Abstract
Multiple changes to the marine environment under climate change can have additive or interactive (antagonistic or synergistic) effects on marine organisms. Prompted by observations of anomalously warm sea temperatures and low chlorophyll concentrations during the 2013–2016 warm “Blob” event in the Northeast Pacific Ocean, we examined the combined effects of thermal stress and a shift in food resources on the development of a larval echinoid (Strongylocentrotus droebachiensis) in the laboratory. A high concentration of phytoplankton yielded faster echinus rudiment development at warm versus historical temperature, indicating a mitigating effect of abundant food on thermal stress; however, low phytoplankton concentration or a shift in diet to suspended kelp detritus, yielded slow development and high mortality at warm temperature. The results indicate a synergistic negative effect of thermal stress and altered food resources on larvae of a keystone marine species.
Introduction
Under climate change, extreme events such as heatwaves are predicted to increase in frequency and intensity1. One such event occurred in the Northeast Pacific from late 2013 through 2016, resulting in anomalously warm sea surface temperatures and low sea level pressure off the west coast of North America. A resultant, persistent mass of anomalously warm surface water was termed the “Blob” by ocean scientists2. The phenomenon led to changes in ocean biology and ecology including diet-shifts and mass-starvation of seabirds, and observations of tropical and subtropical species, such as ocean sunfish (Mola mola), north of their typical geographic range3. Additionally, in the winter and spring of 2014, researchers measured the lowest ocean chlorophyll concentrations observed since 1997, indicating diminished phytoplankton productivity4.
Combined stressors of warm sea temperature and low phytoplankton concentration may impact the planktonic larval stage of benthic marine invertebrates, such as sea urchins, that rely on phytoplankton as a source of food5. On both sides of the North Atlantic and in the Northeast Pacific, recruitment pulses of green sea urchins Strongylocentrotus droebachiensis into kelp beds have led to destructive grazing of kelps, and ecosystem phase shifts from kelp beds to sea urchin barrens6–10. This ecological phase shift is considered a “collapse” of the kelp ecosystem11,12. The impact of heatwaves on recruitment success of S. droebachiensis remains largely unresolved, but has important implications for our ability to predict destructive grazing events and subsequent changes to the structure and functioning of kelp ecosystems. Discordance among studies in terms of an upper thermal threshold for development and survival of larval S. droebachiensis indicates that populations in discrete regions with barriers to dispersal of planktonic larvae may differ in their tolerance to ocean warming13–15. In a study of larvae generated from S. droebachiensis collected at Sandy Cove, Nova Scotia, Canada, Hart and Scheibling13 found that growth rates of larvae increased with temperature from 3 to 9 °C, and were greatest at 14 °C. These results were consistent with observations of a strong sea urchin recruitment pulse in the late 1960s that led to destructive grazing of kelps following anomalously warm spring sea temperatures13. By contrast, Stephens14 studied S. droebachiensis larvae generated from individuals collected at Cape Cod Bay, Massachusetts, USA, and found that larval development declined above a thermal threshold of ~10 °C. Similar to Stephens, Fagerli et al.15 identified a correlation between recruitment failure of S. droebachiensis populations along the mid-coast of Norway and unusually warm sea temperatures (>10 °C). They argued that warming temperatures were limiting larval supply due to temperature-mediated larval mortality15.
The effects of multiple stressors on marine organisms can be examined in laboratory microcosm experiments through simultaneous manipulation of multiple factors, and may yield additive, antagonistic, or synergistic outcomes16. Under climate change, combinations of abiotic stressors resulting from multiple changes to the marine environment (e.g. temperature, salinity, and pH) often result in synergistic negative effects on marine invertebrate larvae17. Here, we test the effects of multiple stressors on larvae of S. droebachiensis by simulating conditions during the “Blob” marine heatwave. We reared larvae in the laboratory under conditions of historical (9 °C) and warm (17 °C) temperatures observed before and during the heatwave, respectively, in combination with high and low rations of phytoplankton food. Additionally, we included a third factor of food type, providing larvae with either phytoplankton or a suspended kelp-derived detritus diet (high and low ration), to examine whether locally abundant, kelp-derived particulate organic matter18,19 provides a suitable diet for larvae at warm temperature in the absence of abundant phytoplankton. We hypothesized that thermal stress and limited availability of food would combine synergistically to reduce larval development and survival. Based on previous observations that kelp detritus rivals a phytoplankton diet in terms of food quality for larval S. droebachiensis at 9 °C20, we predicted that abundant kelp detritus would be a suitable substitute for phytoplankton food under thermally stressed conditions.
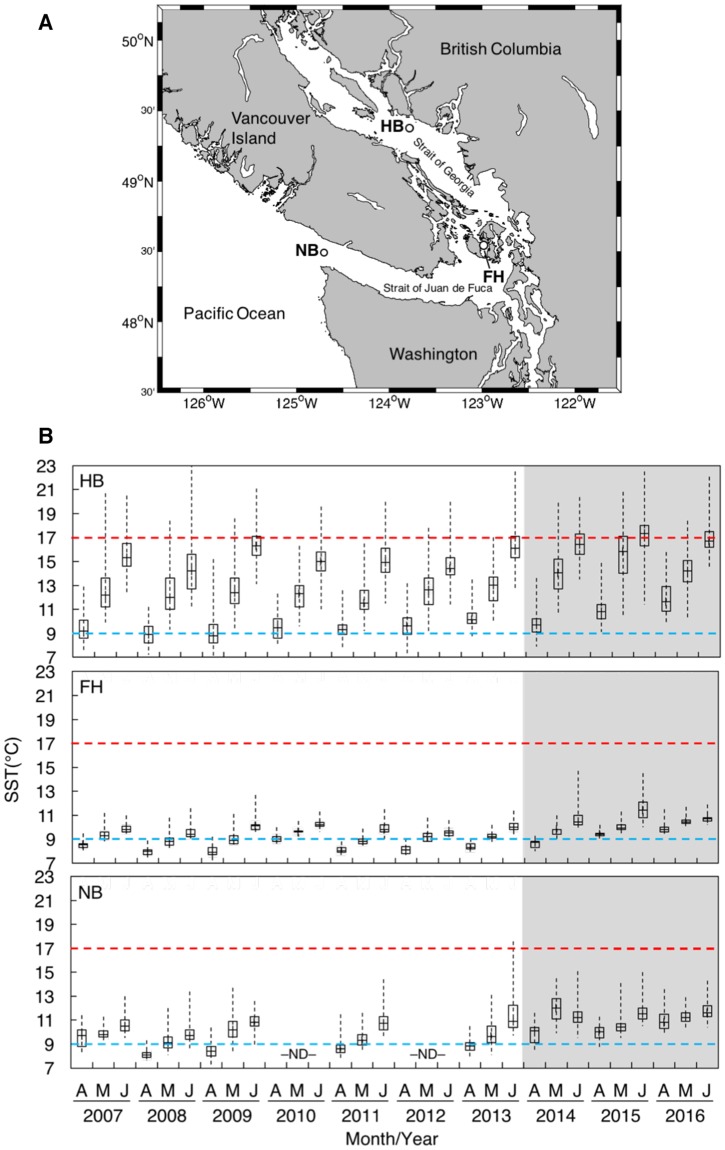
Larvae were generated for the experiment from sea urchins collected in the Salish Sea, Washington, USA, where temperature anomalies of +4 °C were observed during the period of the warm Blob21. To more closely examine the effects of the Blob on sea surface temperatures (SST) in the study region, SST data were acquired from the National Oceanic and Atmospheric Administration (NOAA) National Buoy Data Center (www.ndbc.noaa.gov/) and the Department of Fisheries and Oceans Canada (DFO) Canadian Moored Buoy Historical Data (www.meds-sdmm.dfo-mpo.gc.ca/) for 3 buoy locations spanning the distribution of S. droebachiensis in the Salish Sea (Fig. 1A)22. Given that we were interested in prolonged periods of warm temperature that could affect larval development, we generated a time-series of boxplots to examine the minimum, median, and maximum temperatures in April through June of each year, when S. droebachiensis larvae are expected to be most abundant in the water column23 (Fig. 1B). We found that mean SST in April through June was significantly greater in the presence than in the absence of the Blob at all stations (one-tailed t-tests: FHL, t2 = 24.58, p = 0.002; HB, t2 = 36.34, p < 0.001; and NB, t2 = 20.85, p = 0.002; n = 3–7 years).
Figure 1.
(A) Map of the Salish Sea on the Pacific coast of North America, showing the location of 3 oceanographic buoys (circles) where sea surface temperatures were measured (HB, Halibut Bank; FH, Friday Harbor; NB, Neah Bay). (B) Boxplots of sea surface temperatures (SST, °C) at the 3 buoys over 10 years (2007–2016) in the months of April–June, when larval S. droebachiensis are expected to be most abundant in the water column23. The dashed horizontal blue and red lines indicate the historical (9 °C) and warm (17 °C) temperature treatments applied to larval cultures. The grey band indicates the period of the “Blob” marine heatwave. ND = no data available.
To generate larvae for the experiment, on 26 April 2016 5 adult S. droebachiensis (>30 mm test diameter) were injected with ~1–5 mL of 0.55 M KCl through the peristomial membrane24. Spawning occurred in 1 male and 2 females. A single drop of sperm diluted in 0.37 μm-filtered seawater (FSW) was used to fertilize eggs from individual females (~1 h). Resultant embryos from each parental lineage were intermixed and then transferred into 16 replicate glass culture jars with 2000 mL of FSW, at a concentration of 1 embryo mL−1. Larval cultures were placed in temperature-controlled chambers at either a historical (9 °C) or warm (17 °C) temperature treatment (8 cultures per temperature) and were continuously stirred at 6 RPM with motor-operated plastic paddles mounted to a plastic frame25. The historical treatment approximates the median sea surface temperature observed at the Friday Harbor buoy in April through June of 2007 through 2013 (Fig. 1B). The warm treatment approximates the anomalously warm median sea temperature observed at the Halibut Bank buoy in June of 2015 and 2016 (Fig. 1B). Larval cultures in each temperature treatment were fed 1 of 2 food types (phytoplankton or kelp detritus) at 1 of 2 rations (high or low), yielding 4 possible diets: phytoplankton × high ration, kelp detritus × high ration, phytoplankton × low ration, and kelp detritus × low ration. This resulted in a 3-factor fully-crossed experimental design of combined food type (2 levels), food ration (2 levels), and temperature (2 levels), with n = 2 jars per treatment combination. The phytoplankton food treatment consisted of an ~1:1 mixture (by cell volume) of Dunaliella tertiolecta and Isochrysis galbana. The phytoplankton × high ration diet consisted of 5000 cells mL−1 volume equivalent of D. tertiolecta, while the phytoplankton × low ration diet consisted of 500 cells mL−1 volume equivalent of D. tertiolecta (estimated cell volume ratio of D. tertiolecta:I. galbana of 1:8). Based on an estimated chlorophyll to biomass conversion for D. tertiolecta of 1 pg chlorophyll cell−1 26, these cell concentrations represent chlorophyll concentrations of ~0.5 and 5 mg m3, approximating the values observed in the presence and absence of a typical phytoplankton bloom in the Salish Sea27. The kelp detritus food treatment consisted of particles of 1–2-week old bull kelp Nereocystis luetkeana suspended in FSW. To produce the detritus, fresh N. luetkeana blades were wiped clean and blended in an industrial blender, filtered through a 70 µm mesh to remove large particles, and aged in the dark at ambient sea temperature20. The kelp detritus × high ration diet consisted of 5000 kelp-derived particles mL−1, while the low ration diet consisted of 500 particles mL−1. Kelp-derived detritus can contribute up to 33% of particulate organic matter in the 20 to 63 µm size-fraction in this region19. A previous study has shown that suspended kelp detritus at a concentration of 5000 particles mL−1 rivals an optimal phytoplankton diet of D. tertiolecta and I. galbana in terms of food quality for larval S. droebachiensis under optimal temperature conditions20. Larvae were fed every 2–3 d beginning at age 5 d post-fertilization (early pluteus).
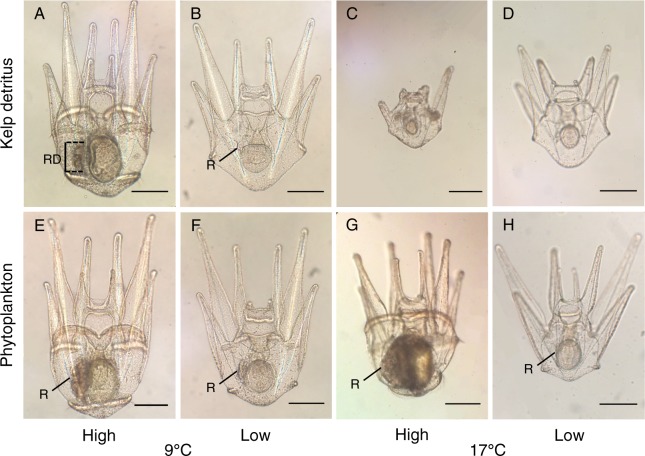
Given that the length of the larval period often determines larval mortality rates due to exposure to predators or risk of offshore advection28, patterns of echinus rudiment formation in preparation for metamorphosis may be the most informative metric in predicting recruitment success of sea urchins. Rudiment diameters of larvae were measured with the ocular micrometer of a compound microscope (5 µm resolution) at ages 5, 9, 13, 16, 19 and 22 d (with d = 0 at fertilization) (Fig. 2). Two larvae were sampled without replacement from each culture jar on each sampling day. Rudiment diameter was measured at first contact of the ectodermal invagination with the hydrocoel, and acts as an indicator of juvenile sea urchin development within a larva and preparation for metamorphosis29.
Figure 2.
Photomicrographs of larval Strongylocentrotus droebachiensis at age 22 d in treatments consisting of combinations of 3 factors: food type (2 levels: kelp detritus, and phytoplankton), food ration (2 levels: high, and low), and temperature (2 levels: 9 °C, and 17 °C). In panel A: RD = echinus rudiment diameter. Rudiments (R) also are indicated in panels B and E–H. Scale bars are 200 µm.
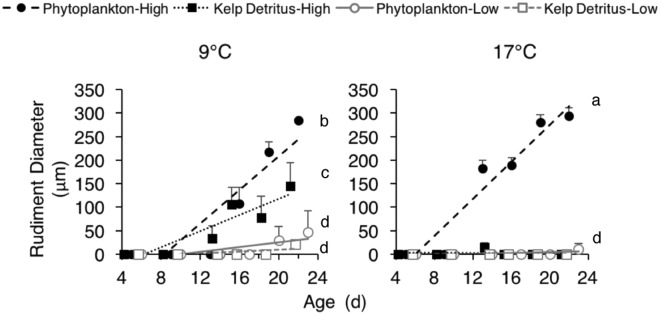
We detected no difference in the rate of rudiment development from age 5 to 22 d for larvae fed a low concentration of phytoplankton or a low concentration of kelp detritus at warm versus historical temperature, with limited to no rudiment formation in these treatments (Fig. 3). This indicates slow development to metamorphosis at low food concentration regardless of food type or temperature. However, at high food concentration, we observed interactive effects of food and temperature on rudiment development. Specifically, we observed faster rudiment development at warm versus historical temperature when larvae were fed a high ration of phytoplankton, and we observed the reverse pattern when larvae were fed a high ration of kelp detritus: larvae developed slower at warm versus historical temperature on a high ration kelp detritus diet (Fig. 3). ANCOVA on rudiment diameters of larvae from age 5 to 22 d showed a significant 4-way interaction between food type, food ration, temperature, and age (covariate), indicating heterogeneity of slopes and an interactive effect of treatments on rudiment development rates (Supplementary Table S1). Paired comparisons of treatments with Tukey’s test (α = 0.05) indicated that the slope was significantly greater for the high ration phytoplankton treatment at 17 °C versus 9 °C (Fig. 3, lowercase letters indicate statistical groupings). Both of these slopes were significantly greater than the slopes of all other treatments. At 17 °C, there was no difference in slopes between the high ration kelp detritus, low ration kelp detritus, and low ration phytoplankton treatments. At 9 °C, the slope of the high ration kelp detritus treatment was significantly greater than the slopes of the low ration kelp detritus and low ration phytoplankton treatments, which did not differ from one another. Additionally, the slope of the high ration kelp detritus treatment was significantly greater at 9 than 17 °C. The slope of the high ration kelp detritus treatment at 9 °C also was significantly greater than the slope of the low ration phytoplankton and low ration kelp detritus treatments at 17 °C. Slopes did not differ among low ration food treatments at 9 versus 17 °C (Fig. 3). These results indicate that a high concentration of phytoplankton mitigated a negative effect of warm temperature on larval development (antagonistic effect), while a high concentration of kelp detritus exacerbated the negative effect of warm temperature on larval development (synergistic effect). The former result suggests that in the presence of abundant phytoplankton, larvae have sufficient energy to compensate for increased metabolic requirements at warm temperature. This interpretation is consistent with a broadly accepted positive relationship between temperature and metabolic energy requirements of marine invertebrates30. In an analogous finding, Yeakel et al.31 observed that tropical corals can achieve high rates of calcification at suboptimal (low) pH when they have access to a nutritionally replete diet. Given that conditions of warm temperature and high phytoplankton concentration did not to our knowledge co-occur in the field during the 2013–2016 marine heatwave (to the contrary, phytoplankton were likely rare; 4), our results should be interpreted with caution. It is likely that a combination of anomalously warm sea temperature and low phytoplankton concentration during the heatwave led to slow development of sea urchin larvae, delaying metamorphosis.
Figure 3.
Rudiment diameters (µm) of larvae at ages 5 to 22 d (d = 0 at fertilization). Treatments are as in Fig. 2. Lines indicate linear relationships. Lowercase letters indicate statistical groupings based on paired comparisons of a significant 4-way interaction in ANCOVA (Tukey’s test, α = 0.05) (Supplementary Table S1). Error bars are +1 SE for n = 4 larvae per treatment, with some errors within the diameter of the symbols. Overlapping data points are shifted by ±1 d for visual clarity.
In addition to slow development of larvae in the warm, high ration kelp detritus treatment, there was complete mortality of larvae in this treatment by day 26 (Supplementary Table S2). Following the observation of complete mortality in the warm, high ration kelp detritus treatment, we measured dissolved oxygen in all larval cultures on day 26 (YSI Model PRO 2030) and found lower dissolved oxygen in this treatment (DO = 96 ± 1%, mean ± SD, n = 2) as compared to all other treatments (DO = 101 ± 2%, n = 14). While this does not provide evidence that the warm, high ration kelp detritus cultures were hypoxic, it does suggest higher rates of respiration in this treatment due to microbes. We posit that opportunistic deleterious microbes associated with the kelp detritus bloomed in this treatment. The presence of deleterious microbes could have contributed to slow growth of larvae due to the energetic cost of mounting an immune response. This hypothesis assumes that deleterious microbes were most abundant in the warm, high ration kelp detritus treatment due to a higher dosage of microbes (i.e. greater volume of kelp detritus added as compared to the low ration diet) and faster growth of microbes at warm versus historical temperature. It is also possible that microbial films on the insides of the jars differed among the kelp detritus and phytoplankton treatments. However, these inferences remain equivocal, as we did not investigate the microbial composition of the kelp detritus before or after addition to the larval cultures. In natural ocean environments, regions of high nutrient concentration have a high concentration of associated deleterious microbes that pose a risk to developing marine invertebrate larvae32. In accordance, in laboratory cultures, the microbiome of larval S. droebachiensis under food-rich conditions shifts towards a state of dysbiosis, leading to mortality32. An alternate hypothesis for our observations is that large microbes inedible to larvae, such as ciliates, bloomed in the warm, high ration kelp detritus cultures, reducing larval feeding efficiency and causing starvation. Decreased feeding efficiency can result from low clearance rates of larvae due to time spent rejecting large, inedible particles33. Previous studies have shown that the presence of large inedible particles reduces larval feeding efficiency in a dosage-dependent manner33,34.
At age 22 d, larval arms were counted for classification of larval stage (4-arm, 6-arm, and 8-arm pluteus) in each treatment. Larval developmental stages observed at age 22 d yielded patterns similar to the results for rudiment development. The high ration kelp detritus and phytoplankton diets resulted in comparable development at historical temperature, with all larvae reaching the 8-arm stage (Fig. 4). However, at warm temperature, larvae in the high ration phytoplankton treatment all reached the 8-arm stage, while larvae in the high ration kelp detritus treatment were less developed, at the 4-arm or 6-arm stage (Fig. 4). This result is consistent with a synergistic negative effect on larval development of combined warm temperature and a high ration kelp detritus diet.
Figure 4.
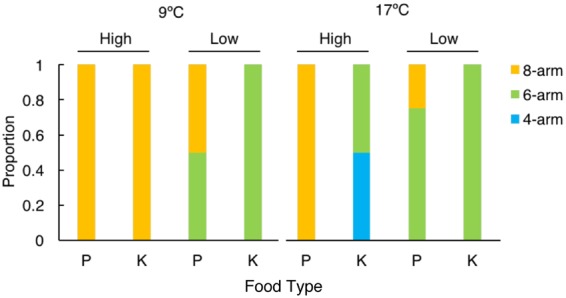
Proportion of larvae at the 4-, 6-, and 8-arm stage at age 22 d. Treatments are as in Fig. 2. P = Phytoplankton, and K = Kelp detritus. Proportions are based on 4 larvae per treatment.
Suspended kelp-derived detritus has been documented in high abundance along coasts in the Northeast Pacific18,19 and may be an alternate food source (to phytoplankton) for larval sea urchins20. However, little is known about the microbial communities that colonize and metabolize this material during the kelp degradation process20. We found that a high concentration of kelp detritus did not mitigate thermal stress in sea urchin larvae in the laboratory, but rather, synergistically reduced larval development and survival when combined with warm temperature. Additional research is needed to determine whether (and how) microbial communities associated with kelp detritus contribute to slow growth and high mortality of larvae, and whether our laboratory observations are representative of interactions between larvae and their food sources in the field.
Electronic supplementary material
Acknowledgements
The paper is dedicated to the memory of Professor Diane K. Adams, a bright, committed, and inspirational scientist, whose untimely passing occurred during the preparation of the manuscript. The authors thank Billie Swalla and Friday Harbor Laboratories for logistical support and facilities access; Katie Dobkowski for collecting sea urchins; Megan Dethier for providing laboratory temperature data; and Michael Brown for providing the map in Fig. 1. CJF was supported by a postdoctoral fellowship from Friday Harbor Laboratories. SY received funding from the Mary Gates Endowment.
Author Contributions
C.J.F., Z.L. and S.Y. designed the laboratory experiment; Z.L. and S.Y. conducted the laboratory experiment; C.J.F. ran statistical analyses and prepared figures; C.J.F. and D.K.A. interpreted the data and wrote the paper. All authors contributed to revising the paper, and C.J.F., Z.L. and S.Y. approved the final version.
Data availability
Figure 1 has associated raw data publicly available from www.ndbc.noaa.gov and www.meds-sdmm.dfo-mpo.gc.ca. All other data analyzed are included in this article (and its supplementary information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30572-w.
References
- 1.Perkins SE, Alexander LV, Nairn J. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys. Res. Lett. 2012;39:L20714. doi: 10.1029/2012GL053361. [DOI] [Google Scholar]
- 2.Bond NA, Cronin MF, Freeland H, Mantua N. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 2015;42:3414–3420. doi: 10.1002/2015GL063306. [DOI] [Google Scholar]
- 3.Cavole LM, et al. Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific: Winners, losers, and the future. Oceanography. 2016;29:273–285. doi: 10.5670/oceanog.2016.32. [DOI] [Google Scholar]
- 4.Whitney FA. Anomalous winter winds decrease 2014 transition zone productivity in the NE Pacific. Geophys. Res. Lett. 2015;42:428–431. doi: 10.1002/2014GL062634. [DOI] [Google Scholar]
- 5.Rumrill SS. Natural mortality of marine invertebrate larvae. Ophelia. 1990;32:163–198. doi: 10.1080/00785236.1990.10422030. [DOI] [Google Scholar]
- 6.Breen PA, Mann KH. Destructive grazing of kelp by sea urchins in eastern Canada. J. Fish. Res. Board. Can. 1976;33:1278–1283. doi: 10.1139/f76-164. [DOI] [Google Scholar]
- 7.Foreman RE. Benthic community modification and recovery following intensive grazing by Strongylocentrotus droebachiensis. Helgolander Wiss. Meeresunters. 1977;30:468–484. doi: 10.1007/BF02207855. [DOI] [Google Scholar]
- 8.Hagen NT. Destructive grazing of kelp beds by sea urchins in Vestfjorden, northern Norway. Sarsia. 1983;68:177–190. doi: 10.1080/00364827.1983.10420570. [DOI] [Google Scholar]
- 9.Scheibling RE, Hennigar AW, Balch T. Destructive grazing, epiphytism, and disease: the dynamics of sea urchin-kelp interactions in Nova Scotia. Can. J. Fish. Aquat. Sci. 1999;56:2300–2314. doi: 10.1139/f99-163. [DOI] [Google Scholar]
- 10.Schultz JA, Cloutier RN, Côté IM. Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ. 2016;4:e1980. doi: 10.7717/peerj.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filbee-Dexter K, Scheibling RE. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 2014;495:1–25. doi: 10.3354/meps10573. [DOI] [Google Scholar]
- 12.Ling SD, et al. Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B. 2015;370(1659):20130269. doi: 10.1098/rstb.2013.0269. [DOI] [Google Scholar]
- 13.Hart MW, Scheibling RE. Heat waves, baby booms, and the destruction of kelp beds by sea urchins. Mar. Biol. 1988;99:167–176. doi: 10.1007/BF00391978. [DOI] [Google Scholar]
- 14.Stephens RE. Studies on the development of the sea urchin Strongylocentrotus droebachiensis. I. Ecology and normal development. Biol. Bull. 1972;142:132–144. doi: 10.2307/1540251. [DOI] [PubMed] [Google Scholar]
- 15.Fagerli CW, Norderhaug KM, Christie HC. Lack of sea urchin settlement may explain kelp forest recovery in overgrazed areas in Norway. Mar. Ecol. Prog. Ser. 2013;488:119–132. doi: 10.3354/meps10413. [DOI] [Google Scholar]
- 16.Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008;11:1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 17.Przeslawski R, Byrne M, Mellin C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Global Change Biol. 2015;21:2122–2140. doi: 10.1111/gcb.12833. [DOI] [PubMed] [Google Scholar]
- 18.Lowe AT, Galloway AWE, Sean Yeung J, Dethier MN, Duggins DO. Broad sampling and diverse biomarkers allow characterization of nearshore particulate organic matter. Oikos. 2014;123:1341–1354. doi: 10.1111/oik.01392. [DOI] [Google Scholar]
- 19.Ramshaw BC, Pakhomov EA, Markel RW, Kaehler S. Quantifying spatial and temporal variations in phytoplankton and kelp isotopic signatures to estimate the distribution of kelp-derived detritus off the west coast of Vancouver Island, Canada. Limnol. Oceanogr. 2017;62:2133–2153. doi: 10.1002/lno.10555. [DOI] [Google Scholar]
- 20.Feehan CJ, Grauman-Boss BC, Strathmann RR, Dethier MN, Duggins DO. Kelp detritus provides high-quality food for sea urchin larvae. Limnol. Oceanogr. 2017;63:S299–S306. doi: 10.1002/lno.10740. [DOI] [Google Scholar]
- 21.Eisenlord ME, et al. Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Phil. Trans. R. Soc. B. 2016;371:20150212. doi: 10.1098/rstb.2015.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry RI, Zhanga Z, Harbob R. Development of the green sea urchin (Strongylocentrotus droebachiensis) fishery in British Columbia, Canada — back from the brink using a precautionary framework. Fish. Res. 2002;55:253–266. doi: 10.1016/S0165-7836(01)00283-1. [DOI] [Google Scholar]
- 23.Strathmann RR. On barriers to hybridization between Strongylocentrotus droebachiensis (O.F. Muller) and S. pallidus (G.O. Sars) J. Exp. Mar. Biol. Ecol. 1981;55:39–47. doi: 10.1016/0022-0981(81)90091-5. [DOI] [Google Scholar]
- 24.Strathmann, M. F. Reproduction and development of marine invertebrates of the northern Pacific coast. Seattle, WA, University of Washington Press (1987).
- 25.Strathmann, R. R. Culturing larvae of marine invertebrates in Developmental biology of the sea urchin and other marine invertebrates: Methods and protocols (ed. Carroll, D.J. & Stricker, S.A. 1–26 (Humana Press, New York, 2014). [DOI] [PubMed]
- 26.LaRoche J, Mortain-Bertrand A, Falkowski PG. Light intensity-induced changes in cab mRNA and light harvesting complex II apoprotein levels in the unicellular chlorophyte Dunaliella tertiolecta. Plant Physiol. 1991;97:147–153. doi: 10.1104/pp.97.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson D, Peña A. Chlorophyll distribution in a temperate estuary: The Strait of Georgia and Juan de Fuca Strait. Estuar. Coast. Shelf. Sci. 2009;82:19–28. doi: 10.1016/j.ecss.2008.12.022. [DOI] [Google Scholar]
- 28.Vaughn D, Allen JD. The peril of the plankton. Integr. Comp. Biol. 2010;50:552–570. doi: 10.1093/icb/icq037. [DOI] [PubMed] [Google Scholar]
- 29.Chia, F. S. & Rice, M. E. Settlement and metamorphosis of marine invertebrate larvae. Proceedings of the American Zoological Society Meeting, Toronto, Canada, Dec 27–28, 1977 (New York, Elsevier, 1978).
- 30.Newell RC, Branch GM. The influence of temperature on the maintenance of metabolic energy balance in marine invertebrates. Adv. Mar. Biol. 1980;17:329–396. doi: 10.1016/S0065-2881(08)60304-1. [DOI] [Google Scholar]
- 31.Yeakel KL, et al. Shifts in coral reef biogeochemistry and resulting acidification linked to offshore productivity. Proc. Natl. Acad. Sci. USA. 2015;112:14512–14517. doi: 10.1073/pnas.1507021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrier TJ, Macrander J, Reitzel AM. A microbial perspective on the life‐history evolution of marine invertebrate larvae: If, where and when to feed. Mar. Ecol. 2018;39:e12490. doi: 10.1111/maec.12490. [DOI] [Google Scholar]
- 33.Strathmann RR. The feeding behavior of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of suspension-feeding. J. Exp. Mar. Biol. Ecol. 1971;6:109–160. doi: 10.1016/0022-0981(71)90054-2. [DOI] [Google Scholar]
- 34.Hansen B, Hansen PJ, Nielsen TG. Effects of large nongrazable particles on clearance and swimming behaviour of zooplankton. J. Exp. Mar. Biol. Ecol. 1991;152:257–269. doi: 10.1016/0022-0981(91)90218-L. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figure 1 has associated raw data publicly available from www.ndbc.noaa.gov and www.meds-sdmm.dfo-mpo.gc.ca. All other data analyzed are included in this article (and its supplementary information files).