Abstract
In the field of plasma medicine, the identification of relevant reactive species in the liquid phase is highly important. To design the plasma generated species composition for a targeted therapeutic application, the point of origin of those species needs to be known. The dominant reactive oxygen species generated by the plasma used in this study are atomic oxygen, ozone, and singlet delta oxygen. The species density changes with the distance to the active plasma zone, and, hence, the oxidizing potential of this species cocktail can be tuned by altering the treatment distance. In both phases (gas and liquid), independent techniques have been used to determine the species concentration as a function of the distance. The surrounding gas composition and ambient conditions were controlled between pure nitrogen and air-like by using a curtain gas device. In the gas phase, in contrast to the ozone density, the singlet delta oxygen density showed to be more sensitive to the distance. Additionally, by changing the surrounding gas, admixing or not molecular oxygen, the dynamics of ozone and singlet delta oxygen behave differently. Through an analysis of the reactive species development for the varied experimental parameters, the importance of several reaction pathways for the proceeding reactions was evaluated and some were eventually excluded.
Introduction
Cold physical plasma jets have shown a high potential in the treatment of chronic wounds1–3 and in cancer as an adjuvant to standard therapy4. Further targets are currently investigated, e.g. atopic eczema5. Common to all these applications is that reactive oxygen species (ROS) have relevant impact via redox signalling processes6,7. Besides electromagnetic radiation (ultraviolet - UV - up to near infrared - NIR - spectral range), atmospheric pressure plasma jets generate various reactive oxygen and reactive nitrogen species (RNS), such as ozone, singlet delta oxygen, hydroxyl radicals, superoxide anion radicals, nitric monoxide, nitrogen dioxide, nitrite, and nitrate8. Most of these species are thought to interact directly or indirectly with biological systems, e.g. cells in a human body. In order to tailor the plasma composition for a given medical application, it is essential to characterize the plasma, the reactive species deposited in liquids, and the subsequent biological effects of those reactive species. Hence, knowing the details of the transfer of the reactive species from the gas phase into liquids helps the interpretation of biological data and is useful to the construction of dedicated plasma sources. It also helps to evaluate the suitability of remote treatment approaches for medical usage that are being studied with increasing emphasis. Although increasing knowledge is available on time- and spatially resolved density distribution of reactive species in the gas phase, their trajectories in a liquid system remain elusive8–11. The formation pathway of many species in the liquids is not clear: liquid phase species can either be generated by a direct interaction of the plasma with the liquid at the interface12, e.g. due to (vacuum)UV-caused photo dissociation of water molecules13,14, or alternatively, via transfer from the gas phase into the liquid15, or even by secondary or tertiary reactions in the bulk liquid16. One of the few species whose formation pathway in the liquids is well studied is hydrogen peroxide in the case of plasma jets fed with humidified argon15,17 and helium18 gases. Shortcomings in specificity or sensitivity limit bar the quantification of most other species in liquids. Short lifetimes or rapid conversions further impede quantification19. Some more recent approaches start to overcome these limits12,19–22. Models predict a large role of the interface layer, with impact on species attachment and solvation into the bulk liquid12,23–27. Yet, knowledge on the species distribution in the bulk liquid, especially when in contact with a biological surface or in presence of organic molecules, is very limited23,24,28. On the other hand, a large body of evidence exists, showing the impact of plasma derived species in biological systems, proving the permeation of the aqueous barriers, like physiologic buffer systems, cell culture media, or body fluids10,11,29–36.
At times, specific plasma-generated species have been attributed to trigger the stimulation, modulation, or execution of the biological effects observed. Among these are hydrogen peroxide (H2O2)15,17, atomic oxygen (•O)37, or nitric oxide (•NO)38 and peroxynitrite (ONOO−)16,22,39. Other species are harder to pinpoint, although present in high amounts in the gas phase. This is valid for plasma generated ozone, O340, and singlet delta oxygen, O2(a1Δg)41, whose full biological impact in plasma medicine is far from being completely known. O3 is a powerful oxidant which is well known for disinfection of drinking water42. In this case, O3 is the precursor for even stronger oxidants such as the hydroxyl radical (•OH)43. The lifetime of O3 is relatively long; in water, O3 has a half-life of seconds up to hours44, depending on the temperature and the pH value45. Hence, in solution, the lifetime of O3 is much longer than the half-life of •OH, which is of the order of ns46. In the gas phase, O3 is even more stable; its half-life is in the range of hours up to days47, depending on the temperature, humidity and air speed. O2(a1Δg) is also a highly reactive ROS. In comparison to O3, it is less stable in both the gas and the liquid phases. Its half-life is 75 minutes in the gas phase41 and several µs in the liquid phase48. Nevertheless, O2(a1Δg) is still more stable than most oxygen radicals. In the presence of superoxide anion radicals, O2•−, O2(a1Δg) is quenched to molecular oxygen49. If H2O2 and hypochlorite (OCl−) are simultaneously present in the solution, O2(a1Δg) can be generated directly in the liquid50. Biological effects of O2(a1Δg) are evident in the context of the photodynamic therapy51.
In the gas phase, the variation of O2(a1Δg) and O3 densities as a function of the O2 admixture to the feed gas has already been shown for several plasma sources41,52–55. Here, the O2(a1Δg) as well as the O3 concentrations are determined, for the first time, in the liquid and in the gas phase under identical conditions. To study the origin and transfer between the gas and liquid phases of plasma generated O3 and O2(a1Δg), a well-characterized argon plasma jet was used (kinpen09)17,20,56,57. By using a curtain gas device, the ambient conditions were controlled58, allowing the modulation of the gas composition around the plasma plume. The O2 content of the working gas was also regulated. This approach allows tuning the reactive species composition in the solution and increases the treatments reproducibility17,59–61, forming the base for the presented data. The final aim of the present paper is to estimate the potential biological impact of these two ROS, O3 and O2(a1Δg), and to evaluate the suitability of direct and indirect treatment procedures.
Results
Reactive oxygen species in the liquid
During the plasma treatment of DPBS (Dulbecco’s phosphate buffered saline solution), several reactive oxygen species are induced in the liquid10: O3, •O, or O2(a1Δg).
In Fig. 1, the concentration of the spin probe TEMPD-HCl adducts is given as a function of the O2 content in the feed gas for two different curtain gas conditions (pure N2 and synthetic air). This spin probe adduct is formed by a reaction with either O3, •O, or O2(a1Δg). The composition of the gas surrounding the plasma plume influences the spin probe adduct concentration. Air-like conditions lead to a lower adduct-concentration than the pure N2 curtain gas. Furthermore, the presence of O2 in the surrounding yields a saturation of the adduct concentration for O2 contents in the argon higher than 0.4%. For a curtain gas of N2, there is always an increase of the adduct concentration, but at a lower rate for higher O2 content in the feed gas – no clear saturation is observed. As this spin probe results in the same EPR spectrum for the three potential reactants (O3, •O, or O2(a1Δg)), further measurements are necessary in order to distinguish the species and identify their origin.
Figure 1.
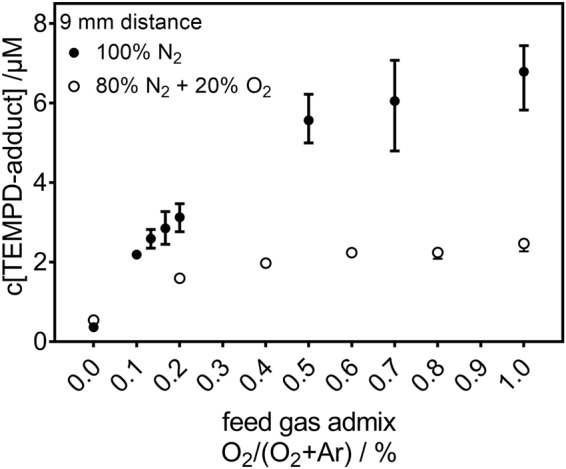
Concentration of spin probe TEMPD-HCl adducts after direct plasma treatment (9 mm, 180 s). In both environmental conditions (N2 or air-like), plasma induces ROS in the liquid. The TEMPD-HCl adduct of O3, •O, O2(a1Δg) is depicted as function of the O2 content in the feed gas of Ar.
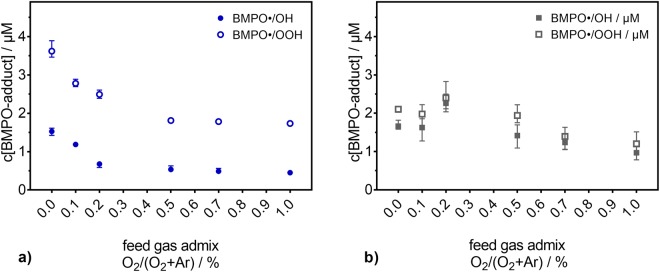
The behaviour of •OH and O2•− for similar experimental conditions was also studied. Using BMPO, •OH and O2•− were detected for pure N2 as curtain gas (see Fig. 2a) and an air-like atmosphere (see Fig. 2b). The resulting spin trap adducts, BMPO-OH and BMPO-OOH respectively, show an entirely different behaviour compared to the TEMPD-HCl adduct. However, the concentrations of both BMPO adducts have the same trend. For pure N2 as curtain gas, their concentrations decrease with increasing O2 content in the feed gas, and the strongest impact on the formation of the BMPO adducts occurs for low amounts of O2: pure argon yields the highest concentration of BMPO adducts, which decreases drastically with the addition of 0.2% of O2 or more. For an air-like atmosphere, the variation of the concentration of BMPO adducts as a function of the O2 admixture into the feed gas is not so pronounced, with maxima concentrations being obtained for 0.2% of O2 and the lowest concentrations for the higher O2 content.
Figure 2.
Concentration of free oxygen radical spin trap (BMPO) adducts after direct plasma treatment (9 mm, 180 s). The formation of the •OH (filled symbols) and O2•− (open symbols) adducts is investigated as a function of the O2 content in the feed gas of Ar under (a) pure N2 or (b) air-like atmospheres.
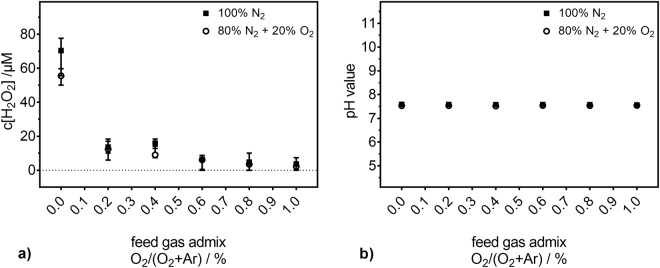
•OH recombines in a diffusion-controlled manner to H2O2. Moreover, O2•− can also disproportionate to H2O2. Therefore, the H2O2 concentration was determined for the two curtain gas compositions. In Fig. 3a, the concentration of H2O2 in the liquid is given as a function of the O2 content in the feed gas. A similar trend to those of the concentrations of •OH and O2•− radicals was observed (if one do not consider the first two points for an air-like atmosphere).
Figure 3.
(a) H2O2 concentration and (b) stability of the pH value after direct plasma treatment (9 mm, 180 s). The values are depicted as a function of the O2 content in the feed gas of Ar under N2 or air-like atmospheres.
Even if the experiments were performed in buffered saline solutions, the pH value was monitored. In Fig. 3b, it is shown that the pH value is stable for all studied conditions, including the untreated controls (not shown).
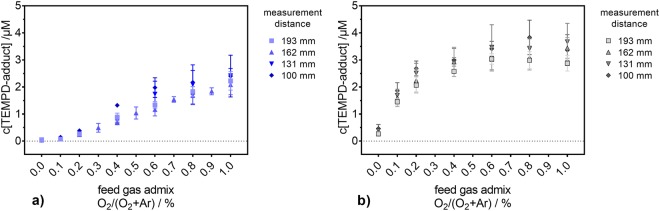
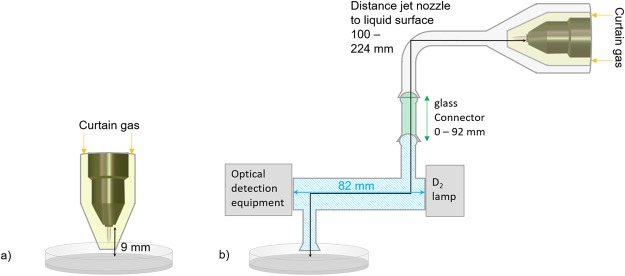
In order to study the formation pathways of the species present in the liquid, measurements in the gas phase are necessary. O2(a1Δg) is one of the possible candidates responsible for the formation of the obtained spin probe TEMPD-HCl adducts. Its density can be determined in the gas phase. Its detection by optical emission spectroscopy in the NIR may be disturbed by background radiation resulting from argon emission from the plasma. Therefore, a different experimental setup was used in order to reduce the argon emission reaching the detector (background noise). This was realized with a piece of bent glass connecting the plasma reactor and the measurement cell (see methods section for details), adversely limiting the shortest possible distance between the plasma jet’s nozzle and the measurement point. Therefore, the species density in the gas phase can only be determined at distances of at least 100 mm from the nozzle of the kinpen09. Glass connectors with different lengths were used, and the TEMPD-HCl adduct measurements in the liquid phase were, thus, repeated for the different distances between the plasma jet’s nozzle and the solutions. In Fig. 4, the obtained data is given in dependence of the O2 content in the feed gas for both curtain gas compositions. As the treatment of the solutions was done by the guided gas exhaust of the plasma jet that passes through the measurement cell, the resulting concentration of TEMPD-HCl adduct was found to be below the detection limit if the treatment time was kept at 180 s, as in the direct plasma treatment. Therefore, the duration of the indirect plasma treatment was increased to 600 s, in order to reach sufficient adduct concentrations. The spin probe adduct concentrations obtained after indirect plasma treatment under pure N2 or air-like atmosphere are given in Fig. 4a and 4b, respectively. Both conditions yield an increase of the concentration with increasing O2 content in the feed gas. Although different trends were found for the two surrounding atmospheres, for each curtain gas the same trend is observed for all treatment distances. In contrast to the concentrations determined after direct plasma treatments at a distance of 9 mm (see Fig. 1), here, the higher concentrations were observed for an air-like curtain gas. Furthermore, for pure N2 curtain gas, the concentrations of TEMPD-HCl adduct obtained after indirect plasma treatments are much lower than those gained after direct plasma treatments.
Figure 4.
Concentration of spin probe adducts after indirect plasma treatment (d > 9 mm, 600 s). In pure N2 (blue, (a)) as well as in air-like (gray, (b)) environments, the plasma induces ROS in the liquid for the various distances between the plasma jet’s nozzle and the liquid studied (100, 131, 162, 193 mm).
Reactive oxygen species in the gas phase
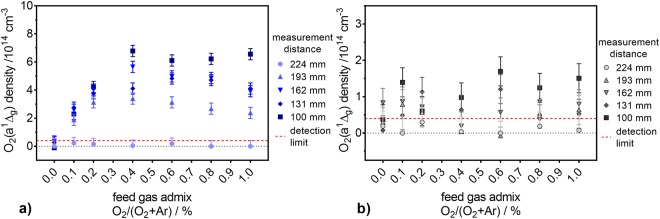
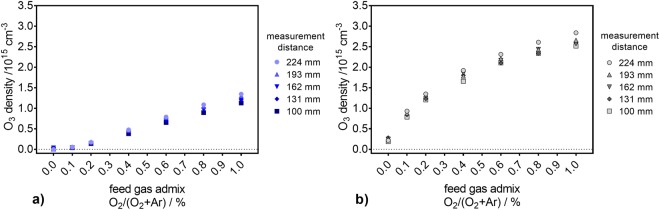
To gain further insight into the species generating the spin probe adducts, gas phase measurements were performed by calibrated infrared emission (O2(a1Δg)) and UV-absorption spectroscopy (O3). The dynamics of the O2(a1Δg) density in the gas phase was studied as a function of the O2 content in the feed gas for different distances between the plasma jet’s nozzle and the measurement cell (see Fig. 5a,b). The O2(a1Δg) density shows a clear dependence on the measurement distance; the shortest (100 mm) gap between the plasma jet’s nozzle and the point of measurement yielded the highest measured densities for both curtain gases. With increasing distance, the densities become lower, and, at the longest distance (224 mm), only small amounts of O2(a1Δg) are still detectable (often below the limit of detection). Noise on low concentration points (below the limit of detection) at longer measurement distances resulted in slightly negative measurement values; these negative data points were zeroed.
Figure 5.
The O2(a1Δg) density in the plasma jet’s exhaust for different measurement distances. The measurements were performed under pure N2 (a) and air-like environments (b) for several amounts of O2 in the feed gas. The O2(a1Δg) density detection limit of 4 1013 cm−3 is indicated by red dashed lines.
In the case of N2 as curtain gas (see Fig. 5a), the addition of O2 yields a strong increase of the O2(a1Δg) gas phase density (noticeable for all distances but 224 mm, where the detected densities were in the range or below the detection limit and, as so, no clear statement can be given). As expected, when working with pure Ar feed gas surrounded by pure N2 curtain gas, only traces of O2(a1Δg), in the range or below the detection limit, were detected regardless of the distance.
For an air-like curtain gas composition (see Fig. 5b), a clear, but less pronounced dependency of the O2(a1Δg) density on the distance between the plasma jet’s nozzle and the measurement cell was also observed. In great contrast to the pure N2 curtain condition, the O2 content in the feed gas when surrounded by an air-like atmosphere seems to have no effect on the O2(a1Δg) gas phase density. Furthermore, the obtained O2(a1Δg) densities are lower here (maximum of 1.7·1014 cm−3) compared to the N2 curtain gas case (maximum of 6.75·1014 cm−3).
In the gas phase measurements, O3 behaves differently than O2(a1Δg): no distance dependency was observed for either gas curtain composition (see Fig. 6a,b). Moreover, the determined O3 densities are an order of magnitude higher than those measured for O2(a1Δg). With increasing O2 content in the feed gas, the O3 density increases under both curtain gases, but a pure N2 curtain gas yields lower O3 densities (maximum of 1.25·1015 cm−3), independently of the O2 content in the feed gas. This is especially the case for the lower O2 admixtures, where the slope of the increase of the O3 density is lower when compared to the fast raise observed under the air-like curtain gas composition. Under this latter condition, the O3 densities reached values of up to 2.5·1015 cm−3. By comparing the maximal attained densities (obtained at 1% of O2 admixture in the feed gas), one can state that the N2 curtain gas yielded only half of the O3 density generated in an air-like surrounding.
Figure 6.
The O3 density in the plasma jet’s exhaust for different measurement distances. The measurements were performed under pure N2 (blue, (a)) and air-like (gray, (b)) environments, for several amounts of O2 in the feed gas.
Discussion
During direct treatment of the liquid (DPBS) with the plasma plume (d = 9 mm, 180 s), a spin probe adduct of TEMPD-HCl can origin from a reaction with either O3, •O or O2(a1Δg), or a combination thereof. This adduct was measured for two different gas surroundings of the plasma plume (see Fig. 1). One of the compositions of the environment was a mixture of 80% of N2 and 20% of O2; the resulting adducts concentration increase as a function of the O2 content in the feed gas, saturating for O2 admixtures higher than 0.4%. The 100% N2 gas curtain results in an even stronger increase of the adducts concentration, which starts to level out at high O2 admixture but without reaching any saturation. It is known from literature that a pure N2 surrounding prevents the quenching of O2(a1Δg), and, therefore, yields higher densities of O2(a1Δg) in the gas phase when O2 is added to the feed gas8. In contrast, the presence of O2 in the surrounding environment results in an increase of the O3 gas density and in a decrease of the O2(a1Δg) gas density8. Assuming a direct solvation of O2(a1Δg) from the gas phase into the liquid, this predicted behaviour of the O2(a1Δg) gas density could directly explain the trends observed in the liquid for the TEMPD-HCl adducts (see Fig. 1). However, other reactive species, such as •OH, O2•−, or H2O2, show a different response to the modified surrounding and the varied O2 content in the feed gas, and •OH, O2•−, and H2O2 can all contribute to the formation of at least O2(a1Δg), according to reactions 1 to 362–65.
| 1 |
| 2 |
| 3 |
In contrast to the TEMPD-HCl adducts (see Fig. 1), H2O2 deposition in the liquid does not strongly respond to changes in the composition of the curtain gas (see Fig. 3a). The H2O2 concentration was only slightly higher in a pure N2 surrounding when no O2 was admixed into the feed gas. If O2 was present in the feed gas, the curtain gas composition did not influence the H2O2 concentration. Furthermore, H2O2, •OH, and O2•− behave similarly: with increasing O2 content in the feed gas their resulting concentrations decrease in a pure N2 surrounding. In fact, their close (chemical) relation to each other is well known50. •OH and O2•− themselves do not yield to a paramagnetic product with TEMPD-HCl. This, together with the different behaviour, indicates that the •OH and O2•− radicals as well as the H2O2 are not dominantly involved in the formation of O3, •O, O2(a1Δg), and, therefore, they did not contribute to the measured TEMPD-HCl adduct. Additionally, as the pH value remained constant at 7.4 during all plasma treatments (see Fig. 3b), reactions containing the hydroperoxyl (HO•2) radical can also be neglected: given its pKa value of 4.850, only 0.25% of the O2•− radical is protonated. This means that O2•− concentrations in the µM range (concentration range measured for H2O2 – see Fig. 3a) result in a low nM HO2• concentration that can be diregarded.
Another possible mechanism of formation of O2(a1Δg) in aqueous solution is through OCl−11 (see equation 4). When the treated liquid contains chloride and the plasma source is producing •O in the gas phase, these two species can react to form OCl− 11,37. Since the investigated solution, DPBS, contains chloride, this reaction cannot be excluded. Nevertheless, due to reaction 4, either H2O2 would be completely consumed in the solution by OCl− or vice versa (depending on which species concentration is higher)11. The lifetime of •O, as a precursor for OCl−, is quite short9 in this plasma jet, so that only low OCl− concentrations are expected to be formed. Furthermore, since H2O2 is still detectable (see Fig. 3a), it is assumed that H2O2 completely consumed OCl−. Hence, the contribution of reaction 4 to the formation of O2(a1Δg) is expected to be negligible.
| 4 |
If O2(a1Δg) is excluded from being responsible for the spin probe adduct formation, O3 is another potential candidate. O3 is a major fraction of the plasma produced ROS in the gas phase, when conditions allow8,23,40,66. O3 is not formed in the liquid; accordingly, it can only originate in the liquid phase through a solvation process from the gas phase.
A reaction of O3 and H2O2 can occur, if both are simultaneously present in the liquid. The respective set of reactions is called peroxone process (see equations 5 to 7)67,68. Additional UV irradiation can enhance this process. Due to the bent glass connector present in the experimental setup used for indirect plasma treatments (see Fig. 8), the amount of UV radiation reaching the liquid, and available for this specific reaction is negligible. Therefore, the UV irradiation does not have a relevant impact compared to the other ongoing processes and reactions. Under acidic conditions, the peroxone process is relatively slow, but it becomes faster for neutral or alkaline pH. Hence, in DPBS with pH 7.4, this process may take place. As the •OH-adduct of BMPO and the H2O2 concentration exhibit the same behaviour for the investigated experimental conditions (see Fig. 2 and Fig. 3a), whose trend is opposite to that of the O3 gas density (see Fig. 6), it can be deduced that the peroxone process is not the dominant mechanism forming •OH.
| 5 |
| 6 |
| 7 |
Figure 8.
Experimental setup of the plasma treatment of liquid samples under controlled environment. Direct plasma treatment (a) was performed for a 9 mm distance between the plasma jet’s nozzle and the liquid surface. In (b), the modified setup for the investigation of O2(a1Δg) and O3 is shown. To avoid direct or scattered light from the plasma interfering with the measurement of O2(a1Δg) and O3, the kinpen09 with its curtain gas device (yellow-shaded area, gas inlet indicated by the yellow arrows) was enclosed in a glass capsule connected to the measurement cell through a bent glass tube. Glass connectors (green-shaded area) with different lengths (up to 92 mm, as indicated by the green double-headed arrow) were added in-between the measurement cell and the bent glass tube. The optical path length of the measurement cell (blue-shaded area) was 82 mm, as indicated by the blue double-headed arrow. The optical detection equipment used for measuring O2(a1Δg) and O3 (InGaAs detector + filter and UV/VIS spectrometer + filter + convex lens, respectively) was placed at one end of the measurement cell. For the indirect plasma treatment (b), the distance between the plasma jet’s nozzle and the liquid surface varied in the range 100–224 mm (as indicated by the black double-headed arrow).
Photolysis of O3 or H2O2 can take place in the case of a treatment at 9 mm of distance, where the plasma plume directly interacted with the liquid. During this direct plasma treatment, O3 can induce the formation of metastable atomic oxygen, O(1D), near the liquid surface (see equation 8). O(1D) can subsequently contribute to the formation of •OH (see equation 9). The photolysis of H2O2 (see equation 10) can also result in the enhancement of the •OH concentration in the liquid phase.
| 8 |
| 9 |
| 10 |
From the products of reactions 8, 9, and 10, only O(1D) could form the TEMPD adduct during direct plasma treatment. The peroxone process (see equations 5 to 7) as well as the photolysis of O3 and/or H2O2 (see equations 8 to 10) ends in the formation of •OH and/or HO2•/O2•−. As these species concentration decrease with increasing O2 content in the feed gas whereas the TEMPD adduct concentration increases, the reactions are assumed to have minor relevance in the system.
The comparison between the behaviour as a function of the measurement distance of the density of the plasma generated species in the gas phase and of the concentration of the reactive species in the liquid phase helps to identify the species responsible for the formation of the TEMPD-HCl adduct. The curtain gas composition which yielded the highest concentration of ROS in the liquid phase was different for the different plasma treatment distances. In fact, while for the 9 mm distance treatments (direct plasma treatments), the pure N2 curtain yielded the highest concentration of the spin probe adducts, for longer distances (≥100 mm, indirect plasma treatment), the highest concentrations of the spin probe adducts were always obtained in the air-like surrounding. It should be noted that the concentrations of the reactive species in the liquid phase determined after direct (9 mm) and indirect (≥100 mm) plasma treatments are not directly comparable, as different treatment times were used. Actually, in order to enhance the signal-to-noise ratio in the EPR measurements, the treatment time was extended from 180 s for the 9 mm treatment gap to 600 s for the distances longer than 100 mm.
The photolysis of O3 or H2O2 can also take place for treatment distances longer than 100 mm, as the necessary photon energy for O3 and H2O2 decomposition requires wavelengths of λ < 280 nm and λ < 300 nm, respectively 67. Radiation below 200 nm does not reach the liquid in the investigated distances, as it is almost immediately absorbed in air. The bent shape of the indirect treatment setup also reduces considerably the incident light. Therefore, the decomposition of H2O2 and O3 by light absorption is assumed to be negligible in the indirect treatments (but it can be relevant in the direct treatments).
For all indirect treatments, the trends of the gas phase density of O2(a1Δg) are different from those observed for the concentration of TEMPD-HCl in the liquid phase (compare Fig. 4 and Fig. 5). Moreover, the O2(a1Δg) gas phase density exhibits a strong dependency on the distance between the plasma jet’s nozzle and the point of measurement, while this behaviour was not observed for the TEMPD-HCl adduct formation in the liquid. Additionally, while the use of a pure N2 curtain gas yielded the highest O2(a1Δg) gas phase densities, the highest spin probe adduct concentrations were obtained with an air-like curtain. Finally, in the latter environment, while the TEMPD-HCl adduct concentration depends on the O2 content in the feed gas, the gas phase O2(a1Δg) density does not seem to be influenced by the O2 admixture into the carrier gas. Thus, it can be concluded that a solvation of gaseous O2(a1Δg) cannot be responsible for the measured TEMPD-HCl adducts. •O is another potential reactant with TEMPD-HCl. However, •O is only present in the plasma plume and vanishes directly after leaving it, according to a model of this plasma source9. Therefore, for long distance indirect treatments TEMPD-HCl, adducts cannot be linked to •O. Hence, the TEMPD-HCl adduct formation can be attributed to the solvation of gaseous O3. In fact, the O3 densities in the gas phase matched remarkably well the TEMPD-HCl adduct concentrations in the liquid phase, as both followed the same trends as a function of the O2 content in the feed gas for both gas environments, and showed higher amounts for the air-like curtain gas composition and no strict distance dependency (compare Fig. 4 and Fig. 6). For a better comparison, the net production rates of O3 in the gas phase and the TEMPD adduct in the liquid phase were calculated. For the gas phase, the net production rate was determined via equation (11):
| 11 |
where the gas phase O3 density (n(O3)) is given in particles per cm3, the factor 4.9·10−4 is the dimensionless Henry constant of O369, Φ1 and Φ2 are the feed gas (3 slm) and the curtain gas (5 slm) flow rates, respectively, and 16.67 is the conversion factor of standard litre per minute into the SI unit cm−3s−1. The net production rate of O3 in the liquid phase was calculated via equation (12):
| 12 |
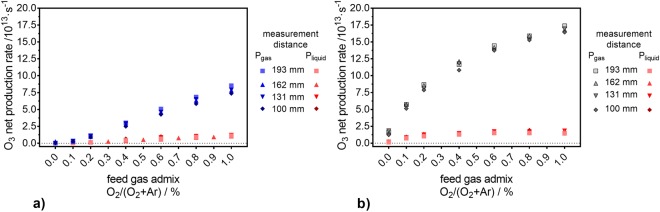
where c(O3) is the concentration of the TEMPD-HCl spin adduct in the liquid, VL the liquid volume (5 mL), NA the Avogadro constant and ttreatment the treatment time. Both net production rates are compared in Fig. 7a for the pure N2 curtain gas condition. The agreement between the gas and liquid net production rates is good, albeit a slightly increasing difference towards higher O2 amounts in the feed gas. This offset is due to the Henry constant of O3, which is relatively low – 1.2·10−7 M/Pa69. Hence, a (local) saturation of the O3 concentration in the liquid phase is reached relatively fast.
Figure 7.
Comparison of the O3 net production rate in the gas and liquid phases. The net production rates in both phases are determined as a function of the O2 content in the feed gas under a pure N2 (a) and an air-like (b) environment, for different measurement distances.
For an air-like surrounding, the net production rates were higher compared to the pure N2 gas curtain (compare Fig. 7a and Fig. 7b). This is especially the case for the O3 net production rate in the gas phase, where the values were more than twice as high (see Fig. 7b), and did not reach a plateau. However, the net production rate of the O3 deposited in the liquid showed a saturation plateau around 1.5·1017 s−1. Hence, for an air-like atmosphere, the trends of both net production rates curves did not agree completely; again, this is caused by the limited solvation of O3 in aqueous solutions.
It can be concluded that the similarity of the net production rates in both phases allows the identification of the oxygen species responsible for the formation of the TEMPD-HCl adducts in the liquid upon indirect plasma treatments. In fact, for treatment distanceslonger than 100 mm, the spin probe adduct is formed by reaction with O3. However, for direct plasma treatments, when the plasma plume interacts directly with the liquid, such statement cannot be given, since the detection of at least one of the potential reactants with TEMPD-HCl, namely O2(a1Δg), is not experimentally feasible at this distance.
| 13 |
| 14 |
| 15 |
| 16 |
| 17 |
| 18 |
| 19 |
| 20 |
| 21 |
The O3 decomposes via ions, hydroxide (OH−)70, chloride (Cl−)71, or O2•− 70 in aqueous or saline solutions71 (see reactions 13 to 21). For instance, •OH (see equations 17 and 19) or even OCl− can be formed by interaction of O3 with chlorite ions (see equations 20 and 21). However, the formation of OCl− due to reaction 21 is subordinated, as only 30% of the formed product yields OCl− 71. It should be noted, though, that, in other publications72,73, especially in Liu et al.74, this pathway is mentioned as the main formation pathway of OCl−. Hence, dissolved O3, as a gaseous precursor of other oxidizing molecules in a plasma treated liquid, is of great relevance for plasma liquid chemistry in the context of biomedical applications of plasmas, especially for jet-like plasmas where a strong stirring of the liquid occurs.
Conclusion
The present study has yielded relevant insight for the medical application of plasma treatment. By varying the treatment distance, the composition, and, therefore, the oxidizing potential of the cocktail of generated species can be modulated. It was shown that during a direct plasma treatment, the role of •O cannot be excluded, but in the case of longer treatment distances, or treatments with just the plasma gas exhaust, O3 is the main active component. O2(a1Δg) is also formed during plasma treatment. The O2(a1Δg) gas density is an order of magnitude lower than the O3 gas density. Moreover, according to their respective Henry constants, also the solubility of O2(a1Δg) is slightly lower. Thus, it was elucidated that, for the investigated experimental conditions, O3 is the dominating species, being originated in the gas phase and subsequently transported to the liquid phase by solvation. By correlating the behaviour of different ROS, several reaction pathways were excluded or their importance for the proceeding reactions was weighted.
Methods
Plasma source
In this study, an argon atmospheric pressure plasma jet was investigated. This plasma source was a commercial plasma jet called kinpen09 (neoplas GmbH, Greifswald, Germany). It consists of a ceramic capillary (1.6 mm in diameter) with a centred rod electrode inside, and a grounded ring electrode close to the end of the capillary. The rod electrode was driven at a frequency of around 1 MHz. The dissipated power in the plasma was of 1.1 W75. The working gas flow was set to 3 standard litres per minute (slm); the carrier gas was argon with up to 1% of molecular oxygen admixture (argon N50, oxygen N48, Air Liquide, Paris, France). A controlled environment during the plasma treatment was provided by the application of a curtain gas device (see yellow-shaded areas in Fig. 8) fed with molecular nitrogen or synthetic air as gases (gas inlet indicated by yellow arrows), at a total gas flow rate of 5 slm (oxygen N48, nitrogen N50, Air Liquide, Paris, France). A detailed study of the effect of the curtain gas on the plasma jet can be found in previous publications58,60.
The liquid treatment took place in Petri dishes of 60 mm in diameter filled with a liquid volume of 5 mL of Dulbecco’s phosphate buffered saline solution (DPBS). The direct treatment was performed for a distance of 9 mm between the liquid surface and the end of the capillary, which is also called the plasma jet’s nozzle (see Fig. 8a), for 180 s.
Gas phase diagnostics
For the gas phase diagnostics, a modified setup had to be used. This setup is schematically shown in Fig. 8b). To avoid scattered light or direct light from the plasma interfering with the measurements, the plasma jet cannot be connected directly to the measurement cell. As so, the plasma jet, including the curtain gas device, was enclosed in a glass capsule connected to the measurement cell through a bent glass tube. The measurement cell has an outlet for the exhaust, which was used to treat the liquid.
In order to study the role of O2(a1Δg) and O3, the distance between the plasma jet’s nozzle and the measurement cell was varied by adding, in-between the measurement cell (blue-shaded area in Fig. 8b) and the bent glass tube, glass connectors (green-shaded area in Fig. 8b) with different lengths (up to 92 mm, as indicated by the green double-headed arrow in Fig. 8). The investigated distances between the end of the capillary of the plasma jet and the point of measurement of O2(a1Δg) and O3 were 100 mm, 131 mm, 162 mm, 193 mm, and 224 mm (as indicated by the black double-headed arrow in Fig. 8).
The O2(a1Δg) density was measured via optical emission spectroscopy in the near infrared (NIR) region at 1270 nm, by using an InGaAs detector (Judson model J22D-M204) and a 19 nm band pass interference filter (Andover 200FC39-25/1270)54. The signal collected by the detector was amplified and monitored with an oscilloscope (Tektronix DPO 2024B). The detector was placed behind a calibrated detection cell (blue-shaded area in Fig. 8b) equipped with quartz windows. This cell has a path length of 82 mm (indicated by the blue double-headed arrow in Fig. 8b)76. The used system has a O2(a1Δg) density detection limit of 4 1013 cm−3.
The O3 density was measured by ultraviolet absorption spectroscopy at 254 nm. A deuterium lamp with a broadband spectrum between 180 and 400 nm was used as UV source (OceanOptics, DH-2000-BAL). The UV radiation was guided through the same measurement cell that was used for the O2(a1Δg) density measurements. For the O3 absorption measurements, this cell was used as an absorption cell (path length of 82 mm) and the intensity of the transmitted radiation was measured by an UV/VIS spectrometer (avantes) with a filter (254 nm) and a convex lens (f = 35) placed in front of it. For deduction of the O3 density, the ratio of the intensity (I) transmitted when the plasma was ignited and the intensity (I0) transmitted when the plasma was not ignited was calculated. The O3 density was calculated from the Beer’s law (equation 22) with the cross section σ = 1.14·10−17 cm² 77 and the path length L = 82 mm. The O3 concentration is assumed to be constant across the absorption path length.
| 22 |
Liquid phase analytic
For the liquid analysis, the pH values were measured electrochemically via a pH meter (SevenMulti™ S47, Mettler-Toledo International Inc., Columbus, OH, USA) equipped with a pH electrode (InLab® Micro, Mettler-Toledo International Inc., Columbus, OH, USA).
The hydrogen peroxide (H2O2) concentration was determined by commercially available test stripes (Merckoquant 110011, Merck) enhanced with a camera read out (digital microscope camera with 1.3 million pixels, zoom factor 10 to 200x, Conrad, Germany). The camera was in a dark box with defined constant light conditions in order to precisely analyse the colour of the sensor part of test stripes by determination of the red-, green- and blue-values17. Prior to each experiment, a calibration was conducted, where the related colour values of hydrogen peroxide concentrations in the range of 0–180 µM (0–6 mg/L) were determined.
The electron paramagnetic resonance (EPR) spectroscope used in this work is an X-band (equals to microwave frequency of 9.87 GHz) EPR (EMXmicro, Bruker BioSpin GmbH, Rheinstetten, Germany) with the resonator ER 4119HS (Bruker BioSpin GmbH, Rheinstetten, Germany). The applied instrument’s parameters of the EPR spectroscope were the same for all measurements: modulation frequency of 100 kHz, modulation amplitude of 0.1 mT, microwave power of 5.024 mW, receiver gain of 30 dB, time constant of 0.01 ms. The magnetic field scan was adjusted according to the sample measured; in this work, a range of 10 mT was used.
The measurement procedure followed always the same protocol, including performing measurements in triplicates for each sample. Prior to a measurement, the spin trap solution was prepared and 50 µL of the untreated solution were taken as control. All samples, treated and untreated, were pipetted into a borosilicate glass tube (125 mm length, 0.8525 mm inner diameter) for the measurement. From the plasma treatment to the measurement, a few minutes were needed due to handling reasons. This delay time was always fixed to four minutes. The spectrum of the untreated control was subtracted from the measured EPR signal. The evaluation of the EPR spectra was performed by assistance of the evaluation software (Xenon software with Xenon Spin Counting module, Bruker BioSpin, Rheinstetten, Germany). As the spectroscope was calibrated, absolute spin numbers could be gained.
In the presented work, 5-tert-Butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO, 2 mM) was used as a spin trap for •OH and O2•− radicals, and 2,2,6,6-tetramethyl-4- piperidone hydrochloride (TEMPD-HCl, 100 mM) was used as a spin probe for •O, O2(a1Δg), or O3. The used BMPO concentration was chosen based on previous studies19 using the exactly same plasma source, where higher concentrations yielded similar adduct concentrations in the µM range. Therefore, the chosen concentration of 2 mM is high enough to trap all detectable radicals. The TEMPD-HCl concentration used in the study was based on literature78; furthermore, the used concentrations were far higher than the detected adduct concentration, so that the spin trap was offered in excess.
Acknowledgements
Authors are grateful to Dr. Jörn Winter, Dr. Ansgar Schmidt-Bleker, Dana Sponholz, Melanie Schulz and Julia Koch for their help. This work was funded by the German Federal Ministry of Education and Research (BMBF) (Grant No. 03Z2DN12 & 03Z22DN12), as well as by the Ministry of Education, Science and Culture of the State of Mecklenburg-Vorpommern (Grant No: AU 15 001).
Author Contributions
H.J., J.S.S. and S.R. conceived and designed the experiments. H.J. performed all liquid analytic experiments as well as the O3 measurements in the gas phase. J.S.S. and S.R. performed and analysed the O2(a1Δg) measurements in the gas phase. K.W. provided assistance with the chemical interpretation. All authors discussed the results. H.J. conceived and wrote the main manuscript and prepared all figures. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kristian Wende and Stephan Reuter contributed equally to this work.
References
- 1.Hasse S, et al. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin Exp Dermatol. 2016;41:202–209. doi: 10.1111/ced.12735. [DOI] [PubMed] [Google Scholar]
- 2.Daeschlein G, et al. In Vitro Susceptibility of Multidrug Resistant Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD) Plasma Process Polym. 2014;11:175–183. doi: 10.1002/ppap.201300070. [DOI] [Google Scholar]
- 3.Isbary, G. et al. Reasons why we need cold atmospheric plasmas in bacteria related diseases in medicine. Plasma Medicine, 10.1615/PlasmaMed.2014006273 (2014).
- 4.Weltmann KD, von Woedtke T. Plasma medicine-current state of research and medical application. Plasma Physics and Controlled Fusion. 2017;59:014031. doi: 10.1088/0741-3335/59/1/014031. [DOI] [Google Scholar]
- 5.Emmert S, et al. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clinical Plasma Medicine. 2013;1:24–29. doi: 10.1016/j.cpme.2012.11.002. [DOI] [Google Scholar]
- 6.Lu X, et al. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Physics Reports. 2016;630:1–84. doi: 10.1016/j.physrep.2016.03.003. [DOI] [Google Scholar]
- 7.Schmidt A, von Woedtke T, Bekeschus S. Periodic Exposure of Keratinocytes to Cold Physical Plasma: An In Vitro Model for Redox-Related Diseases of the Skin. Oxid Med Cell Longev. 2016;2016:9816072. doi: 10.1155/2016/9816072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Bleker A, Bansemer R, Reuter S, Weltmann K-D. How to produce an NOx- instead of Ox-based chemistry with a cold atmospheric plasma jet. Plasma Process Polym. 2016;13:1120–1127. doi: 10.1002/ppap.201600062. [DOI] [Google Scholar]
- 9.Schmidt-Bleker A, Winter J, Bösel A, Reuter S, Weltmann K-D. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Science and Technology. 2015;25:015005. doi: 10.1088/0963-0252/25/1/015005. [DOI] [Google Scholar]
- 10.Jablonowski H, von Woedtke T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clinical Plasma Medicine. 2015;3:42–52. doi: 10.1016/j.cpme.2015.11.003. [DOI] [Google Scholar]
- 11.Wende K, et al. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases. 2015;10:029518. doi: 10.1116/1.4919710. [DOI] [PubMed] [Google Scholar]
- 12.Bruggeman PJ, et al. Plasma-liquid interactions: a review and roadmap. Plasma Sources Sci T. 2016;25:053002. doi: 10.1088/0963-0252/25/5/053002. [DOI] [Google Scholar]
- 13.Jablonowski, H. et al. Plasma Jet (V) UV-Radiation Impact on Biologically Relevant Liquids and Cell Suspension. Bulletin of the American Physical Society59 (2014).
- 14.Attri P, et al. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci Rep. 2015;5:9332. doi: 10.1038/srep09332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter J, et al. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J Phys D Appl Phys. 2014;47:285401. doi: 10.1088/0022-3727/47/28/285401. [DOI] [Google Scholar]
- 16.Lukes P, Dolezalova E, Sisrova I, Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Science and Technology. 2014;23:015019. doi: 10.1088/0963-0252/23/1/015019. [DOI] [Google Scholar]
- 17.Winter J, et al. Feed gas humidity: a vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J Phys D Appl Phys. 2013;46:295401. doi: 10.1088/0022-3727/46/29/295401. [DOI] [Google Scholar]
- 18.Gorbanev Y, O’Connell D, Chechik V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chemistry. 2016;22:3496–3505. doi: 10.1002/chem.201503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tresp H, Hammer MU, Winter J, Weltmann KD, Reuter S. Quantitative detection of plasma-generated radicals in liquids by electron paramagnetic resonance spectroscopy. J Phys D Appl Phys. 2013;46:435401. doi: 10.1088/0022-3727/46/43/435401. [DOI] [Google Scholar]
- 20.Jablonowski H, et al. Impact of plasma jet vacuum ultraviolet radiation on reactive oxygen species generation in bio-relevant liquids. Physics of Plasmas. 2015;22:122008. doi: 10.1063/1.4934989. [DOI] [Google Scholar]
- 21.Gorbanev Y, Stehling N, O’Connell D, Chechik V. Reactions of nitroxide radicals in aqueous solutions exposed to non-thermal plasma: limitations of spin trapping of the plasma induced species. Plasma Sources Sci T. 2016;25:055017. doi: 10.1088/0963-0252/25/5/055017. [DOI] [Google Scholar]
- 22.Girard F, et al. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. Rsc Advances. 2016;6:78457–78467. doi: 10.1039/C6RA12791F. [DOI] [Google Scholar]
- 23.Norberg SA, Tian W, Johnsen E, Kushner MJ. Atmospheric pressure plasma jets interacting with liquid covered tissue: touching and not-touching the liquid. J Phys D Appl Phys. 2014;47:475203. doi: 10.1088/0022-3727/47/47/475203. [DOI] [Google Scholar]
- 24.Tian W, Kushner MJ. Atmospheric pressure dielectric barrier discharges interacting with liquid covered tissue. J Phys D Appl Phys. 2014;47:165201. doi: 10.1088/0022-3727/47/16/165201. [DOI] [Google Scholar]
- 25.van Gils CAJ, Hofmann S, Boekema BKHL, Brandenburg R, Bruggeman PJ. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. Journal of Physics D: Applied Physics. 2013;46:175203. doi: 10.1088/0022-3727/46/17/175203. [DOI] [Google Scholar]
- 26.Chen C, et al. A Model of Plasma-Biofilm and Plasma-Tissue Interactions at Ambient Pressure. Plasma Chemistry and Plasma Processing. 2014;34:403–441. doi: 10.1007/s11090-014-9545-1. [DOI] [Google Scholar]
- 27.Alexander DL, David BG, Steven CS. Fully coupled simulation of the plasma liquid interface and interfacial coefficient effects. Journal of Physics D: Applied Physics. 2016;49:235204. doi: 10.1088/0022-3727/49/23/235204. [DOI] [Google Scholar]
- 28.Amanda ML, Mark JK. Air plasma treatment of liquid covered tissue: long timescale chemistry. Journal of Physics D: Applied Physics. 2016;49:425204. doi: 10.1088/0022-3727/49/42/425204. [DOI] [Google Scholar]
- 29.Bekeschus S, et al. Cold physical plasma selects for specific T helper cell subsets with distinct cells surface markers in a caspase-dependent and NF-κB-independent manner. Plasma Process Polym. 2016;13:1144–1150. doi: 10.1002/ppap.201600080. [DOI] [Google Scholar]
- 30.Schmidt A, et al. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process Polym. 2016;13:1179–1188. doi: 10.1002/ppap.201600090. [DOI] [Google Scholar]
- 31.von Woedtke, T. et al. In 20th Int. Symp. on Plasma Chemistry (ISPC-20) (2011).
- 32.von Woedtke T, Metelmann HR, Weltmann KD. Clinical Plasma Medicine: State and Perspectives ofin VivoApplication of Cold Atmospheric Plasma. Contributions to Plasma Physics. 2014;54:104–117. doi: 10.1002/ctpp.201310068. [DOI] [Google Scholar]
- 33.Chen ZT, Lin L, Cheng XQ, Gjika E, Keidar M. Effects of cold atmospheric plasma generated in deionized water in cell cancer therapy. Plasma Process Polym. 2016;13:1151–1156. doi: 10.1002/ppap.201600086. [DOI] [Google Scholar]
- 34.Collet G, et al. Plasma jet-induced tissue oxygenation: potentialities for new therapeutic strategies. Plasma Sources Sci T. 2014;23:012005. doi: 10.1088/0963-0252/23/1/012005. [DOI] [Google Scholar]
- 35.Nasruddin, et al. A Simple Technique to Improve Contractile Effect of Cold Plasma Jet on Acute Mouse Wound by Dropping Water. Plasma Process Polym. 2015;12:1128–1138. doi: 10.1002/ppap.201400236. [DOI] [Google Scholar]
- 36.Tanaka H, et al. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci Rep. 2016;6:36282. doi: 10.1038/srep36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekeschus S, et al. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci Rep. 2017;7:2791. doi: 10.1038/s41598-017-03131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasilets VN, Shekhter AB, Guller AE, Pekshev AV. Air plasma-generated nitric oxide in treatment of skin scars and articular musculoskeletal disorders: Preliminary review of observations. Clinical Plasma Medicine. 2015;3:32–39. doi: 10.1016/j.cpme.2015.05.001. [DOI] [Google Scholar]
- 39.Oehmigen K, et al. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process Polym. 2011;8:904–913. doi: 10.1002/ppap.201000099. [DOI] [Google Scholar]
- 40.Winter J, et al. Aspects of UV-absorption spectroscopy on ozone in effluents of plasma jets operated in air. J Phys D Appl Phys. 2012;45:385201. doi: 10.1088/0022-3727/45/38/385201. [DOI] [Google Scholar]
- 41.Sousa JS, et al. Cold atmospheric pressure plasma jets as sources of singlet delta oxygen for biomedical applications. J Appl Phys. 2011;109:123302. doi: 10.1063/1.3601347. [DOI] [Google Scholar]
- 42.Glaze WH, Kang JW, Chapin DH. The Chemistry of Water-Treatment Processes Involving Ozone, Hydrogen-Peroxide and Ultraviolet-Radiation. Ozone-Sci Eng. 1987;9:335–352. doi: 10.1080/01919518708552148. [DOI] [Google Scholar]
- 43.Hoigne J, Bader H. Ozonation of Water - Role of Hydroxyl Radicals as Oxidizing Intermediates. Science. 1975;190:782–784. doi: 10.1126/science.190.4216.782. [DOI] [Google Scholar]
- 44.Hoigné, J. The Chemistry of Ozone in Water. 121–141, 10.1007/978-1-4684-8556-1_11 (1988).
- 45.Pan G, Chen C-L, Chang H-M, Gratzl J. Studies on ozone bleaching. I. The effect of pH, temperature, buffer systems and heavy metal-ions on stability of ozone in aqueous solution. Journal of wood chemistry and technology. 1984;4:367–387. doi: 10.1080/02773818408070655. [DOI] [Google Scholar]
- 46.Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClurkin, J. & Maier, D. Half-life time of ozone as a function of air conditions and movement. Julius-Kühn-Archiv, 381, 10.5073/jka.2010.425.167.326 (2010).
- 48.Wilkinson F, Helman WP, Ross AB. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet-State of Molecular-Oxygen in Solution - an Expanded and Revised Compilation. Journal of Physical and Chemical Reference Data. 1995;24:663–1021. doi: 10.1063/1.555965. [DOI] [Google Scholar]
- 49.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine. (Oxford University Press, 2007).
- 51.Riethmüller M, Burger N, Bauer G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biology. 2015;6:157–168. doi: 10.1016/j.redox.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nayak G, Sousa JS, Bruggeman PJ. Singlet delta oxygen production in a 2D micro-discharge array in air: effect of gas residence time and discharge power. Journal of Physics D: Applied Physics. 2017;50:105205. doi: 10.1088/1361-6463/aa5764. [DOI] [Google Scholar]
- 53.Santos Sousa, J., Bauville, G., Lacour, B., Puech, V. & Touzeau, M. Atmospheric pressure generation of O-2(a(1)Delta(g)) by microplasmas. European Physical Journal-Applied Physics47, 10.1051/epjap/2009103 (2009).
- 54.Santos Sousa, J. et al. O(2)(a(1)Delta(g)) production at atmospheric pressure by microdischarge. Appl Phys Lett93, 10.1063/1.2957032 (2008).
- 55.Sousa JS, Bauville G, Puech V. Arrays of microplasmas for the controlled production of tunable high fluxes of reactive oxygen species at atmospheric pressure. Plasma Sources Sci T. 2013;22:035012. doi: 10.1088/0963-0252/22/3/035012. [DOI] [Google Scholar]
- 56.Reuter S, et al. Detection of ozone in a MHz argon plasma bullet jet. Plasma Sources Sci T. 2012;21:034015. doi: 10.1088/0963-0252/21/3/034015. [DOI] [Google Scholar]
- 57.Schmidt-Bleker A, et al. Propagation mechanisms of guided streamers in plasma jets: the influence of electronegativity of the surrounding gas. Plasma Sources Sci T. 2015;24:035022. doi: 10.1088/0963-0252/24/3/035022. [DOI] [Google Scholar]
- 58.Reuter S, et al. Controlling the Ambient Air Affected Reactive Species Composition in the Effluent of an Argon Plasma Jet. Ieee T Plasma Sci. 2012;40:2788–2794. doi: 10.1109/TPS.2012.2204280. [DOI] [Google Scholar]
- 59.Jablonowski H, et al. Plasma jet’s shielding gas impact on bacterial inactivation. Biointerphases. 2015;10:029506. doi: 10.1116/1.4916533. [DOI] [PubMed] [Google Scholar]
- 60.Reuter S, et al. From RONS to ROS: Tailoring Plasma Jet Treatment of SkinCells. Ieee T Plasma Sci. 2012;40:2986–2993. doi: 10.1109/TPS.2012.2207130. [DOI] [Google Scholar]
- 61.Tresp H, Hammer MU, Weltmann K-D, Reuter S. Effects of Atmosphere Composition and Liquid Type on Plasma-Generated Reactive Species in Biologically Relevant Solutions. Plasma Medicine. 2013;3:45–55. doi: 10.1615/PlasmaMed.2014009711. [DOI] [Google Scholar]
- 62.Bauer G. The Antitumor Effect of Singlet Oxygen. Anticancer Res. 2016;36:5649–5663. doi: 10.21873/anticanres.11148. [DOI] [PubMed] [Google Scholar]
- 63.Takamatsu T, et al. Microbial Inactivation in the Liquid Phase Induced by Multigas Plasma Jet. PloS one. 2015;10:e0132381. doi: 10.1371/journal.pone.0132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarr M, Valenzeno DP. Singlet oxygen: the relevance of extracellular production mechanisms to oxidative stress in vivo. Photochem Photobiol Sci. 2003;2:355–361. doi: 10.1039/b211778a. [DOI] [PubMed] [Google Scholar]
- 65.Liu DX, et al. Aqueous reactive species induced by a surface air discharge: Heterogeneous mass transfer and liquid chemistry pathways. Sci Rep. 2016;6:23737. doi: 10.1038/srep23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaens WV, et al. Numerical analysis of the effect of nitrogen and oxygen admixtures on the chemistry of an argon plasma jet operating at atmospheric pressure. New J Phys. 2015;17:033003. doi: 10.1088/1367-2630/17/3/033003. [DOI] [Google Scholar]
- 67.Parvulescu, V. I., Magureanu, M. & Lukes, P. Plasma Chemistry and Catalysis in Gases and Liquids. (Wiley, 2013).
- 68.Merenyi G, Lind J, Naumov S, Sonntag C. Reaction of ozone with hydrogen peroxide (peroxone process): a revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ Sci Technol. 2010;44:3505–3507. doi: 10.1021/es100277d. [DOI] [PubMed] [Google Scholar]
- 69.Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmospheric Chemistry and Physics. 2015;15:4399–4981. doi: 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- 70.Staehelin J, Hoigne J. Decomposition of Ozone in Water - Rate of Initiation by Hydroxide Ions and Hydrogen-Peroxide. Environmental Science & Technology. 1982;16:676–681. doi: 10.1021/es00104a009. [DOI] [Google Scholar]
- 71.Razumovskii SD, Konstantinova ML, Grinevich TV, Korovina GV, Zaitsev VY. Mechanism and kinetics of the reaction of ozone with sodium chloride in aqueous solutions. Kinetics and Catalysis. 2010;51:492–496. doi: 10.1134/S0023158410040051. [DOI] [Google Scholar]
- 72.Knipping E, Dabdub D. Modeling Cl~ 2 formation from aqueous NaCl particles: Evidence for interfacial reactions and importance of Cl~ 2 decomposition in alkaline solution. Journal of geophysical research-all series- 2002;107:ACH 8–ACH 8. [Google Scholar]
- 73.Rao B, Anderson TA, Redder A, Jackson WA. Perchlorate formation by ozone oxidation of aqueous chlorine/oxy-chlorine species: role of ClxOy radicals. Environ Sci Technol. 2010;44:2961–2967. doi: 10.1021/es903065f. [DOI] [PubMed] [Google Scholar]
- 74.Liu, Z. C. et al. Chemical Kinetics and Reactive Species in Normal Saline Activated by a Surface Air Discharge. Plasma Process Polym14, n/a-n/a, 10.1002/ppap.201600113 (2017).
- 75.Schmidt-Bleker A, Reuter S, Weltmann KD. Quantitative schlieren diagnostics for the determination of ambient species density, gas temperature and calorimetric power of cold atmospheric plasma jets. J Phys D Appl Phys. 2015;48:175202. doi: 10.1088/0022-3727/48/17/175202. [DOI] [Google Scholar]
- 76.Santos Sousa J, Puech V. Diagnostics of reactive oxygen species produced by microplasmas. J Phys D Appl Phys. 2013;46:464005. doi: 10.1088/0022-3727/46/46/464005. [DOI] [Google Scholar]
- 77.Orphal J. A critical review of the absorption cross-sections of O3 and NO2 in the ultraviolet and visible. Journal of Photochemistry and Photobiology A: Chemistry. 2003;157:185–209. doi: 10.1016/S1010-6030(03)00061-3. [DOI] [Google Scholar]
- 78.Hideg E, et al. Pure forms of the singlet oxygen sensors TEMP and TEMPD do not inhibit Photosystem II. Biochim Biophys Acta. 2011;1807:1658–1661. doi: 10.1016/j.bbabio.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.