. Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson's disease. Parkinsonism Relat Disord 2016;25:45–51. 10.1016/j.parkreldis.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 2017;8:456–462. 10.1212/WNL.0000000000003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeda T, Iijima M, Uchihara T, et al. TDP‐43 pathology progression along the olfactory pathway as a possible substrate for olfactory impairment in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 2015;74:547–556. 10.1097/NEN.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 10. Hawkes CH, Shephard BC, Geddes JF, et al. Olfactory disorder in motor neuron disease. Exp Neurol 1998;150:248–253. 10.1006/exnr.1997.6773. [DOI] [PubMed] [Google Scholar]
- 11. Elian M. Olfactory impairment in motor neuron disease: a pilot study. J Neurol Neurosurg Psychiatry 1991;54:927–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pilotto A, Rossi F, Rinaldi F, et al. Exploring olfactory function and its relation with behavioral and cognitive impairment in amyotrophic lateral sclerosis patients: a cross‐sectional study. Neurodegener Dis 2016;16:411–416. 10.1159/000446802. [DOI] [PubMed] [Google Scholar]
- 13. Spielman AI. Chemosensory function and dysfunction. Crit Rev Oral Biol Med 1998;9:267–291. [DOI] [PubMed] [Google Scholar]
- 14. Balendra R, Jones A, Jivraj N, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS functional rating scale. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:279–284. 10.3109/21678421.2014.897357. [DOI] [PubMed] [Google Scholar]
- 15. Doty RL, Shaman P, Applebaum SL, et al. Smell identification ability: changes with age. Science 1984;226:1441–1443. [DOI] [PubMed] [Google Scholar]


