Abstract
Background:
Evidence exists showing that various aspects of diet are implicated in the etiology of prostate cancer, although results across studies remain inconsistent.
Methods:
We examined the ability of the dietary inflammatory index (DII) to predict prostate cancer in a case-control study conducted in Kingston, Ontario, Canada between 1997 and 1999. Cases were 72 incident primary prostate cancer patients and controls were 302 urology clinic patients who had prostate conditions other than prostate cancer. The DII was computed based on intake of 18 nutrients assessed using a 67-item food frequency questionnaire. Univariate and multivariate logistic regression models were used to estimate odds ratios (OR).
Results:
Men with higher DII scores were at increased risk of prostate cancer using DII score fit both as a continuous (OR=1.58, 95% confidence interval, CI, 1.05–2.38) and categorical variable [compared to men in the lowest DII quartile men in the highest quartile were at elevated risk (OR = 3.50, 95% CI 1.25–9.80; p-trend=0.02)]. There was no significant heterogeneity by weight status but stronger association was observed in men with BMI >25kg/m2 versus <25 kg/m2.
Conclusion:
These findings suggest that a pro-inflammatory diet, as indicated by increasing DII score, is a risk factor for prostate cancer.
Keywords: dietary inflammatory index, diet, inflammation, prostate cancer risk, case-control, Ontario
Introduction
Prostate cancer is the most common cancer among Canadian males, with an incidence rate of 114.7 cases per 100,000 per year in 2016(1). Considerable evidence is accumulating on the role of chronic inflammation in prostate cancer (2–4). Typically, the body responds to any kind of tissue insult or injury by releasing inflammatory cytokines, which leads to wound healing and successfully mounting immune responses to fight infections (5, 6). These responses include immune surveillance to identify and destroy early cancers (7, 8). By contrast, chronic low-grade systemic inflammation is a persistent condition resulting in tissue destruction and repair occurring simultaneously over a long period of time (9, 10). This involves continuous recruitment of pro-inflammatory cytokines associated with increased blood flow to the injured tissue, due to histamine released by damaged mast cells (5).
Results from a case-control study in Australia showed that men with prostate cancer have higher levels of the pro-inflammatory marker, interleukin-6 (IL-6), compared to men with benign prostate neoplasms (11). Similarly in a case-control study in Korea statistically significantly higher levels of C-reactive protein (CRP), another pro-inflammatory marker, were seen in men with prostate cancer compared to men with benign prostatic hypertrophy (12). These results are consistent with the hypothesis that innate immunity and inflammation play a role in prostate cancer (13).
There is growing evidence that specific dietary components influence both acute and chronic inflammation (14–17). Diet represents a complex set of exposures that often interact, and cumulative effects may modify both inflammatory responses and health outcomes (18). Although many studies have been conducted to discern the relationship between diet and prostate cancer, results are inconclusive (19–21). According to the Second Expert Report from the World Cancer Research Fund, there is probable evidence that being overweight and obese is a strong risk factor for advanced prostate cancer, and there is limited suggestive evidence that dairy products, diets high in calcium and low in alpha-tocopherol and selenium increase prostate cancer risk (18).
Previous research among Kingston, Ontario men showed that those with a dietary pattern higher in processed foods that includes pro-inflammatory items such as processed red and organ meats, refined grains, soft drinks, vegetable oils and juices were at higher risk of prostate cancer (22). The literature-derived dietary inflammatory index (DII™) (23), which has been validated with various inflammatory markers, including C-reactive protein (24, 25), IL-6 (26, 27), and homocysteine (26), has been positively associated with risk of prostate cancer in Italy and Jamaica (28, 29). The objective of this case-control study, conducted in Kingston, Ontario, was to determine the association between DII scores and prostate cancer risk.
Methods
Participants were men with no previous cancer diagnosis aged 50–80 years scheduled for prostate core biopsy or attending a urology clinic from 1997–1999 in Kingston, Ontario, Canada. Of 676 eligible men, 46 refused and 20 were lost to follow-up. Of the 610 men recruited to the study, mailed questionnaires were completed by 90% of this sample prior to diagnosis. These included questions on family history of prostate cancer, physical activity as a teenager, education, weight at age 40, height and dietary information. Of the 241 men undergoing biopsy, 80 cases of incident primary prostate cancer were identified. After excluding those with prostatic intraepithelial neoplasia (n=25) and missing or abnormal PSA results (>4ng/ml) (n=52), controls included 136 men who were biopsy negative for prostate cancer and 198 men who visited the same urologists and were not scheduled for a biopsy, for a total of 334 controls. Controls were diagnosed with erectile dysfunction, prostatitis, benign prostatic hyperplasia and various other urinary conditions. Finally, 32 controls and 8 cases were excluded due to at least one missing dietary variable; therefore, the analyses are based on 72 cases and 302 controls. A detailed description of methods and earlier results for this case-control study have been published previously (30, 31). The study was approved by the Health Sciences Research Ethics Board at Queen’s University and Kingston General Hospital.
Participants were asked to recall their average consumption of 67 food and beverage items two years prior to interview using a validated food frequency questionnaire (FFQ) adapted from Byers et al (32). These dietary items were chosen as they account for the majority of the variability in intake of various macronutrients and vitamins regarded as important in cancer, including total energy intake, carbohydrates, protein, fat, cholesterol, fiber and vitamin A (33). The questionnaire also was modified to take into account foods routinely consumed by the Canadian population. The FFQ recorded the consumption of food items in 6 categories: never or less than once per month, 1–3 times per month, 1 or 2 times per week, 3 or 4 times per week, 5 or 6 times per week, or daily. Seven categories were included for beverages: none or less than once per week, 1–6 times per week, 1 time per day, 2 times per day, 3 times per day, 4 times per day or 5 or more times per day. Serving sizes for foods and beverages were recorded as small, medium and large. The Canadian Nutrient File (34) was used to estimate nutrient intake based on the participant’s reported consumption.
To calculate DII scores, dietary consumption data were linked to a world database that provided a global estimate of a mean and standard deviation for each dietary parameter (nutrients, food items and flavonoids) (24). The global database, consisting of data from 11 countries, was developed to provide a reference for intake during the design phase of the DII in 2014 (24). This was achieved by subtracting the “standard global mean” from the intake reported in the FFQ and dividing this by the standard deviation (both calculated from the world database) to obtain ‘z’ scores. To minimize the effect of “right skewing”, these ‘z’ scores were then converted to a centered proportion score by doubling the z-score expressed on a scale of 0 to 1 and then subtracting 1. The centered percentile score for each food parameter for each individual was then multiplied by the respective food parameter effect score (inflammatory potential for each food parameter), which was derived from the literature review, in order to obtain a food parameter-specific DII score for an individual. All of the food parameter-specific DII scores (n=18) were then summed to create the overall DII score for each participant in the study (24). Details of the steps involved in calculating the DII are described in Figure 1. A description of validation work, including both dietary recalls and the 7-day dietary recall also is available (35). Previously, we did not observe an attenuated association between DII and C-reactive protein > 3mg/l when we moved from an exhaustive list of 44 food parameters derived from 24-hour recalls to 28 from the 7-day dietary recall, a structured questionnaire similar to an FFQ, (OR = 1·08; 95% CI 1·01, 1·16, P = 0·035 for the 24HR; and OR = 1·10; 95% CI 1·02, 1·19, P = 0·015 for 7-day dietary recall) (35).
Figure 1.
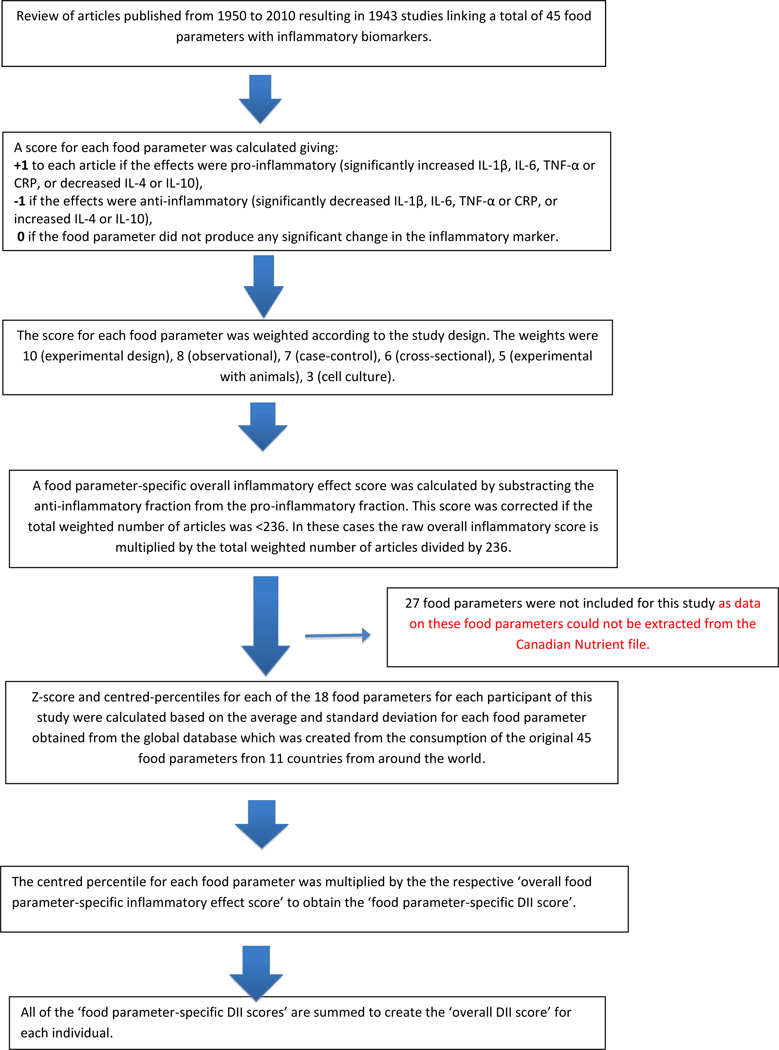
Sequence of steps in creating the dietary inflammatory index in the Canadian Prostate case-control study
All of the 18 food parameters used for DII calculation were nutrients and out of these 18 food parameters 8 (energy, carbohydrate, total fat, cholesterol, vitamin-B12, iron, protein and saturated fat) have pro- inflammatory scores. The remaining 10 (mono-unsaturated fat, poly-unsaturated fat, omega-3, niacin, thiamin, riboflavin, vitamin-B6, zinc, vitamin A, folic acid) have anti-inflammatory scores which are derived from extensive literature review (24). A higher DII score indicates a more pro-inflammatory diet and a lower DII score indicates a more anti-inflammatory diet. The 27 food parameters missing from this study are: omega-6 fatty acid, trans fat, vitamin- C, D, E, magnesium, selenium, alcohol, beta carotene, fiber, anthocyanidins eugenol, flavan3ol, flavones, flavonols, flavonones, isoflavones, caffeine, garlic, ginger, onion, saffron, turmeric, pepper, thyme, rosemary and tea. Data on these food parameters were not available because Canadian Nutrient file that was used to extract nutrient values for this study did not have information on these 27 food parameters.
DII scores were analyzed both as continuous and categorized by quartiles of exposure. DII (as quartiles) was examined across the following characteristics: age, education, BMI, tobacco smoking, and family history of prostate cancer using ANOVA for continuous variables or χ2 test for categorical ones. To understand the dietary profile for each quartile of DII, we examined the distribution of various food groups across quartiles of DII. An ANOVA was used to test for differences. Odds ratio (OR) and corresponding 95% confidence intervals (95% CI) were estimated using logistic regression models. A parsimonious model predicting prostate cancer risk was identified from these potential covariates: age, income, ethnicity, education, family history of a first-degree relative with prostate or breast cancer, medical history, smoking, physical activity as a teenager, energy intake and body mass index (BMI). The exposure time window for physical activity and cancer is usually at least 10 or 20 years prior to at the time of cancer diagnosis, and may be much longer for prostate cancer. Current physical activity would capture the wrong exposure time window since cancer has already occurred (36). After a backward selection screening procedure, age, physical activity as a teenager, energy intake, and family history of prostate cancer were retained in the model at the 0.20 significance level. Linear tests for trend were performed using the median value within each quartile as an ordinal variable. Two sensitivity analyses were conducted; in the first controls were restricted to those who had a negative biopsy; and in the second cases were restricted to those men with a Gleason score ≥7. Statistical tests were performed using SAS® 9.3 (SAS Institute Inc., Cary, NC), and all hypotheses tests were two-sided. The study power to examine the relationship between 72 prostate cancer cases and 302 controls using a logistic model was 88% (37) and the effect size for this is based on the DII and prostate cancer study in Jamaica (28).
Results
The distribution of prostate cancer cases and controls according to age, education, and other variables is presented in Table 1. The only statistically significant difference between cases and controls was for physical activity as a teenager, where cases more frequently report “low” than controls.
Table 1.
Distribution of 72 prostate cancer cases and 302 controls according to selected characteristics, Kingston, Ontario, Canada, 1997–1999.
| Characteristica | Cases (n=72) | Controls (302) | p-value |
|---|---|---|---|
| Age (years) Mean (SD) |
65.1 (6.0) | 63.5 (6.9) | 0.06 |
| Income per person (%) | |||
| Lower | 39 (54.2) | 154 (51.0) | 0.63 |
| Higher | 33 (45.8) | 148 (49.0) | |
| Ethnic background (%) | 0.57 | ||
| British | 53 (73.6) | 212 (70.2) | |
| Other | 19 (26.4) | 90 (29.8) | |
| Education (%) | |||
| ≤ High school | 39 (54.2) | 156 (51.7) | 0.70 |
| > High school | 33 (45.8) | 146 (48.3) | |
| Body mass index (kg/m2) at age 40 Mean (SD) |
24.9 (2.8) | 24.8 (2.9) | 0.82 |
| Smoking (%) | 0.98 | ||
| Ever smoker | 23 (31.9) | 97 (32.1) | |
| Non smoker | 49 (68.1) | 205 (67.9) | |
| Family history of prostate cancerb (%) | |||
| No | 50 (69.4) | 229 (75.8) | 0.26 |
| Yes | 22 (30.6) | 73 (24.2) | |
| Physical activity as a teenager (%) | 0.02 | ||
| Low | 16 (22.2) | 113 (37.4) | |
| Moderate | 37 (51.4) | 145 (48.0) | |
| Strenuous | 19 (26.4) | 44 (14.6) |
Mean (SD) for continuous or frequency (%) for categorical variables.
In first-degree relatives.
Men with higher DII scores (higher quartiles) were younger compared to men in lower quartiles (p<0.01) (Table 2). There also was a higher percentage of men with less than a high school education in quartile 4 compared to quartile 1 (p=0.01). A significant reduction in the consumption of vegetables, fruits, egg, poultry and seafood across quartile of DII scores also was observed (Table 3).
Table 2.
Participants’ characteristics across quartiles of dietary inflammatory index (DII) among 302 controls, Kingston, Ontario, Canada, 1997–1999.
| Characteristicsc | DII quartiles | ||||
|---|---|---|---|---|---|
| < −0.52 | −0.52,−0.20 | −0.21,0.68 | >0.68 | p-valuec | |
| Age (yr) a | 65.9±6.6 | 63.4±6.0 | 62.7±6.6 | 62.2±7.9 | 0.001 |
| Ethnicity | 0.12 | ||||
| British | 50 (68.5) | 52 (67.5) | 65 (80.2) | 45 (63.4) | |
| Others | 23 (31.5 | 25 (32.5) | 16 (19.8) | 26 (36.6) | |
| Education (years) | 0.01 | ||||
| ≤ High school | 34 (46.6) | 28 (36.4) | 39 (48.1) | 45 (63.4) | |
| > High school | 39 (53.4) | 49 (63.6) | 42 (51.9) | 26 (36.6) | |
| Income | 0.50 | ||||
| Lower | 38 (52.0) | 32 (41.6) | 41 (50.6) | 37 (52.1) | |
| Higher | 35 (48.0) | 45 (58.4) | 40 (49.4) | 34 (47.9) | |
| Body mass index (kg/m2)a | 25.1±3.4 | 24.3±2.5 | 24.7±2.6 | 25.2±3.3 | 0.65 |
| Smoking | 0.26 | ||||
| Ever smoker | 25 (34.2) | 28 (36.7) | 28 (34.6) | 16 (22.5) | |
| Non smoker | 48 (65.8) | 49 (63.6) | 53 (65.4) | 55 (77.5) | |
| Family history of prostate cancerb | 0.24 | ||||
| No | 50 (68.5) | 62 (80.5) | 65 (80.2) | 52 (73.2) | |
| Yes | 23 (31.5) | 15 (19.5) | 16 (19.8) | 19 (26.8) | |
| Physical Activity as a teenager | 0.06 | ||||
| Low | 28 (38.4) | 30 (39.0) | 29 (35.8) | 26 (36.6) | |
| Moderate | 25 (34.2) | 38 (49.3) | 43 (53.1) | 30 (43.3) | |
| Strenuous | 18 (24.7) | 8 (10.4) | 8 (9.9) | 10 (14.1) | |
Mean± standard deviation.
In first-degree relatives.
t test for continuous variables and chi-square test for categorical variables.
Table 3.
Distribution of food groups across quartiles of the dietary inflammatory index (DII) among 302 controls, Kingston, Ontario, Canada, 1997–1999.
| Food groups | DII quartile, Servings/week±standard deviation |
P-valuea | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Fruits | 14.0±3.4 | 11.7±3.0 | 10.6±2.6 | 8.5±2.4 | <0.0001 |
| Vegetables | 26.3±3.7 | 23.4±2.6 | 21.3±2.8 | 18.7±3.2 | <0.0001 |
| French fries | 2.1±0.9 | 2.2±0.9 | 2.2±0.8 | 2.2±0.8 | 0.45 |
| Butter | 2.8±2.0 | 3.3±2.2 | 3.1±2.1 | 3.0±2.0 | 0.87 |
| Egg | 3.0±0.9 | 2.8±0.8 | 2.9±0.9 | 2.6±1.2 | 0.02 |
| Poultry | 3.1±0,8 | 2.9±0.7 | 2.8±0.7 | 2.4±0.8 | <0.0001 |
| Ice cream | 2.3±1.1 | 2.4±1.0 | 2.3±1.1 | 2.1±1.1 | 0.20 |
| Broccoli | 3.0±0.9 | 2.6±0.8 | 2.2±0.8 | 1.8±0.7 | <0.0001 |
| Sea food | 3.8±1.1 | 3.2±0.8 | 3.2±0.7 | 2.7±0.7 | <0.0001 |
| Chocolate | 2.1±0.9 | 1.9±0.9 | 2.0±0.9 | 2.9±1.3 | 0.66 |
ANOVA was used for all the food groups.
An association was seen for continuous DII in multivariable analysis, with an OR of 1.58 (95% CI 1.05–2.38); i.e., a one unit increase in DII, corresponding to ~7% of its global range (+7.98 to −8.87) was associated with a 58% increase in prostate cancer risk (Table 4). Associations between quartiles of DII exposure and risk of prostate cancer also are shown in Table 3. In addition to a statistically significant increased risk for quartiles 3 and 4, there was evidence of a linear trend of increasing risk as the inflammatory index increases (ptrend=0.02). In the first sensitivity analysis where controls were restricted to those who had a negative biopsy, the OR for one unit increase in DII was 2.02 (95% CI 1.13–3.59), an even higher magnitude of risk. In the second sensitivity analysis in which cases were restricted to those with a Gleason score ≥7 (n=37), the OR per unit change of DII was 1.60 (95% CI 0.94–2.72). While this result is in the same range as the confidence intervals of the previous results from the whole sample, this analysis was adversely affected by the much smaller sample size.
Table 4.
Odds ratios (OR) and corresponding confidence intervals (CI) for quartiles of DII and prostate cancer risk, Kingston, Ontario, Canada, 1997–1999.
| DII quartiles | ptrend | OR (95% CI)c |
||||
|---|---|---|---|---|---|---|
| < −0.52 | −0.52,−0.20 | −0.21,0.68 | >0.68 | |||
| Cases/controls | 14/73 | 22/77 | 18/81 | 18/71 | ||
| Multivariable OR (95% CI)a | 1 b | 2.41 (1.06–5.51) |
2.25 (0.93–5.52) |
3.50 (1.25–9.80) |
0.02 | 1.58 (1.05–2.38) |
Adjusted for age, family history of prostate cancer, physical activity as a teenager and energy intake.
Reference category.
Continuous OR for one unit increment of the DII, corresponding to ≈7% of its global range.
Results obtained within strata of BMI (<25 vs ≥25kg/m2) at 40 years of age are presented in Table 5. There was no significant heterogeneity (p =0.20) by weight status, but a stronger association was seen for DII quartiles among those whose BMI was ≥25 kg/m2, especially in the highest quartile of DII (OR=5.77, 95% CI=1.09–30.49). For the continuous measure of DII among those whose BMI was ≥25 kg/m2, increased risk similar to that seen in the overall population was observed (OR=1.94; 95% CI=1.02–3.67).
Table 5.
Odds ratios (OR) of prostate cancer risk and corresponding 95% confidence intervals (CI) according to quartiles of dietary inflammatory index (DII), among 72 cases and 302 controls by BMI at age 40, Kingston, Ontario, Canada, 1997–1999.
| Cases/ Controls |
DII quartiles, OR (95% CIa | DII conitnuous | p value for interaction | ||||
|---|---|---|---|---|---|---|---|
| < −0.52 | −0.52,−0.20 | −0.21,0.68 | >0.68 | ||||
| BMI at age 40 (kg/m2)a | 0.20 | ||||||
| <25 | 40/170 | 1.0 b | 1.40 (0.49, 3.97) | 1.67 (0.55, 5.07) | 2.48 (0.64, 9.64) | 1.29 (0.76, 2.19) | |
| ≥25 | 32/132 | 1.0 b | 4.97 (1.18, 20.87) | 3.64 (0.78, 17.07) | 5.77 (1.09, 30.49) | 1.94 (1.02, 3.67) | |
Adjusted for age, family history of prostate cancer, physical activity as a teenager and energy intake.
Reference category.
Discussion
Using data from a case-control study on diet and prostate cancer conducted in Ontario, Canada we demonstrated a positive association between DII score and prostate cancer risk, with statistically significant risk estimates for DII as a continuous variable, and for exposure expressed as quartiles of DII with an indication of a trend. We also observed that men in the highest DII quartile consumed the lowest amount of plant-based foods, including vegetables and fruits. These results support the hypothesis that men with a pro-inflammatory diet are at higher risk of prostate cancer (13). Consistency of findings for this hypothesis is improving. For example, in a large case-control study in Italy, there was a modest increased risk in quartiles 3 and 4 relative to quartile 1 of the DII (ORQuartile 3 v. 1 1.32, 95% CI =1.03–1.69 and ORQuartile 4 v. 1 1.33, 95% CI 1.01–1.76; ptrend = 0.04) (29). In another case-control study in Jamaica, men in the highest quartile of the DII were at higher risk of prostate cancer (OR = 2.39; 95% CI= 1.14–5.04) (28). A positive association of similar magnitude was observed in a prospective study in France, (HRQuartile 4 v. 1 2.08, 95% CI 1.06–4.09) (38). These results show that the DII can be applied to a variety of populations, using any competent dietary assessment tool, including different types of validated FFQs.
It is interesting to note that we observed a stronger association between DII scores and prostate cancer among men with BMI ≥25 kg/m2 at 40 years of age compared to men with BMI <25 kg/m2. Because obesity is, itself, a pro-inflammatory state, this association may reflect synergy between adiposity and diet-associated inflammation (39). However, these results should be viewed with caution because the test for heterogeneity was not significant.
Some studies have shown that dietary factors exert an array of effects that increase the risk of prostate cancer (e.g., dietary fat, meat, and carbohydrate intake) (40, 41); while others, such as isoflavones (42), soy (43), coffee, flavonoids and tea, have been shown to reduce risk (41, 44). Out of these 18 food parameters used to compute DII scores in this study, carbohydrates, saturated fat and vitamin B12 have pro-inflammatory effects and results in increased risk while omega-3 fatty acids have an anti-inflammatory effect and reduce the risk of prostate cancer (45).
The positive association of the DII with prostate cancer in this case-control study is of specific interest as the results provide further evidence showing that a pro-inflammatory diet is associated with an increase in the risk of prostate cancer (46, 47). One of the possible mechanisms responsible for this association is the effect of a pro-inflammatory diet on systemic inflammation and insulin resistance (48, 49). Along this line, a diet characterized by a high glycemic load has been related to increased prostate cancer risk (50). A diet rich in pro-inflammatory constituents, such as saturated fat, also causes cell proliferation, inflammation, and oxidative stress that can lead to benign prostatic hyperplasia, prostatitis, and possibly cancer of the prostate (51).
Typically, human diets consist of both pro-inflammatory and anti-inflammatory foods, nutrients, and other food constituents. The influence of diet on cancer is difficult to estimate, and challenges in dietary exposure assessment are normally greatest in case-control studies, where disease-related information bias is a special concern (18). A major strength of this study is that subjects going to biopsy completed the questionnaire prior to knowledge of their disease status, thus avoiding disease-related recall and interviewer biases, a common pitfall of case-control studies. This design feature strengthens the internal validity of the results. However, we acknowledge that recall of past diet might be influenced by current diet leading to some non-differential misclassification bias, which would tend to attenuate the observed associations towards the null. Another major strength of this study is that selection bias is avoided because we included as controls only those men with normal PSA and DRE results within the year prior to enrollment (non-biopsied urology controls), as well as men who were assessed for prostate cancer and had negative biopsy results (biopsy-confirmed healthy controls). Therefore, controls were similar to cases in their health-seeking behavior and all participants were screened by PSA to exclude latent prostate carcinoma.
It also should be made clear that 18 of the 45 food parameters mentioned in the DII development paper were used to calculate DII scores (24). This is a limitation of this study; however, in previous validation studies with inflammatory markers as outcomes, we have shown that there is no decrease in the predictive ability when we reduce the number of food parameters to 28 (35). In a study conducted in Belgium, for example, the DII derived from only 17 food parameters was associated with inflammatory markers (26). While we have not conducted a validation study to examine how reducing food parameters to 18 would affect predictability, some of the missing parameters here are not commonly consumed in large quantities in the Kingston, Ontario population (e.g., turmeric, ginger, garlic, saffron); hence, we think that these missing food parameters do not pose a severe limitation. The global database for the DII was created to include dietary consumption of the 45 food parameters from 11 countries in order to obtain a wide spectrum of consumption: USA, Mexico, England, Denmark, India, Australia, New Zealand, Bahrain, Scotland, South Korea, and Japan (24), and mean values of the food parameters from this database should be representative of the average consumption of these parameters across the world. However, we acknowledge that a limitation of the study is the lack of information on inflammatory markers that could be validated with these DII scores. Another important limitation of the study is that exposure window period for PA and BMI was different than for diet and this may have had an influence on our results.
Our findings of a positive association of DII with prostate cancer are biologically plausible and could be related to immune factors (51–53). In conclusion, this study on prostate cancer and DII indicates a possible role of diet on prostate cancer risk through the process of inflammation. However, there is a need for other studies to be conducted in different populations and with prospective cohorts to more firmly establish cause and effect.
Acknowledgements:
Financial support: Dr Aronson was supported by a Career Scientist Award from the Ministry of Health and Long-Term Care (Ontario), and she and Dr Walker were supported by the Canadian Institutes of Health Research (CIHR) at the time of original data collection, which was also funded by an operating grant from the CIHR. Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Disclosure: JRH owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. NS is an employee of CHI.
References.
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. In: Statistics CC, editor. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Kopp TI, Friis S, Christensen J, Tjonneland A, Vogel U. Polymorphisms in genes related to inflammation, NSAID use, and the risk of prostate cancer among Danish men. Cancer Genet. 2013. July 20;20(13):00084–7. PubMed PMID: . Eng. [DOI] [PubMed] [Google Scholar]
- 3.Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005. December 15;65(24):11779–84. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]
- 4.Nakai Y, Nonomura N. Inflammation and prostate carcinogenesis. Int J Urol. 2013. February;20(2):150–60. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]
- 5.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15(17):1949–55. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009. June 10;57(11):4467–77. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. Journal of Internal Medicine. 2000;248(3):171–83. [DOI] [PubMed] [Google Scholar]
- 8.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–69. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002. December 19–26;420(6917):860–7. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004. December;14(6):433–9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Tindall EA, Severi G, Hoang HN, Southey MC, English DR, Hopper JL, et al. Interleukin-6 promoter variants, prostate cancer risk, and survival. Prostate. 2012. December 1;72(16):1701–7. PubMed PMID: . Epub 2012/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Jeon Y, Lee H, Lee D, Shim B. The Prostate Cancer Patient Had Higher C-Reactive Protein Than BPH Patient. Korean J Urol. 2013. February;54(2):85–8. PubMed PMID: . eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazma R, Mefford JA, Cheng I, Plummer SJ, Levin AM, Rybicki BA, et al. Association of the innate immunity and inflammation pathway with advanced prostate cancer risk. PLoS ONE. 2012;7(12):e51680 PubMed PMID: . Pubmed Central PMCID: 3522730. Epub 2012/12/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, et al. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011. November;54(11):2755–67. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]
- 15.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011. October;8(10):2868–75. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]
- 16.Luciano M, Mottus R, Starr JM, McNeill G, Jia X, Craig LC, et al. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun. 2012. July;26(5):717–20. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2005. January;14(1):138–47. PubMed PMID: . Epub 2005/01/26. eng. [PubMed] [Google Scholar]
- 18.Second Expert Report Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. World Cancer Research Fund / American Institute for Cancer Research., 2007.
- 19.Lin PH, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC medicine. 2015;13:3 PubMed PMID: . Pubmed Central PMCID: 4286914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labbe DP, Zadra G, Ebot EM, Mucci LA, Kantoff PW, Loda M, et al. Role of diet in prostate cancer: the epigenetic link. Oncogene. 2015. September 3;34(36):4683–91. PubMed PMID: . Pubmed Central PMCID: 4476943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VH. Nutrition and prostate cancer: an overview. Expert review of anticancer therapy. 2014. November;14(11):1295–304. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Walker M, Aronson KJ, King W, Wilson JWL, Fan W, Heaton JPW, et al. Dietary patterns and risk of prostate cancer in Ontario, Canada. International Journal of Cancer. 2005;116(4):592–8. [DOI] [PubMed] [Google Scholar]
- 23.Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, et al. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2014. February;56(2):145–8. PubMed PMID: . Pubmed Central PMCID: 3922825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition. 2014. August;17(8):1689–96. PubMed PMID: . Pubmed Central PMCID: 3925198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert JR, Shivappa N, Tabung FK, Steck SE, Wirth MD, Hurley TG. On the use of the dietary inflammatory index in relation to low-grade inflammation and markers of glucose metabolism in the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. The American journal of clinical nutrition. 2014. June;99(6):1520 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner-McGrievy GM, Wirth MD, Shivappa N, Wingard EE, Fayad R, Wilcox S, et al. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutrition research. 2015. February;35(2):97–106. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015. January;45(1):177–83. PubMed PMID: . Pubmed Central PMCID: 4190104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Jackson MD, Bennett F, Hébert JR. Increased Dietary Inflammatory Index (DII) Is Associated With Increased Risk of Prostate Cancer in Jamaican Men. Nutrition and Cancer. 2015. 2015/08/18;67(6):941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Association between dietary inflammatory index and prostate cancer among Italian men. The British journal of nutrition. 2015. January 28;113(2):278–83. PubMed PMID: . Pubmed Central PMCID: 4433863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronson KJ, Wilson JW, Hamel M, Diarsvitri W, Fan W, Woolcott C, et al. Plasma organochlorine levels and prostate cancer risk. Journal of exposure science & environmental epidemiology. 2010. July;20(5):434–45. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi LC, Limburg H, Miao Q, Woolcott C, Bedard LL, Massey TE, et al. Folate intake, alcohol consumption, and the methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism: influence on prostate cancer risk and interactions. Frontiers in oncology. 2012;2:100 PubMed PMID: . Pubmed Central PMCID: 3418632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byers T, Marshall J, Fiedler R, Zielezny M, Graham S. Assessing nutrient intake with an abbreviated dietary interview. American journal of epidemiology. 1985. July;122(1):41–50. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Randall E, Marshall JR, Graham S, Brasure J. Patterns in Food Use and Their Associations with Nutrient Intakes. Am J Clin Nutr. 1990. October;52(4):739–45. PubMed PMID: WOS:A1990DZ79500029. English. [DOI] [PubMed] [Google Scholar]
- 34.Brault D, Caron-Lahaie L. Nutritive value of foods. St. Lambert: Societe Brault-Lahaie; 1994. [Google Scholar]
- 35.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public health nutrition. 2014. August;17(8):1825–33. PubMed PMID: . Pubmed Central PMCID: 3983179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rideout CA, McKay HA, Barr SI. Self-reported lifetime physical activity and areal bone mineral density in healthy postmenopausal women: the importance of teenage activity. Calcified tissue international. 2006. October;79(4):214–22. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Dupont WD, Plummer WD Jr. Power and sample size calculations for studies involving linear regression. Controlled clinical trials. 1998. December;19(6):589–601. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, et al. The Dietary Inflammatory Index Is Associated with Prostate Cancer Risk in French Middle-Aged Adults in a Prospective Study. J Nutr. 2016. March 9 PubMed PMID: . Pubmed Central PMCID: 4807649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2016. November;17(11):1067–79. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Pelser C, Mondul AM, Hollenbeck AR, Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013. April;22(4):697–707. PubMed PMID: . Pubmed Central PMCID: 4129658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drake I, Sonestedt E, Gullberg B, Ahlgren G, Bjartell A, Wallstrom P, et al. Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmo Diet and Cancer cohort. Am J Clin Nutr. 2012. December;96(6):1409–18. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 42.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009. June 2;100(11):1817–23. PubMed PMID: . eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hebert JR, Hurley TG, Olendzki B, Ma Y, Teas J, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J Natl Cancer Inst. 1998;90:1637–47. [DOI] [PubMed] [Google Scholar]
- 44.Geybels MS, Verhage BAJ, Arts ICW, van Schooten FJ, Goldbohm RA, van den Brandt PA. Dietary Flavonoid Intake, Black Tea Consumption, and Risk of Overall and Advanced Stage Prostate Cancer. American journal of epidemiology. 2013. June 15;177(12):1388–98. PubMed PMID: WOS:000320062700008. English. [DOI] [PubMed] [Google Scholar]
- 45.Masko EM, Allott EH, Freedland SJ. The relationship between nutrition and prostate cancer: is more always better? Eur Urol. 2013. May;63(5):810–20. doi: 10.1016/j.eururo.2012.11.012. Epub Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan JM, Weinberg V, Magbanua MJ, Sosa E, Simko J, Shinohara K, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Cause Control. 2011. January;22(1):141–50. PubMed PMID: WOS:000285360700016. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metast Rev. 2002. March;21(1):3–16. PubMed PMID: WOS:000177874900002. English. [DOI] [PubMed] [Google Scholar]
- 48.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary Patterns and Markers of Systemic Inflammation among Iranian Women. The Journal of Nutrition. 2007. April 1, 2007;137(4):992–8. [DOI] [PubMed] [Google Scholar]
- 49.Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000. July 4, 2000; 102(1):42–7. [DOI] [PubMed] [Google Scholar]
- 50.Augustin LS, Galeone C, Dal Maso L, Pelucchi C, Ramazzotti V, Jenkins DJ, et al. Glycemic index, glycemic load and risk of prostate cancer. Int J Cancer. 2004. November 10;112(3):446–50. PubMed PMID: . Epub 2004/09/24. eng. [DOI] [PubMed] [Google Scholar]
- 51.Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S. High-fat diet increases NF-kappaB signaling in the prostate of reporter mice. Prostate. 2011. February 1;71(2):147–56. PubMed PMID: . eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandey M, Gupta S. Green tea and prostate cancer: from bench to clinic. Front Biosci (Elite Ed). 2009;1:13–25. PubMed PMID: . eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001. February;60(1):91–106. PubMed PMID: . eng. [DOI] [PubMed] [Google Scholar]


